Abstract
Oxytocin is thought to play a central role in promoting close social bonds via influence on social interactions. The current investigation targeted interactions involving expressed gratitude between members of romantic relationships because recent evidence suggests gratitude and its expression provides behavioral and psychological ‘glue’ to bind individuals closer together. Specifically, we took a genetic approach to test the hypothesis that social interactions involving expressed gratitude would be associated with variation in a gene, CD38, which has been shown to affect oxytocin secretion. A polymorphism (rs6449182) that affects CD38 expression was significantly associated with global relationship satisfaction, perceived partner responsiveness and positive emotions (particularly love) after lab-based interactions, observed behavioral expression of gratitude toward a romantic partner in the lab, and frequency of expressed gratitude in daily life. A separate polymorphism in CD38 (rs3796863) previously associated with plasma oxytocin levels and social engagement was also associated with perceived responsiveness in the benefactor after an expression of gratitude. The combined influence of the two polymorphisms was associated with a broad range of gratitude-related behaviors and feelings. The consistent pattern of findings suggests that the oxytocin system is associated with solidifying the glue that binds adults into meaningful and important relationships.
Keywords: close relationships, gratitude, emotion expression, CD38, oxytocin
INTRODUCTION
For centuries, scholars have pondered the nature of the ties that bind people together. In the 1950s, psychologists Harlow (1958) and Bowlby (1958) emphasized the necessity of maternal emotional warmth and comfort for healthy child development. More recently, theorists have posited that the biological, behavioral and psychological systems that co-evolved in support of parent–child attachments have come to support close adult relationships as well (e.g. Hazan and Diamond, 2000; Diamond, 2004). One approach to illuminating the mechanisms through which people form and maintain these important relationships has been to shine the empirical spotlight on common social interactions that occur within them. Recent evidence strongly implicates the emotion and expression of gratitude in the adult bonding process (Algoe, 2012; Algoe et al., 2013). In this study, we test whether oxytocin may support the behavior and acute psychological response surrounding the expression of gratitude in real time. We do this by using a genetic indicator of oxytocin secretion, variation in the CD38 gene, and by creating a meaningful psychological context in which oxytocin is likely to reveal its influence: live interactions between members of ongoing romantic relationships.
THE BONDING EFFECTS OF GRATITUDE
Recent work regarding the emotion of gratitude supports the theory that this momentary emotional response to someone else’s kind gesture has evolved to connect people more closely to others who would make high-quality relationship partners (see review in Algoe, 2012). Earlier models of gratitude’s role in social life focused on economic factors like costs to the benefit-provider (i.e. benefactor), value to the recipient and repayment of the benefactor (e.g. Trivers, 1971; McCullough et al., 2001). However, the most recent evidence clarifies that, rather than merely facilitating interpersonal accounting of resources, gratitude works through a particular relational currency that is associated with intimacy, perceived partner responsiveness (Algoe et al., 2008). Perceived partner responsiveness underlies relational intimacy and is associated with feeling understood, accepted and cared for (Reis et al., 2004); it is an important situational trigger for feeling gratitude upon receipt of a benefit (Algoe et al., 2008; Algoe and Stanton, 2012). In turn, the grateful individual is likely to demonstrate responsiveness for the benefactor’s own needs and preferences in the future (e.g. Kubacka et al., 2011). In short, the positive emotion of gratitude provides fuel for upward spirals of mutual responsiveness between dyad members, thereby promoting the quality of the relationship over time.
In light of these strong connections between experienced emotion and relational outcomes, more recent work has begun to focus in on how one person’s experienced gratitude might translate to relational growth for each dyad member. One candidate behavior that follows from emotion theory is the expression of gratitude; indeed, in situations that would cause gratitude, acknowledging a benefit is the most frequently reported motive of the benefit recipient (Algoe and Haidt, 2009). Recent studies provide suggestive evidence that, for the grateful individual, expressing gratitude is necessary to reap the relational benefits (e.g. Lambert et al., 2010; Algoe and Stanton, 2012). For example, participants who were randomly assigned to ‘go the extra mile’ to express gratitude to a friend over the course of 3 weeks reported greater communal relationship strength with that friend by the end of the study, even compared with a condition in which people were assigned to focus on thinking about things they appreciate about the friend over the 3-week period (Lambert et al., 2010).
Beyond the grateful person, though, because of gratitude’s ‘other-praising’ nature (Algoe and Haidt, 2009), expressed gratitude may provide psychological rewards to the target of the expression (i.e. the original benefactor) that act as a hook to keep the benefactor engaged in and satisfied with the relationship with the grateful individual. Critically, this is most likely to be true if the expresser is perceived to be responsive when thanking the original benefactor (Algoe et al., 2013). Specifically, a recent study used a naturalistic interaction in which one romantic partner expressed gratitude to the other in the lab; for targets of the expressions who perceived their romantic partner as being particularly responsive—that is, understanding, validating and caring (Reis et al., 2004)—when expressing gratitude to them, the target’s satisfaction with the relationship improved over the subsequent 6 months (Algoe et al., 2013). In fact, effects of perceived partner responsiveness after the partner expressed gratitude to the target in the lab even held when statistically controlling for perceptions of the partner’s responsiveness after participating in two different types of common couple interactions that have been documented to be important for relationship well-being (i.e. providing support for positive or negative events; Gable et al., 2006). These findings highlight the unique and powerful role that expressions of gratitude can play in social bonding, beyond the other types of behaviors that may foster relationship quality. Identifying potential biological contributors to these effects stands to open doors for improved understanding of social bonding processes.
CLOSE RELATIONSHIPS AND OXYTOCIN
Since the seminal discovery that oxytocin facilitates monogamous bonds in rodents (Williams et al., 1994), a rapidly growing body of research in humans has sought to determine if oxytocin is involved in human social connections, broadly defined. Accordingly, oxytocin has been associated with everything from parenting behavior (e.g. Feldman et al., 2012) to behavior toward a new acquaintance (e.g. Kosfeld et al., 2005). However, for the question of close social bonds, it is notable that the adult romantic relationship context is unique in its qualitative experience as well as its impact on health and well-being (e.g. Berscheid, 1999; Hazan and Diamond, 2000). Because the connection between oxytocin and psychosocial processes appears to be highly dependent on the behavioral and relational context (see also Bartz et al., 2011), it is important to study psychosocial correlates of oxytocin directly in the romantic relationship context.
