Abstract
Theory-of-mind (ToM) ability is foundational for successful social relationships, and dependent on a neurocognitive system, which includes temporoparietal junction and medial prefrontal cortex. Schizophrenia is associated with ToM impairments, and initial studies demonstrate similar, though more subtle deficits, in unaffected first-degree relatives, indicating that ToM deficits are a potential biomarker for the disorder. Importantly, the social consequences of ToM deficits could create an additional vulnerability factor for individuals at familial high risk (FHR). However, behavioral studies of ToM are inconsistent and virtually nothing is known about the neural basis of ToM in FHR or the relationship between ToM and social functioning. Here, FHR and non-FHR control participants underwent functional MRI scanning while reasoning about a story character’s thoughts, emotions or physical appearance. Afterwards, participants completed a 28-day online ‘daily-diary’ questionnaire in which they reported daily social interactions and degree of ToM reasoning. FHR participants demonstrated less neural activity in bilateral temporoparietal junction when reasoning about thoughts and emotions. Moreover, across all participants, the degree of neural activity during ToM reasoning predicted several aspects of daily social behavior. Results suggest that vulnerability for schizophrenia is associated with neurocognitive deficits in ToM and the degree of deficit is related to day-to-day social functioning.
Keywords: theory of mind, social functioning, schizophrenia, familial high risk, fMRI
INTRODUCTION
Social dysfunction is arguably one of the most important and least understood risk factors for schizophrenia. Young people with a first-degree relative with schizophrenia [i.e. those at familial high risk (FHR)] have less social interest and activities, worse peer relationships, and fewer friends than people without familial risk (Dworkin et al., 1991, 1993; Cornblatt et al., 1992; Hans et al., 2000; Miller et al., 2002; Johnstone et al., 2005; Glatt et al., 2006). These social problems are apparent in childhood/pre-adolescence and prospectively predict schizophrenia diagnosis (Tarbox and Pogue-Geile, 2008). Pre-morbid social deficits might not only indicate underlying neurobiological abnormalities associated with schizophrenia pathology but might also contribute to illness progression. Lack of social support exposes vulnerable individuals to the negative impact of stressful life-events, and both interpersonal conflicts and social isolation precipitate and exacerbate psychotic symptoms (Horan et al., 2006; Hoffman, 2007; Hooley, 2007). Together the evidence suggests that, among FHR, a population already at elevated risk for psychosis (Gottesman, 1991), social functioning could serve as a marker of vulnerability and a target for preventive intervention. However, most social functioning assessments reflect the long-term outcome of social interactions overtime, and little is known about the behavioral and neural mechanisms that influence day-to-day social interactions and their contribution to functional outcome in FHR. As a result, it is unclear what social phenomenon would best indicate psychosis-risk and provide the greatest benefit if improved through preventive intervention.
Theory of mind (ToM), the ability to identify the mental states of others (i.e. beliefs, intentions, emotions) and understand how those mental states motivate behavior, is foundational for successful social interactions. ToM skills can be assessed with laboratory-based experiments, such as the False-Belief task, that require predicting a person’s behavior based on their mental state (Wimmer and Perner, 1983). Such tasks require mental state reasoning (i.e. using mental state information to predict future mental states or actions) which is a skill that engages different cognitive and neural processes than mental state decoding (i.e. detecting mental states based on currently available information, such as facial affect) (Tager-Flusberg and Sullivan, 2000; Sabbagh, 2004). Mental state reasoning recruits a network of neural regions, including medial prefrontal cortex (MPFC), temporoparietal junction (TPJ) [including posterior superior temporal sulcus (STS) and superior temporal gyrus), and precuneus (PC) (Van Overwalle, 2009; Mar, 2011). Individuals with schizophrenia exhibit marked behavioral impairment in ToM, particularly mental state reasoning (Sprong et al., 2007; Bora et al., 2009a), and its concomitant neural substrates, particularly TPJ and MPFC (Brunet et al., 2003; Brune et al., 2008; Lee et al., 2011; Das et al., 2012; Dodell-Feder et al., 2014). Among individuals with schizophrenia, behavioral performance on ToM tasks significantly predicts functional outcome (Fett et al., 2011), and neural deficits in TPJ and MPFC are related to both performance on ToM tasks and engagement in ToM to enhance relationships in daily life (Hooker et al., 2011).
Behavioral impairment in ToM has also been observed in individuals at clinical high risk (Kim et al., 2011; Green et al., 2012; Thompson et al., 2012) and FHR for schizophrenia (Janssen et al., 2003; Versmissen et al., 2008; Anselmetti et al., 2009; de Achaval et al., 2010; Montag et al., 2012). Preliminary evidence also exists that individuals at clinical high risk exhibit neural abnormalities in the network supporting mental state reasoning (Brune et al., 2011). Together, these data suggest that ToM is not only an intermediate phenotype for schizophrenia-spectrum disorders (Gottesman and Gould, 2003; Bora et al., 2009b) but also a potentially remediable social cognitive skill that influences social interactions and their cumulative effect on functional outcome (Kurtz and Richardson, 2012).
Although ToM has been proposed as a vulnerability marker (Bora and Pantelis, 2013), the current data in FHR and other HR populations are inconclusive. Several studies found no behavioral ToM differences between FHR and non-FHR (Kelemen et al., 2004; Gibson et al., 2010; Bora and Pantelis, 2013). Furthermore, no FHR studies have linked ToM processing to social functioning, or neural measures to either ToM performance or social functioning. One problem is that most behavioral ToM measures are not adequately challenging for adults without severe mental illness, and, therefore, not sensitive enough to accurately characterize the range of individual abilities or detect subtle group differences when they exist (see Dodell-Feder et al., 2013 for a discussion). Additionally, FHR may be differentially impaired depending on the type of ToM task used. For example, a recent meta-analysis (Bora and Pantelis, 2013) indicated that FHR are more likely to exhibit impairment on mental state reasoning tasks than mental state decoding tasks (e.g. de Achaval et al., 2010; Eack et al., 2010b).
Presumably, ToM deficits in FHR stem from genetic influences on neural systems that support ToM processing, so neural measures may be more sensitive than behavioral measures. Nonetheless, focusing exclusively on group differences between FHR and non-FHR is problematic. Only a small percentage of FHR individuals will develop psychosis (Gottesman and Shields, 1982; McGuffin et al., 2004) or exhibit deficits on intermediate phenotypes (Keshavan et al., 2010), such as ToM. FHR individuals may also develop unique compensatory strategies that conceal behavioral deficits and/or obscure neuroimaging group effects. Consequently, although meaningful abnormalities exist, the group averages for FHR and non-FHR may not be significantly different.
The only FHR study that investigated neural function during ToM reasoning (Marjoram et al., 2006) found no neural activity differences between FHR and non-FHR controls. However, compared with FHR with past or current psychotic symptoms, FHR without psychotic symptoms had greater activity in dorsal MPFC as well as several regions not typically associated with ToM. FHR without psychotic symptoms also activated most regions more than non-FHR controls, suggesting that this subgroup of FHR recruited compensatory neural mechanisms (i.e. non-ToM processes) and over-activated regions due to additional effort and/or inefficient neural processing. These findings illustrate the variability of ToM-related neural dysfunction and the difficulty identifying its source. As FHR individuals demonstrate neural abnormalities in multiple brain regions associated with several cognitive domains, including language (Francis et al., 2012), working memory (Whitfield-Gabrieli et al., 2009) and cognitive-control (Becker et al., 2008), neural abnormalities during a ToM task could arise for a variety of reasons and have different effects on behavior. To understand the potential value of ToM assessments, it is essential to isolate neural activity specific to ToM processing (rather than activity reflecting a secondary process) and measure how variation in ToM-related activity predicts variation in real-life social behaviors that are supported by ToM skills.
This study used a multi-method approach that optimized assessment of individual differences to investigate whether ToM-related neural activity prospectively predicts social behavior over a 4-week period. While undergoing functional MRI (fMRI), young adult FHR and non-FHR control participants performed a mental state reasoning task that involved reading short vignettes about another person’s thoughts, emotions, or appearances, and used this information to judge the likelihood of future behavior (Saxe and Powell, 2006). Behavioral responses were made purposefully easy in order to avoid confounds which complicate data interpretation, such as between-group performance differences (Price and Friston, 1999). Appearance stories contained information about people and thus allowed us to control for the presence of general social information. ToM-related neural processing was isolated by measuring activity during thought and emotion stories relative to appearance stories; thus, yielding neural measures of cognitive ToM (i.e. reasoning about beliefs) and affective ToM (i.e. reasoning about emotions). Comparing emotion stories vs thought stories measured activity specific to affective ToM.
To obtain the best estimate of each individual’s ToM-related neural processing, we used the False-Belief task, which robustly activates the ToM network, to localize exact neural coordinates of ToM processing in each participant (Saxe and Kanwisher, 2003). Although brain regions recruited for ToM are well-defined, the coordinates of peak activity within those regions vary across individuals (Saxe et al., 2006). Thus, the use of individually tailored regions-of-interest (ROIs) allowed us to test our hypotheses in regions that were functionally defined as being selective for mental state information in each participant. We expected that ToM-related activity in the specific region that the participant uses for ToM would best predict that individual’s social behavior. After the scan, participants completed a 28-day structured daily-diary questionnaire in which, every evening, they answered questions about the quantity and quality of social encounters that day. Assessing social behavior the day it occurs is more accurate and ecologically valid than commonly used retrospective reports covering prior weeks or months (Myin-Germeys et al., 2009). Furthermore, the repeated assessment, each day for 28 days, provides a stable and reliable measure of each social phenomenon (Myin-Germeys et al., 2009). Daily-diary questions assessed engagement in ToM skills, such as perspective-taking, and other social behaviors that are, theoretically, influenced by ToM skills. We predicted: (i) FHR would have less ToM-related neural activity than non-FHR in key regions of the ToM network, including bilateral TPJ and MPFC; and (ii) across all participants, ToM-related neural activity will predict daily engagement in ToM processing and enhanced social functioning.
METHODS
Participants
Nineteen FHR and 18 non-FHR individuals participated for monetary compensation (Table 1). FHR status was defined as between 15 and 32 years of age (age-range of greatest psychosis-risk) with two or more affected relatives (at least one first-degree relative with schizophrenia or schizoaffective disorder and a second relative with a psychotic disorder). Requiring two affected relatives made it less likely that one ill relative was an environmental phenocopy or had a de novo mutation, thus increasing the certainty of genetic liability. FHR individuals were recruited through community advertisements and National Alliance on Mental Illness support meetings. Non-FHR had no family history of psychotic disorder, psychiatric hospitalization or suicide.
Table 1.
