Abstract
Humans are willing to punish norm violations even at a substantial personal cost. Using fMRI and a variant of the ultimatum game and functional magnetic resonance imaging, we investigated how the brain differentially responds to fairness in loss and gain domains. Participants (responders) received offers from anonymous partners indicating a division of an amount of monetary gain or loss. If they accept, both get their shares according to the division; if they reject, both get nothing or lose the entire stake. We used a computational model to derive perceived fairness of offers and participant-specific inequity aversion. Behaviorally, participants were more likely to reject unfair offers in the loss (vs gain) domain. Neurally, the positive correlation between fairness and activation in ventral striatum was reduced, whereas the negative correlations between fairness and activations in dorsolateral prefrontal cortex were enhanced in the loss domain. Moreover, rejection-related dorsal striatum activation was higher in the loss domain. Furthermore, the gain–loss domain modulates costly punishment only when unfair behavior was directed toward the participants and not when it was directed toward others. These findings provide neural and computational accounts of increased costly norm enforcement under adversity and advanced our understanding of the context-dependent nature of fairness preference.
Keywords: fairness, costly norm enforcement, ultimatum game, fMRI, computational modeling
INTRODUCTION
The concept of fairness and equity in social interaction has occupied the mind of philosophers (Rawls, 1971; Rousseau, 1754/2011; Aristotle, 1998) and economists (Kahneman et al., 1986; Buchanan, 1987) throughout recorded history. As the political philosopher John Rawls noted, ‘the fundamental idea in the concept of justice is fairness’ (Rawls, 1958). The maintenance of fairness typically requires some forms of punishment, as certain individuals are inevitably tempted by their self-interest to violate the norm of fairness in social encounters (Sober and Wilson, 1999; Fehr and Gächter, 2002; Montague and Lohrenz, 2007). Indeed, humans are willing to enforce the fairness norm by punishing violations even at substantial personal costs (Boyd et al., 2003; Henrich et al., 2006).
A classical way to elicit inequality aversion and costly norm enforcement in laboratory settings is the ‘ultimatum game’ (UG) in which one player (the proposer) is endowed with an amount of money (e.g. $10) and proposes a division (e.g. keep $7/offer $3) to another player (the responder). The responder can either accept or reject the offer, with the corresponding consequence of both parties receiving the proposed shares or getting nothing (Güth et al., 1982). Behaviorally, responders routinely reject unfair or unequal divisions of resources, with the rejection rate increasing as the unfairness or the inequality of the offer increases (Camerer, 2003). This behavioral preference for fairness is observed in domestic dogs (Range et al., 2009) and non-human primates (Proctor et al., 2013), suggesting a long evolutionary history to the human sense of fairness. In humans, neuroimaging studies with UG show that unfair division of resources elicits negative affect and interoceptive responses represented in the insula (Sanfey et al., 2003; Güroğlu et al., 2010; Hollmann et al., 2011; Kirk et al., 2011), and that rejection of an unfair offer, and thus punishment of norm violations, activates reward-related brain regions such as the dorsal striatum (DS; de Quervain et al., 2004; Baumgartner et al., 2008). Moreover, rejection of unfair offer usually comes at a personal cost to the punisher and thus requires the inhibition of selfish impulses, subserved by dorsolateral prefrontal cortex (DLPFC; Knoch et al., 2006, 2008; Buckholtz et al., 2008; Baumgartner et al., 2011, 2012).
However, most of the previous studies were conducted in a gain domain, leaving aside the situations in which individuals have to share a certain amount of loss. The latter situations are common in human society, such as the liquidation of a bankrupt company. Are people more or less willing to bear the cost to enforce the fairness norm by punishing violators in liability sharing than in gain sharing? As potential losses tend to have a greater impact than equivalent gains upon individuals’ choices (Tversky and Kahneman, 1981, 1991; Tom et al., 2007), fairness preference and costly norm enforcement in liability sharing might not be the same as in gain sharing. In a recent study, we (see also Buchan et al., 2005; Leliveld et al., 2009; Zhou and Wu, 2011) extended the classic UG to the loss domain, in which participants had to decide whether to accept or to reject a division of a certain amount of monetary loss [e.g. 10 monetary units (MUs)] proposed by an anonymous partner in a one-shot manner. As in the classic UG, if the participants accept the offer, both parties bear the proposed loss; otherwise both sides had to pay the whole price (i.e. losing 10 U each). We found that participants were more likely to reject unfair offers in the loss than in the gain domain, suggesting a higher propensity of norm enforcement under adversity. This finding cannot be explained solely by strategic comparisons in making decisions between the two domains as this effect was present regardless of whether the gain–loss frame was manipulated within- or between-participants (Zhou and Wu, 2011).
Two possible motives may underlie this behavioral pattern. One is ‘a legitimate passion for equality that incites men to want to be strong and esteemed’ and the other ‘a depraved taste for equality … that leads the weak to want to bring the strong down to their level’ (Tocqueville, 1835/2010). According to the first theory, costly norm enforcement is driven by fairness preference, i.e. ‘something equal should be given to those who are equal’ (Aristotle, 1998; Fehr and Schmidt, 1999). Neurally, it has been demonstrated that the preference for fairness is implemented in the brain valuation system (Bossaerts et al., 2009; Bartra et al., 2013), most notably the ventral striatum (VS) (Tabibnia et al., 2008; Tricomi et al., 2010). The increased demand for fairness and the increased costly norm enforcement under adversity may therefore be associated with enhanced subjective value of fairness and its representation in VS.
Alternatively, costly norm enforcement may be driven by satisfaction through the punishment of norm violations (Dufwenberg and Kirchsteiger, 2004). Punishing those who have behaved unfairly is intrinsically rewarding to the punisher (Seymour et al., 2007; Espin et al., 2012). This motive is especially strong when the potential punisher is the victim of the norm violations. In this case, punishment is likely to be driven by the desire for revenge or retaliation. Neuroimaging studies have observed activation of DS when punishing breaches of trust (de Quervain et al., 2004), unfair divisions of resources (Sanfey et al., 2003; Baumgartner et al., 2011; Strobel et al., 2011; Crockett et al., 2013) and aggressive behavior (Krämer et al., 2007). Thus, the loss frame may increase costly norm enforcement by enhancing the satisfaction of punishment and associated neural processes in DS.