Within this context, the oxytocin system has been hypothesized to both facilitate bonding processes and change in response to social interactions (see Carter and Porges, 2013). In this work, we focus on the former type of research question, regarding the facilitation of bonding (for examples of the latter, see Grewen et al., 2005; Holt-Lunstad et al., 2008; Smith et al., 2013). The few studies that have addressed whether or how oxytocin might facilitate bonding processes in the context of romantic relationships do show concurrent associations between the oxytocin system and a handful of relationship-relevant behaviors, as observed in well-defined laboratory interactions between couple-members. Specifically, intranasal administration of oxytocin led to an increase in positive communication during a conflict discussion (Ditzen et al., 2009); similarly, higher levels of plasma oxytocin have been associated with greater positive behaviors during a conversation involving one person’s expression of worry or concern (Gouin et al., 2010), as well as while discussing a shared positive experience (Schneiderman et al., 2012). Integrating across these diverse methodologies, these studies suggest that higher synaptic levels of oxytocin promote relationship building behaviors. Finally, variation in the oxytocin receptor gene (OXTR), which theoretically alters the impact of oxytocin signaling, was associated with greater use of affiliative cues during a partner’s expression of worry (Kogan et al., 2011). These studies suggest that in general, the oxytocin system is involved in relationship-relevant processes. However, the mixture of methods used to measure or manipulate the oxytocin system combined with theory and evidence from relationship science suggesting that the various relationship behaviors investigated across these studies play different or unique roles in the promotion, maintenance and prevention-of-deterioration of the relationship (see Gable and Reis, 2001; Gable et al., 2012; McNulty and Fincham, 2012), mean it is too early to draw broad conclusions about oxytocin’s role in facilitating processes central to the promotion of close relationships.1 In short, there is much more to learn.
As reviewed, within the adult close relationship context, there has been surprisingly little examination of early assertions (Uvnas-Moberg, 1996, 1998; Carter, 1998) regarding oxytocin’s role in facilitating human social bonds—assertions which have been recently recapitulated (Carter and Porges, 2013). We believe a focus on social interactions involving expressed gratitude provides a prime opportunity to test associations between the oxytocin system and social processes involved in promoting adult human pair bonds because of the unique role of expressed gratitude in this process (Algoe et al., 2013). Moreover, we go beyond the behavior of expressed gratitude to specify some of the acute psychological impacts of such interactions that should be particularly instrumental in the bonding process, namely positive emotions and perceived partner responsiveness.
GENETIC ASSOCIATIONS WITH RELATIONSHIP PROCESSES
As mentioned earlier, prior research suggests that genetic association methodology is a viable method for gaining clues to potential neurochemical underpinnings of social bonding related processes in romantic couples (Kogan et al., 2011). We adopt a similar approach here. However, the cellular mechanism explaining how the previously discussed OXTR genetic variant could impact cellular signaling has not yet been identified, and a recent meta-analysis reported no association with a broad spectrum of socially relevant behaviors (Bakermans-Kranenburg and van IJzendoorn, 2014). Instead, we studied functional variation in a different gene, CD38, which is a key regulator of oxytocin release. Mice with deletion of the CD38 gene exhibit marked reductions of oxytocin within the cerebrospinal fluid and the plasma (Jin et al., 2007), indicating that CD38 is necessary for oxytocin secretion. Unable to release oxytocin, these mice exhibit profound deficits in basic social processes such as social recognition and memory (Jin et al., 2007).
In humans, CD38 appears to have a role in oyxtocin signaling and social processes as well. Levels of CD38 gene expression in peripheral cells are positively correlated with plasma levels of oxytocin (Kiss et al., 2011), suggesting that CD38 facilitates oxytocin release. Commonly occurring variation in CD38 (rs3796863) has also been associated with plasma oyxtocin levels (Feldman et al., 2012). Therefore, CD38 is an important regulator of oxytocin signaling.
Consistent with a role in regulating oxytocin release, CD38 is also associated with social processes. Accordingly, levels of peripheral CD38 gene expression are related to clinician-observed social skills in autistic patients (Kiss et al., 2011; Riebold et al., 2011). The previously mentioned single nucleotide polymorphism (SNP) (rs3796863) has also been associated with either low functioning autism (Lerer et al., 2010) or high functioning autism (Munesue et al., 2010). In the non-clinical context, a study of parenting behavior showed that rs3796863 was associated with reduced parental touch of infants (Feldman et al., 2012), suggesting that CD38 affects social engagement. Thus, there is an emerging literature tying rs3796863 to social processes. However, the molecular pathway by which this SNP could affect CD38 expression or function has not been identified.
A different polymorphism in CD38 that is located at the opposite end (5′) of the CD38 gene from rs3796863 has a more clearly delineated molecular pathway by which it can affect CD38 expression. This SNP, rs6449182, is located in intron 1, which is a regulatory control region of the CD38 gene (Ferrero et al., 1999) and differentially affects transcription factor binding (Saborit-Villarroya et al., 2011). Accordingly, this polymorphism has been associated with differences in relative levels of CD38 messenger RNA (Jamroziak et al., 2009), protein (Jamroziak et al., 2009) and enzymatic activity (Polzonetti et al., 2012). Thus, rs6449182 has functional effects on expression of CD38 and, by extension, extracellular oxytocin levels in the brain and plasma. Although the mechanism by which rs6449182 affects CD38 function is better delineated than for rs3796863, it has been less studied in a psychological context. Therefore in this study, we examine the association of rs6449182 as well as rs3796863 with gratitude and its social bonding effects. Because these two SNPs are located in different portions of the CD38 gene, and genotypes at each locus are potentially independent (meaning that knowledge of one genotype does not necessarily predict the other), we also studied their combined effect by summing genotypes to create a putative index of CD38 gene expression (see Chapman et al., 2003 for this approach).