Participant characteristics and task performance
| Non-FHR | FHR | Between-group difference | |
|---|---|---|---|
| n | 18 | 19 | |
| Gender (male/female) | 4/14 | 5/14 | χ2(1, N = 37) = 0.08, P = 0.77 |
| Age | 26.2 (4.0) | 27.4 (3.9) | t(35) = 0.97, P = 0.34 |
| Education (years) | 16.3 (0.8) | 16.1 (1.5) | t(35) = 0.71, P = 0.48 |
| IQa | 118.3 (11.4) | 117.0 (9.8) | t(34) = 0.38, P = 0.71 |
| BDI-IIb | 1.9 (3.3) | 5.5 (5.4) | t(32) = 2.28, P = 0.03 |
| STAI-Stateb | 25.7 (5.6) | 27.7 (7.3) | t(32) = 0.89, P = 0.38 |
| STAI-Traitb | 29.1 (7.3) | 34.3 (8.7) | t(32) = 1.88, P = 0.07 |
| Lifetime Axis-I diagnosis (number of participants) | 2 | 9 | |
| MDD | — | 4 | |
| ADHD | — | 1 | |
| Substance abuse | 2 | — | |
| Comorbid diagnosesc | — | 4 | |
| SIPSd | |||
| Positive | 0.12 (0.33) | 2.83 (2.53) | U = 49.5, P < 0.001e |
| Negative | 0.06 (0.24) | 1.78 (1.96) | U = 64.0, P = 0.003 |
| Disorganized | 0.35 (0.61) | 2.00 (1.57) | U = 55.5, P = 0.001 |
| General | 0.24 (0.56) | 1.67 (1.78) | U = 76.0, P = 0.010 |
| Person-Description taska | |||
| Thought accuracy (%) | 90.2 (7.3) | 87.6 (12.3) | t(34) = 0.76, P = 0.45 |
| Emotion accuracy (%) | 96.5 (5.0) | 93.5 (9.7) | t(34) = 1.19, P = 0.25 |
| Appearance accuracy (%) | 94.0 (10.2) | 88.1 (13.9) | t(34) = 1.44, P = 0.16 |
| Thought RT (s) | 2.9 (0.4) | 3.2 (0.4) | t(34) = 1.79, P = 0.08 |
| Emotion RT (s) | 2.5 (0.5) | 2.7 (0.5) | t(34) = 1.19, P = 0.24 |
| Appearance RT (s) | 2.9 (0.5) | 3.0 (0.5) | t(34) = 1.27, P = 0.22 |
| False-Belief task | |||
| False-Belief accuracy (%) | 90.1 (10.7) | 88.5 (11.0) | t(35) = 0.47, P = 0.64 |
| False-Photo accuracy (%) | 90.0 (12.8) | 86.7 (13.1) | t(35) = 0.78, P = 0.44 |
| False-Belief RT (s) | 2.9 (0.5) | 3.3 (0.6) | t(35) = 2.55, P = 0.02 |
| False-Photo RT (s) | 3.0 (0.5) | 3.1 (0.5) | t(35) = 0.92, P = 0.37 |
When applicable, values represent means with standard deviations in parentheses. MDD, major depressive disorder; ADHD, attention deficit hyperactivity disorder. aData were not collected for one FHR participant. bData were not collected for two FHR and one non-FHR participant. cIndividuals were diagnosed with the following: MDD and anxiety (n = 2), MDD and ADHD (n = 1), MDD, anxiety, ADHD and substance abuse (n = 1). dData were not collected for 1 FHR and 1 non-FHR participant. eBecause these data were non-normally distributed, these variables were compared with nonparametric Mann–Whitney U-tests.
The Diagnostic Interview for Genetic Studies (Nurnberger et al., 1994) and Family Interview for Genetic Studies (Maxwell, 1996) were used to identify personal and family history of psychopathology. Exclusion criteria for all participants: past/current DSM-IV Axis-I psychotic disorder (schizophrenia, schizoaffective disorder, psychosis-NOS, substance-induced psychosis, or bipolar/major depressive disorder with psychotic features); past/current treatment with antipsychotics or mood stabilizers; IQ < 70, non-native English speaker, MRI contraindicators. FHR is associated with elevated rates of multiple psychiatric disorders (Erlenmeyer-Kimling et al., 1997; Kendler and Gardner, 1997; Chang et al., 2002; Keshavan et al., 2004); thus, to increase external validity of our sample, FHR and non-FHR participants were not excluded for past/current psychopathology other than disorders listed above.
Mood state was assessed with the Beck Depression Inventory-II (BDI-II) (Beck et al., 1996) and State-Trait Anxiety Inventory (STAI) (Speilberger, 1983). Psychotic-like symptoms and traits were measured with the Structured Interview for Prodromal Syndromes (SIPS) (Miller et al., 2003). IQ was assessed with Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). Participants gave written informed consent in accordance with Harvard University Institutional Review Board.
FMRI tasks
Person-Description
During scanning, participants read short stories1 describing either a protagonist’s thoughts, emotions or physical appearance (without reference to beliefs or emotions), and then judged (Yes/No) whether a statement made sense in the context of the story (Table 2) (Saxe and Powell, 2006). Stories were matched on low-level linguistic features including number of words/sentences, word frequency and Flesch reading-level (Flesch, 1948). Each trial included the story (10 s), Yes/No judgment (5 s), and fixation-cross/rest-period (12 s). Ten stories per condition were divided evenly across five functional runs.
Table 2.
Sample stimuli from the scanner tasks
| Task/Condition | Story | Judgment |
|---|---|---|
| Person-Description task | ||
| Thought | Ethan knew that his sister’s flight from San Francisco was delayed 10 h. Only one flight was delayed so much that night, so when he got to the airport, he knew that flight was hers. | Ethan’s sister is unlikely to use that airline again (Yes/No) |
| Emotion | Jessica really misses her father who had been sick in the hospital. When she goes to visit, the doctor informs her that her dad had died the night before. Jessica cries out in disbelief. | After calming down, Jessica goes to see her dad’s body (Yes/No) |
| Appearance | Hank was a heavy-set man with a gut that fell over his belt. He was balding and combed his blonde hair over the top of his head. His face was pleasant, with large brown eyes. | Because of his large gut, Hank’s back was always slightly bent forward (Yes/No) |
| False-Belief task | ||
| False-Belief | The morning of the high school dance, Barbara placed her high heel shoes under the dress and went shopping. That afternoon, her sister borrowed the shoes and later put them under Barbara’s bed. | Barbara gets ready assuming her shoes are under the dress (True/False) |
| False-Photograph | Old maps near the islands near Titan are displayed in the Maritime museum. Erosion has since taken its toll, leaving only the three largest islands. | Near Titan today, there are many islands (True/False) |
False-Belief
After Person-Description, participants completed two runs of the False-Belief task (Dodell-Feder et al., 2011) that required participants to read and make True/False judgments on 10 False-Belief and 10 False-Photograph/Map stories (Table 2) (Saxe and Kanwisher, 2003). False-Belief and False-Photograph stories both require the representation of false content. In False-Belief stories, the false content is beliefs; in False-Photograph stories, the false content is physical information. Each trial included the story (11 s), True/False question (6 s), and fixation-cross/rest-period (12 s). Each participant’s results from this task were used to identify individually defined ROIs.
For each task, stories were presented in a pseudo-randomized order; condition order was counterbalanced across runs and participants with two predetermined sequences. Stimuli were presented in white font on a black background with Matlab 7.6 using Psychophysics Toolbox extensions (Brainard, 1997; Kleiner et al., 2007). Accuracy and reaction time (RT) data were collected. Participants were instructed to perform as accurately as possible.
MRI acquisition and analysis
Data were collected on a 3Tesla Siemens scanner at Harvard University using a 12-channel head coil, and analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). Anatomical images were acquired using a T1-weighted multi-echo MPRAGE sequence (176 sagittal slices, slice thickness = 1.0 mm). Functional data were collected with echo-planar images acquired in the oblique-axial plane with whole-brain coverage (40 interleaved slices, 3 × 3 × 3 mm voxels, TE = 30 ms, TR = 2560 ms, flip angle = 85°, FOV = 216 mm × 216 mm, matrix size = 72 × 72). The first four volumes consisted of dummy scans discarded prior to analysis to ensure steady-state magnetization. Preprocessing included realignment to the mean functional image, coregistration of the anatomical image to the mean functional image, normalization to Montreal Neurological Institute (MNI) template-space, resampling into 2 × 2 × 2 mm voxels and smoothing with a 6 mm Gaussian kernel.
Person-Description
For each subject, hemodynamic response was modeled at the onset of each story (10 s duration) and convolved with the canonical hemodynamic response function. Yes/No judgments and movement parameters were included as nuisance regressors. Data were high-pass filtered (128 s). Contrast files were created for Thought > Appearance, Emotion > Appearance, Emotion > Thought and each condition vs baseline (which consisted of a central, white fixation-cross on black background).
We investigated recruitment of the ToM network within each group by conducting separate one-sample t-tests within FHR and within non-FHR participants for Thought > Appearance, Emotion > Appearance and Emotion > Thought. Group*condition interactions were investigated with 2 × 2 mixed-design analyses of variance (ANOVAs) using SPM8 full factorial models. Three ANOVAs were conducted to identify group differences for Thought > Appearance, Emotion > Appearance and Emotion > Thought. As the neural basis of ToM in FHR is a new field, analyses of between-group differences were conducted at threshold of P < 0.001, k > 10, uncorrected for multiple comparisons to achieve a better balance between Type I and Type II error rates (Lieberman and Cunningham, 2009). Regions that survive a correction for multiple comparisons at P < 0.001, corrected at the cluster-level to P < 0.05 using the CorrClustTh tool (http://www-personal.umich.edu/∼nichols/JG5/CorrClusTh.m), are indicated in Table 3 with asterisks (*).
Table 3.