These two theories provide different predictions about punishment behaviors in different social contexts. Retaliation motivates punishment of unfair behavior inflicted on oneself (second-party punishment) but not on others (third-party punishment), whereas fairness preference motivates punishment of unfair behavior directed both toward oneself and toward others (Fehr and Fischbacher, 2004). Therefore, if gain–loss context regulates fairness preferences, loss should increase both second- and third-party punishment, and neuroimaging should reveal enhanced fairness-related responses in the medial prefrontal cortex (MPFC) and VS, which are related to the social utility of fairness (Tabibnia et al., 2008; Tricomi et al., 2010). On the other hand, if gain–loss context regulates retaliatory motives, loss should increase second-, but not third-party punishment and enhance the responses in the DS, anterior cingulate cortex and insula, which are related to reactive aggression and retaliation (Sanfey et al., 2003; de Quervain et al., 2004; Crockett et al., 2013; White et al., 2014).
Here, combining our previous behavioral paradigm with functional magnetic resonance imaging (fMRI), we investigated the neural basis of increased costly norm enforcement by recording the brain hemodynamic responses while participants, as responders in the UG game, were exposed to fair/unfair offers in both loss and gain domains (see also Guo et al., 2013). Using a formal inequality aversion model (Messick and McClintock, 1968; Fehr and Schmidt, 1999), we obtained subjective utility (SU) associated with each offer for each individual participant and a parameter (i.e. the ‘envy’ parameter) that quantifies how much a particular participant cares about inequality. We then applied these parameters to fMRI data (Wright et al., 2011). A key advantage of using model-based approach over more conventional neuroimaging approaches is that the former can ‘provide insights into how a particular cognitive process is implemented in a specific brain area as opposed to merely identifying where a particular process is located’ (O’Doherty et al., 2007). Moreover, the model parameters have more specific psychological meanings as compared with rejection rates (Messick and McClintock, 1968; Fehr and Schmidt, 1999). In our imaging analysis, brain activity was time-locked to the presentation of the offers. Based on previous studies, we hypothesized that the mesolimbic areas, such as VS and ventromedial prefrontal cortex (VMPFC), would be activated by monetary gain and fairness (Tabibnia et al., 2008; Haber and Knutson, 2010; Tricomi et al., 2010). DLPFC, anterior cingulate cortex (ACC) and insula, in contrast, would be activated by monetary loss and inequality (Sanfey et al., 2003; Baumgartner et al., 2011, 2012; Chang and Sanfey, 2013). Critically, we predicted that the gain–loss frame would modulate the neural processing of monetary interest and fairness implemented in these brain structures, which may give rise to the behavioral observation of increased costly norm enforcement under adversity.
We also conducted a behavioral experiment to differentiate to what extent the brain activations observed in the fMRI experiment were related to the general fairness preference or to the desire of retaliation. In particular, we asked participants to act as a third-party who could use their own endowment to punish the unfair proposers by reducing the proposers’ payoff at a 1:5 ratio. Given that it is established that the third-party punishment is primarily driven by fairness preferences (Fehr and Fischbacher, 2004), this experiment allowed us to address the crucial question as to whether the increased costly punishment in the loss domain is due to fairness preference or desire of retaliation.
METHODS
Participants
Twenty-eight healthy volunteers participated in the neuroimaging study in return for payment. Ten participants were excluded from data analysis either due to excessive head motion (>3 mm; three persons) or pure rational response (accepting essentially all offers; seven persons). The remaining 18 (13 females) participants had a mean age of 21.6 ± 1.8 years. Another 31 volunteers (18 females; mean age: 21.0 ± 1.4 years) participated in the behavioral third-party punishment experiment. All the participants were right-handed and were screened for psychiatric or neurological problems. The experiment protocols were approved by the Ethics Committee of the Department of Psychology at Peking University. All participants provided written informed consent after the procedures had been fully explained, in accordance with the Declaration of Helsinki.
Design and procedures
Prior to the experiment, participants were informed of the rules for the UG game. They were told that they would play the game with a group of anonymous students whom they did not know and who had made proposals, in a pilot session, on how to share a 10 MU gain or loss between them and the participants. In each round, the participants and a randomly drawn proposer would be endowed with 10 MUs (in the gain domain) or had to pay for a 10 MU penalty (in the loss domain); the participant’s task was to either accept or reject the offer made by the proposer. Upon acceptance, the gain or loss was split as proposed. Upon rejection, the participants and the proposer would receive nothing for the round of endowment or would pay out 10 MUs each for the round of penalty. The participants were informed that they would get 60 Chinese yuan for participating in the fMRI scanning and an additional 0–40 yuan for bonus, the amount of which was dependent on their performance in the experiment. They were also led to believe that the games would be played for real with the set of proposers they encountered during the experiment and that their decision in each trial was directly related to their own and the corresponding proposer’s final payoff. Participants were debriefed and thanked before they left the testing room.
Unknown to the participants, the offer in each round was predetermined by the experimenter. A two (domain: gain vs loss) by five (fairness level: 5:5, 4:6, 3:7, 2:8, 1:9) factorial design was used. Division schemes were 5/5, 4/6, 3/7, 2/8, 1/9 for the gain domain, and −5/−5, −6/−4, −7/−3, −8/−2 and −9/−1 for the loss domain, with the number before the slash indicating the offered amount to the responder and the number after the slash indicating the amount left to the proposer. Prior to scanning, each participant was familiarized with the task through 20 out-of-scanner practice trials which had the same composition of experimental conditions as the formal experiment in the scanner.
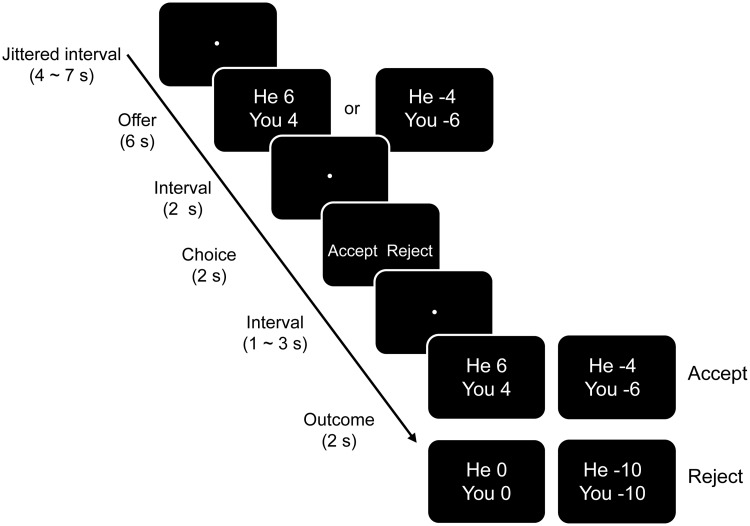
At the beginning of each trial, a fixation dot was presented at the center of the screen for a jittered duration (4–7 s; Figure 1). The participant then saw the offer (e.g. ‘you 2, he 8’ in the gain domain, and ‘you −8, he −2’ in the loss condition). The offer screen remained on the screen for 6 s, during which the participants evaluated the offer without making a response. Then the offer screen disappeared and the fixation dot reappeared for another 2 s, followed by a response screen also presented for 2 s. This screen, with the word ‘Accept’ on the left and the word ‘Reject’ on the right, counterbalanced over participants, prompted the participant to make either an acceptance or rejection decision by pressing a corresponding button using the right index or middle finger of the right hand. After a varied duration of 1–3 s, the outcome for the participant and the proposer (e.g. ‘you −10, he −10’) was displayed for 2 s (Figure 1). Thus, on average, a single round lasted for 18 s. There were 14 trials for each type of offer, with the 10 types of offers being presented in pseudorandom sequence for each participant. The 140 trials were divided into two equal-length runs, resulting in a total scanning time of ∼45 min. Each type of offer was equally divided into the two sessions.