THIS STUDY
The current investigation focuses on whether variation in CD38 is associated with social interactions involving the expression of gratitude. Evidence for the potential relational benefits from expressing gratitude (e.g. Lambert et al., 2010) as well as receiving an expression of gratitude (Grant and Gino, 2010; Algoe et al., 2013) suggests that the oxytocin system may influence both social roles: the expresser of gratitude and the recipient of gratitude expression. Moreover, we specify the relational and affective consequences of such interactions that are most closely related with high-quality, close, social connections: perceived partner responsiveness and positive emotions (see Fredrickson, 1998 for theory and Kok et al., 2013 for the role of positive emotions in building social resources, over time). In addition, work that differentiates among positive emotions in momentary social function (e.g. Algoe and Haidt, 2009; Shiota et al., 2011) suggests that it is worthwhile to further explore whether a specific active emotional ingredient in the romantic bonding context—love—is relevant (and see Gonzaga et al., 2006 for links between romantic love and oxytocin). Thus, after receiving an expression of gratitude and after expressing gratitude, we examined perceptions of partner responsiveness as well as positive and negative emotions experienced; we also examined grateful behavior itself, observed in the lab and as gathered from daily self-reports.
METHOD
Participants
Each member of 77 heterosexual romantic couples participated in a study on ‘Everyday experiences and feelings of people in romantic relationships’ (aka the Carolina Couples Study; see additional information in Algoe et al., 2013). Couples were recruited from the region surrounding Chapel Hill, North Carolina, and must have been romantically involved for a minimum of 6 months. The current sample consists of 128 individuals who provided saliva for genotyping (83.1% of sample; 63 women, 65 men across 69 heterosexual couples; aged 18–57, M = 28.5, s.d. = 8.65). The majority of participants self-identified as White/Caucasian (76.2%), and the remaining participants were Black/African-American (11.9%), East or South Asian (4%), and multi-racial or of an unidentified racial category (7.9%); 3.2% identified as Hispanic. Couples had been together for about 4 years (M = 4.2 years), and were either dating exclusively (55.8%) or committed for life (i.e. married, engaged to be married or living as married; 44.2%).
Overview of design and procedure
The larger Carolina Couples Study was an observational investigation consisting of two visits to the lab, 2 weeks apart, with brief nightly questionnaires completed for each of 14 nights between lab visits. The current investigation focuses on one pair of 5 min interactions between couple-members at the second lab visit, in which participants were each given the opportunity to express gratitude to the partner, as well as nightly self-reported gratitude expression.
Expressed gratitude behavioral task
Each participant was asked to choose something specific, big or small, that the partner did for him or her, for which the participant felt grateful (see Algoe et al., 2013). After noting the event, one participant took up to 5 min to thank the partner, after which they both self-reported their reactions to the interaction via private online questionnaires. The interaction and self-report were repeated with the second partner. Whether the male or female participant thanked the partner first was counterbalanced. Note that each participant in the study was part of two interactions: one in which he or she expressed gratitude, and one in which he or she received an expression of gratitude. Responses to each are important to our investigation because they reflect giving and receiving of positively valenced, other-focused attention, respectively.
Measures
At study entry, each participant reported on global relationship satisfaction using a well-validated seven-item measure (Hendrick, 1988; α = 0.82).
After each interaction, participants rated their agreement with several items on scales ranging from ‘not at all true/never true’ (0) to ‘very true/true all of the time’ (6). First, each participant responded to 24 items to measure emotional response. To reduce demand effects, we included a broad range of items and information that any given situation may produce its own set of emotions. The 11 positive emotion terms (e.g. peaceful, loving, amused, proud) were averaged into a positive emotions composite (α = 0.90, 0.86 after receiving an expression of gratitude and providing an expression, respectively). Note that the composite scores of the 13 negative emotion terms had extremely low ratings after each interaction (M < 0.31), consistent with the nature of the interactions, and we did not consider them further. Next, each participant responded to 10 items to measure the core components of perceived responsiveness (Gable et al., 2006), which include understanding, validating and caring (10-item composite α = 0.93, 0.95 after receiving an expression of gratitude and providing an expression, respectively). Data analysis included three measures both after expressing gratitude and after receiving an expression of gratitude: composite scores of perceived partner responsiveness and positive emotions, and, to probe specificity regarding theory, one targeted positive emotion term, ‘loving’.
Grateful behavior was indexed by a participant’s degree of praising of the partner’s actions within the expression of gratitude (for theory, see Algoe and Haidt, 2009), and encompassed both verbal and non-verbal behaviors to guard against counting insincere praise. The behavior was reliably observed by four judges who rated the degree of praising on a scale ranging from 1 (no or little praise) to 5 (excellent degree of benefactor praiseworthiness; α = 0.78), and participants varied in their spontaneous use of praising behavior when expressing gratitude, with the average of judges’ scores ranging from 1.25 to 5 (M = 3.26; s.d. = 0.73).
Finally, one question from the 14 nightly reports completed prior to the lab visit measured grateful behavior in a different way: each night, participants responded to the item, ‘I thanked my partner for something he/she did that I appreciated’ with a yes or no (coded as 1 and 0 for data analysis). This second measure provides the opportunity to test convergent and ecological validity of our hypothesis.
