Abstract
Several brain regions are important for processing self-location and first-person perspective, two important aspects of bodily self-consciousness. However, the interplay between these regions has not been clarified. In addition, while self-location and first-person perspective in healthy subjects are associated with bilateral activity in temporoparietal junction (TPJ), disturbed self-location and first-person perspective result from damage of only the right TPJ. Identifying the involved brain network and understanding the role of hemispheric specializations in encoding self-location and first-person perspective, will provide important information on system-level interactions neurally mediating bodily self-consciousness. Here, we used functional connectivity and showed that right and left TPJ are bilaterally connected to supplementary motor area, ventral premotor cortex, insula, intraparietal sulcus and occipitotemporal cortex. Furthermore, the functional connectivity between right TPJ and right insula had the highest selectivity for changes in self-location and first-person perspective. Finally, functional connectivity revealed hemispheric differences showing that self-location and first-person perspective modulated the connectivity between right TPJ, right posterior insula, and right supplementary motor area, and between left TPJ and right anterior insula. The present data extend previous evidence on healthy populations and clinical observations in neurological deficits, supporting a bilateral, but right-hemispheric dominant, network for bodily self-consciousness.
Keywords: self-location, first-person perspective, temporoparietal junction, insula, multisensory integration
INTRODUCTION
Cognitive neuroscience has studied both high-level cognitive (Northoff et al., 2006) and low-level sensory aspects of the self, using visual (Heatherton et al., 2006), auditory (Perrin et al., 2005) and somatosensory stimulation (Laureys and Tononi, 2009). More recently, the association between multisensory bodily stimuli and conscious aspects of the self has been investigated (Christoff et al., 2011). Thus, different components of bodily self-consciousness have been identified and experimentally manipulated using multisensory conflicts (for review see Blanke, 2012). In particular, visuotactile conflicts have been used to manipulate the sense of body ownership and induce illusory ownership of a fake hand (Botvinick and Cohen, 1998) or distort the perceived location of one’s own hand (Tsakiris and Haggard, 2005). However, bodily self-consciousness is associated not only with such localized body-part specific components but also with more global and unitary aspects related to the whole body (Blanke and Metzinger, 2009). To understand these global aspects of bodily self-consciousness, two components have been experimentally manipulated using visuotactile conflicts (Ehrsson, 2007; Lenggenhager et al., 2007; Aspell et al., 2009): self-location, defined as ‘the experience of where I am in the world’ (Aspell et al., 2010) and first-person perspective, defined as ‘the experience of where I perceive the world from’ (Petkova et al., 2011b).
What are the neural correlates of self-location and first-person perspective? An increasing, but still limited, number of investigations have addressed this issue (review in Ionta et al., 2011a). In a recent study using functional magnetic resonance imaging (fMRI), experimentally induced changes of first-person perspective were associated with predictable changes in perceived self-location and were reflected in the activity of the bilateral temporoparietal junction (TPJ) (Ionta et al., 2011b). TPJ activity has been linked to self-regulation (Heatherton, 2011), self/other discrimination (Farrer et al., 2003a; Frith, 2005; Salomon et al., 2009), Theory-of-Mind (Frith and Frith, 2006; Andrews-Hanna, 2012) and saliency detection (Kucyi et al., 2012), with distinct sub-regions within TPJ that may account for such different functions (Mars et al., 2012). However, whether the different activation patterns in TPJ relate to the activity in other regions encoding self-location and first-person remains an open question. The main aim of this study was to provide new data concerning the role of hemispheric specialization in self-location and first-person perspective in order to understand whether TPJ encodes these components though working in isolation or as part of a broader network.
One possibility for improving understanding of the interplay between different regional activity profiles is provided by functional connectivity (FC) fMRI, a tool of proven efficacy for self-referential research, e.g. the neural correlates of self-other distinction (e.g. David et al., 2007; Salomon et al., 2013). In addition, BOLD fluctuations correlate with the power modulation of the local field potentials both in the primate (Pan et al., 2013) and the human brain (Keller et al., 2013), as well as with anatomical connectivity (Greicius et al., 2009). These findings support the theory that the neuronal-hemodynamic correlation constitutes a property of FC. On this basis, we used FC-fMRI to identify the network whose activity correlates with TPJ BOLD modulations induced by experimentally manipulated changes in self-location and first-person perspective. At least three methodological motivations support the choice of FC-fMRI. First, it can identify brain networks (Fox and Raichle, 2007) with high topographical similarity to staining techniques (in monkeys; Kelly et al., 2010) or diffusion tensor imaging (in humans; Skudlarski et al., 2008; Greicius et al., 2009). Second, FC-fMRI can be used to investigate experimental condition-dependent changes in the interplay across different brain areas (Hampson et al., 2004, 2006; Salomon et al., 2011). Third, important information on condition-independent FC in block-designed fMRI datasets can be achieved by removing the contribution of condition-dependent effects from intrinsic BOLD fluctuations (Fair et al., 2007; Gavrilescu et al., 2008; Hasson et al., 2009; Jones et al., 2010). Therefore, FC-fMRI is an excellent analytic tool to investigate both condition-dependent and condition-independent synchronization between different brain regions.
First-person perspective has been repeatedly associated not only with activity in TPJ (Ruby and Decety, 2001; Vogeley and Fink, 2003; Vogeley et al., 2004; McCleery et al., 2011) but also with prefrontal (David et al., 2006) and ventral premotor and intraparietal cortex (Petkova et al., 2011a). Similarly, self-location does not activate only TPJ but also other multisensory regions including the premotor and intraparietal cortex (Petkova et al., 2011a) as well as medial frontal, prefrontal and sensorimotor cortex (Lenggenhager et al., 2011) and insula (for local components; Tsakiris, 2010). Based on these findings, we hypothesized that within the condition-independent network TPJ would be coupled with prefrontal, intraparietal and insular regions. Furthermore, additional regions might include the occipitotemporal and posterior parietal cortex, due to their strong association with other aspects of bodily self-consciousness such as the senses of agency (David et al., 2007) and body ownership (Giummarra et al., 2011). Next, we anticipated that changes in self-location and first-person perspective would modulate the connectivity between TPJ and insula (Craig, 2009). Finally, based on clinical data (review in Blanke, 2012), we predicted finding a network with a right-hemispheric predominance.
METHODS
Similarly to previous studies that differentially analyzed the same dataset to address multiple research questions, i.e. brain activity and FC (cf. de Lange et al., 2007 versus de Lange et al., 2010; and Delnooz et al., 2012 versus Delnooz et al., 2013), we performed FC analysis on a pre-recorded dataset. The complete experimental procedure and BOLD signal modulation have been previously reported (Ionta et al., 2011b; see also Supplementary materials). Only the features relevant to the FC-fMRI analysis will be detailed here.
Twenty-two right-handed (Briggs and Nebes, 1975), naïve, male volunteers (mean age = 25.4 years; SD = 5.7 years) with normal vision participated in the experiment. All subjects signed the informed consent and the local ethics committee approved the experimental protocol, which was carried out in accordance with the 1964 Declaration of Helsinki.
Stimuli
The visual stimuli consisted in videos of the back view of a human virtual body (‘body’ condition) being stroked on the back by a rod. Control videos showed only the moving rod (‘no-body’ condition). During the visual stimulation, a robotic device moved a rod along the subjects’ back delivering the tactile stimulation. The trajectory of the tactile stimulation either matched (synchronous) or not (asynchronous) the displayed position of the virtual rod (see also Supplementary Materials).