Whole-brain analysis results from the Person-Description task
| Region | BA | Cluster size | MNI coordinates x y z | Peak voxel t-value |
|---|---|---|---|---|
| Non-FHR | ||||
| Thought > Appearance | ||||
| L anterior STS* | 21 | 1370 | −52 0 −26 | 12.07 |
| L posterior STS* | 21 | — | −54 −30 −6 | 7.99 |
| R anterior STS* | 21 | 3442 | 52 −2 −20 | 10.80 |
| R TPJ* | 40 | — | 58 −48 28 | 10.44 |
| L TPJ* | 39 | 2034 | −50 −54 24 | 9.70 |
| PC* | — | 2400 | −6 −52 44 | 9.38 |
| R parahippocampal gyrus* | 30 | 161 | 24 −30 −18 | 8.01 |
| L superior frontal gyrus* | 6 | 152 | −6 16 64 | 6.95 |
| L middle frontal gyrus* | 6 | 367 | −38 2 54 | 6.23 |
| L parahippocampal gyrus* | 30 | 190 | −18 −38 −12 | 6.11 |
| R middle frontal gyrus* | 44 | 193 | 38 20 32 | 5.99 |
| R cerebellum | — | 45 | 8 −50 −48 | 5.70 |
| R cerebellum | — | 80 | 20 −74 −28 | 5.62 |
| R IFG | 38 | 25 | 48 28 −10 | 5.56 |
| R superior frontal gyrus* | 6 | 220 | 10 16 64 | 5.50 |
| L cerebellum* | — | 138 | −20 −76 −30 | 5.29 |
| L cerebellum | — | 43 | −6 −50 −44 | 5.25 |
| L IFG | 48 | 54 | −58 20 8 | 5.12 |
| R IFG | 45 | 76 | 58 24 10 | 4.81 |
| R middle frontal gyrus | 9 | 60 | 24 26 46 | 4.71 |
| R dorsal MPFC | 32 | 59 | 6 38 34 | 4.52 |
| L dorsal MPFC | 32 | 35 | −4 46 24 | 4.47 |
| R superior frontal gyrus | 10 | 34 | 22 60 16 | 4.33 |
| L thalamus | — | 11 | −8 −10 2 | 4.32 |
| Emotion > Appearance | ||||
| R TPJ* | 42 | 4401 | 60 −48 26 | 14.35 |
| R anterior STS* | 21 | — | 52 −2 −18 | 12.53 |
| L PC* | — | 2764 | −8 −52 46 | 12.95 |
| L superior anterior temporal sulcus* | 21 | 1313 | −48 −2 −20 | 10.76 |
| L TPJ* | 22 | 2086 | −56 −54 26 | 9.41 |
| R cingulate gyrus* | 23 | 177 | 4 −20 40 | 8.50 |
| R ventral MPFC* | 32 | 1725 | 2 52 14 | 7.66 |
| R cerebellum* | — | 117 | 22 −72 −30 | 6.31 |
| R superior frontal gyrus | 8 | 59 | 10 20 64 | 6.10 |
| L cerebellum* | — | 208 | −20 −76 −36 | 5.98 |
| L middle frontal gyrus | 9 | 47 | −40 22 48 | 5.83 |
| R superior temporal gyrus | 38 | 66 | −38 20 −24 | 5.73 |
| R cerebellum | — | 52 | 10 −52 −50 | 5.50 |
| R middle frontal gyrus | 44 | 18 | 38 20 34 | 5.41 |
| L superior temporal gyrus | 38 | 36 | −48 14 −18 | 5.28 |
| L IFG | 45 | 64 | −50 22 2 | 5.20 |
| R thalamus | — | 59 | 20 −24 4 | 5.11 |
| L cerebellum | — | 48 | −8 −54 −50 | 4.94 |
| R thalamus | — | 17 | 10 −18 14 | 4.90 |
| R putamen | — | 22 | 20 6 2 | 4.84 |
| R thalamus | — | 15 | 8 −28 2 | 4.70 |
| R rostral anterior cingulate cortex | 25 | 19 | 6 22 −12 | 4.66 |
| R middle frontal gyrus | 9 | 33 | 24 26 46 | 4.58 |
| L IFG | 38 | 23 | −40 22 −14 | 4.45 |
| L ventral MPFC | 11 | 38 | −4 34 −4 | 4.44 |
| R middle frontal gyrus | 6 | 44 | 40 6 42 | 4.41 |
| L cerebellum | — | 11 | −12 −42 −34 | 4.25 |
| L ventral MPFC | 11 | 14 | −2 46 −18 | 3.84 |
| Emotion > Thought | ||||
| R precentral gyrus* | 4 | 455 | 46 −12 38 | 7.13 |
| R postcentral gyrus | 3 | — | 48 −24 60 | 6.59 |
| L IFG | 48 | 88 | −52 2 10 | 6.97 |
| R dorsal MPFC | 10 | 62 | 8 60 20 | 5.88 |
| L postcentral gyrus* | 3 | 140 | −40 −24 46 | 5.88 |
| R somatosensory related cortex | — | 69 | 68 −34 28 | 5.82 |
| R posterior STS | 21 | 75 | 48 −48 6 | 5.54 |
| L dorsal MPFC* | 10 | 158 | −2 58 26 | 5.42 |
| L supramarginal gyrus* | 42 | 224 | −60 −32 20 | 5.30 |
| R superior temporal gyrus | 48 | 33 | −36 4 −18 | 5.19 |
| L insula | 48 | 31 | −40 4 2 | 4.99 |
| R insula | 48 | 20 | 34 −18 10 | 4.98 |
| L rostral anterior cingulate cortex | 11 | 36 | −4 38 −2 | 4.76 |
| L IFG | 45 | 10 | −40 24 14 | 4.49 |
| L middle occipital gyrus | 19 | 14 | −44 −74 4 | 4.47 |
| R postcentral gyrus | — | 25 | 18 −40 70 | 4.45 |
| R orbital frontal cortex | 11 | 39 | 2 38 −24 | 4.44 |
| R posterior middle temporal gyrus | 39 | 16 | 44 −78 18 | 4.37 |
| R cingulate gyrus | 23 | 53 | 4 −12 38 | 4.36 |
| L middle frontal gyrus | 46 | 11 | −34 40 32 | 4.28 |
| Lingual gyrus | — | 15 | 0 −78 −8 | 4.17 |
| L precentral gyrus | 4 | 35 | −42 −22 64 | 4.02 |
| FHR | ||||
| Thought > Appearance | ||||
| L TPJ* | 22 | 3131 | −54 −54 28 | 9.36 |
| L posterior STS* | 21 | — | −62 −22 −8 | 7.52 |
| L anterior STS* | 21 | — | −52 6 −24 | 6.74 |
| R TPJ* | 39 | 2754 | 48 −52 26 | 8.93 |
| R posterior STS* | 20 | — | 52 −18 −10 | 6.17 |
| L middle frontal gyrus* | 9 | 862 | −26 24 40 | 7.70 |
| R middle frontal gyrus* | — | 1159 | 28 32 48 | 7.14 |
| R dorsal MPFC* | 8 | — | 4 36 44 | 6.59 |
| L ventral MPFC* | 32 | — | −10 48 18 | 5.80 |
| R ventral MPFC* | 32 | 294 | 14 48 12 | 6.36 |
| R PC* | — | 1878 | 10 −48 38 | 6.27 |
| R IFG* | 45 | 155 | 60 24 14 | 5.60 |
| L IFG* | 48 | 182 | −54 22 10 | 5.19 |
| L dorsal MPFC | 10 | 48 | −16 60 22 | 4.88 |
| R cerebellum | — | 35 | 18 −74 −26 | 4.60 |
| R IFG | 47 | 62 | 52 42 −10 | 4.52 |
| R middle frontal gyrus | 9 | 54 | 44 20 50 | 4.35 |
| L parahippocampal gyrus | 30 | 19 | −22 −38 −14 | 4.07 |
| Emotion > Appearance | ||||
| L TPJ* | — | 3148 | −60 −58 24 | 9.81 |
| L posterior STS* | 20 | — | −56 −20 −10 | 7.60 |
| R TPJ* | 22 | 3060 | 52 −50 24 | 8.02 |
| R anterior STS* | 21 | — | 50 10 −22 | 7.04 |
| L PC* | — | 1646 | −10 −52 40 | 7.65 |
| L ventral MPFC* | 32 | 544 | −10 50 16 | 5.94 |
| L middle frontal gyrus | — | 124 | −26 22 38 | 5.66 |
| L IFG* | 48 | 182 | −58 20 10 | 5.50 |
| L cerebellum* | — | 220 | −18 −82 −30 | 5.24 |
| R cerebellum* | — | 188 | 20 −74 −30 | 5.02 |
| Rostral anterior cingulate cortex | 11 | 15 | 0 30 −6 | 4.68 |
| R superior frontal gyrus | 8 | 36 | 6 20 62 | 4.62 |
| L middle frontal gyrus | 8 | 36 | −34 6 56 | 4.58 |
| L superior frontal gyrus | 8 | 43 | −8 32 60 | 4.48 |
| R thalamus | — | 20 | 8 −30 4 | 4.21 |
| R superior frontal gyrus | 8 | 18 | 14 26 52 | 3.96 |
| L ventral MPFC | 11 | 12 | −8 46 −10 | 3.89 |
| Emotion > Thought | ||||
| L IFG | 48 | 19 | −28 32 10 | 4.55 |
| L cerebellum | — | 15 | −30 −54 −44 | 4.49 |
| R cingulate gyrus | — | 14 | 10 −24 28 | 4.43 |
| R sub-gyral temporal lobe | 48 | 15 | 34 −4 −14 | 4.35 |
| L ventral MPFC | — | 20 | −6 46 −6 | 4.29 |
| R fusiform gyrus | 20 | 10 | 44 −32 −18 | 4.04 |
| L sub-gyral temporal lobe | 36 | 10 | −32 2 −26 | 3.93 |
| Non-FHR > FHR | ||||
| Thought > Appearance | ||||
| R TPJ | 48 | 54 | 50 −44 30 | 4.17 |
| R TPJ | 39 | 18 | 44 −56 18 | 3.39 |
| R STS | 20 | 24 | 60 −30 −10 | 4.02 |
| L PC | — | 11 | −4 −54 42 | 3.48 |
| Emotion > Appearance | ||||
| R STS | 20 | 24 | 58 −30 −10 | 3.95 |
| R TPJ | 48 | 23 | 50 −44 30 | 3.78 |
| R TPJ | 39 | 10 | 56 −58 26 | 3.40 |
| L PC | — | 14 | −6 −54 42 | 3.63 |
| L inferior temporal gyrus | 20 | 11 | −56 −22 −24 | 3.52 |
| Emotion > Thought | ||||
| R postcentral gyrus | 3 | 121 | 48 −24 60 | 4.16 |
| R putamen | 48 | 19 | 24 16 4 | 3.92 |
| R ventral MPFC/orbitofrontal cortex | 11 | 25 | 12 36 −20 | 3.61 |
| FHR > Non-FHR | ||||
| Thought > Appearance | ||||
| L ventral MPFC/orbitofrontal cortex | 11 | 16 | −10 44 −16 | 4.39 |
| L IFG | 45 | 56 | −42 30 10 | 4.10 |
| L postcentral gyrus | 2 | 12 | −56 −26 50 | 3.65 |
| R postcentral gyrus | 2 | 11 | 34 −44 66 | 3.52 |
| R IFG | 45 | 13 | 48 30 10 | 3.49 |
| Emotion > Appearance | ||||
| L supramarginal gyrus | 41 | 22 | −38 −44 30 | 4.72 |
| L inferior temporal lobe | 20 | 26 | −38 −28 −14 | 4.25 |
| Emotion > Thought | ||||
| L inferior temporal lobe | 37 | 42 | 38 −36 −12 | 4.80 |
Statistical threshold is P < 0.001, k = 10/80 mm, uncorrected for multiple comparisons. (−) in the cluster size column indicates that the region is included in the larger cluster listed above. Regions with an asterisk (*) survive correction for multiple comparisons at P < 0.001, corrected at the cluster-level to P < 0.05. BA, Brodmann area; R, right, L, left.