Fig. 1.
Sequence of events and timing in a trial. Each trial began by presenting the offer to the participant for 6 s. The participant was told to evaluate the offer but not to press any button at this moment. After a 2 s interval, the participant had to decide whether to accept or to reject the offer by pressing one of two buttons. After another interval, the duration of which varied from 1 to 3 s, the outcome of this trial was presented. Upon acceptance, the amount of gain or loss would be divided according to the proposer’s offer. Upon rejection, both the participant and the proposer would get nothing (in the gain domain) or have to pain the full price (in the loss domain).
In the third-party punishment task, participants observed multiple rounds of one-shot UG. They were told that a number of players had participated in an UG. The responders could either reject or accept the offers by the proposer, or they could give up their right to make a decision. The division schemes for the participants who gave up their decision were presented to the participants who were asked to make third-party decision as to whether to punish the proposers. On each trial they were endowed with 1 yuan, which they could use to reduce the proposer’s payoff at a 1:5 ratio. Participants could use 0.2, 0.4, 0.6, 0.8 or 1.0 yuan from their endowment and reduce the proposer’s payoff up to 5.0 yuan.
The proposer’s division schemes were identical to the second-party punishment paradigm, i.e. 5/5, 4/6, 3/7, 2/8, 1/9 for the gain domain, and −5/−5, −6/−4, −7/−3, −8/−2 and −9/−1 for the loss domain. Each type of division was presented six times in a pseudo-random sequence, rendering 60 critical trials. The participants were instructed that five rounds would be randomly selected and the payoffs to them, the proposer and the responder were computed according to the choices in these rounds. The dependent variable was then the amount of money spent on reducing the proposer’s payoff. Since a credible scenario is critical, as it ensures that the third-party experiment and the second-part one can actually be compared, we took three measures to ensure credibility: (i) we excluded any participants who had previously encountered economic games from participation in the study, (ii) we asked each participant after the experiment whether he/she believed the game was real (no participants were suspicious of the scenario) and (iii) we asked each participant to withhold the content of the experiment until we finished data collection (after which, we notified the participant that the data collection was complete and the experiment could be discussed).
fMRI data acquisition
Functional images were acquired on a 3T Siemens Trio system at the Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, using a T2-weighted echo planar imaging sequence (48 sagittal slices, 3 mm thickness; TR = 2400 ms; TE = 25 ms; flip angle = 90°; field of view = 224 × 224 mm2 ; voxel size = 3 × 3.5 × 3.5 mm3). The first five volumes were discarded to account for magnetic equilibration. Two runs of 535 volumes were collected from each participant.
Behavioral modeling
We examined the correspondence between the prediction of a formal economic model and participants’ choices (Messick and McClintock, 1968; Fehr and Schmidt, 1999). The aim of this analysis was 2-fold: the first was to explicitly distinguish between the SU of an offer to a particular participant and the degree to which he/she cared about the inequality in that offer. These two psychologically different factors are otherwise implicitly embedded in the participant’s choice. The second purpose was to derive model parameters that could bridge the external choices on the one hand and the underlying neural mechanisms on the other (see below).
Following the procedure of Wright et al. (2011), we fitted the behavioral data (i.e. the acceptance rate at each fairness level) using a psychometric model,
where z is the fairness level and b0 and b1 are free model parameters. We estimated the model separately for the gain and loss domains. Assuming that the acceptance rate is a sigmoid function of SU associated with each offer (Wright et al., 2011), the above psychometric equation can be re-written as:
where the SU (U) is defined according to an influential economic theory of fairness and inequality aversion (Fehr and Schmidt, 1999):
Instead of denoting direct payoff derived from the offers (e.g. 4 or −8), xself and xother here denote the generalized payoff, i.e. the additional amount of money the proposer (xother) and the responder (xself) would get when the responder accepts relative to rejects offer. In the gain domain, these values are equal to the proposed division. In the loss domain, these values equal to 10 plus the proposed division (i.e. 1, 2, 3, 4, 5 for the responder and 9, 8, 7, 6, 5 for the proposer). This transformation, while keeping the shape of the regression curves, and thus keeping the model parameters unchanged, aligns the curve in the loss domain with that in the gain domain in a Cartesian two-dimensional space (i.e. generalized payoff as x-axis and acceptance rate as y-axis) so that a direct comparison between gain and loss is made easy. The ‘envy’ parameter α reflects the degree to which an individual cares about inequality, and the ‘temperature’ parameter λ reflects decision randomness. We optimized participant-specific α and λ, separately for gain trials and loss trials, according to the acceptance rate in each condition using the glmfit function implemented in Matlab (Table 1).
Table 1.