Self-location task
We evaluated self-location after each block of visuotactile stimulation by asking subjects to imagine releasing a ball and to estimate the time the ball would need to ‘hit’ the ground. Response times in this task varied as a function of the perceived self-location: longer RT for higher self-location and shorter RTs for lower self-location (Lenggenhager et al., 2009; see also Supplementary Materials).
Experimental protocol
The experiment consisted of two runs of 8 blocks, each lasting 78 s. Each block consisted of three elements presented in the following order: exposure to visuotactile stimulation (39 s); three executions of the self-location task triggered by the auditory cue (15 s); observation of a white cross on a black screen without stroking as a ‘baseline’ (24 s). The experimental conditions (body/synchronous, body/asynchronous, no-body/synchronous and no-body/asynchronous) were presented four times during the experiment in a pseudo-randomized order.
Subjects’ self-reports indicated that they experienced two different directions of first-person perspective during the experiment. Thus, one group of subjects had the impression of looking upwards and were accordingly included in the ‘up group’; the remaining subjects reported the impression of looking downwards and comprised the ‘down group’. This led to a 2 × 2 × 2 design with eight conditions resulting from the interaction of perspective (up; down) as between-subject factor, and object (body; no-body) and stroking (synchronous; asynchronous) as within-subject factors (see also Ionta et al., 2011b).
Data
Using a 3T Siemens Trio scanner, we ran a magnetization-prepared rapid acquisition gradient echo sequence (MPRAGE) to collect T1-weighted anatomical images (1 mm isotropic voxels, 160 sagittal slices, TR = 9.7 ms, TE = 4 ms), and a gradient echo EPI sequence to collect functional images (slices = 28; thickness = 3.5 mm; TR = 3 s, TE = 60 ms, 64 × 64 image matrix, 3.5 × 3.5 mm in-plane resolution). Using SPM8 (www.fil.ion.ucl.ac.uk/spm), data were corrected for head movements (Friston et al., 1995b), normalized to the MNI brain template (Mazziotta et al., 1995), re-sampled to 1 × 1 × 1 mm voxel size and spatially smoothed with a Gaussian kernel of 6-mm FWHM (Friston et al., 1995a).
Region selection
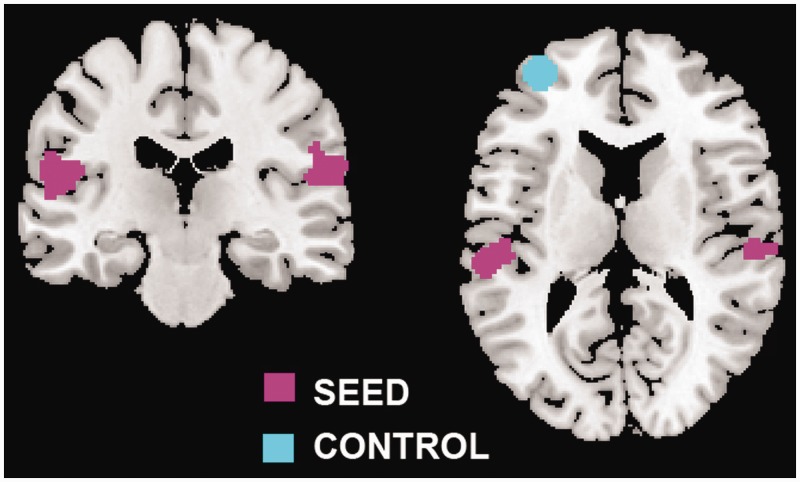
As seed regions for the FC-fMRI analyses, we selected the clusters where the activity reflected changes in self-location and first-person perspective (Ionta et al., 2011b): right and left TPJ (rTPJ and lTPJ, respectively; Figure 1). Both rTPJ (MNI: 57, −29, 21) and lTPJ (MNI: −54, −30, 19) seed regions were centerd on the superior temporal gyrus and included also the supramarginal gyrus. To test the specificity of the FC-fMRI results, we selected a control region of comparable size with respect to the seed regions: the (anterior and contralateral) left dorsolateral prefrontal cortex (lDLPFC; MNI: −34, 52, 7), defined as a sphere (7-mm radius). At least two main motivations justified the selection of the lDLPFC as control region. First, rather than being involved in encoding self-location and first-person perspective, its activity has been classically associated with working memory (Fletcher et al., 1998). Second, it is located in the anterior part of the left hemisphere, while the neural response related to bodily self-consciousness is typically associated with activity in the posterior part of the right hemisphere.
Fig. 1.
Seed and control regions. Localization of the seed (pink) and control (blue) regions on the standard brain.
Functional connectivity
Pre-processing
Seed-driven FC-fMRI was computed using the CONN software (http://www.nitrc.org/projects/conn; Whitfield-Gabrieli and Nieto-Castanon, 2012) implemented in Matlab (TheMathWorksInc., Natick, MA, USA). To remove the non-region-specific BOLD fluctuations, we regressed out from the data several nuisance components, including: motion parameters and average signals of white matter, gray matter and cerebrospinal fluid. To remove the effects of the experimental conditions on the BOLD signal, we removed from the data the main effects of conditions estimated by means of a general linear model using the hemodynamic response function and its temporal derivative as basic functions. We considered the temporal derivative to better account for the variability of the hemodynamic response function across brain regions.
Single subject analysis
The residual signals were low-pass filtered (cut-off frequency 0.1 Hz; Lowe et al., 1998) and the average time course over each seed region was separated into epochs associated with each experimental condition (Whitfield-Gabrieli and Nieto-Castanon, 2012). To take into account the hemodynamic delay, the epochs associated with the same experimental condition were concatenated across all sessions and weighted by the value of the time series of the regressor associated with each experimental condition (Whitfield-Gabrieli and Nieto-Castanon, 2012). After this step, each experimental condition included 52 time points (36 of which had weighting greater than 0.75 for the computation of the FC). Next, FC maps were computed for each experimental condition and each seed region separately, using the simple correlation method between the time course of the signal in the seed regions and the signal of each voxel in the brain. Thus, for each subject, we obtained four FC maps for each seed region, representing the correlation of the activity in each voxel in the brain with the average signal of the specific seed region.
Group analysis
Inference on population was obtained by means of a second level analysis. Correlation values were Fisher transformed to improve normality (Hays, 1981). The group analysis comprised three different steps. First, previous studies identified specific functionally connected brain networks by analyzing the correlations of condition-independent BOLD fluctuations (Biswal et al., 1995; Hampson et al., 2002; Greicius et al., 2003; Mantini et al., 2007; Seeley et al., 2007). Thus, to investigate the brain network connected to the right and left TPJ, for each seed region we computed the average connectivity maps across conditions (P < 0.001; 200 mm3 cluster threshold).