False-Belief
Each participant’s False-Belief > False-Photograph contrast, generated from a first-level GLM, was used to identify three ROIs in the ToM network: right temporoparietal junction (RTPJ), left temporoparietal junction (LTPJ) and MPFC. According to recent meta-analyses and review articles, these three regions are the most consistently active in fMRI studies of ToM processing in healthy individuals (Van Overwalle, 2009; Van Overwalle and Baetens, 2009; Mar, 2011). Identifying functional ROIs in these three anatomical areas allowed us to characterize ToM-related processing in the specific regions that each individual subject recruited while engaging in mental state reasoning. Because these regions were defined as being selective for ToM, group differences in the response of these ROIs to the Person-Description task should be associated with mental state reasoning, rather than a peripheral cognitive process that may be affected by familial-risk status (Poldrack, 2006; Saxe et al., 2006). Furthermore, the use of an independent task for functional localization allowed us to investigate ToM-related neural activity in a dataset not biased by the selection criteria (i.e. being functionally selective for mental state vs non-mental state information) (Kriegeskorte et al., 2009). MNI coordinates of the individually defined ROIs averaged across participants are as follows: RTPJ = [55 −53 25], LTPJ = [−54 −58 23], MPFC = [2 51 34].
ROIs were defined as all voxels within a 9 mm radius around the peak voxel identified at P < 0.001, k > 10, uncorrected for multiple comparisons. If unidentifiable at P < 0.001, the threshold was lowered iteratively (P < 0.005, 0.01, then 0.05) until the peak was visible (Bedny et al., 2011, 2012). We used this procedure, as opposed to discarding data from participants who did not activate a region at P < 0.001 (e.g. Saxe and Powell, 2006), in order to characterize the full range of variation in these regions associated with mental state reasoning.
Contrast estimates of Person-Description conditions relative to the fixation-cross/rest-period (i.e. Thought > Baseline, Emotion > Baseline, and Appearance > Baseline) were extracted from each participant’s individually defined ROIs. Contrast values in each voxel that defined the ROI were averaged together to derive a single value that represented neural activity in that ROI. The difference between conditions was calculated (i.e. Thought-Appearance, Emotion-Appearance, Emotion-Thought). These values were compared between groups with independent-sample t-tests, which resulted in nine comparisons (three contrasts from three ROIs). Statistical significance was set at P < 0.05 (two-tailed). We then used the adaptive Benjamini–Hochberg correction (Benjamini and Hochberg, 2000), which controls the false-discovery rate, to evaluate whether group differences remained significant (P < 0.05) after correcting for multiple comparisons (i.e. nine tests conducted). Neural activity difference for each contrast (i.e. Thought − Appearance, etc.) was used to investigate the relationship between neural activity and daily-diary ratings.
Daily-diary
The online daily-diary was a questionnaire completed each evening (before bed) for 28 days after the scan. Individuals with <17 diary-days were excluded. Each day, participants were asked whether they had social interactions or interpersonal conflicts (Yes/No), and rated (one-to-five scale: 1 = not at all; 5 = extremely) the following aspects of ToM engagement and social functioning: perspective-taking, empathy, perspective-taking and empathy during interpersonal conflict, social motivation and enjoyment of socializing. Diary questions are listed in Table 4.
Table 4.
Daily-diary variables, individual items, and average reports on constructs from the daily-diary online questionnaire
| Construct and items | Alphaa | All subjects Mean (s.d.)b | Non-FHR Mean (s.d.) | FHR Mean (s.d.) |
|---|---|---|---|---|
| Perspective-taking | 0.80 | 2.3 (0.9) | 2.2 (0.9) | 2.5 (0.8) |
| I made an effort to understand another person’s experience or point of view | ||||
| I tried to imagine what another person may be thinking or feeling | ||||
| I tried to see things from multiple different points of view | ||||
| Empathy | 0.69 | 2.7 (0.9) | 2.6 (0.9) | 2.8 (0.9) |
| I had warm affectionate feelings for someone else | ||||
| I felt empathy or sympathy (e.g. tender and concerned feelings) for someone else because of their difficult circumstances | ||||
| I felt compassion for someone else’s situation | ||||
| Social motivation | 0.62 | 3.6 (0.8) | 3.7 (0.8) | 3.5 (0.9) |
| I wanted to be with people and felt like reaching out to others | ||||
| I wanted to be alone. [reversed scored] | ||||
| Enjoyment of socializing | 0.86 | 3.8 (0.8) | 4.9 (0.8) | 3.6 (0.8) |
| I socialized with other people and I enjoyed it | ||||
| I socialized with other people and I look forward to socializing again in the near future | ||||
| I socialized with other people and I felt like other people liked me | ||||
| Perspective-taking and empathy during interpersonal conflict | 0.65 | 2.3 (0.8) | 2.3 (0.8) | 2.4 (0.8) |
| During the encounter I expressed empathy for the other person’s feelings | ||||
| During the encounter I tried to see things from the other person’s point of view |
Descriptive information for the online daily-diary questionnaire, including daily-diary variables, alpha coefficients for internal consistency and average ratings. All questions were rated on a one-to-five scale (1 = not at all; 5 = extremely). aStandardized alphas are reported. bSeventeen non-FHR and 15 FHR completed the diary.
Analysis of neural activity and daily-diary data
To investigate whether ToM-related neural activity predicted daily ToM engagement and social behavior, we used hierarchical linear models (HLMs), which are appropriate for hierarchically organized data. The structure of the diary-data was such that diary-day was nested within participants and participants were nested within groups. HLM allows the simultaneous assessment of variability at each level of the data, both within- and between-participants. These analyses were performed in SAS using the MIXED procedure.
Our hypothesis was that across all participants, ToM-related activity in individually defined ROIs would positively predict daily-diary reports of ToM engagement and social functioning. Thus, data were analyzed at the within-participants level to generate estimates of each participant’s average level of a diary variable and the relationship between neural activity and a diary variable across each day of the diary. To evaluate whether the relationship between neural activity for ToM and social functioning was qualitatively different between non-FHR and FHR, we tested whether social functioning was predicted by the interaction of neural activity and group status. Said otherwise, we examined whether the within-participant relationships between neural activity and diary variable were a function of between-participant variables, namely group membership and extent of ROI activity (i.e. high vs low activity).
Neural activity in the three individually defined ROIs for Thought > Appearance, Emotion > Appearance and Emotion > Thought was entered (separately) as predictors for each daily-diary variable. For interaction models, neural data were grand-mean centered. Significant interactions were further explored with simple slopes analysis at the different levels of each between-participant variable; that is, high/low levels of neural activity (±1 s.d. from the mean) within FHR and non-FHR (Aiken and West, 1991). This analysis allowed us to investigate whether (i) differing levels of neural activity modulated social functioning within in each group, and (ii) group status modulated social functioning at high and low levels of neural activity.
The distribution of the data was inspected for normality and outliers (±2.5 s.d. from the mean) prior to analysis. Sharpio–Wilk tests confirmed that the daily diary constructs did not significantly deviate from normality (all Ps > 0.29) (Supplementary Table S1). Two non-FHR neural values were identified as outliers and winsorized. Model residuals were visually inspected for homoscedasticity and normally distributed errors. All residuals were normally distributed.
Regression models were used to investigate the relationship between each neural predictor (i.e. three contrasts from three ROIs) and each one of the five daily-diary social functioning variables, resulting in a total of 45 tests. The data were analyzed in this way—instead of combining all ROI data into a single regression model—because it is not clear from the extant literature how individual regions within the ToM-network, which may be supporting different aspects of mental state attribution, relate to different aspects of social behavior. Analyzing the relationship between individual ROIs and social behavior provides a more detailed account of these associations, which we hope future work will build upon. Furthermore, a regression model combining all ROI data would have resulted in substantial multicollinearity.
Regression results are considered statistically significant at P < 0.05 (two-tailed). We then evaluated whether these results remained significant after correcting for the number of tests conducted. Multiple test correction was implemented using the adaptive Benjamini–Hochberg procedure (Benjamini and Hochberg, 2000), which we used to control the false-discovery rate at P < 0.05 in two ways. First, we corrected for the number of diary variables predicted by each ROI, resulting in a correction for five tests. Second, we corrected for the total number of HLMs performed, resulting in a correction for 45 tests. Analyses that survive each correction are indicated in Table 5 and Supplementary Tables S3 and S4.
Table 5.
Significant results from the HLMs predicting daily-diary ratings of social functioning
| b | F | P | |
|---|---|---|---|
| Neural activity predicting (→) social functioning | |||
| Emotion > Appearance | |||
| RTPJ → enjoyment of socializing | 0.11 | 4.04 | 0.05 |
| LTPJ → enjoyment of socializing | 0.11 | 5.13 | 0.03 |
| MPFC → enjoyment of socializing | 0.10 | 4.20 | 0.05 |
| LTPJ → perspective-taking and empathy during interpersonal conflict | 0.04 | 3.99 | 0.05 |
| MPFC → perspective-taking and empathy during interpersonal conflict | 0.09 | 4.58 | 0.04 |
| RTPJ → social motivation | 0.10 | 4.27 | 0.05 |
| Emotion > Thought | |||
| RTPJ → empathy | 0.26 | 10.84 | 0.003a |
| MPFC → empathy | 0.13 | 5.51 | 0.03 |
| RTPJ → perspective-taking | 0.17 | 4.70 | 0.04 |
| Interaction of group and neural activity predicting (→) social functioning | |||
| Emotion > Thought | |||
| MPFC → social motivation | −0.10 | 4.57 | 0.04 |
aCorrected P < 0.05 controlling the false-discovery rate for five comparisons.
RESULTS
Participant characteristics and behavioral performance
There were no differences between FHR and non-FHR on demographic characteristics or IQ (Table 1). FHR reported higher depression on the BDI-II. As is characteristic of this population, FHR had more psychotic-like symptoms on the SIPS (Tarbox and Pogue-Geile, 2011). On the Person-Description task, accuracy was high and there was no between-group difference. On the False-Belief task, FHR individuals were slower making True/False judgments on False-Belief stories.