Genotyping
DNA was extracted from saliva collected using Oragene kits (DNA Genotek; www.dnagenotek.com) according to the manufacturer’s instructions. Both SNPs were genotyped using Taqman SNP Genotyping Assays (rs6449182: C___1216863_10; rs3796863: C___1216944_10) from Applied Biosystems using an ABI 7300 Sequence Detection System. All samples were successfully genotyped on two separate occasions with complete concordance. Haploview (version 4.2; Barrett et al., 2005) was used to calculate Hardy–Weinberg Equilibrium values using the exact test (Wigginton et al., 2005) as well as the normalized measure of allelic association (D′; Lewontin, 1964) and coefficient of determination (r2; Hill and Robertson, 1968). The rs3796863 SNP was coded in a dominant manner [CC genotype = 0; A allele carriers (AC and AA) = 1] as in prior work (Feldman et al., 2012, 2013; Sauer et al., 2012). The CC genotype, which is present in approximately 40% of the Caucasian and East Asian populations, has been associated with lower CD38 expression (Lerer et al., 2010) and lower plasma oxytocin levels (Feldman et al., 2012). With respect to rs6449182, the G allele is generally associated with greater CD38 expression, which is reflected in higher levels of CD38 mRNA, protein, and enzymatic activity (Jamroziak et al., 2009; Polzonetti et al., 2012; but see Riebold et al., 2011). This evidence on CD38 expression suggests that the G allele functions in an additive, not dominant manner, with the GG genotype (∼5–10% of the Caucasian population) associated with the greatest expression. Therefore, the rs6449182 polymorphism was coded in an additive manner (CC = 0, CG = 1; GG = 2). To create a cumulative index of putative CD38 expression, the two SNPs were summed according to their effects on gene expression, as coded earlier.
RESULTS
Analysis plan
Table 1 contains descriptive statistics for all dependent measures. Dependent measures violate assumptions of independence required for typical ANOVA (analysis of variance) because each member of the couple provided reports on the same outcomes. Therefore, we use multilevel modeling (using HLM; Raudenbush et al., 1996) for all analyses. Dependent measures from lab-based interactions were tested with two-level models (i.e. person nested within couple), and daily grateful behavior was tested with a three-level model (i.e. day within person within couple). (See Supplementary Table S1 containing correlations among dependent measures as well as intraclass correlations between dyads on each variable.) The distribution of genotypes for the two SNPs did not deviate from Hardy–Weinberg equilibrium (Ps > 0.19; for rs6449182- CC n = 83, CG n = 37, GG n = 8; for rs3796863, CC n = 62, A carriers n = 66). Models were run with rs6449182, rs3796863, or their combination as a continuous predictor variable for that person’s own behavior or response to an interaction. None of the effects reported below was moderated by participant sex, nor were conclusions altered when controlling for ethnicity.
Table 1.
Descriptive statistics for all dependent measures
| Mean | s.d. | |
|---|---|---|
| Global relationship satisfaction | 6.07 | 0.48 |
| Receiving a gratitude expression | ||
| Perceived partner responsiveness | 5.06 | 0.75 |
| Positive emotions | 3.78 | 1.08 |
| Loving | 4.92 | 0.97 |
| Expressing gratitude | ||
| Perceived partner responsiveness | 5.14 | 0.79 |
| Positive emotions | 4.11 | 0.91 |
| Loving | 5.00 | 1.01 |
| Praising behavior | 3.25 | 0.54 |
| Daily expressed gratitude | 0.79 | 0.12 |
Mean and s.d. reflect intercept and standard deviation of r, respectively, in unconditional HLM models where the listed variable was the dependent variable. For daily expressed gratitude, the intercept accounts for Bernoulli distribution of the outcome (0 or 1) and standard error (not deviation) is reported.
Similarity of rs6449182 and rs3796863
As expected due to their distinct locations in the CD38 gene, there was a relatively low degree of association between the two SNPs (in the primary ethnic group: D′ = 0.50, 95% CI [0.09, 0.79]; overall: D′ = 0.45, 95% CI [0.08, 0.75]; r2 = 0.026). In other words, rs6449182 status is not correlated with rs3796863 status [r (Spearman’s rho) = −0.004, P = 0.97].
rs6449182
The first section of columns in Table 2 presents the results of analyses for the nine targeted outcomes, showing that rs6449182 was significantly associated with eight of the nine. All associations were in the same direction, indicating that CC individuals had more positive psychological outcomes or behaviors. Specifically, rs6449182 was significantly associated with global evaluation of relationship satisfaction assessed at study entry; after receiving an expression of gratitude, rs6449182 was associated with the perception the expresser was responsive as well as with the specific emotion, loving; after providing an expression of gratitude rs6449182 was associated with the perception that the benefactor was responsive as well as experienced positive emotions (generally) and the specific emotion, loving. rs6449182 was also associated with the behavioral expression of gratitude, either as observed in the videorecorded lab interactions by external judges or as reported by the participant across 14 days: the odds ratio demonstrates that people with the CC genotype were most likely to report thanking the partner for an appreciated event on any given day (see Figure 1 for illustrative data).
Table 2.
Regression coefficients, significance levels and effect sizes from tests of rs6449182, rs3796863 and putative CD38 expression on the nine targeted outcomes
|
CD38 |
CD38 |
CD38 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs6449182 | rs3796863 | expression | ||||||||||||
| B | 95% CI | P | d | B | 95% CI | P | d | B | 95% CI | P | d | |||
| Relationship satisfaction | −0.15 | −0.29, −0.01 | 0.03 | 0.39 | −0.03 | −0.24, 0.17 | 0.77 | 0.05 | −0.11 | −0.23, 0.01 | 0.07 | 0.33 | ||
| Receiving a gratitude expression | ||||||||||||||
| Perceived responsiveness | −0.24 | −0.45, −0.02 | 0.03 | 0.40 | −0.14 | −0.45, 0.18 | 0.39 | 0.16 | −0.20 | −0.37, −0.04 | 0.02 | 0.44 | ||
| Positive emotions | −0.22 | −0.55, 0.11 | 0.19 | 0.24 | −0.19 | −0.66, 0.28 | 0.43 | 0.15 | −0.20 | −0.48, 0.08 | 0.16 | 0.26 | ||
| Loving | −0.37 | −0.69, −0.05 | 0.02 | 0.42 | −0.09 | −0.58, 0.40 | 0.71 | 0.07 | −0.26 | −0.55, 0.02 | 0.07 | 0.33 | ||
| Expressing gratitude | ||||||||||||||
| Perceived responsiveness | −0.19 | −0.35, −0.03 | 0.02 | 0.42 | −0.37 | −0.68, −0.06 | 0.02 | 0.43 | −0.26 | −0.41, −0.12 | 0.001 | 0.65 | ||
| Positive emotions | −0.31 | −0.62, −0.001 | 0.05 | 0.36 | −0.30 | −0.69, 0.09 | 0.13 | 0.28 | −0.31 | −0.54, −0.07 | 0.01 | 0.47 | ||
| Loving | −0.58 | −0.92, −0.24 | 0.001 | 0.62 | −0.25 | −0.62, 0.12 | 0.18 | 0.24 | −0.46 | −0.71, −0.22 | 0.000 | 0.69 | ||
| Observed praising behavior | −0.21 | −0.42, −0.001 | 0.05 | 0.39 | 0.03 | −0.20, 0.26 | 0.79 | 0.05 | −0.12 | −0.31, 0.06 | 0.19 | 0.26 | ||
| Daily expressed gratitude | 0.70 | 0.53, 0.91 | 0.009 | n/a | 0.73 | 0.96, 1.94 | 0.09 | n/a | 0.70 | 0.57, 0.88 | 0.002 | n/a | ||
B is the unstandardized regression coefficient from multilevel models with CD38 status as a predictor of the outcome of interest; 95% CI is the 95% confidence interval; P is statistical significance level, and the effect size (absolute value) is Cohen’s d. For daily expressed gratitude, the model accounts for Bernoulli distribution of the outcome (0 or 1), and we therefore present the odds ratio rather than B.