Results of behavioral model fitting
| Participant | Gain |
Loss |
||||
|---|---|---|---|---|---|---|
| α | λ | Log-likelihood | α | λ | Log-likelihood | |
| 1 | 0.12 | 0.02 | −3.60 | 0.12 | 0.02 | −5.75 |
| 2 | 0.42 | 0.74 | −18.15 | 0.94 | 0.77 | −12.15 |
| 3 | 0.53 | 0.74 | −16.30 | 0.71 | 0.89 | −16.96 |
| 4 | 0.74 | 0.03 | −8.38 | 0.75 | 0.03 | −9.12 |
| 5 | 0.13 | 0.02 | −3.60 | 0.73 | 0.03 | −3.60 |
| 6 | 0.33 | 0.02 | −5.74 | 0.54 | 0.47 | −9.39 |
| 7 | 0.12 | 0.02 | −9.56 | 0.13 | 0.02 | −5.74 |
| 8 | 0.12 | 0.02 | −5.74 | 0.33 | 0.02 | −5.74 |
| 9 | 0.12 | 0.02 | −8.38 | 0.13 | 0.02 | −9.56 |
| 10 | 0.17 | 0.85 | −20.71 | 0.13 | 0.02 | −7.28 |
| 11 | 0.56 | 0.81 | −17.36 | 1.11 | 0.03 | −0.00 |
| 12 | 0.33 | 0.03 | −9.12 | 0.47 | 0.43 | −9.38 |
| 13 | 0.33 | 0.50 | −14.04 | 0.44 | 0.73 | −17.34 |
| 14 | 0.34 | 0.03 | −3.60 | 0.34 | 0.03 | −3.60 |
| 15 | 0.74 | 0.03 | −5.75 | 0.76 | 0.03 | −5.74 |
| 16 | 0.21 | 0.02 | −0.00 | 0.32 | 0.93 | −23.41 |
| 17 | 0.13 | 1.11 | −24.70 | 0.20 | 1.14 | −26.02 |
| 18 | 0.34 | 0.03 | −7.27 | 1.07 | 0.71 | −9.39 |
The ‘envy’ parameter α reflects the degree to which an individual cares about inequality, and the ‘temperature’ parameter λ reflects decision randomness. Log-likelihoods show the goodness of fit (the closer to zero, the better the fit).
fMRI data analysis
Functional data were analyzed using standard procedures in SPM8 (Statistical Parametric Mapping; http://www.fil.ion.ucl.ac.uk/spm). Images were slice-time corrected, motion corrected, re-sampled to 3 × 3 × 3 mm3 isotropic voxel, normalized to MNI space (Montreal Neurology Institute), spatially smoothed with an 8 mm FWHM Gaussian filter, and temporally filtered using a high-pass filter with 1/128 Hz cutoff frequency. Statistical analyses based on general linear model (GLM) were performed first at the participant level and then at the group level.
For the individual participant level analysis, we built a parametric model and a factorial model. In the parametric model (GLM 1), we separately modeled the offer presentation, response cue, motor response and outcome in the gain and loss domains with boxcar functions spanning the whole event convolved with a canonical hemodynamic response function. The regressors corresponding to the offer presentation screen in both the gain and the loss domains were further modulated by the estimated SU that was computed with the above modeling procedures. (We also built a parametric model in which the defined fairness level, instead of the SU, served as the parametric modulation. Essentially, the same pattern of activations was obtained.) We checked the correlations between the regressors and found that the correlation between the offer stage and the decision stage was 0.19 and the correlation between the decision stage and the outcome stage was 0.13. These correlations were tolerable high in an event-related fMRI design. In the factorial model (GLM 2), the offer presentation events were assigned to four regressors according to the gain/loss domain and the participants’ choice (acceptance vs rejection). Another six regressors were included corresponding to the onset and duration of the response cue, motor response and outcome in both gain and loss domains. To extract regional activation strength (i.e. beta estimates), a third model was built (GLM 3), in which the offer presentation corresponding to each fairness level was modeled in separate regressors. The six rigid body parameters were also included in all the three models to account for head motion artifact.
For the group level analysis, a full factorial model with the parametric regressors in the gain and loss domains was built. This model allowed us to identify the brain regions that showed differential or similar association with SU in the loss and the gain domains. For the commonalities, we defined a conjunction between the positive effect of SU in the gain and the loss domains, and a conjunction between the negative effect of SU in the gain and the loss domains. For the differential effect, we first defined four contrasts with an exclusive mask approach in parametric analysis (Pochon et al., 2002; Seidler et al., 2002; Roggeman et al., 2011; Chen and Zhou, 2013): (i) positive effect of the parametric regressor of SU in the gain domain exclusively masked by positive effect of the parametric regressor of SU in the loss domain, i.e. Gain+ [masked (excl.) by Loss+], (ii) negative effect of the parametric regressor of SU in the loss domain exclusively masked by negative effect of the parametric regressor of SU in the gain domain Loss− [masked (excl.) by Gain−]; (iii) and (iv) the reversed contrast of (i) and (ii), i.e. Loss+ [masked (excl.) by Gain+] and Gain− [masked (excl.) by Loss−]. The mask image was thresholded at P < 0.01 uncorrected. The Gain+[masked (excl.) by Loss+] contrast, for example, will show brain areas that positively correlate with SU in the gain domain (at P < 0.001) but not positively correlate with SU in the loss domain (even at P < 0.01). This difference in significance, however, should not be taken as significant difference (Nieuwenhui et al., 2011). For a formal test for significant difference in the association with SU and fairness level, we extracted from two regions of interests (ROIs), i.e. the VS and the right DLPFC, the beta values corresponding to all the 10 offer types (based on GLM 3) and subjected them to repeated measures of analysis of variance (ANOVA). The coordinates of the ROIs were defined based on the exclusive mask procedure. Because the criteria for ROI selection (based on GLM 1) and ROI data extraction and statistical analyses (based on GLM 3) were independent, we believe this procedure controlled for the ‘double dipping’ problem (Kriegeskorte et al., 2009).
To reveal the interaction between gain–loss frame and participants’ behavioral choice, the contrast corresponding to this interaction [Loss (rej–acc) − Gain (rej–acc)] was defined using the one sample t-test in SPM8 based on GLM 2.
We reported only those clusters that survive cluster-level correction for multiple comparison (family wise error, FWE; P < 0.05) either over the whole brain or over a priori ROIs (cluster-level correction after voxel-level thresholding at P < 0.005; Lieberman and Cunningham, 2009). The a priori ROI of DS (MNI coordinates: −16, 2, 14) was derived from Crockett et al. (2013), that of VS (MNI coordinates: −9, 12, −6) and VMPFC (MNI coordinates: −9, 39, −9) were derived from Tricomi et al. (2010), and that of the anterior cingulate cortex (MNI coordinates: −8, 26, 28) was derived from Sanfey et al. (2003). Statistical analyses over the ROIs were conducted using the small-volume correction (SVC) method implemented in SPM8. Specifically, spherical search space with 8 mm radius was defined surrounding the independently defined coordinates reported above.