FMRI results
ROI analysis
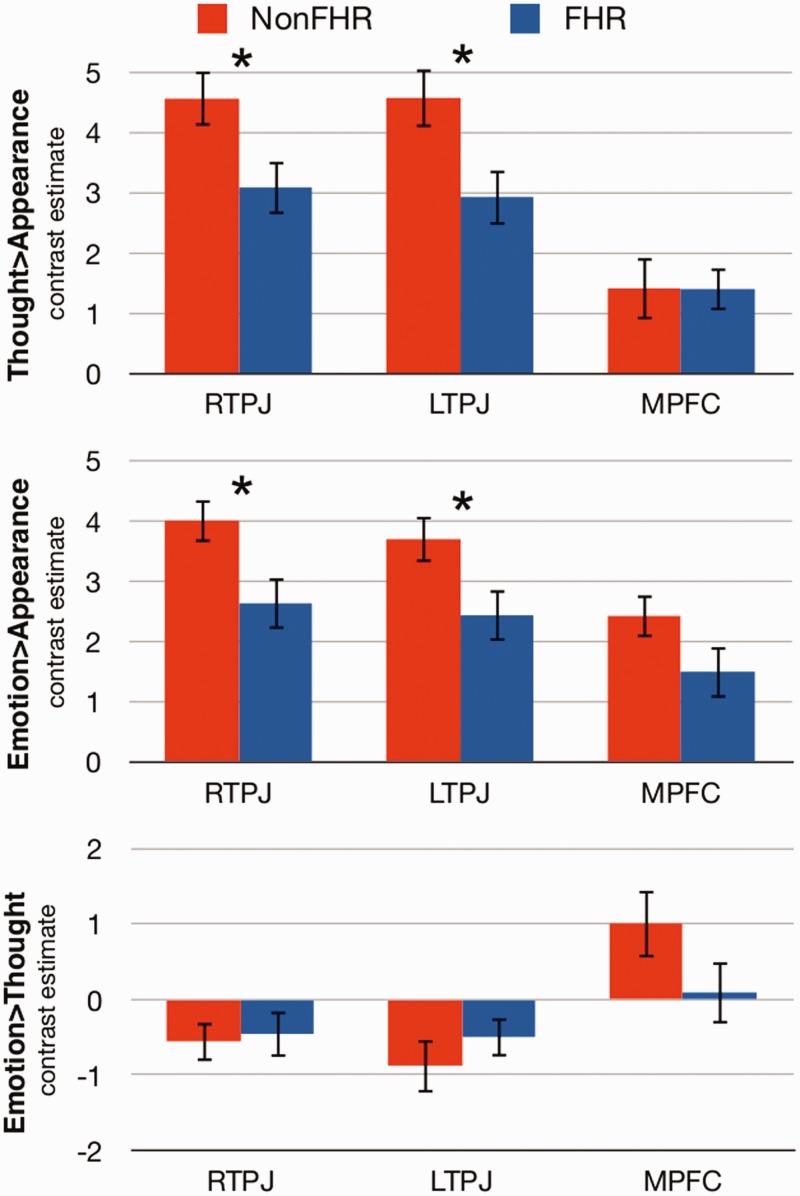
Our main analyses focused on whether there existed group differences in a priori regions of the ToM network, namely bilateral TPJ and MPFC. Toward that end, we investigated neural activity in regions that were individually defined from the False-Belief task for each contrast of the Person-Description task. FHR exhibited a significantly smaller magnitude of difference than that of non-FHR for Thought > Appearance in RTPJ, t(35) = 2.29, P = 0.028, d = 0.75 and LTPJ, t(35) = 2.42, P = 0.021, d = 0.80, but not MPFC, t(35) = 0.02, P = 0.985, d = 0.01 (Figure 1). Similarly, FHR showed a significantly smaller magnitude of difference for Emotion > Appearance in RTPJ, t(35) = 2.42, P = 0.021, d = 0.80, and LTPJ, t(35) = 2.18, P = 0.036, d = 0.72, but not MPFC, t(35) = 1.65, P = 0.114, d = 0.53. The differences in bilateral TPJ for Thought and Emotion > Appearance survived correction for multiple comparisons. No group differences were observed between-groups for Emotion > Thought in any ROI (all Ps > 0.147).
Fig. 1.
Results of the ROI analyses comparing neural activity for Thought > Appearance (top panel), Emotion > Appearance (middle panel) and Emotion > Thought (bottom panel) between non-FHR (red bars) and FHR (blue bars). *P < 0.05.
Whole-brain analysis
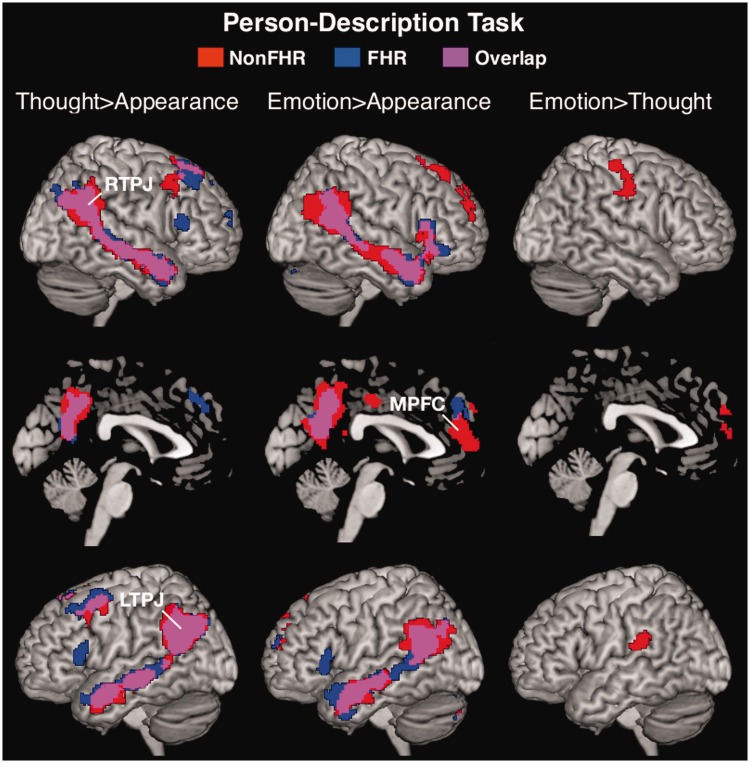
One-sample t-tests confirmed that the Thought > Appearance and Emotion > Appearance contrasts robustly recruited the ToM network, including RTPJ, LTPJ and MPFC within each group (Table 3, Figure 2). In non-FHR, the Emotion > Thought contrast revealed significant activations in regions associated with emotion processing including MPFC, right precentral gyrus, bilateral postcentral gyrus and left supramarginal gyrus. FHR did not activate these regions for Emotion > Thought.
Fig. 2.
Whole-brain random-effects analysis (one sample t-test) of the Person-Description task within non-FHR (red activations), FHR (blue activations) and their overlap (purple activations) for the Thought > Appearance (left panel), Emotion > Appearance (middle panel) and Emotion > Thought (right panel) contrasts. Maps are displayed at P < 0.001 corrected for multiple comparisons to P < 0.05 at the cluster-level.
Group*condition ANOVA results demonstrated reduced recruitment of the ToM network in FHR at an uncorrected threshold (Table 3, Supplementary Figure S2). Specifically, compared with non-FHR, FHR demonstrated reduced recruitment of RTPJ for Thought > Appearance and Emotion > Appearance. FHR also showed reduced recruitment of ToM and emotion processing regions, for Emotion > Thoughts in right ventral MPFC/orbitofrontal cortex, right postcentral gyrus and right putamen. Regions that showed an interaction in the unexpected direction are reported in Table 3. None of these differences remained significant when correcting for multiple comparisons.
Daily-diary
Fifteen FHR and 17 non-FHR had complete daily-diary data (four FHR declined participation; one non-FHR completed <17 days). Number of diary-days did not differ between-groups [M(s.d.): non-FHR = 25(3), FHR = 24(3), t(30) = 1.58, P = 0.12]. Daily-diary averages are reported in Table 4. Analyses examined three questions.
Does FHR status predict daily social behavior?
Group status did not significantly predict any of the daily-diary variables. There was a trend-level relationship showing that FHR reported less enjoyment from socializing (P = 0.07). Otherwise, there was no indication that FHR, as a group, had worse social functioning than that of non-FHR (Supplementary Table S2).
Does ToM-related neural activity predict daily social behavior?
Neural activity for each contrast in each ROI was investigated as a separate predictor of daily-diary ratings of social behavior. Results are shown in Table 5, Figure 3, Supplementary Tables S3 and S4.
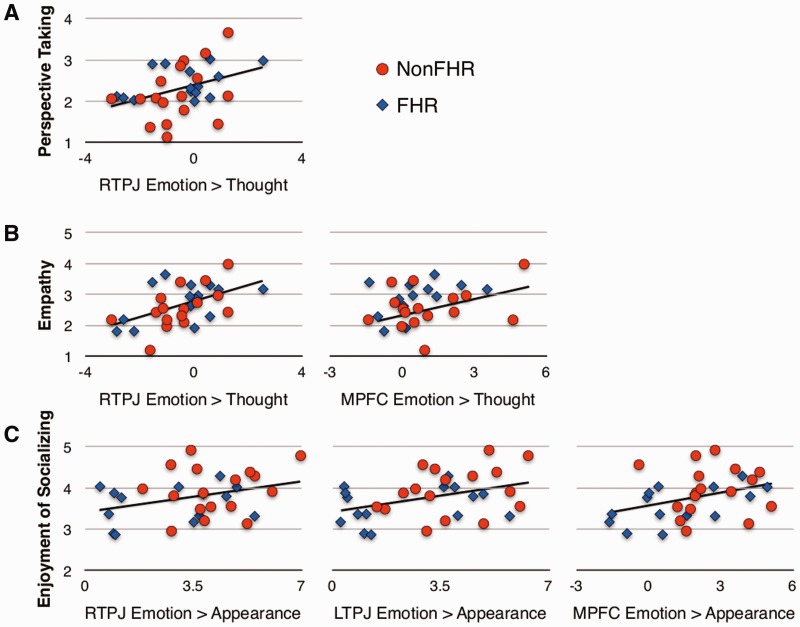
Fig. 3.
Scatter plots illustrating the relationship between neural activity and social functioning. Average ratings across 28 days for the daily-diary social functioning variables (perspective-taking in panel A; empathy in panel B; enjoyment of socializing in panel C) are plotted on the y-axis and contrast estimates from the ROIs are plotted on the x-axis. Possible scores on the social functioning variables range from 1 (not at all) to 5 (extremely). Red circles represent non-FHR; blue diamonds represent FHR.