The second step resulted in the identification of a subset of regions—hereafter labeled as ‘target' regions—defined as the clusters where the connectivity with the seed regions reflected stroking-dependent changes in self-location and first-person perspective. Based on previous evidence also showing that condition-independent BOLD fluctuations can be specifically modulated by several factors, including cognitive tasks (Dodel et al., 2005; Hampson et al., 2006) or mood states (Harrison et al., 2008), we tested whether the FC within the clusters connected with the seed regions was modulated by our experimental conditions. The analysis of these condition-dependent changes in FC was restricted to the network showing a reliable FC with the seed regions and based on a mixed-model analysis of variance (ANOVA) with perspective as between-subject factor, and object and stroking as within-subject factors (P < 0.005; 200 mm3 cluster threshold). Therefore, the number of multiple comparisons has been reduced with respect to the first step, i.e. the identification of the condition-independent connectivity. Based on this, and in accordance with previous studies (Beauchamp, 2005; Martuzzi et al., 2010; Indovina et al., 2013), the significant differences across conditions were identified using a more liberal threshold (P < 0.005, 200 mm3 cluster threshold). Third, to understand the directionality of the 3-way interaction, we computed the average Fisher-transformed correlation coefficient (hereafter referred to as ‘correlation index’) for each experimental condition. Then, we tested the nature of the modulation of the connectivity index as a function of conditions using post hoc comparisons (Newman–Keuls test; P < 0.05). To evaluate the FC specificity between the seed and the target regions, we tested the coupling of the control region with all the voxels in the brain and then we applied to the control-target FC the same ANOVA model we used for the seed-target FC. To automatically localize and visualize the target regions, we used the BrainShow software (Galati et al., 2008; see also Supplementary Materials). The BrainShow software was used also to project the clusters onto the PALS atlas (Van Essen, 2005), and to superimpose them to the standard brain cortex. In particular, BrainShow allows one to superimpose the statistical maps on the cortical surface of the MNI canonical brain and to automatically identify the anatomical structures comprised in a specific cluster, including the percentage of the cluster’s voxels that belongs to each included anatomical structure (Tzourio-Mazoyer et al., 2002).
RESULTS
First, we report the average FC of each seed region across all conditions. Then, we show how this connectivity was further modulated by the experimental conditions. Finally, we demonstrate that the connectivity modulation was specific to the seed-target complex.
Condition-independent connectivity
The regions resulting from the first analysis correspond to the network functionally connected to the seed regions across conditions.
Right TPJ seed region
rTPJ was bilaterally connected to the insular, occipitotemporal, medial frontal and intraparietal cortex. Unilaterally, the rTPJ was connected to the right medial parietal, the right ventral premotor (PMv) and the left inferior frontal cortex (Figure 2A).
Fig. 2.
Condition-independent network. Blue-to-red scale represents the statistics (t20) of the voxel-wise analyses testing whether the average connectivity across conditions with right TPJ (A) and left TPJ (B) seed regions (contoured in black) was statistically significant. Vertical white arrows indicate the location of the central sulcus (CS). Horizontal white arrows indicate the anterior (ANT) and posterior (POST) pole of the brain.
Left TPJ seed region
Similarly to rTPJ, the lTPJ was bilaterally connected to insular, medial frontal and intraparietal cortices. Unilaterally the lTPJ was connected to the right precentral gyrus, and the left occipitotemporal and PMv cortices (Figure 2B).
Condition-dependent connectivity between seed and target regions
Here, we show the network in which the connectivity with the seed regions was modulated as a function of body-selective, synchrony-related and perspective-dependent changes in self-location.
Right TPJ seed region
Within the network connected to rTPJ, the connectivity values (correlation index) were modulated as a function of the three experimental factors only in right insula (rIns1) and right supplementary motor area (rSMA; Figure 3A; Table 1). The rIns1 target region had 90% of the voxels within the insular cortex. The rSMA target region had 86% of the voxels within the SMA.
Fig. 3.
Condition-dependent network. White outlines indicate right TPJ (A) and left TPJ (B) seed regions. Blue outlines indicate the condition-independent networks. Red cluster indicate regions with significant experiment-related modulation of the connectivity index.
Table 1.
Condition-dependent FC
| Region (label) | Hemisphere | T(1,20) score | Cluster size (voxels) | MNI coordinates |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Precentral gyrus (rSMA) | Right | 4.76 | 504 | 7 | 15 | 70 |
| Insula (rIns1) | Right | 4.20 | 508 | 47 | −15 | 19 |
| Insula (rIns2) | Right | 3.89 | 342 | 46 | 2 | 0 |
| Basal Ganglia (lBG) | Left | 4.64 | 496 | −29 | −21 | 9 |
Clusters’ anatomical definition (label), statistics, size and MNI coordinates.
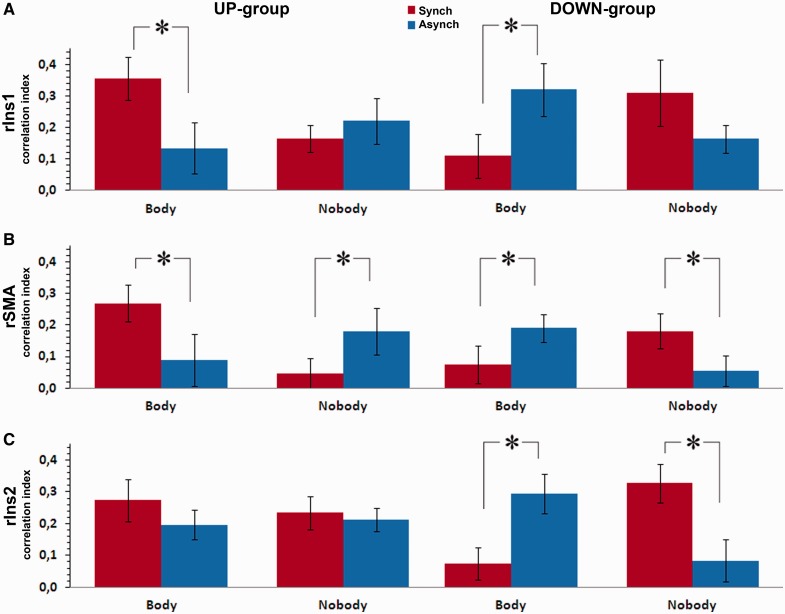
Between rTPJ seed region and rIns1 target region (Figure 4A), for both the up- and down- groups, the connectivity was significantly different between synchronous and asynchronous stroking only in the body conditions [F(1,20) = 14.8; P < 0.001]. In addition, these body-specific and stroking-dependent differences were modulated in the opposite fashion by first-person perspective. The post-hoc comparisons showed that for the up-group the correlation index was higher (P < 0.01) during synchronous (0.36) than asynchronous stroking (0.13); the opposite was found for the down-group: the correlation index was lower (P < 0.02) during synchronous (0.11) than asynchronous stroking (0.32). No other interactions or main effects were observed (all P > 0.27). This pattern of correlation index suggests that the connectivity between rTPJ and rIns1 reflected changes in self-location that further depended on the experienced direction of the first-person perspective, visuotactile stimulation and the presence of a body.
Fig. 4.
Connectivity patterns. Correlation index between right TPJ and right Insula (A), right TPJ and right SMA (B), left TPJ and right Insula (C). Synchronous (red) and asynchronous stroking (blue) are represented for each condition. Asterisks and error bars represent significant differences and standard errors, respectively.
The analysis of the correlation index between rTPJ seed region and rSMA target region revealed a significant interaction between perspective, object and stroking [F(1,20) = 25.1; P < 0.0001]. In particular, in the body conditions the correlation index for the up-group was higher (P < 0.004) during synchronous (0.27) than asynchronous stroking (0.09; Figure 4B), whereas for the down-group it was lower (P < 0.05) in the synchronous (0.07) than the asynchronous condition (0.19). However, in contrast to the pattern found between rTPJ and rIns1, the correlation index between rTPJ and rSMA was significantly different also in the no-body conditions, and thus was not body-specific. For the up-group, the correlation index was lower (P < 0.025) during the synchronous (0.046) than the asynchronous condition (0.17). For the down-group, it was higher (P < 0.03) during synchronous (0.18) than asynchronous stroking (0.05). No other interactions or main effects were observed (all P > 0.28). This pattern of correlation index indicates that the connectivity between rTPJ and rSMA is not body-specific, but depends on visuotactile synchrony and is affected by the direction of first-person perspective.