RESULTS
Behavioral results
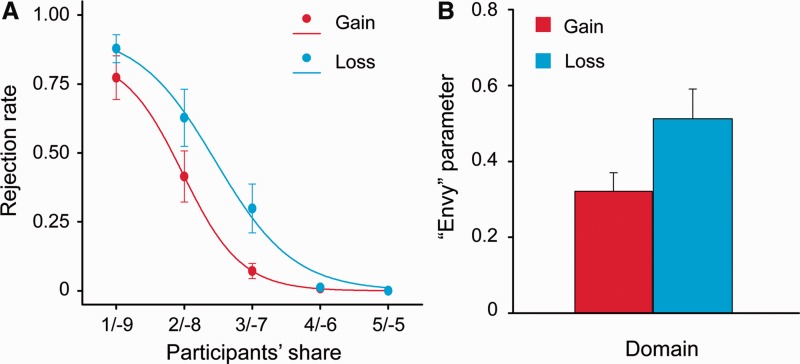
We first examined the behavioral effects of gain–loss frame on costly norm enforcement in the UG (Figure 2A). Not surprisingly, participants were increasingly likely to reject offers as the level of unfairness increased, Greenhouse–Geisser adjusted F(4, 68) = 61.73, P < 0.001. Importantly, replicating our previous study (Zhou and Wu, 2011), the overall rejection rate was higher for the offers in the loss domain (mean rejection rate = 36.3%, s.d. = 4.1%) than in the gain domain (mean rejection rate = 25.4%, s.d. = 3.4%), F(1, 17) = 35.20, P < 0.001. Moreover, the interaction between domain and fairness level was significant, Greenhouse–Geisser adjusted F(4, 68) = 4.06, P < 0.05, such that the difference in rejection rate tended to be the largest for the moderately unfair conditions (3:7 and 2:8).
Fig. 2.
Behavioral and modeling results from the UG. (A) Points indicate rejection rates in gain (red) and loss domains (blue), respectively. Lines are rejection rates as a logistic function fitted to those points. (B) The ‘envy’ parameter (α) derived from the computational model (Wright et al., 2011) in gain and loss domains. Error bars represent s.e.m.
Results of behavioral modeling
Although the α parameter was significantly larger than zero in both domains (αgain = 0.32 ± 0.21, range, 0.12–0.74, t17 = 6.60, P < 0.001; αloss = 0.51 ± 0.33, range, 0.12–1.11, t17 = 6.58, P < 0.001), the value was significantly larger in the loss than in the gain domain (t17 = 3.37, P < 0.01; Table 1, Figure 2B). The ‘temperature’ parameter λ (λgain = 0.28 ± 0.09, range, 0.01–1.11; λloss = 0.35 ± 0.10, range, 0.02–1.14) did not significantly differ between gain and loss domains (t17 < 1, P > 0.1; Table 1). These results confirmed our prediction that people are generally inequality aversive, and more so in the loss than in the gain domain.
Brain areas tracking subjective utility (SU)
Compared with offers in the loss domain, offers in the gain domain elicited activations in the mesolimbic dopaminergic regions (based on GLM 1), including VMPFC, ventral tegmental area (VTA) and posterior cingulate cortex (PCC) (Table 2). The reversed contrast revealed activations in bilateral DLPFC, inferior parietal lobule (IPL) and superior parietal lobule (Table 2). A conjunction analysis showed that in both the loss and the gain domains, VMPFC, VS and PCC were more activated as the utility of the offers increased (Table 3). In contrast, activations in bilateral DLPFC, ACC, anterior insula (AI), thalamus and midbrain negatively correlated with the SU, irrespective of domain (Table 3).
Table 2.
Brain activations in the gain vs loss contrast
| Regions | Hemisphere | Max T-value | Cluster size (voxels) | Cluster level corrected PFWE | MNI coordinates |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Gain–loss | |||||||
| VMPFC | R | 6.85 | 155 | <0.001 | 6 | 47 | −11 |
| PCC | R | 4.37 | 60 | 0.019 | 9 | −10 | −11 |
| VTA | L | 6.73 | 167 | <0.001 | −6 | −52 | 16 |
| Loss–gain | |||||||
| Putamen | R | 5.50 | 52 | 0.034 | 30 | 5 | 7 |
| DLPFC | L | 7.01 | 138 | <0.001 | −36 | 2 | 34 |
| Rolandic | R | 7.01 | 112 | 0.001 | 45 | −10 | 22 |
| IPL/angular | R | 5.32 | 153 | <0.001 | 27 | −61 | 46 |
| SOG | L | 6.54 | 58 | 0.022 | −21 | −67 | 40 |
| Calcarine | L | 8.15 | 554 | <0.001 | −18 | −73 | 16 |
SOG, superior occipital gyrus.
Table 3.
Brain activations in parametric contrast (conjunction of gain and loss domains)
| Regions | Hemisphere | Max T-value | Cluster size (voxels) | Cluster level corrected PFWE | MNI coordinates |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Increase with SU | |||||||
| VMPFC | L/R | 4.55 | 181 | 0.021 | 6 | 53 | −17 |
| VS | L/R | 3.78 | 10 | 0.039 a | −3 | 8 | −11 |
| Parahippocampus | R | 4.02 | 151 | 0.044 | 18 | −28 | −10 |
| Fusiform | L | 5.65 | 361 | <0.001 | −33 | −28 | −19 |
| Precuneus | L | 4.09 | 178 | 0.022 | −9 | −55 | 13 |
| Decrease with SU | |||||||
| ACC | L/R | 3.99 | 17 | 0.025 a | −6 | 32 | 25 |
| DLPFC | R | 5.36 | 246 | 0.004 | 39 | 26 | 28 |
| L | 5.90 | 437 | <0.001 | −42 | 20 | 31 | |
| Putamen/insula | R | 4.58 | 149 | 0.047 | 30 | 20 | 1 |
| PAG | R | 5.12 | 278 | 0.002 | 3 | −22 | −14 |
| IPL | R | 6.13 | 1342 | <0.001 | 24 | −55 | 34 |
| L | 5.66 | −24 | −51 | 40 | |||
| IOG | L | 4.60 | 674 | <0.001 | −42 | −76 | −8 |
PAG, periaqueductal gray; IOG, inferior occipital gyrus. aSVC based on independently defined ROI (see Methods).