Thought > Appearance
Neural activity for Thought > Appearance in individually defined ROIs did not predict any of the daily-diary social functioning variables (Supplementary Table S3).
Emotion > Appearance
As expected, ToM-related neural activity for Emotion > Appearance was related to better social functioning. Specifically, greater RTPJ activity for Emotion > Appearance predicted higher daily-diary ratings for enjoyment of socializing and social motivation. Greater LTPJ activity predicted more enjoyment of socializing and more perspective-taking and empathy during conflict. Greater MPFC activity also predicted more enjoyment of socializing and more perspective-taking and empathy during conflict.
Results that survive correction for multiple comparisons by controlling the false-discovery rate at P < 0.05 are indicated in Table 5.
Emotion > Thought
Higher levels of activity for Emotion > Thought (i.e. affective ToM vs cognitive ToM) in RTPJ and MPFC were related to more daily empathy. Additionally, greater RTPJ activity predicted more daily perspective-taking.
Is the relationship between neural activity and social functioning different for FHR and non-FHR?
Thought > Appearance
The interaction of group and neural activity from Thought > Appearance did not predict any social variables (Supplementary Table S4).
Emotion > Appearance
The interaction of group and neural activity from Emotion > Appearance did not predict any social variables (Supplementary Table S4).
Emotion > Thought
The interaction of group and MPFC activity for Emotion > Thought was significantly related to social motivation. The interaction was further examined using simple slopes analyses (Aiken and West, 1991). Results showed that MPFC activity was moderately related to social motivation for FHR (b = 0.12, t = 1.87, P = 0.07); FHR participants with more MPFC activity had more social motivation. However, MPFC activity did not significantly predict social motivation for non-FHR participants (b = 0.08, t = 1.16, P = 0.25). Among people with low MPFC activity, FHR participants had significantly less social motivation than that of non-FHR (b = 0.25, t = 2.07, P = 0.05). Among people with high MPFC activity, there was no difference in social motivation between FHR and non-FHR (b = 0.12, t = 0.96, P = 0.35).
Because FHR reported higher BDI-II scores, analyses were conducted a second-time controlling for depression; these results are reported in Supplementary Tables S5 and S6. Briefly, results from the ROI analyses were unchanged with one exception: controlling for depression revealed that FHR had lower activity than that of non-FHR in MPFC for Emotion > Appearance (P = 0.02) and Emotion > Thought (P = 0.057). We did not observe these differences when depression was not used as a covariate. In the daily-diary analysis, the relationship between MPFC and LTPJ (Emotion > Appearance) and perspective-taking and empathy during conflict was no longer significant. All other findings were unchanged suggesting that, by and large, group differences and the relationship between neural activity for ToM and daily social functioning are independent of mood.
DISCUSSION
This study found that being at FHR for schizophrenia is associated with disruption to the underlying neural network for ToM and that recruitment of this network for affective ToM (i.e. reasoning about emotions) is associated with real-world social behavior. Analysis of neural activity in individually defined ROIs demonstrated that FHR had reduced recruitment of the ToM network, most notably, bilateral TPJ when processing thoughts and emotions compared with physical appearances. Across all participants, neural activity during affective ToM prospectively predicted social behavior over the following 28 days, including daily ratings of perspective-taking, empathy, social motivation, enjoyment of socializing and using ToM skills during interpersonal conflict. None of these social behaviors was predicted by FHR group status. The linear relationship between ToM-related activity and social functioning across all participants, with FHR in the lower-end of the distribution, was the most consistent finding; however, social motivation was predicted by the interaction of group and neural activity with a pattern indicating that low ToM-related neural activity had a more detrimental effect on social motivation for FHR than non-FHR participants.
These results suggest that the genetic vulnerability for schizophrenia manifests as disruption to brain regions recruited for ToM and that disruption to this neural circuit is a measurable contributing factor to the social problems characteristic of FHR and shown to predict psychosis (Tarbox and Pogue-Geile, 2008). The findings provide foundational evidence that ToM has potential as a marker of psychosis-risk and functional outcome. Despite prior evidence that social deficits, particularly in FHR, robustly predict schizophrenia-spectrum disorders (Tarbox and Pogue-Geile, 2008), it has been unclear how to implement this information in early identification and prevention efforts. Most measures of social deficits assess broad indicators of functioning, which are influenced by multiple factors and easily biased by retrospective reports. Our results demonstrate that ToM is a proximal, quantifiable neurocognitive process that varies dimensionally and predicts meaningful aspects of social behavior. Considered alongside ToM dysfunction in schizophrenia (Hooker et al., 2011), autism (Chung et al., 2013) and other disorders (Bora et al., 2009b), the findings indicate that, eventually, identifying the dimensional boundaries of normal and abnormal ToM-related neural function could inform the assessment and treatment of social deficits among healthy, disordered and at-risk populations.
In this study, the fMRI lab-based neural measure of affective ToM processing predicted real-life ToM processing, including the daily use of ToM skills, such as perspective-taking to understand others’ points-of-view, as well as the emotions generated after using those skills, such as compassion for another’s distress. Neural activity in all three ROIs during the affective ToM condition predicted real-life ToM processing as measured in the daily-diary. RTPJ and MPFC showed the strongest relationships. Across all participants, RTPJ activity for affective ToM vs cognitive ToM was related to daily perspective-taking and empathy; MPFC activity for this contrast also predicted daily empathy, and MPFC activity for affective ToM vs appearance predicted the use of perspective-taking and expression of empathy during interpersonal conflict. These data indicate that our lab-based assessment of affective ToM accurately estimated the use of ToM processing to enhance relationships in real-life. The findings also suggest that affective ToM, which predicted perspective-taking, empathy (and nearly all other social behaviors we measured), facilitates social relationships more than cognitive ToM.
These findings add to a growing literature, primarily with healthy adults, showing that greater RTPJ and MPFC activity during affective ToM is related to more perspective-taking (Hooker et al., 2008), empathic accuracy (Zaki et al., 2009) and empathic/prosocial behavior in daily-life (Masten et al., 2011; Rameson et al., 2012). Similarly, among individuals with schizophrenia, those with more gray matter volume (GMV) in MPFC self-reported more perspective-taking, were clinician-rated as having better perspective-taking skills and more empathy in their interpersonal relationships, and performed better on an affective ToM task (Hooker et al., 2011).
Our other daily-diary measures were developed with the idea that good ToM skills create more rewarding social interactions, and thus more motivation to socialize. Results are consistent with this idea. Greater RTPJ, LTPJ and MPFC activity during affective ToM predicted more enjoyment from socializing, including feeling liked by others and looking forward to socializing again. Greater RTPJ activity during affective ToM also predicted more motivation to socialize, regardless of social opportunities. These findings do not speak to the temporal sequence of social events, so additional research is needed to identify whether successful ToM processing leads to greater social reward and motivation. Nonetheless, our findings are consistent with prior research showing that individuals with greater GMV in right posterior STS/TPJ and MPFC have larger social networks (Lewis et al., 2011; Kanai et al., 2012b) and report less loneliness (Kanai et al., 2012a).
Though we found the brain–behavior relationships to be largely the same for FHR and non-FHR, the relationship between MPFC activity and social motivation differed between groups. Specifically, MPFC activity influenced motivation to socialize more for FHR than non-FHR, and FHR with low MPFC activity had less motivation than that of non-FHR with low MPFC activity. This suggests that FHR with low MPFC activity may be especially vulnerable to social withdrawal and isolation; a contributing risk factor for schizophrenia (Tarbox and Pogue-Geile, 2008). However, only one such interaction was found, which did not survive correction for multiple comparisons. Thus, this relationship should be interpreted cautiously, as most results showed a direct relationship between activity and social functioning.
Although the functional ROI approach probably enhanced our ability to reveal relationships between ToM neural processing and social behavior, it also has limitations. The False-Belief task engages cognitive ToM processes, so emotion regions, engaged during affective ToM or mental state decoding tasks, were not identified as ROIs and thus not investigated in our analyses. Similarly, regions specific to schizophrenia pathology could influence social behavior but would not be identified by a ToM localizer. Notably, whole-brain analyses revealed group differences in two regions that have been implicated in both emotion processing and schizophrenia pathology, although at uncorrected thresholds. Right postcentral gyrus exhibited less differentiation between emotions and thoughts in FHR than non-FHR. Somatosensory deficits and inferior parietal abnormalities are implicated in schizophrenia pathology (Chang and Lenzenweger, 2005; Torrey, 2007). Recruitment of right postcentral gyrus has been consistently implicated in aspects of emotion processing (Adolphs et al., 2000; Adolphs, 2002; Pitcher et al., 2008) and increased recruitment of this region post-cognitive training is associated with improved emotion recognition in schizophrenia (Hooker et al., 2012). In addition, we found an unexpected interaction in the inferior frontal gyrus (IFG). Non-FHR demonstrated greater activation to the appearance vs thought condition, whereas FHR did not differentiate between the two. As IFG is recruited during mental state decoding with emotional faces (Sabatinelli et al., 2011), this interaction may reflect FHR failing to engage regions associated with decoding, which is consistent with other work showing abnormalities in IFG during mental state decoding in individuals with schizophrenia (Russell et al., 2000; Mier et al., 2010; de Achaval et al., 2012) and FHR (de Achaval et al., 2012). With that said, these group differences in the whole-brain analysis were not significant after correction for multiple comparisons and so should be interpreted cautiously.
Finally, our aim in using the daily-diary was to provide a detailed analysis of how neural activity is related to a range of social behaviors. Although the benefit is a nuanced picture of brain–behavior relationships, a limitation is the numerous tests conducted on a relatively small sample. When results for each neural predictor were corrected for the five tests used to examine all diary variables, the relationship between RTPJ and empathy remained significant, but the others did not, and none of the brain-behavior relationships remained significant when accounting for all tests performed. Additionally, although the daily-diary assessment is more ecologically valid than most social functioning measures, it is a self-report measure subject to self-perception biases. Future research may benefit from having friends or family report on the participant’s behavior in way that addresses both frequency (e.g. how often the participant engaged in a social interaction or an empathic exchange) and performance (e.g. how well the participant performed in the social interaction or how accurate their attempts at empathy and validation were). Finally, there was a higher prevalence of psychopathology in the FHR group. This has the potential to complicate interpretation of the group differences as a function of familial risk vs manifest psychopathology. With that said, given the shared variance between the familial at-risk state and psychopathology, the exclusion of these individuals would have substantially undermined the external validity of this study. Furthermore, the analyses covarying out the effect of depression minimally changed the results of the ROI and daily-diary findings.