Fig. 1.
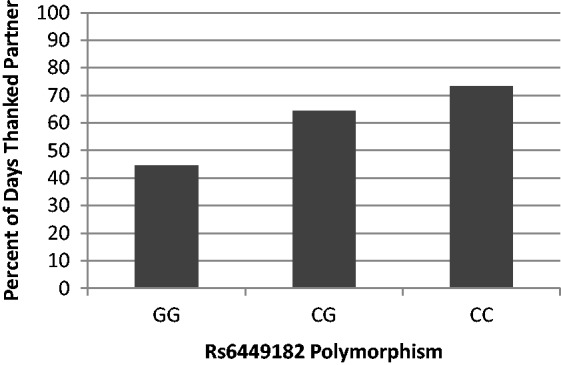
Illustration of linear trend in the percent of days, across 2 weeks, participants reported thanking the partner, by rs6449182 polymorphism. Note that raw data are used in this figure for illustration, but analyses reported in text control for dependence in data across couple-members.
rs3796863
The second section of columns in Table 2 presents the results of analyses for the nine targeted outcomes, showing that rs3796863 was significantly associated with one of the nine. Specifically, after providing an expression of gratitude, rs3796863 was significantly associated with the perception that the benefactor was responsive, in the direction indicating that those with the CC genotype were most likely to perceive responsiveness in the benefactor after having expressed gratitude to him or her. In addition, a marginally significant effect emerged for self-reported thanking behavior across 14 days in the same direction, such that those with the CC genotype tended to be most likely to report thanking the partner on a given day. From these rs3796863 analyses, though all coefficients were in the same direction, no other associations exceeded the 0.05 significance threshold.
Combined CD38 markers as an index of likelihood of oxytocin influence
The third section of columns in Table 2 presents results of analyses for the nine targeted outcomes, showing that putative CD38 expression was significantly associated with five of the nine (and an additional two approach statistical significance: relationship satisfaction and perception that the partner was loving when receiving an expression of gratitude). All significant (and non-significant) associations were in the same direction, indicating that individuals with presumptive lower CD38 expression had more positive psychological outcomes or behaviors. Specifically, after receiving an expression of gratitude, CD38 expression was significantly associated with the perception that the benefactor was responsive; after providing an expression of gratitude, the CD38 expression variable was associated with the perception that the benefactor was responsive as well as experienced positive emotions (generally) and the specific emotion, loving. Finally, adding ecological validity, from measures across 14 days, lower CD38 expression was associated with a greater likelihood of reporting having thanked the partner for an appreciated event on any given day.2
Alternative explanation
We tested the same pattern of analyses for relational and affective responses to a different conversation topic: disclosure of a personal positive event. Such interactions are positive in valence and responses to them have been associated with global relationship quality (see Gable et al., 2006 for task description and evidence). However, the primary positive emotional theme is joy/enthusiasm rather than gratitude; research demonstrates socially functional distinctions between these emotions (Algoe and Haidt, 2009), and that perceived responsiveness after a partner expressed gratitude better captured the variance in growth in the listener’s relationship satisfaction (i.e. promoting bonds) than did perceived responsiveness of the partner after responding to a positive event disclosure (Algoe et al., 2013). No self-report measures after the positive event disclosure interaction (i.e. positive emotions—including loving—or perceived partner responsiveness) was significantly predicted by CD38 status, either when sharing a positive event or when hearing a partner share an event (rs6449181 Ps = 0.21–0.98, M P-value = 0.55; rs3796863 Ps = 0.06–0.87, M P-value = 0.60; CD38 expression Ps = 0.09–0.98, M P-value = 0.60). This suggests that the results for the gratitude expression are not solely due to the positivity of the interaction, and is consistent with theorizing that oxytocin has context-specific effects (Bartz et al., 2011; Feldman, 2012), with the focus of the current investigation on one meaningful context thought to actively promote social bonding.
DISCUSSION
Adult human pair bonds have been posited to be supported by the co-evolution of biological, behavioral and psychological processes (e.g. Hazan and Diamond, 2000; Diamond, 2004; Feldman, 2012). Resting on this assumption, our investigation focused on a putative marker of oxytocin secretion, the behavioral expression of gratitude and psychological responses associated with interactions involving expressions of gratitude, all of which have been closely implicated in promoting the quality of social bonds. In laboratory interactions between people in romantic relationships, and from self-reported behavior in daily life, we found that functional genetic variation in CD38 was associated with the quality and frequency of grateful behavior toward the partner. In addition, this variation also predicted the psychological impact of providing or receiving an expression of gratitude, as well as global relationship satisfaction. Of the two polymorphisms, rs6449182 was associated with a broader spectrum of the building blocks by which gratitude facilitates social bonds. Moreover, when these uncorrelated markers of oxytocin release were combined into a putative index of CD38 expression, five of nine targeted effects remained, including thanking behavior in everyday life.