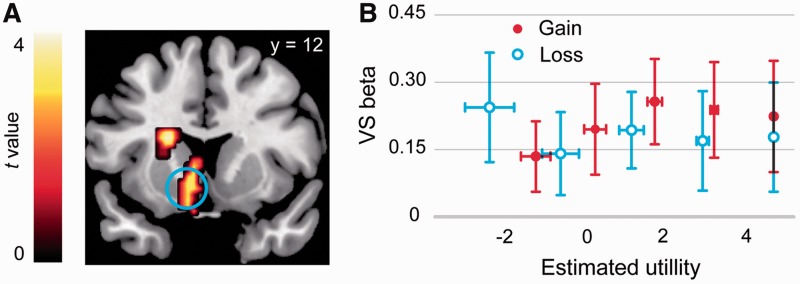
Examining the correlations between brain activations associated with the offer presentation stage and fairness (i.e. SU) of the offers, we found significant correlation in the VS, DS and VMPFC in the gain domain but not in the loss domain (Figure 3A, Table 4). We extracted the beta estimates in VS corresponding to each of the 10 offer type (based on GLM 3) and subjected these values to a formal test for significant interaction. A repeated-measures ANOVA with domain and fairness level as within participant factor showed a significant interaction between domain (gain vs loss) and fairness level, F(4, 68) = 2.48, P = 0.05, confirming the pattern observed in the whole-brain analysis (Figure 3B). It can be seen from the figure that in the gain domain the VS activation increased with the increase of offer utility, but this trend was not apparent in the loss domain.
Fig. 3.
Positive effect of SU modulated by frame. (A) Whole-brain exploratory analysis of the contrast ‘Gain+ [masked (excl.) by Loss+]’. (B) Beta values corresponding to 10 types of offers (based on GLM 3) extracted from the VS peak. Error bars represent s.e.m.
Table 4.
Brain activations in parametric contrast (domain-specific activations)
| Regions | Hemisphere | Max T-value | Cluster size (voxels) | Cluster level corrected PFWE | MNI coordinates |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Positive association with SU only in gain domain | |||||||
| VMPFC | L | 5.02 | 378 | <0.001 | −6 | 62 | 1 |
| Caudate | L | 4.62 | 673 | <0.001 | −18 | 14 | 19 |
| MTG | R | 4.23 | 154 | 0.041 | 51 | −22 | −11 |
| Fusiform | L | 5.10 | 232 | 0.006 | −60 | −43 | −8 |
| Angular | R | 5.16 | 182 | 0.020 | 60 | −52 | 25 |
| Negative association with SU only in loss domain | |||||||
| LOFC | L | 4.93 | 170 | 0.027 | −48 | 47 | 1 |
| DLPFC | R | 5.17 | 428 | <0.001 | 54 | 11 | 28 |
| ACC | L/R | 3.74 | 17 | 0.025 a | −6 | 26 | 31 |
| Precentral | L | 4.35 | 155 | 0.040 | −33 | −1 | 43 |
| IPL | L | 5.46 | 257 | 0.003 | −48 | −25 | 40 |
MTG, middle temporal gyrus. aSVC based on independently defined ROI (see Methods).
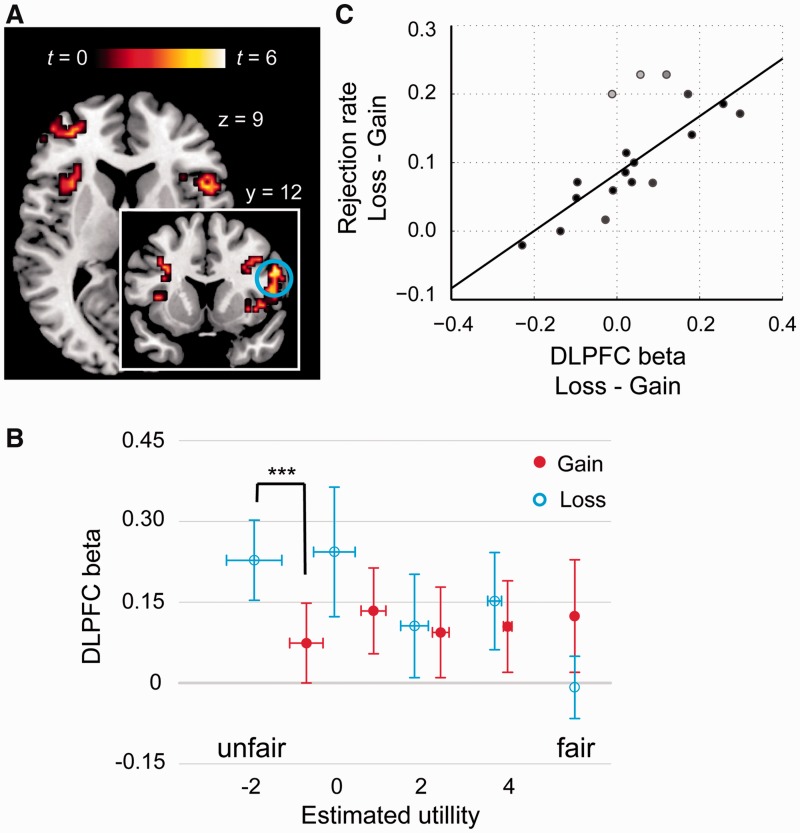
In contrast, we found that the activations in bilateral AI, ACC, right DLPFC, and left lateral orbitofrontal cortex (LOFC) showed negative correlations with fairness (i.e. SU) in the loss domain (Figure 4A, Table 4) but not in the gain domain. The beta estimates (based on GLM 3) in right DLPFC showed a significant interaction between domain (gain vs loss) and fairness level, F(4, 68) = 2.62, P < 0.05, confirming the pattern observed in the whole-brain analysis (Figure 4B). Specifically, the increase of DLPFC activation in the loss relative to the gain domain was most evident in the most unfair condition (1:9; t(17) = 4.43, P < 0.001), whereas a reversed trend was observed in the fairest condition (5:5; t(17) = −1.82, P = 0.087). Moreover, the difference in the mean activation in DLPFC (averaged across all offer levels) in the loss relative to the gain domain predicted the increased rejection rate in the same comparison (robust regression coefficient r = 0.80, P < 0.001; Figure 4C) (Wager et al., 2005).
Fig. 4.
Negative effect of SU modulated by frame. (A) Whole-brain level exploratory analysis of the contrast ‘Loss− [masked (excl.) by Gain−]’. (B) Beta values corresponding to 10 types of offers (based on GLM 3) extracted from the DLPFC peak. (C) The difference in the mean beta values in the gain and loss domain predicted the differences in rejection rates between the loss and gain domain (r = 0.80, P < 0.001). ***P < 0.001 (two-tailed). Error bars represent s.e.m.