The relatively short time-frame of the study prohibits conclusions about causal relationships between neural deficits and social behavior. Nonetheless, the findings are consistent with proposals of an ongoing dynamic exchange between neural function and social experience (Hoffman, 2007; Subramaniam et al., 2012). Theoretically, neural dysfunction in ToM, associated with FHR, could precipitate a cascade of events, including diminished interest and enjoyment of social relationships, ultimately, leading to social withdrawal. Absence of social contact could, in turn, cause deleterious neuroplastic reorganization in the ToM network, which further compromises social capacity and potentiates vulnerability to illness progression (Hoffman, 2007). Research on the neural basis of ToM in individuals at risk for psychosis is in its infancy and more information is needed to understand brain–behavior dynamics over time. However, research in schizophrenia indicates that regions associated with ToM, such as MPFC, demonstrate neuroplastic response to social cognitive interventions (Eack et al., 2010a; Hooker et al., 2012) which predicts social functioning improvement (Lee et al., 2006; Subramaniam et al., 2012). These findings suggest that, for psychosis-risk and perhaps other populations, behavioral interventions that engage ToM processing might promote neuroplastic changes that improve ToM-related neural function, enhance social competence, and, ultimately, yield more fulfilling social relationships.
FUNDING
This work was supported by an award from the Milton Fund to C.I.H., a training grant from the National Institutes of Health Blueprint for Neuroscience Research [T90DA022759/R90DA023427] and the Sackler Scholar Programme in Psychobiology to D.D.-F., and, indirectly, by an National Institute of Mental Health Award R21MH083205 to L.E.D. that helped create the recruitment infrastructure. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or any other funding agency.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
The authors would like to thank Gul Jabbar, Ashley Proal, Cheryl Best, Matthew Yung, Sarah Hope Lincoln and Laura M. Tully for help with subject recruitment and data collection, and the staff at Harvard University’s Center for Brain Sciences for support with the fMRI data acquisition and analysis. They would also like to thank the participants for their involvement and dedication to this research.
Footnotes
1 The experiment included an additional condition that was not analyzed.
REFERENCES
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002;12(2):169–77. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. Journal of Neuroscience. 2000;20(7):2683–90. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- Anselmetti S, Bechi M, Bosia M, et al. ‘Theory’ of mind impairment in patients affected by schizophrenia and in their parents. Schizophrenia Research. 2009;115(2–3):278–85. doi: 10.1016/j.schres.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonia, TX: Psychological Corporation; 1996. [Google Scholar]
- Becker TM, Kerns JG, Macdonald AW, III, Carter CS. Prefrontal dysfunction in first-degree relatives of schizophrenia patients during a Stroop task. Neuropsychopharmacology. 2008;33(11):2619–25. doi: 10.1038/sj.npp.1301673. [DOI] [PubMed] [Google Scholar]
- Bedny M, Caramazza A, Pascual-Leone A, Saxe R. Typical neural representations of action verbs develop without vision. Cerebral Cortex. 2012;22(2):286–93. doi: 10.1093/cercor/bhr081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M, Pascual-Leone A, Dodell-Feder D, Fedorenko E, Saxe R. Language processing in the occipital cortex of congenitally blind adults. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(11):4429–34. doi: 10.1073/pnas.1014818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. On the adaptive control of the false discovery fate in multiple testing with independent statistics. Journal of Educational and Behavioral Statistics. 2000;25(1):60–83. [Google Scholar]
- Bora E, Pantelis C. Theory of mind impairments in first-episode psychosis, individuals at ultra-high risk for psychosis and in first-degree relatives of schizophrenia: systematic review and meta-analysis. Schizophrenia Research. 2013;144(1–3):31–6. doi: 10.1016/j.schres.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophrenia Research. 2009a;109(1–3):1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Theory of mind impairment: a distinct trait-marker for schizophrenia spectrum disorders and bipolar disorder? Acta Psychiatrica Scandinavica. 2009b;120(4):253–64. doi: 10.1111/j.1600-0447.2009.01414.x. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10(4):433–6. [PubMed] [Google Scholar]
- Brune M, Lissek S, Fuchs N, et al. An fMRI study of theory of mind in schizophrenic patients with “passivity” symptoms. Neuropsychologia. 2008;46(7):1992–2001. doi: 10.1016/j.neuropsychologia.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Brune M, Ozgurdal S, Ansorge N, et al. An fMRI study of “theory of mind” in at-risk states of psychosis: comparison with manifest schizophrenia and healthy controls. Neuroimage. 2011;55(1):329–37. doi: 10.1016/j.neuroimage.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Brunet E, Sarfati Y, Hardy-Bayle MC, Decety J. Abnormalities of brain function during a nonverbal theory of mind task in schizophrenia. Neuropsychologia. 2003;41(12):1574–82. doi: 10.1016/s0028-3932(03)00119-2. [DOI] [PubMed] [Google Scholar]
- Chang BP, Lenzenweger MF. Somatosensory processing and schizophrenia liability: proprioception, exteroceptive sensitivity, and graphesthesia performance in the biological relatives of schizophrenia patients. Journal of Abnormal Psychology. 2005;114(1):85–95. doi: 10.1037/0021-843X.114.1.85. [DOI] [PubMed] [Google Scholar]
- Chang CJ, Chen WJ, Liu SK, et al. Morbidity risk of psychiatric disorders among the first degree relatives of schizophrenia patients in Taiwan. Schizophrenia Bulletin. 2002;28(3):379–92. doi: 10.1093/oxfordjournals.schbul.a006947. [DOI] [PubMed] [Google Scholar]
- Chung YS, Barch D, Strube M. A meta-analysis of mentalizing impairments in adults with schizophrenia and autism spectrum disorder. Schizophrenia Bulletin. 2013;40(3):602–16. doi: 10.1093/schbul/sbt048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Lenzenweger MF, Dworkin RH, Erlenmeyer-Kimling L. Childhood attentional dysfunctions predict social deficits in unaffected adults at risk for schizophrenia. British Journal of Psychiatry. 1992;161(Supplement 18):59–64. [PubMed] [Google Scholar]
- Das P, Lagopoulos J, Coulston CM, Henderson AF, Malhi GS. Mentalizing impairment in schizophrenia: a functional MRI study. Schizophrenia Research. 2012;134(2–3):158–64. doi: 10.1016/j.schres.2011.08.019. [DOI] [PubMed] [Google Scholar]
- de Achaval D, Costanzo EY, Villarreal M, et al. Emotion processing and theory of mind in schizophrenia patients and their unaffected first-degree relatives. Neuropsychologia. 2010;48(5):1209–15. doi: 10.1016/j.neuropsychologia.2009.12.019. [DOI] [PubMed] [Google Scholar]
- de Achaval D, Villarreal MF, Costanzo EY, et al. Decreased activity in right-hemisphere structures involved in social cognition in siblings discordant for schizophrenia. Schizophrenia Research. 2012;134(2–3):171–9. doi: 10.1016/j.schres.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Dodell-Feder D, Koster-Hale J, Bedny M, Saxe R. fMRI item analysis in a theory of mind task. Neuroimage. 2011;55(2):705–12. doi: 10.1016/j.neuroimage.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Dodell-Feder D, Lincoln SH, Coulson JP, Hooker CI. Using fiction to assess mental state understanding: a new task for assessing theory of mind in adults. PLoS One. 2013;8(11):e81279. doi: 10.1371/journal.pone.0081279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodell-Feder D, Tully LM, Lincoln SH, Hooker CI. The neural basis of theory of mind and its relationship to social functioning and social anhedonia in individuals with schizophrenia. NeuroImage: Clinical. 2014;4:154–63. doi: 10.1016/j.nicl.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, Bernstein G, Kaplansky LM, et al. Social competence and positive and negative symptoms: a longitudinal study of children and adolescents at risk for schizophrenia and affective disorder. American Journal of Psychiatry. 1991;148(9):1182–8. doi: 10.1176/ajp.148.9.1182. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Cornblatt BA, Friedmann R, et al. Childhood precursors of affective vs. social deficits in adolescents at risk for schizophrenia. Schizophrenia Bulletin. 1993;19(3):563–77. doi: 10.1093/schbul/19.3.563. [DOI] [PubMed] [Google Scholar]
- Eack SM, Hogarty GE, Cho RY, et al. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: results from a 2-year randomized controlled trial. Archives of General Psychiatry. 2010a;67(7):674–82. doi: 10.1001/archgenpsychiatry.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Mermon DE, Montrose DM, et al. Social cognition deficits among individuals at familial high risk for schizophrenia. Schizophrenia Bulletin. 2010b;36(6):1081–8. doi: 10.1093/schbul/sbp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Adamo UH, Rock D, et al. The New York High-Risk Project. Prevalence and comorbidity of axis I disorders in offspring of schizophrenic parents at 25-year follow-up. Archives of General Psychiatry. 1997;54(12):1096–102. doi: 10.1001/archpsyc.1997.01830240052008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neuroscience and Biobehavioral Reviews. 2011;35(3):573–88. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Flesch R. A new readability yardstick. The Journal of Applied Psychology. 1948;32(3):221–33. doi: 10.1037/h0057532. [DOI] [PubMed] [Google Scholar]
- Francis AN, Seidman LJ, Jabbar GA, et al. Alterations in brain structures underlying language function in young adults at high familial risk for schizophrenia. Schizophrenia Research. 2012;141(1):65–71. doi: 10.1016/j.schres.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CM, Penn DL, Prinstein MJ, Perkins DO, Belger A. Social skill and social cognition in adolescents at genetic risk for psychosis. Schizophrenia Research. 2010;122(1–3):179–84. doi: 10.1016/j.schres.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt SJ, Stone WS, Faraone SV, Seidman LJ, Tsuang MT. Psychopathology, personality traits and social development of young first-degree relatives of patients with schizophrenia. British Journal of Psychiatry. 2006;189:337–45. doi: 10.1192/bjp.bp.105.016998. [DOI] [PubMed] [Google Scholar]
- Gottesman I, Shields J. Schizophrenia: The Epigenetic Puzzle. New York, NY: Cambridge University Press; 1982. [Google Scholar]
- Gottesman II. Schizophrenia Genesis: The Origins of Madness. New York, NY: WH Freeman/Times Books/Henry Holt, Co; 1991. [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Green MF, Bearden CE, Cannon TD, et al. Social cognition in schizophrenia, Part 1: performance across phase of illness. Schizophrenia Bulletin. 2012;38(4):854–64. doi: 10.1093/schbul/sbq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans SL, Auerbach JG, Asarnowm JR, Styr B, Marcus J. Social adjustment of adolescents at risk for schizophrenia: the Jerusalem infant development study. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1406–14. doi: 10.1097/00004583-200011000-00015. [DOI] [PubMed] [Google Scholar]