Left TPJ seed region
Within the network functionally connected to the lTPJ seed region, the correlation index was modulated as a function of the three experimental factors in the (contralateral) right insula [rIns2; F(1,20) = 19.2; P < 0.0003] and the (ipsilateral) left basal ganglia [lBG; F(1,20) = 21.1; P < 0.0002]. The rIns2 target region had 56% of the voxels within the insula, 19% within the rolandic operculum and 25% within the inferior frontal gyrus. The lBG target region had 95% of the voxels within the lBG (Table 1).
For the down-group the correlation index between lTPJ seed region and rIns2 target region (Figure 4C) was significantly different during synchronous and asynchronous stroking in both the body and the no-body conditions. Thus, in the body conditions the correlation index was lower (P < 0.001) during synchronous (0.07) than asynchronous stroking (0.29). The opposite was found in the no-body conditions [synchronous (0.33) higher (P < 0.001) than asynchronous (0.08)]. These comparisons were not significantly different in the up-group (all P > 0.16). These results show that the FC between lTPJ and rIns2 only in the down-group reflected stroking related differences that differed between body and no-body (control) conditions.
The significant interaction between perspective, object and stroking for the connectivity between lTPJ seed region and lBG target region was driven by the highest correlation index for the down-group during the synchronous stroking in the no-body condition (0.31), with respect to all the other conditions (all other correlation indices < 0.17; all P < 0.05). As the stroking-related difference in the correlation index between lTPJ and lBG was found only in the down-group and only in the no-body condition, we conclude that the connectivity between these two clusters does not reflect changes in self-location and first-person perspective.
FC between target and control regions
To test the specificity of the connectivity pattern between the seed and the target regions, we selected a control region (lDLPFC) and analyzed the correlation index between this control region and all the voxels in the brain, including the target regions identified previously. There were no significant main effects or interactions for the correlation index of lDLPFC with rIns1, rSMA, rIns2 or lBG (all P > 0.08). Thus, the control region did not show the condition-dependent pattern of connectivity that characterized the relationship between seed and target regions, i.e. the interaction between perspective, object and stroking.
DISCUSSION
Condition-independent connectivity
We found that rTPJ and lTPJ were bilaterally connected to partially overlapping brain networks including SMA, PMv, intraparietal cortex, occipitotemporal cortex and insula. Several of these regions have been linked to bodily self-consciousness (see Blanke, 2012 for review). Intraparietal and PM cortices process multisensory visuotactile and proprioceptive information both in primate (Duhamel et al., 1998; Graziano et al., 2000, respectively) and human brain (Macaluso et al., 2003; Baier and Karnath, 2008, respectively). Interestingly, the PMv regions reported here, stereotaxically correspond to the PMv regions associated with illusory body ownership due to multisensory conflicts (Petkova et al., 2011a). Illusory body ownership is further associated with intraparietal activity (Ehrsson et al., 2004; Lloyd et al., 2006; Kanayama et al., 2009; Evans and Blanke, 2013) and is reduced due to Transcranial magnetic stimulation over the intraparietal cortex (Kammers et al., 2009) or lesion of the PM cortex (Zeller et al., 2011). The regions that we defined as occipitotemporal cortex overlapped with the extrastriate body area (Astafiev et al., 2004), which encodes viewpoint (Chan et al., 2004; Saxe et al., 2006), visual self-recognition (Myers and Sowden, 2008) and mental own-body transformations (Blanke et al., 2010). The present FC-fMRI data show that processing of self-related multisensory bodily information recruits a bilateral network centered at TPJ and included the premotor, intraparietal and occipitotemporal cortices.
A partially overlapping network is involved in (visuospatial) attention reorienting (review in Corbetta and Shulman, 2011). Indeed stimulus-driven attentional processing activates not only TPJ (Shulman et al., 2010) but also insula, and inferior and medial frontal cortices (Corbetta and Shulman, 2002). However, visual attentional tasks have been associated also with decreased activity in TPJ (Shulman et al., 1997; Gusnard and Raichle, 2001). In addition, neurological patients suffering from visuospatial neglect due to lesions of TPJ (Karnath et al., 2001) show impairments in perspective taking as well as deficits in stimulus-driven reorienting attention (Rengachary et al., 2011). According to these data, though mechanisms related to bodily self-consciousness and attention reorienting seem to recruit common neural substrates, further investigations are required to disentangle their reciprocal influence.
Condition-dependent connectivity
Within the network functionally connected to left and right TPJ, we found that in right insula (rIns1 and rIns2 clusters) and SMA (rSMA cluster) the strength of connectivity was modulated by the experimental conditions, i.e. the level of correlated activity depended on self-location and first-person perspective. The sensitivity of FC to experimental manipulations (Hampson et al., 2004, 2006) and pathological conditions (Irwin et al., 2004; Waites et al., 2006; Negishi et al., 2011; Salomon et al., 2011; Salomons and Kucyi, 2011) has already been demonstrated. In this study, we found that the (ipsilateral) coupling of rTPJ activity with rIns1—and to a lesser extent with rSMA—reflected changes in experimentally manipulated self-location and first-person perspective. Thus, rIns1 showed a body-specific modulation of FC with right TPJ, further depending on the direction of first-person perspective. lTPJ–rIns2 (contralateral) coupling was less selective and only reflected changes in self-location and first-person perspective for down-looking participants. We note that the rIns1 target region was stereotaxically located in a more posterior part of the insular cortex with respect to the (more anterior) rIns2 target region. Finally, the rTPJ–rSMA connectivity pattern differed qualitatively with respect to the rTPJ-rIns1 pattern. In particular, the synchrony-dependent connectivity between rTPJ and rSMA varied as a function of the stroking not only in the body condition but also in the no-body control condition. Based on these data, we conclude that rSMA showed a first-person perspective dependent (but not body-specific) modulation of connectivity with rTPJ.
Right TPJ—right (posterior) insula
The insular cortex is anatomically connected to TPJ and premotor cortex (Augustine, 1996; Dijkerman and de Haan, 2007). Similarly to SMA, posterior insula (PI) is involved in several self-related processes, including self-attribution (Farrer and Frith, 2002), agency (Farrer et al., 2003b), self-recognition (Devue et al., 2007), first-person perspective (Vogeley et al., 2004) and body ownership (Tsakiris et al., 2007). Furthermore, impairments in self-attribution are associated with insular lesions (Baier and Karnath, 2005; Berti et al., 2005; Karnath et al., 2005). The present study reveals that the connectivity between right TPJ and right PI is body-selective and modulated by changes in self-location and first-person perspective. On the basis of their co-activation, the coupling between right TPJ and right PI has been considered important for self-orientation (Bottini et al., 1994a, 1994b; Bucher et al., 1998; Bense et al., 2001) and egocentric frame of reference (Fink et al., 2003). The present data support that the network involved in processing self-location and first-person perspective comprises TPJ and PI, and that there is a right-hemispheric dominance of these self-related mechanisms, confirming previous theories (Keenan et al., 2005; Vallar and Ronchi, 2009).