Gain–loss domain modulates rejection-related activation in the dorsal striatum (DS)
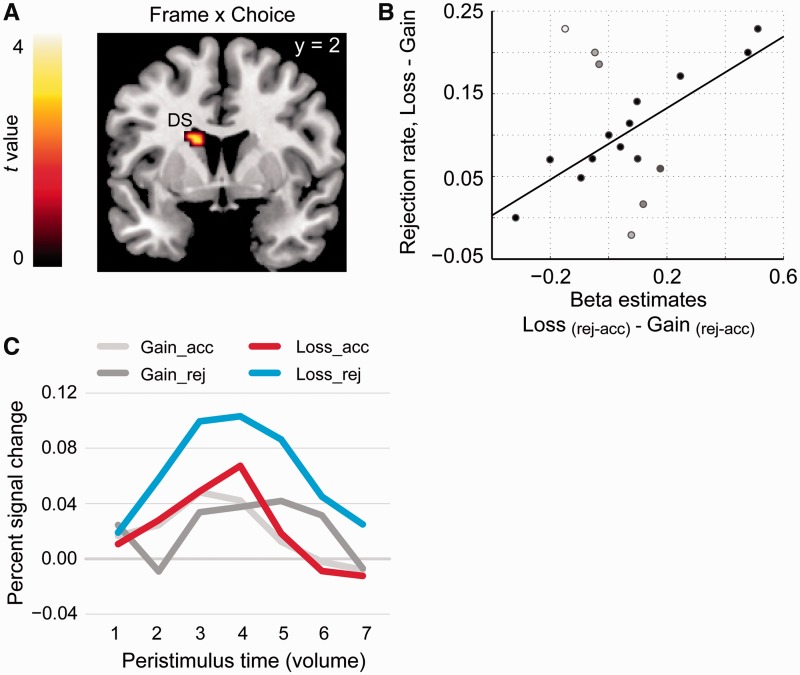
To test the hypothesis that loss enhanced the rejection-related activation in the DS, we built a factorial model (GLM 2) which separately modeled the offer presentation events according to the gain/loss domain and the participants’ choice (acceptance vs rejection). Confirming our hypothesis, the contrast ‘Loss (rej-acc) − Gain (rej-acc)’ revealed a significant cluster in left DS [MNI coordinates: −15, 2, 22; PFWE (SVC) < 0.05, k = 10; Figure 5A]. Percent signal change data extracted using Marsbar software (available online at http://marsbar.sourceforge.net/) from a 6 mm radius sphere around the independently defined DS coordinates (Crockett et al., 2013) were plotted to illustrate the direction of the interaction (Figure 5B). As can be seen, the interaction was driven by the increased activation during rejection relative to acceptance in the loss domain.
Fig. 5.
Neural effects of interaction between choice and frame. (A) ROI-based analysis of the contrast ‘Loss (rej-acc) − Gain (rej-acc)’. SVC revealed an activation cluster in the left DS, whose rejection-induced activation was higher in the loss compared with gain domain. (B) Activation timecourse extracted from a 6 mm sphere around the maximum coordinates indicates that this interaction effect was driven by the amplified activation difference in the loss relative to the gain domain. (C) The differences in beta estimates extracted from the activation maximum (Loss − Gain) predicted the increases in rejection rate in the loss relative to the gain domain (r = 0.67, P < 0.05). Note, the white and grey dots are outliers identified by robust regression and they are down-weighted in computing the correlation coefficients (Wager et al., 2005).
Given that rejection predominantly occurred in the most unfair condition and acceptance in the fairest condition, it may be argued that the observed interaction between participants’ choice and domain is driven by the differential effects associated with the two extreme conditions. To test this possibility, we extracted the beta values from the peak voxel of DS and found that the interaction between fairness level (1:9 vs 5:5) and domain was not significant, F(1, 17) < 1, P > 0.1, indicating that the observed effect was not solely due to the differential effect of these two conditions.
We then examined whether increases in DS activation during rejection in the loss domain (vs gain domain) were correlated with increases in rejection rate. We found that participants with the greatest increases in left DS activation during rejection in the loss domain showed the greatest increases in rejection rate in the loss domain (robust regression coefficient r = 0.67, P < 0.05; Figure 5C).
Gain–loss domain and third-party punishment
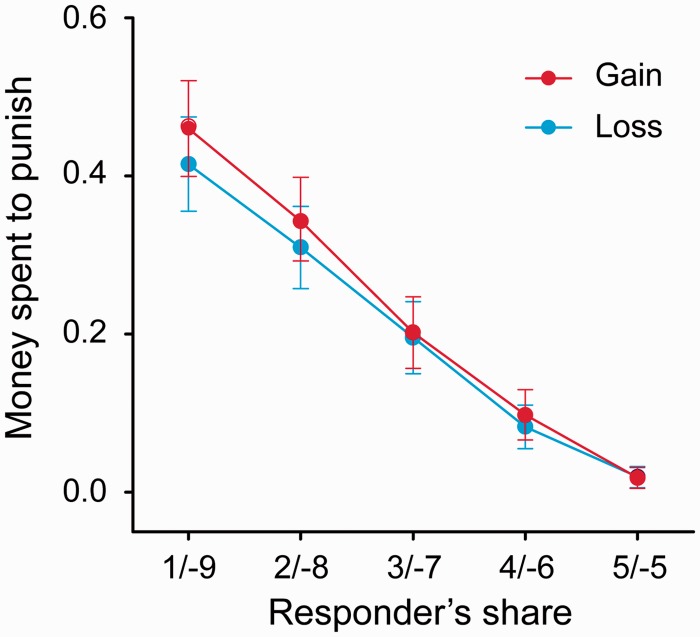
The neuroimaging findings suggest that the loss domain increased second-party punishment by enhancing retaliatory motives, while at the same time reducing fairness preferences. Lending support to these findings, we found that gain–loss domain did not regulate third-party punishment, which primarily relied on fairness preference rather than retaliatory motives (Figure 6). Specifically, participants paid more to punish proposers as their offers became increasingly unfair (main effect of fairness, F(4, 120) = 48.87, P < 0.001). We did not observe any effects of domain on the amount paid to reduce the proposer’s payoff [domain: F(1, 30) = 1.30, P = 0.264; domain-by-fairness: F(4, 120) = 1.03, P = 0.395].
Fig. 6.
Effect of fairness and domain on third-party punishment behavior. The amount spent on punishment increased as the offer fairness decreased. Gain–loss domain did not modulate third-party punishment. Error bars represent s.e.m.