- Hoffman RE. A social deafferentation hypothesis for induction of active schizophrenia. Schizophrenia Bulletin. 2007;33(5):1066–70. doi: 10.1093/schbul/sbm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Bruce L, Fisher M, Verosky SC, Miyakawa A, Vinogradov S. Neural activity during emotion recognition after combined cognitive plus social cognitive training in schizophrenia. Schizophrenia Research. 2012;139(1–3):53–9. doi: 10.1016/j.schres.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Bruce L, Lincoln SH, Fisher M, Vinogradov S. Theory of mind skills are related to gray matter volume in the ventromedial prefrontal cortex in schizophrenia. Biological Psychiatry. 2011;70(12):1169–78. doi: 10.1016/j.biopsych.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Germine LT, Knight RT, D’Esposito M. Mentalizing about emotion and its relationship to empathy. Social Cognitive and Affective Neuroscience. 2008;3(3):204–17. doi: 10.1093/scan/nsn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley JM. Expressed emotion and relapse of psychopathology. Annual Review of Clinical Psychology. 2007;3:329–52. doi: 10.1146/annurev.clinpsy.2.022305.095236. [DOI] [PubMed] [Google Scholar]
- Horan WP, Subotnik KL, Snyder KS, Nuechterlein KH. Do recent-onset schizophrenia patients experience a “social network crisis”? Psychiatry. 2006;69(2):115–29. doi: 10.1521/psyc.2006.69.2.115. [DOI] [PubMed] [Google Scholar]
- Janssen I, Krabbendam L, Jolles J, van Os J. Alterations in theory of mind in patients with schizophrenia and non-psychotic relatives. Acta Psychiatrica Scandinavica. 2003;108(2):110–7. doi: 10.1034/j.1600-0447.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Ebmeier KP, Miller P, Owens DG, Lawrie SM. Predicting schizophrenia: findings from the Edinburgh High-Risk Study. British Journal of Psychiatry. 2005;186:18–25. doi: 10.1192/bjp.186.1.18. [DOI] [PubMed] [Google Scholar]
- Kanai R, Bahrami B, Duchaine B, Janik A, Banissy MJ, Rees G. Brain structure links loneliness to social perception. Current Biology. 2012a;22(20):1975–9. doi: 10.1016/j.cub.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Bahrami B, Roylance R, Rees G. Online social network size is reflected in human brain structure. Proceedings. Biological Sciences. 2012b;279(1732):1327–34. doi: 10.1098/rspb.2011.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemen O, Keri S, Must A, Benedek G, Janka Z. No evidence for impaired ‘theory of mind’ in unaffected first-degree relatives of schizophrenia patients. Acta Psychiatrica Scandinavica. 2004;110(2):146–9. doi: 10.1111/j.1600-0047.2004.00357.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. The risk for psychiatric disorders in relatives of schizophrenic and control probands: a comparison of three independent studies. Psychological Medicine. 1997;27(2):411–9. doi: 10.1017/s003329179600445x. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Montrose DM, Stanley JA, Pettegrew JW. Premorbid characterization in schizophrenia: the Pittsburgh High Risk Study. World Psychiatry. 2004;3(3):163–8. [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Kulkarni SR, Bhojraj T, et al. Premorbid cognitive deficits in young relatives of schizophrenia patients. Frontiers in Human Neuroscience. 2010;3(62):1–14. doi: 10.3389/neuro.09.062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Shin NY, Jang JH, et al. Social cognition and neurocognition as predictors of conversion to psychosis in individuals at ultra-high risk. Schizophrenia Research. 2011;130(1–3):170–5. doi: 10.1016/j.schres.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli D. What’s new in Psychtoolbox-3? Perception. 2007;36:14–14. [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience. 2009;12(5):535–40. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz MM, Richardson CL. Social cognitive training for schizophrenia: a meta-analytic investigation of controlled research. Schizophrenia Bulletin. 2012;38(5):1092–104. doi: 10.1093/schbul/sbr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Quintana J, Nori P, Green MF. Theory of mind in schizophrenia: exploring neural mechanisms of belief attribution. Social Neuroscience. 2011;6(5–6):569–81. doi: 10.1080/17470919.2011.620774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K-H, Brown WH, Engleston PN, et al. A functional magnetic resonance imaging study of social cognition in schizophrenia during an acute episode and after recovery. American Journal of Psychiatry. 2006;163(11):1926–33. doi: 10.1176/ajp.2006.163.11.1926. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Rezaie R, Brown R, Roberts N, Dunbar RI. Ventromedial prefrontal volume predicts understanding of others and social network size. Neuroimage. 2011;57(4):1624–9. doi: 10.1016/j.neuroimage.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4(4):423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar RA. The neural bases of social cognition and story comprehension. Annual Review of Psychology. 2011;62:103–34. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- Marjoram D, Job DE, Whalley HC, et al. A visual joke fMRI investigation into Theory of Mind and enhanced risk of schizophrenia. Neuroimage. 2006;31(4):1850–8. doi: 10.1016/j.neuroimage.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Masten CL, Morelli SA, Eisenberger NI. An fMRI investigation of empathy for ‘social pain’ and subsequent prosocial behavior. Neuroimage. 2011;55(1):381–8. doi: 10.1016/j.neuroimage.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Maxwell M. Family Instrument for Genetic Studies (FIGS) Washington, DC: Clinical Neurogenetic Branch, Intramural Research Program, National Institute of Mental Health; 1996. [Google Scholar]
- McGuffin P, Owen MJ, Gottesman II. Psychiatric Genetics and Genomics. Oxford and New York: Oxford University Press; 2004. [Google Scholar]
- Mier D, Sauer C, Lis S, et al. Neuronal correlates of affective theory of mind in schizophrenia out-patients: evidence for a baseline deficit. Psychological Medicine. 2010;40(10):1607–17. doi: 10.1017/S0033291709992133. [DOI] [PubMed] [Google Scholar]
- Miller P, Byrne M, Hodges A, Lawrie SM, Owens DG, Johnstone EC. Schizotypal components in people at high risk of developing schizophrenia: early findings from the Edinburgh High-Risk Study. British Journl of Psychiatry. 2002;180:179–84. doi: 10.1192/bjp.180.2.179. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophrenia Bulletin. 2003;29(4):703–15. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Montag C, Neuhaus K, Lehmann A, et al. Subtle deficits of cognitive theory of mind in unaffected first-degree relatives of schizophrenia patients. European Archives of Psychiatry and Neurological Sciences. 2012;262(3):217–26. doi: 10.1007/s00406-011-0250-2. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, Oorschot M, Collip D, Lataster J, Delespaul P, van Os J. Experience sampling research in psychopathology: opening the black box of daily life. Psychological Medicine. 2009;39(9):1533–47. doi: 10.1017/S0033291708004947. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of General Psychiatry. 1994;51(11):849–59. doi: 10.1001/archpsyc.1994.03950110009002. discussion: 863–44. [DOI] [PubMed] [Google Scholar]
- Pitcher D, Garrido L, Walsh V, Duchaine BC. Transcranial magnetic stimulation disrupts the perception and embodiment of facial expressions. Journal of Neuroscience. 2008;28(36):8929–33. doi: 10.1523/JNEUROSCI.1450-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences. 2006;10(2):59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Scanning patients with tasks they can perform. Human Brain Mapping. 1999;8(2–3):102–8. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<102::AID-HBM6>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameson LT, Morelli SA, Lieberman MD. The neural correlates of empathy: experience, automaticity, and prosocial behavior. Journal of Cognitive Neuroscience. 2012;24(1):235–45. doi: 10.1162/jocn_a_00130. [DOI] [PubMed] [Google Scholar]
- Russell TA, Rubia K, Bullmore ET, et al. Exploring the social brain in schizophrenia: left prefrontal underactivation during mental state attribution. American Journal of Psychiatry. 2000;157(12):2040–2. doi: 10.1176/appi.ajp.157.12.2040. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Fortune EE, Li Q, et al. Emotional perception: meta-analyses of face and natural scene processing. Neuroimage. 2011;54(3):2524–33. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Sabbagh MA. Understanding orbitofrontal contributions to theory-of-mind reasoning: implications for autism. Brain and Cognition. 2004;55(1):209–19. doi: 10.1016/j.bandc.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Saxe R, Brett M, Kanwisher N. Divide and conquer: a defense of functional localizers. Neuroimage. 2006;30(4):1088–96. doi: 10.1016/j.neuroimage.2005.12.062. discussion: 1097–9. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19(4):1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It’s the thought that counts: specific brain regions for one component of theory of mind. Psychological Science. 2006;17(8):692–9. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Speilberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Sprong M, Schothorst P, Vos E, Hox J, van Engeland H. Theory of mind in schizophrenia: meta-analysis. British Journal of Psychiatry. 2007;191:5–13. doi: 10.1192/bjp.bp.107.035899. [DOI] [PubMed] [Google Scholar]
- Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73(4):842–53. doi: 10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H, Sullivan K. A componential view of theory of mind: evidence from Williams syndrome. Cognition. 2000;76(1):59–90. doi: 10.1016/s0010-0277(00)00069-x. [DOI] [PubMed] [Google Scholar]
- Tarbox SI, Pogue-Geile MF. Development of social functioning in preschizophrenia children and adolescents: a systematic review. Psychological Bulletin. 2008;134(4):561–83. doi: 10.1037/0033-2909.34.4.561. [DOI] [PubMed] [Google Scholar]
- Tarbox SI, Pogue-Geile MF. A multivariate perspective on schizotypy and familial association with schizophrenia: a review. Clinical Psychology Review. 2011;31(7):1169–82. doi: 10.1016/j.cpr.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A, Papas A, Bartholomeusz C, et al. Social cognition in clinical “at risk” for psychosis and first episode psychosis populations. Schizophrenia Research. 2012;141(2–3):204–9. doi: 10.1016/j.schres.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophrenia Research. 2007;97(1–3):215–25. doi: 10.1016/j.schres.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Human Brain Mapping. 2009;30(3):829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48:564–84. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Versmissen D, Janssen I, Myin-Germeys I, et al. Evidence for a relationship between mentalising deficits and paranoia over the psychosis continuum. Schizophrenia Research. 2008;99(1–3):103–10. doi: 10.1016/j.schres.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio: Psychological Corporation; 1999. [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(4):1279–84. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer H, Perner J. Beliefs about beliefs: representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition. 1983;13(1):103–28. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]
- Zaki J, Weber J, Bolger N, Ochsner K. The neural bases of empathic accuracy. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(27):11382–7. doi: 10.1073/pnas.0902666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.