Left TPJ—right (anterior) insula
The right anterior insula (AI) is involved in encoding different physiological internal states (review in Craig, 2009), as well as interoceptive awareness (Craig, 2002), subjective feelings (de Greck et al., 2008) and self-recognition (Devue et al., 2007). On this basis, right AI has been considered part of a self-reflective network (Sridharan et al., 2008), important for maintaining a coherent sense of self (Craig, 2009). The present data show that the contralateral connectivity between left TPJ and right AI is modulated by changes in self-location due to visuotactile multisensory stimulation only in the down-looking perspective group and independent of whether a body was shown or not. Interestingly this experimentally induced downward perspective due to multisensory conflict (Ionta et al., 2011b) corresponds to the illusory perception reported by neurological patients suffering from out-of-body experiences (Blanke et al., 2004) and may point to an implication of right AI in encoding related multisensory information (Bushara et al., 2001). The coupling between TPJ and AI is crucial in self-related multisensory mechanisms linking sensory stimulation to conscious awareness (Corbetta et al., 2008). The data of this study support the right-lateralized predominance of the TPJ–AI complex in processing self-location and first-person perspective. Such right-lateralized predominance has been associated with self-awareness (Critchley et al., 2004), supporting the role of right AI in self-related processing (Dijkerman and de Haan, 2007; Craig, 2009).
Right TPJ—right SMA
Similarly to TPJ, SMA is involved in self-awareness (Boly et al., 2007; Owen et al., 2007; Monti et al., 2010) as well as in a wide range of self-related mechanisms, including memory (Macrae et al., 2004), language (Esslen et al., 2008), and personality (Kjaer et al., 2002). Abnormalities in SMA activity are associated with deficits in self-consciousness (Heydrich et al., 2010; Lopez et al., 2010). In addition, thanks to its multimodal nature (Mukamel et al., 2010) and similarly to TPJ (Rodriguez Moreno et al., 2010), SMA encodes global body-related visuotactile multisensory conflicts inducing illusory self-location (Lenggenhager et al., 2011). The present FC-fMRI data further extend these findings by showing that the condition-dependent modulation of connectivity between right TPJ and right SMA reflects synchrony-related and first-person-dependent processing of conflicting multisensory visuotactile information. This suggests that the coupling between right TPJ and SMA reflects changes in the conscious first-person perspective.
Taken together, our findings point to a specialized network involved in computations of bodily self-consciousness. The approach of using condition-independent connectivity based upon regions of interest derived from functional (condition-related) activations has several advantages. First, it allows investigating, in the same subjects, brain networks which are functionally connected, decoupled from the activations associated with the experimental design. This has been shown to be effective in detecting cortical networks related to non-bodily self-related processes like episodic memory or personal preferences (e.g. Sestieri et al., 2011; Salomon et al., 2013), but not tested for bodily self-consciousness. Second, the use of activity-defined ROIs derived from the same participants allows guiding the analysis of condition-independent connectivity, which is typically performed using data-driven methods (e.g. Greicius et al., 2008; Jafri et al., 2008). This procedure allows targeting specific regions related to particular cognitive processes, within subjects. In contrast to traditional fMRI activation analysis, the FC method used here allowed us to identify the network involved in processing specific components of bodily self-consciousness. In this vein, our findings showed that the network condition-dependently connected to bilateral TPJ included insula and SMA, which were shown to be involved in multisensory processing associated with self-location (review in Blanke, 2012) but so far were not included in a broader network. FC has been suggested to relate to structural-anatomical connectivity (Sporns et al., 2000; Greicius et al., 2009; Honey et al., 2009); hence, the correlations revealed in this study may indicate a functional structural network involved in the formation of bodily self-consciousness. The condition-related modulations reported here suggest that within a generalized network, heightened correlated fluctuations between specific nodes may be related to distinctive components of bodily self-consciousness.
CONCLUSIONS
Here, we report three main findings. First, independent of changes in self-location and first-person perspective, TPJ activity is coupled with a bilateral brain network including SMA as well as premotor, parietal, occipitotemporal and insular cortices. Second, a specific brain network encodes two components of self-consciousness, namely self-location and first-person perspective. Third, this network is right-hemispheric predominant and comprises bilateral TPJ, right insula and right SMA.
Based on these data, we propose that a right-lateralized brain network is responsible for processing multisensory information associated with self-location and first-person perspective as sub-components of self-consciousness, and that the modulations in the intensity of experimentally induced changes in bodily self-consciousness are reflected in the coupling within a specific brain network including bilateral TPJ, right insula and right SMA. We further suggest that in bodily illusions leading to body-specific changes in self-location and first-person perspective, the right insula exchanges information with both left and right TPJ. Conversely, when encoding coherent visuo-tactile multisensory information regardless the presence of a human body, the right SMA is connected with only the right TPJ.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This work was supported by the Bertarelli Foundation, the Swiss National Science Foundation (grant #513225), the Leenaards and the Jeantet Foundations, and the Centre d'Imagerie BioMédicale (CIBM) of the University of Lausanne (UNIL), the Swiss Federal Institute of Technology Lausanne (EPFL), the University of Geneva (UniGe), the Centre Hospitalier Universitaire Vaudois (CHUV), the Hôpitaux Universitaires de Genève (HUG).
Footnotes
The first two authors contributed equally to this work.
REFERENCES
- Andrews-Hanna JR. The brain's default network and its adaptive role in internal mentation. Neuroscientist. 2012;18(3):251–70. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspell JE, Lavanchy T, Lenggenhager B, Blanke O. Seeing the body modulates audiotactile integration. European Journal of Neuroscience. 2010;31(10):1868–73. doi: 10.1111/j.1460-9568.2010.07210.x. [DOI] [PubMed] [Google Scholar]
- Aspell JE, Lenggenhager B, Blanke O. Keeping in touch with one's self: multisensory mechanisms of self-consciousness. PLoS One. 2009;4(8):e6488. doi: 10.1371/journal.pone.0006488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astafiev SV, Stanley CM, Shulman GL, Corbetta M. Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nature Neuroscience. 2004;7(5):542–8. doi: 10.1038/nn1241. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research. Brain Research Reviews. 1996;22(3):229–44. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Baier B, Karnath HO. Incidence and diagnosis of anosognosia for hemiparesis revisited. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76(3):358–61. doi: 10.1136/jnnp.2004.036731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier B, Karnath HO. Tight link between our sense of limb ownership and self-awareness of actions. Stroke. 2008;39(2):486–8. doi: 10.1161/STROKEAHA.107.495606. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS. Statistical criteria in FMRI studies of multisensory integration. Neuroinformatics. 2005;3(2):93–113. doi: 10.1385/NI:3:2:093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI) Journal of Neurophysiology. 2001;85(2):886–99. doi: 10.1152/jn.2001.85.2.886. [DOI] [PubMed] [Google Scholar]