This consistent pattern of findings indirectly linking the oxytocin system with the quality and quantity of expressed gratitude adds to the small but growing body of evidence regarding the behavioral and psychological mechanisms by which oxytocin may influence human pair bonds. Recent theory in relationship science postulates that behaviors associated with prevention of negative outcomes (e.g. fighting well) or alleviation of negative emotions (e.g. receiving social support) are not tapping into the same psychological processes as behaviors associated with the promotion of positive outcomes or emotions (Gable and Reis, 2001). Empirically, negative emotions were very mild in these interactions, whereas positive emotions were relatively high. Consistent with theory, variations in rs6449182 in particular predicted differential positive evaluations of the partner and interaction, whether after providing an expression of gratitude or receiving an expression. Perceived partner responsiveness and positive emotions (e.g. love) after either interaction may have been greater due to consideration of the partner’s positive qualities or the partner’s actual behaviors in the interaction. Regardless, in so far as CD38 gene expression affects oxytocin signaling, our results implicate the oxytocin system in the psychological reactions to expressions of gratitude which serve to reward the person for remaining in the relationship. As recent work highlights, it is the acute psychological impact of such interactions that can forecast change in relationship quality (Algoe et al., 2013).
More broadly, our findings are important because the social roles of receiving and providing other-directed positive attention, here enacted through receiving and providing an expression of gratitude, have each recently been linked with consequential downstream outcomes. First, perceiving a partner’s responsiveness upon receipt of an expression of gratitude has been associated with downstream relational health benefits (Algoe et al., 2013). The findings regarding the impact of providing an expression of gratitude are interesting in light of the growing evidence for the importance of other-focus for mental health as well as longevity (e.g. Crocker et al., 2009; Konrath et al., 2012). We see our data, examined at the level of a live social interaction, as providing important basic research on potential biological, behavioral and psychological mechanisms for such effects.
Methodologically, integrating live social interactions with measures from daily life, as was done here, represents a valuable path forward for improving understanding of how oxytocin is related to creating social bonds. As reviewed in the introduction, a few studies of structured social interactions in the laboratory generally indicate that oxytocin is associated with beneficial relationship-relevant behaviors. Yet other studies that assess a longer relationship time frame have suggested that oxytocin is associated with distress in the pair-bond relationship (see Taylor et al., 2010). Resolving such controversies will require not only studying the same construct, such as gratitude, across multiple contexts, but also employing different methodologies and manipulations of the oxytocin system to provide convergent validity. In this vein, the current investigation draws attention to the utility of focusing on the gene CD38. There is a well-delineated molecular pathway by which rs6449182 can impact cellular signaling, which stands in contrast to commonly studied polymorphisms in the oxytocin receptor gene, such as rs53576, for which there has not been a clearly identified molecular mechanism that could explain how the polymorphism influences signaling. Though the small sample size requires interpretation of these findings as preliminary until replicated, the pattern of associations across a variety of measures that have long been posited to be associated with oxytocin—other-directed positive behavior, intimacy and warm positive feelings (cf. Carter, 1998)—suggests that CD38 is a useful marker for future research investigating the role of oxytocin system in the promotion of social bonds.
In addition to being involved in processes other than oxytocin secretion, a limitation of the genetic approach using CD38 is that because genetic variants are indirect measures of neurotransmitter signaling, it is not yet possible to draw conclusions regarding whether the reported associations with gratitude are due to greater or lesser release of oxytocin in the brain. Mice that do not express CD38 have low extracellular oxytocin levels (Jin et al., 2007). Similarly, humans with low levels of expression in lymphocytes have lower plasma oxytocin levels (Kiss et al., 2011). In this study, the genotypes were coded based on previously reported effects on CD38 expression, suggesting that lower CD38 expression is associated with greater expression of gratitude and greater resulting psychological effects. However, taking the next step to infer that the measures in this study are associated with lower levels of oxytocin release should be made cautiously for several reasons. First, the studies associating rs6449182 and rs3796863 with CD38 expression (e.g. Jamroziak et al., 2009) were conducted in cells derived from peripheral tissues rather than brain tissue. Yet, it is presumably alterations in CD38 expression within the brain that are leading to the behavioral and psychological effects seen here. Second, the effects of rs6449182 on gene expression could be influenced by nutritional and cellular factors that were not measured in this study. Third, there are likely to be complex compensatory mechanisms that might counteract the lifelong effects of these polymorphisms. For example, even though mice with deletion of the CD38 gene have very low levels of oxytocin in the plasma, they actually have very high levels of oxytocin inside the cell because the oxytocin is unable to be released without CD38 (Jin et al., 2007). Therefore, it will be important in future studies to link these polymorphisms to levels of oxytocin in the cerebrospinal fluid of humans to gain a better understanding of how they might be related to oxytocin signaling and how the aforementioned moderators might impact the effect of rs6449182 on CD38 expression.
In sum, this research presents the first evidence regarding the biological underpinnings of expressed gratitude in the formation of adult human bonds. Moreover, CD38 status was associated with behavior and psychological reactions within live interactions, as well as global relationship quality. As an index of oxytocin release, the reliable pattern of results for CD38 variants across several distinct and theoretically consistent measures provides intriguing evidence for the oxytocin system in solidifying the ‘glue’ that brings close adult relationship partners closer together.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This work was supported by a grant from the National Institute of Mental Health (MH59615) and a grant from the National Center for Advancing Translational Sciences (8KL2TR000112-05). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank Ms Sara DeMaria for her excellent technical assistance, as well as the exceptional team of 30 research assistants who helped with the Carolina Couples Study, lead by Jenny Bridgers (full list available on first author’s website). Barbara Fredrickson, B. Keith Payne and Crystal Schiller provided insightful feedback on an earlier manuscript draft.
Footnotes
1 To add complexity, another body of work tests concurrent associations between plasma oxytocin and self-reported assessment of global relationship evaluation rather than specific social behavior or its immediate impact in a live and meaningful context. Presumably, the quality of specific interpersonal interactions adds up to comprise the overall evaluation of the relationship. Yet in this line of work, researchers sometimes find negative associations between oxytocin and global relationship evaluation (e.g. Taylor, Saphire-Bernstein, & Seeman, 2010; Smith et al., 2013). Though not the primary focus of the current investigation, we include a measure of global relationship satisfaction.