- Berti A, Bottini G, Gandola M, et al. Shared cortical anatomy for motor awareness and motor control. Science. 2005;309(5733):488–91. doi: 10.1126/science.1110625. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blanke O. Multisensory brain mechanisms of bodily self-consciousness. Nature Reviews. Neuroscience. 2012;13(8):556–571. doi: 10.1038/nrn3292. [DOI] [PubMed] [Google Scholar]
- Blanke O, Ionta S, Fornari E, Mohr C, Maeder P. Mental imagery for full and upper human bodies: common right hemisphere activations and distinct extrastriate activations. Brain Topography. 2010;23(3):321–32. doi: 10.1007/s10548-010-0138-x. [DOI] [PubMed] [Google Scholar]
- Blanke O, Landis T, Spinelli L, Seeck M. Out-of-body experience and autoscopy of neurological origin. Brain. 2004;127(Pt 2):243–58. doi: 10.1093/brain/awh040. [DOI] [PubMed] [Google Scholar]
- Blanke O, Metzinger T. Full-body illusions and minimal phenomenal selfhood. Trends in Cognitive Sciences. 2009;13(1):7–13. doi: 10.1016/j.tics.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Boly M, Coleman MR, Davis MH, et al. When thoughts become action: an fMRI paradigm to study volitional brain activity in non-communicative brain injured patients. Neuroimage. 2007;36(3):979–92. doi: 10.1016/j.neuroimage.2007.02.047. [DOI] [PubMed] [Google Scholar]
- Bottini G, Corcoran R, Sterzi R, et al. The role of the right hemisphere in the interpretation of figurative aspects of language. A positron emission tomography activation study. Brain. 1994a;117(Pt 6):1241–53. doi: 10.1093/brain/117.6.1241. [DOI] [PubMed] [Google Scholar]
- Bottini G, Sterzi R, Paulesu E, et al. Identification of the central vestibular projections in man: a positron emission tomography activation study. Experimental Brain Research. 1994b;99(1):164–9. doi: 10.1007/BF00241421. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Cohen J. Rubber hands ‘feel' touch that eyes see. Nature. 1998;391(6669):756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex. 1975;11(3):230–8. doi: 10.1016/s0010-9452(75)80005-0. [DOI] [PubMed] [Google Scholar]
- Bucher SF, Dieterich M, Wiesmann M, et al. Cerebral functional magnetic resonance imaging of vestibular, auditory, and nociceptive areas during galvanic stimulation. Annals of Neurology. 1998;44(1):120–5. doi: 10.1002/ana.410440118. [DOI] [PubMed] [Google Scholar]
- Bushara KO, Grafman J, Hallett M. Neural correlates of auditory-visual stimulus onset asynchrony detection. The Journal of Neuroscience. 2001;21(1):300–4. doi: 10.1523/JNEUROSCI.21-01-00300.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AW, Peelen MV, Downing PE. The effect of viewpoint on body representation in the extrastriate body area. Neuroreport. 2004;15(15):2407–10. doi: 10.1097/00001756-200410250-00021. [DOI] [PubMed] [Google Scholar]
- Christoff K, Cosmelli D, Legrand D, Thompson E. Specifying the self for cognitive neuroscience. Trends in Cognitive Sciences. 2011;15(3):104–12. doi: 10.1016/j.tics.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews. Neuroscience. 2002;3(3):201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu Rev Neurosci. 2011;34:569–99. doi: 10.1146/annurev-neuro-061010-113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews. Neuroscience. 2002;3(8):655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews. Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- David N, Bewernick BH, Cohen MX, et al. Neural representations of self versus other: visual-spatial perspective taking and agency in a virtual ball-tossing game. Journal of Cognitive Neuroscience. 2006;18(6):898–910. doi: 10.1162/jocn.2006.18.6.898. [DOI] [PubMed] [Google Scholar]
- David N, Cohen MX, Newen A, et al. The extrastriate cortex distinguishes between the consequences of one's own and others' behavior. Neuroimage. 2007;36(3):1004–14. doi: 10.1016/j.neuroimage.2007.03.030. [DOI] [PubMed] [Google Scholar]
- de Greck M, Rotte M, Paus R, et al. Is our self based on reward? Self-relatedness recruits neural activity in the reward system. Neuroimage. 2008;39(4):2066–75. doi: 10.1016/j.neuroimage.2007.11.006. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Roelofs K, Toni I. Increased self-monitoring during imagined movements in conversion paralysis. Neuropsychologia. 2007;45(9):2051–8. doi: 10.1016/j.neuropsychologia.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Toni I, Roelofs K. Altered connectivity between prefrontal and sensorimotor cortex in conversion paralysis. Neuropsychologia. 2010;48(6):1782–8. doi: 10.1016/j.neuropsychologia.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Delnooz CC, Helmich RC, Medendorp WP, Van de Warrenburg BP, Toni I. Writer's cramp: Increased dorsal premotor activity during intended writing. Human Brain Mapping. 2013;34(3):613–25. doi: 10.1002/hbm.21464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delnooz CC, Helmich RC, Toni I, van de Warrenburg BP. Reduced parietal connectivity with a premotor writing area in writer's cramp. Movement Disorders. 2012;27(11):1425–31. doi: 10.1002/mds.25029. [DOI] [PubMed] [Google Scholar]
- Devue C, Collette F, Balteau E, et al. Here I am: the cortical correlates of visual self-recognition. Brain Research. 2007;1143:169–82. doi: 10.1016/j.brainres.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Dijkerman HC, de Haan EH. Somatosensory processes subserving perception and action. The Behavioural and Brain Science. 2007;30(2):189–201; discussion 201-39. doi: 10.1017/S0140525X07001392. [DOI] [PubMed] [Google Scholar]
- Dodel S, Golestani N, Pallier C, Elkouby V, Le Bihan D, Poline JB. Condition-dependent functional connectivity: syntax networks in bilinguals. Philosphical Transactions of the Royal Society of London Series B Biological Sciences. 2005;360(1457):921–35. doi: 10.1098/rstb.2005.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. Journal of Neurophysiology. 1998;79(1):126–36. doi: 10.1152/jn.1998.79.1.126. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH. The experimental induction of out-of-body experiences. Science. 2007;317(5841):1048. doi: 10.1126/science.1142175. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Spence C, Passingham RE. That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science. 2004;305(5685):875–7. doi: 10.1126/science.1097011. [DOI] [PubMed] [Google Scholar]
- Esslen M, Metzler S, Pascual-Marqui R, Jancke L. Pre-reflective and reflective self-reference: a spatiotemporal EEG analysis. Neuroimage. 2008;42(1):437–49. doi: 10.1016/j.neuroimage.2008.01.060. [DOI] [PubMed] [Google Scholar]
- Evans N, Blanke O. Shared electrophysiology mechanisms of body ownership and motor imagery. Neuroimage. 2013;64:216–28. doi: 10.1016/j.neuroimage.2012.09.027. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, et al. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35(1):396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer C, Franck N, Georgieff N, Frith CD, Decety J, Jeannerod M. Modulating the experience of agency: a positron emission tomography study. Neuroimage. 2003a;18(2):324–33. doi: 10.1016/s1053-8119(02)00041-1. [DOI] [PubMed] [Google Scholar]
- Farrer C, Franck N, Paillard J, Jeannerod M. The role of proprioception in action recognition. Consciousness and Cognition. 2003b;12(4):609–19. doi: 10.1016/s1053-8100(03)00047-3. [DOI] [PubMed] [Google Scholar]
- Farrer C, Frith CD. Experiencing oneself vs another person as being the cause of an action: the neural correlates of the experience of agency. Neuroimage. 2002;15(3):596–603. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Weiss PH, et al. Performing allocentric visuospatial judgments with induced distortion of the egocentric reference frame: an fMRI study with clinical implications. Neuroimage. 2003;20(3):1505–17. doi: 10.1016/j.neuroimage.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Frith CD, Frackowiak RS, Dolan RJ. The functional roles of prefrontal cortex in episodic memory. II. Retrieval. Brain. 1998;121(Pt 7):1249–56. doi: 10.1093/brain/121.7.1249. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews. Neuroscience. 2007;8(9):700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage. 1995a;2(2):166–72. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, et al. Analysis of fMRI time-series revisited. Neuroimage. 1995b;2(1):45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Frith C. The self in action: lessons from delusions of control. Consciousness and Cognition. 2005;14(4):752–70. doi: 10.1016/j.concog.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50(4):531–4. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Galati G, Committeri G, Spitoni G, et al. A selective representation of the meaning of actions in the auditory mirror system. Neuroimage. 2008;40(3):1274–86. doi: 10.1016/j.neuroimage.2007.12.044. [DOI] [PubMed] [Google Scholar]
- Gavrilescu M, Stuart GW, Rossell S, et al. Functional connectivity estimation in fMRI data: influence of preprocessing and time course selection. Human Brain Mapping. 2008;29(9):1040–52. doi: 10.1002/hbm.20446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giummarra MJ, Bradshaw JL, Nicholls ME, Hilti LM, Brugger P. Body integrity identity disorder: deranged body processing, right fronto-parietal dysfunction, and phenomenological experience of body incongruity. Neuropsychology Review. 2011;21(4):320–33. doi: 10.1007/s11065-011-9184-8. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Cooke DF, Taylor CS. Coding the location of the arm by sight. Science. 2000;290(5497):1782–6. doi: 10.1126/science.290.5497.1782. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Kiviniemi V, Tervonen O, et al. Persistent default-mode network connectivity during light sedation. Human Brain Mapping. 2008;29(7):839–47. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral Cortex. 2009;19(1):72–8. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews. Neuroscience. 2001;2(10):685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. The Journal of Neuroscience. 2006;26(51):13338–43. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Olson IR, Leung HC, Skudlarski P, Gore JC. Changes in functional connectivity of human MT/V5 with visual motion input. Neuroreport. 2004;15(8):1315–19. doi: 10.1097/01.wnr.0000129997.95055.15. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Human Brain Mapping. 2002;15(4):247–62. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Ortiz H, Fornito A, Pantelis C, Yucel M. Modulation of brain resting-state networks by sad mood induction. PLoS One. 2008;3(3):e1794. doi: 10.1371/journal.pone.0001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Nusbaum HC, Small SL. Task-dependent organization of brain regions active during rest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(26):10841–6. doi: 10.1073/pnas.0903253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays WL. Statistics. 3rd edn. New York: Holt, Rinehart, and Winston; 1981. [Google Scholar]
- Heatherton TF. Neuroscience of self and self-regulation. Annual Review of Psychology. 2011;62:363–90. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience. 2006;1(1):18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydrich L, Dieguez S, Grunwald T, Seeck M, Blanke O. Illusory own body perceptions: case reports and relevance for bodily self-consciousness. Consciousness and Cognition. 2010;19(3):702–10. doi: 10.1016/j.concog.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, et al. Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(6):2035–40. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indovina I, Maffei V, Pauwels K, Macaluso E, Orban GA, Lacquaniti F. Simulated self-motion in a visual gravity field: sensitivity to vertical and horizontal heading in the human brain. Neuroimage. 2013;71:114–24. doi: 10.1016/j.neuroimage.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Ionta S, Gassert R, Blanke O. Multi-sensory and sensorimotor foundation of bodily self-consciousness—an interdisciplinary approach. Frontiers in Psychology. 2011a;2:383. doi: 10.3389/fpsyg.2011.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionta S, Heydrich L, Lenggenhager B, et al. Multisensory mechanisms in temporo-parietal cortex support self-location and first-person perspective. Neuron. 2011b;70(2):363–74. doi: 10.1016/j.neuron.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Irwin W, Anderle MJ, Abercrombie HC, Schaefer SM, Kalin NH, Davidson RJ. Amygdalar interhemispheric functional connectivity differs between the non-depressed and depressed human brain. Neuroimage. 2004;21(2):674–86. doi: 10.1016/j.neuroimage.2003.09.057. [DOI] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39(4):1666–81. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TB, Bandettini PA, Kenworthy L, et al. Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. Neuroimage. 2010;49(1):401–14. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammers MP, Verhagen L, Dijkerman HC, Hogendoorn H, De Vignemont F, Schutter DJ. Is this hand for real? Attenuation of the rubber hand illusion by transcranial magnetic stimulation over the inferior parietal lobule. Journal of Cognitive Neuroscience. 2009;21(7):1311–20. doi: 10.1162/jocn.2009.21095. [DOI] [PubMed] [Google Scholar]
- Kanayama N, Sato A, Ohira H. The role of gamma band oscillations and synchrony on rubber hand illusion and crossmodal integration. Brain and Cognition. 2009;69(1):19–29. doi: 10.1016/j.bandc.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Baier B, Nagele T. Awareness of the functioning of one's own limbs mediated by the insular cortex? The Journal of Neuroscience. 2005;25(31):7134–38. doi: 10.1523/JNEUROSCI.1590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO, Ferber S, Himmelbach M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature. 2001;411(6840):950–3. doi: 10.1038/35082075. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Rubio J, Racioppi C, Johnson A, Barnacz A. The right hemisphere and the dark side of consciousness. Cortex. 2005;41(5):695–704. doi: 10.1016/s0010-9452(08)70286-7. discussion 731–4. [DOI] [PubMed] [Google Scholar]
- Keller CJ, Bickel S, Honey CJ, et al. Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. The Journal of Neuroscience. 2013;33(15):6333–42. doi: 10.1523/JNEUROSCI.4837-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Uddin LQ, Shehzad Z, et al. Broca's region: linking human brain functional connectivity data and non-human primate tracing anatomy studies. European Journal of Neuroscience. 2010;32(3):383–98. doi: 10.1111/j.1460-9568.2010.07279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage. 2002;17(2):1080–6. [PubMed] [Google Scholar]
- Kucyi A, Hodaie M, Davis KD. Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience- and attention-related brain networks. Journal of Neurophysiology. 2012;108(12):3382–92. doi: 10.1152/jn.00674.2012. [DOI] [PubMed] [Google Scholar]
- Laureys S, Tononi G. The Neurology of Consciousness: Cognitive Neuroscience and Neuropathology. Amsterdam/Boston: Elsevier/Academic Press; 2009. [Google Scholar]
- Lenggenhager B, Halje P, Blanke O. Alpha band oscillations correlate with illusory self-location induced by virtual reality. European Journal of Neuroscience. 2011;33(10):1935–43. doi: 10.1111/j.1460-9568.2011.07647.x. [DOI] [PubMed] [Google Scholar]
- Lenggenhager B, Mouthon M, Blanke O. Spatial aspects of bodily self-consciousness. Consciousness and Cognition. 2009;18(1):110–7. doi: 10.1016/j.concog.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Lenggenhager B, Tadi T, Metzinger T, Blanke O. Video ergo sum: manipulating bodily self-consciousness. Science. 2007;317(5841):1096–9. doi: 10.1126/science.1143439. [DOI] [PubMed] [Google Scholar]
- Lloyd D, Morrison I, Roberts N. Role for human posterior parietal cortex in visual processing of aversive objects in peripersonal space. Journal of Neurophysiology. 2006;95(1):205–14. doi: 10.1152/jn.00614.2005. [DOI] [PubMed] [Google Scholar]