2 Four dependent measures contained outliers (i.e. > 3 s.d. beyond mean). These analyses were re-run with outliers removed, using each index of CD38 expression as predictor; perceived responsiveness after receiving an expression of gratitude became non-significant as predicted by rs6449182 (P = 0.11); all other conclusions remained the same.
REFERENCES
- Algoe SB. Find, remind, and bind: the functions of gratitude in everyday relationships. Social and Personality Psychology Compass. 2012;6(6):455–69. [Google Scholar]
- Algoe SB, Fredrickson BL, Gable SL. The social functions of the emotion of gratitude via expression. Emotion. 2013;13(4):605–9. doi: 10.1037/a0032701. [DOI] [PubMed] [Google Scholar]
- Algoe SB, Haidt J. Witnessing excellence in action: the “other-praising” emotions of elevation, gratitude, and admiration. Journal of Positive Psychology. 2009;4(2):105–27. doi: 10.1080/17439760802650519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algoe SB, Haidt J, Gable SL. Beyond reciprocity: gratitude and relationships in everyday life. Emotion. 2008;8:425–9. doi: 10.1037/1528-3542.8.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algoe SB, Stanton AL. Gratitude when it is needed most: social functions of gratitude in women with metastatic breast cancer. Emotion. 2012;12(1):163–8. doi: 10.1037/a0024024. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. A sociability gene? Meta-analysis of oxytocin receptor genotype effects in humans. Psychiatric Genetics. 2014;24(2):45–51. doi: 10.1097/YPG.0b013e3283643684. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011;15(7):301–9. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Berscheid E. The greening of relationship science. American Psychologist. 1999;54(4):260–6. doi: 10.1037//0003-066x.54.4.260. [DOI] [PubMed] [Google Scholar]
- Bowlby J. The nature of the child’s ties to his mother. International Journal of Psych-Analysis. 1958;39:350–73. [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23(8):779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Porges SW. The biochemistry of love: an oxytocin hypothesis. EMBO Reports. 2103;14:12–6. doi: 10.1038/embor.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JM, Cooper JD, Todd JA, Clayton DG. Detecting disease associations due to linkage disequilibrium using haplotype tags: a class of tests and the determinants of statistical power. Human Heredity. 2003;56:18–31. doi: 10.1159/000073729. [DOI] [PubMed] [Google Scholar]
- Crocker J, Olivier M-A, Nuer N. Self-image goals and compassionate goals: costs and benefits. Self & Identity. 2009;8:251–69. doi: 10.1080/15298860802505160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond LM. Emerging perspectives on distinctions between romantic love and sexual desire. Current Directions in Psychological Science. 2004;13(3):116–9. [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry. 2009;65(9):728–31. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Feldman R. Oxytocin and social affiliation in humans. Hormones and Behavior. 2012;61:380–91. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Influs M, Gutbir T, Ebstein RP. Parental oxytocin and early caregiving jointly shape children's oxytocin response and social reciprocity. Neuropsychopharmacology. 2013;38(7):1154–62. doi: 10.1038/npp.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Zagoory-Sharon O, Weisman O, et al. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biological Psychiatry. 2012;72:175–81. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- Ferrero E, Saccucci F, Malavasi F. The human CD38 gene: polymorphism, CpG island, and linkage to the CD157 (BST-1) gene. Immunogenetics. 1999;49(7–8):597–604. doi: 10.1007/s002510050654. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL. What good are positive emotions? Review of General Psychology. 1998;2:300–19. doi: 10.1037/1089-2680.2.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable SL, Gonzaga GC, Strachman A. Will you be there for me when things go right? Supportive responses to positive event disclosures. Journal of Personality and Social Psychology. 2006;91:904–17. doi: 10.1037/0022-3514.91.5.904. [DOI] [PubMed] [Google Scholar]
- Gable SL, Gosnell CL, Maisel NC, Strachman A. Safely testing the fire alarm: close others’ responses to personal positive events. Journal of Personality and Social Psychology. 2012;103(6):963–81. doi: 10.1037/a0029488. [DOI] [PubMed] [Google Scholar]
- Gable SL, Reis HT. Appetitive and aversive social interaction. In: Harvey J, Wenzel A, editors. Close Romantic Relationships: Maintenance and Enhancement. Mahwah, NJ: Lawrence Erlbaum; 2001. pp. 169–94. [Google Scholar]
- Gonzaga GC, Turner RA, Keltner D, Campos B, Altemus M. Romantic love and sexual desire in close relationships. Emotion. 2006;6(2):163–79. doi: 10.1037/1528-3542.6.2.163. [DOI] [PubMed] [Google Scholar]
- Gouin J-P, Carter CS, Pournajafi-Nazarloo H, et al. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology. 2010;35:1082–90. doi: 10.1016/j.psyneuen.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AM, Gino F. A little thanks goes a long way. Explaining why gratitude expression motivate prosocial behavior. Journal of Personality and Social Psychology. 2010;98(6):946–55. doi: 10.1037/a0017935. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosomatic Medicine. 2005;67(4):531–8. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Harlow HF. The nature of love. American Psychologist. 1958;13:673–85. doi: 10.1037/h0029383. [DOI] [PubMed] [Google Scholar]
- Hazan C, Diamond LM. The place of attachment in human mating. Review of. General Psychology. 2000;4(2):186–204. [Google Scholar]
- Hendrick SS. A generic measure of relationship satisfaction. Journal of Marriage and the Family. 1988;50:93–8. [Google Scholar]