- Lopez C, Heydrich L, Seeck M, Blanke O. Abnormal self-location and vestibular vertigo in a patient with right frontal lobe epilepsy. Epilepsy and Behaviour. 2010;17(2):289–92. doi: 10.1016/j.yebeh.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7(2):119–32. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Driver J, Frith CD. Multimodal spatial representations engaged in human parietal cortex during both saccadic and manual spatial orienting. Current Biology. 2003;13(12):990–9. doi: 10.1016/s0960-9822(03)00377-4. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14(6):647–54. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(32):13170–5. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Sallet J, Schuffelgen U, Jbabdi S, Toni I, Rushworth MF. Connectivity-based subdivisions of the human right “temporoparietal junction area”: evidence for different areas participating in different cortical networks. Cerebral Cortex. 2012;22(8):1894–903. doi: 10.1093/cercor/bhr268. [DOI] [PubMed] [Google Scholar]
- Martuzzi R, Ramani R, Qiu M, Rajeevan N, Constable RT. Functional connectivity and alterations in baseline brain state in humans. Neuroimage. 2010;49(1):823–34. doi: 10.1016/j.neuroimage.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM) Neuroimage. 1995;2(2):89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- McCleery JP, Surtees AD, Graham KA, Richards JE, Apperly IA. The neural and cognitive time course of theory of mind. The Journal of Neuroscience. 2011;31(36):12849–54. doi: 10.1523/JNEUROSCI.1392-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti MM, Vanhaudenhuyse A, Coleman MR, et al. Willful modulation of brain activity in disorders of consciousness. The New England Journal of Medicine. 2010;362(7):579–89. doi: 10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. Single-neuron responses in humans during execution and observation of actions. Current Biology. 2010;20(8):750–6. doi: 10.1016/j.cub.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A, Sowden PT. Your hand or mine? The extrastriate body area. Neuroimage. 2008;42(4):1669–77. doi: 10.1016/j.neuroimage.2008.05.045. [DOI] [PubMed] [Google Scholar]
- Negishi M, Martuzzi R, Novotny EJ, Spencer DD, Constable RT. Functional MRI connectivity as a predictor of the surgical outcome of epilepsy. Epilepsia. 2011;52(9):1733–40. doi: 10.1111/j.1528-1167.2011.03191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Using functional magnetic resonance imaging to detect covert awareness in the vegetative state. Archives of Neurology. 2007;64(8):1098–102. doi: 10.1001/archneur.64.8.1098. [DOI] [PubMed] [Google Scholar]
- Pan WJ, Thompson GJ, Magnuson ME, Jaeger D, Keilholz S. Infraslow LFP correlates to resting-state fMRI BOLD signals. Neuroimage. 2013;74:288–97. doi: 10.1016/j.neuroimage.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Maquet P, Peigneux P, et al. Neural mechanisms involved in the detection of our first name: a combined ERPs and PET study. Neuropsychologia. 2005;43(1):12–9. doi: 10.1016/j.neuropsychologia.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Petkova VI, Bjornsdotter M, Gentile G, Jonsson T, Li TQ, Ehrsson HH. From part-to whole-body ownership in the multisensory brain. Current Biology. 2011a;21(13):1118–22. doi: 10.1016/j.cub.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Petkova VI, Khoshnevis M, Ehrsson HH. The perspective matters! Multisensory integration in ego-centric reference frames determines full-body ownership. Frontiers in Psychology. 2011b;2:35. doi: 10.3389/fpsyg.2011.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengachary J, He BJ, Shulman GL, Corbetta M. A behavioral analysis of spatial neglect and its recovery after stroke. Frontiers in Human Neuroscience. 2011;5:29. doi: 10.3389/fnhum.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Moreno D, Schiff ND, Giacino J, Kalmar K, Hirsch J. A network approach to assessing cognition in disorders of consciousness. Neurology. 2010;75(21):1871–8. doi: 10.1212/WNL.0b013e3181feb259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nature Neuroscience. 2001;4(5):546–50. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Salomon R, Bleich-Cohen M, Hahamy-Dubossarsky A, et al. Global functional connectivity deficits in schizophrenia depend on behavioral state. The Journal of Neuroscience. 2011;31(36):12972–81. doi: 10.1523/JNEUROSCI.2987-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R, Levy DR, Malach R. Deconstructing the default: cortical subdivision of the default mode/intrinsic system during self-related processing. Human Brain Mapping. 2013 doi: 10.1002/hbm.22268. doi: 10.1002/hbm.22268 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R, Malach R, Lamy D. Involvement of the intrinsic/default system in movement-related self recognition. PLoS One. 2009;4(10):e7527. doi: 10.1371/journal.pone.0007527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Kucyi A. Does meditation reduce pain through a unique neural mechanism? The Journal of Neuroscience. 2011;31(36):12705–7. doi: 10.1523/JNEUROSCI.2843-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Jamal N, Powell L. My body or yours? The effect of visual perspective on cortical body representations. Cerebral Cortex. 2006;16(2):178–82. doi: 10.1093/cercor/bhi095. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. The Journal of Neuroscience. 2011;31(12):4407–20. doi: 10.1523/JNEUROSCI.3335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Corbetta M, Buckner RL, et al. Top-down modulation of early sensory cortex. Cerebral Cortex. 1997;7(3):193–206. doi: 10.1093/cercor/7.3.193. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Pope DL, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. The Journal of Neuroscience. 2010;30(10):3640–51. doi: 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Calhoun VD, Hampson M, Skudlarska BA, Pearlson G. Measuring brain connectivity: diffusion tensor imaging validates resting state temporal correlations. Neuroimage. 2008;43(3):554–61. doi: 10.1016/j.neuroimage.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Edelman GM. Theoretical neuroanatomy: relating anatomical and functional connectivity in graphs and cortical connection matrices. Cerebral Cortex. 2000;10(2):127–41. doi: 10.1093/cercor/10.2.127. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(34):12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Rosen HJ, Allison S, Miller BL, Levenson RW. Self-conscious emotion deficits in frontotemporal lobar degeneration. Brain. 2006;129(Pt 9):2508–16. doi: 10.1093/brain/awl145. [DOI] [PubMed] [Google Scholar]
- Tsakiris M. My body in the brain: a neurocognitive model of body-ownership. Neuropsychologia. 2010;48(3):703–12. doi: 10.1016/j.neuropsychologia.2009.09.034. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Haggard P. The rubber hand illusion revisited: visuotactile integration and self-attribution. Journal of Experimental Psychology. Human Perception and Performance. 2005;31(1):80–91. doi: 10.1037/0096-1523.31.1.80. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Hesse MD, Boy C, Haggard P, Fink GR. Neural signatures of body ownership: a sensory network for bodily self-consciousness. Cerebral Cortex. 2007;17(10):2235–44. doi: 10.1093/cercor/bhl131. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vallar G, Ronchi R. Somatoparaphrenia: a body delusion. A review of the neuropsychological literature. Experimental Brain Research. 2009;192(3):533–51. doi: 10.1007/s00221-008-1562-y. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28(3):635–62. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Fink GR. Neural correlates of the first-person-perspective. Trends in Cognitive Sciences. 2003;7(1):38–42. doi: 10.1016/s1364-6613(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR. Neural correlates of first-person perspective as one constituent of human self-consciousness. Journal of Cognitive Neuroscience. 2004;16(5):817–27. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- Waites AB, Briellmann RS, Saling MM, Abbott DF, Jackson GD. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Annals of Neurology. 2006;59(2):335–43. doi: 10.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity. 2012;2(3):125–41. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Zeller D, Gross C, Bartsch A, Johansen-Berg H, Classen J. Ventral premotor cortex may be required for dynamic changes in the feeling of limb ownership: a lesion study. The Journal of Neuroscience. 2011;31(13):4852–7. doi: 10.1523/JNEUROSCI.5154-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.