- Hill W, Robertson A. Linkage disequilibrium in finite populations. Theoretical and Applied Genetics. 1968;38(6):226–31. doi: 10.1007/BF01245622. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Birmingham WA, Light KC. Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosomatic Medicine. 2008;70(9):976–85. doi: 10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- Jamroziak K, Szemraj Z, Grzybowska-Izydorczyk O, et al. CD38 gene polymorphisms contribute to genetic susceptibility to B-cell chronic lymphocytic leukemia: evidence from two case-control studies in polish caucasians. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, . 2009 doi: 10.1158/1055-9965.EPI-08-0683. Cosponsored by the American Society of Preventive Oncology, 18(3), 945–53. [DOI] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446(7131):41–5. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- Kiss I, Levy-Gigi E, Keri S. CD 38 expression, attachment style and habituation of arousal in relation to trust-related oxytocin release. Biological Psychology. 2011;88(2–3):223–6. doi: 10.1016/j.biopsycho.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Kogan A, Saslow LR, Impett EA, Oveis C, Keltner D, Saturn SR. Thin-slicing study of the oxytocin receptor (OXTR) gene and the evaluation and expression of the prosocial disposition. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(48):19189–92. doi: 10.1073/pnas.1112658108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok BE, Coffey KA, Cohn MA, et al. How positive emotions build physical health: perceived positive social connections account for the upward spiral between positive emotions and vagal tone. Psychological Science. 2013;24(7):1123–32. doi: 10.1177/0956797612470827. [DOI] [PubMed] [Google Scholar]
- Konrath S, Fuhrel-Forbis A, Lou A, Brown S. Motives for volunteering are associated with mortality risk in older adults. Health Psychology. 2012;31(1):87–96. doi: 10.1037/a0025226. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–6. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kubacka KE, Finkenauer C, Rusbult CE, Keijsers L. Maintaining close relationships: gratitude as a motivator and a detector of maintenance behavior. Personality and Social Psychology Bulletin. 2011;37:1362–75. doi: 10.1177/0146167211412196. [DOI] [PubMed] [Google Scholar]
- Lambert NM, Clark MS, Durtschi J, Fincham FD, Graham SM. Benefits of expressing gratitude: expressing gratitude to a partner changes one’s view of the relationship. Psychological Science. 2010;21:574–80. doi: 10.1177/0956797610364003. [DOI] [PubMed] [Google Scholar]
- Lerer E, Levi S, Israel S, et al. Low CD38 expression in lymphoblastoid cells and haplotypes are both associated with autism in a family-based study. Autism Research: Official Journal of the International Society for Autism Research. 2010;3(6):293–302. doi: 10.1002/aur.156. [DOI] [PubMed] [Google Scholar]
- Lewontin RC. The interaction of selection and linkage. I. General considerations; heterotic models. Genetics. 1964;49:49–67. doi: 10.1093/genetics/49.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ME, Kilpatrick SD, Emmons RA, Larson DB. Is gratitude a moral affect? Psychological Bulletin. 2001;127(2):249–66. doi: 10.1037/0033-2909.127.2.249. [DOI] [PubMed] [Google Scholar]
- McNulty JK, Fincham FD. Beyond positive psychology? Toward a contextual view of psychological processes and well-being. The American Psychologist. 2012;67(2):101–10. doi: 10.1037/a0024572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munesue T, Yokoyama S, Nakamura K, et al. Two genetic variants of CD38 in subjects with autism spectrum disorder and controls. Neuroscience Research. 2010;67(2):181–91. doi: 10.1016/j.neures.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Polzonetti V, Carpi FM, Micozzi D, Pucciarelli S, Vincenzetti S, Napolioni V. Population variability in CD38 activity: correlation with age and significant effect of TNF-alpha -308G>A and CD38 184C>G SNPs. Molecular Genetics and Metabolism. 2012;105(3):502–7. doi: 10.1016/j.ymgme.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong YF, Congdon RT. Hierarchical Linear and Nonlinear Modeling. Chicago: Scientific Software International; 1996. HLM 5. [Google Scholar]
- Reis H, Clark MS, Holmes JG. Perceived partner responsiveness as an organizing construct in the study of intimacy and closeness. In: Mashek DJ, Aron AP, editors. Handbook of Closeness and Intimacy. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 2004. pp. 201–25. [Google Scholar]
- Riebold M, Mankuta D, Lerer E, et al. All-trans retinoic acid upregulates reduced CD38 transcription in lymphoblastoid cell lines from autism spectrum disorder. Molecular Medicine (Cambridge, Mass.) 2011;17(7–8):799–806. doi: 10.2119/molmed.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saborit-Villarroya I, Vaisitti T, Rossi D, et al. E2A is a transcriptional regulator of CD38 expression in chronic lymphocytic leukemia. Leukemia : Official Journal of the Leukemia Society of America, Leukemia Research Fund. 2011 doi: 10.1038/leu.2010.291. U.K, 25(3), 479–88. [DOI] [PubMed] [Google Scholar]
- Sauer C, Montag C, Worner C, Kirsch P, Reuter M. Effects of a common variant in the CD38 gene on social processing in an oxytocin challenge study: possible links to autism. Neuropsychopharmacology. 2012;37(6):1474–82. doi: 10.1038/npp.2011.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin during the initial stages of romantic attachment: relations to couples’ interactive reciprocity. Psychoneuroendocrinology. 2012;37:1277–85. doi: 10.1016/j.psyneuen.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota MN, Neufeld SL, Yeung WH, Moser SE, Perea EF. Feeling good: autonomic nervous system responding in five positive emotions. Emotion. 2011;11(6):1368–78. doi: 10.1037/a0024278. [DOI] [PubMed] [Google Scholar]
- Smith TW, Uchino BN, MacKenzie J, et al. Effects of couple interactions and relationship quality on plasma oxytocin and cardiovascular reactivity: empirical findings and methodological considerations. International Journal of Psychophysiology. 2103;88:271–81. doi: 10.1016/j.ijpsycho.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychological Science. 2010;21(1):3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- Trivers RL. The evolution of reciprocal altruism. Quarterly Review of Biology. 1971;46:35–57. [Google Scholar]
- Uvnas-Moberg K. Neuroendocrinology of the mother-child interaction. Trends in Endocrinology and Metabolism. 1996;7(4):126–31. doi: 10.1016/1043-2760(96)00036-7. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23(8):819–35. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. The American Journal of Human Genetics. 2005;76(5):887–93. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) Journal of Neruoendocrinology. 1994;6:247–50. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