DISCUSSION
Using fMRI and a variant of the UG, we provide evidence for a neural and behavioral account of how gain–loss frame modulates costly norm enforcement. Our findings are generally in-line with a recent neuroimaging study (Guo et al., 2013) which adopted our previous paradigm (Zhou and Wu, 2011). However, it should be noted that this study focused on the brain responses to subjective value of offers and on the association between brain activations and behavioral measures, such as acceptance rate and subjective value. With the aid of these behavior–brain correlations, we were better able to interpret our neuroimaging results in terms of psychological and economic factors.
Replicating our previous behavioral finding (Zhou and Wu, 2011), participants in the current experiment rejected more in the loss than in the gain domain. Parallel with this, results evidenced a higher response to offers that would be rejected than to those that would be accepted, and critically, the difference was amplified in the loss domain. This raised the possibility that the loss context increased the motivation to reject an unfair offer and thus punish the proposer. Reinforcement learning literature showed that the DS plays a unique role in learning about actions and their reward consequences (Balleineet al., 2007). In more complex social context, the DS has been implicated in punishing norm violations (de Quervain et al., 2004; Krämer et al., 2007; Baumgartner et al., 2008, 2012; Strobel et al., 2011; Crockett et al., 2013). Indeed, Guo et al. (2013) also found that the rejection (vs acceptance) of unfair offers elicited higher DS activation in the loss than in the gain domain. Together with these findings, our results suggest that loss may have increased the motivation for punishing norm enforcement, rather than the fairness preferences. This argument is strengthened by the finding that loss did not increase punishment in a third-party context (Figure 6), where punishment was primarily motivated by fairness preferences.
Our results also suggest a context-dependent nature of fairness preference (see also Guo et al., 2013). Previous studies have implicated VS in processing the subjective value of fairness and cooperation (Rilling et al., 2002; Hsu et al., 2008; Tricomi et al., 2010). If the loss frame increases costly norm enforcement by enhancing the salience of fairness preferences, we should have observed increased association between VS activation and fairness in the loss relative to the gain domain. But what we observed was just the opposite. Behaviorally, the model output indicated that the same level of unfairness corresponded to lower subjective value in the loss relative to the gain domain. Neurally, loss has actually blunted the association between the VS activation and fairness. Taken together, our results suggest that loss reduces, rather than enhances the salience of fairness preference.
The increased punishment of norm violations in the loss domain was associated with stronger negative correlations between fairness and the activation in the right DLPFC, which has consistently been implicated in the implementation of second-party punishment such as that in the UG. Existing studies (Knoch et al., 2006, 2008) have shown that the DLPFC (especially the right DLPFC) is causally involved in the implementation of costly norm enforcement, perhaps by overriding selfish impulses and thus making the rejection of unfair offers easier (Knoch et al., 2006). We found that the negative correlation between right DLPFC activation and fairness increased in the loss relative to the gain domain. The difference between gain and loss in DLPFC activation was most evident in the most unfair conditions, where participants substantially rejected the offers and incurred a cost to themselves. Compared with not getting anything, which is the consequence of rejection in the gain domain, losing 10 MU by rejecting stingy offers in the loss domain seemed to be a more difficult decision for the participants (Tversky and Kahneman, 1991) and may thus require stronger inhibition of selfish impulses. The enhanced negative association between fairness and the neural activations in the DLPFC indicated that heightened inhibition of selfish impulses may contribute to the behavioral effect of increasing demand of fairness and norm enforcement under adversity.
Our finding of the effect of gain–loss domain on costly punishment echoes the evidence from a recent pharmacological functional neuroimaging (Crockett et al., 2013). There the authors temporarily depleted participants’ serotonin level and found that this manipulation increased participants’ rejection to unfair offers in the second-party UG, but did not affect the punishment decision in the third-party UG. Moreover, serotonin depletion decreased the VS responses to fairness and increased DS responses associated with retaliation. Given that serotonin functions to reduce impulsivity and aggression in human social and economic behaviors (Bjork et al., 1999; Crockett et al., 2008; Rogers, 2011; Kiser et al., 2012), it is conceivable that depletion of serotonin increased impulsive and aggressive behaviors, i.e. retaliating norm violators in UG, rather than increase the fairness preferences. Analogously, we could argue that the loss domain selectively increases competitive, spiteful punishment, which is positively correlated with impulsive choice, rather than moralistic, fairness-based punishment, which is negatively correlated with impulsive choice (Espín et al., 2012).
It should be noted, however, that we did not equate the activation of VS with fairness preference, the activation of DS with motivation to punishment, and so on. This kind of informal inference from brain activation to mental processes may commit the reverse inference fallacy, which refers to a way of inference in neuroimaging study that ‘reason backward from patterns of activation to infer the engagement of specific mental processes’ (Poldrack, 2006, 2011). However, as the UG has been investigated with fMRI a substantial number of times and converging evidence concerning the functional link between specific brain regions and mental process (e.g. dopamine system and fairness preference) has been well established (Tabibnia et al., 2008; Tricomi et al., 2010; Crockett et al., 2013; Guo et al., 2013), our inference in this context is much safer than a pure explorative attempt.
In conclusion, our findings provide neural and computational accounts for the behavioral effect of increasing demand of fairness and norm enforcement under adversity. Our neuroimaging data imply that loss reduces the responsiveness of the dopamine system to fairness and while enhancing the motivation for rejection. This process is complemented by increased responses of the prefrontal regions to insultingly unfair offers. Taken together, the computation of social utility and the fairness-related decisions are context-dependent and are modulated by gain–loss status.
Acknowledgments
This study was supported by National Basic Research Program (973 Program: 2010CB833904), by grants from the Natural Science Foundation of China (30110972, 91232708, 71172216, 71132001, 31100744) and from the China Postdoctoral Science Foundation (20100470156, 2012T50023). Part of the findings reported in this article has been presented on the Peking University–New York University Joint Symposium on Neuroeconomics and Social Cognition (28–29 June 2010, Beijing) and on the Second International Conference on Neuroeconomics and Neuromanagement, Zhejiang University (27–28 October 2010, Hangzhou). The authors thank Ms Lihui Wang and Dr Luke Chang for neuroimaging data analysis, Ms Yunyan Duan for model estimation, and Mr Philip Blue and two anonymous reviewers for their constructive comments on an earlier version of the manuscript.
REFERENCES
- Aristotle . The Nicomachean Ethics. Oxford, England: Oxford University Press; 1998. [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. Journal of Neuroscience. 2007;27(31):8161–5. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–27. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Götte L, Gügler R, Fehr E. The mentalizing network orchestrates the impact of parochial altruism on social norm enforcement. Human Brain Mapping. 2012;33(6):1452–69. doi: 10.1002/hbm.21298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58(4):639–50. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Knoch D, Hotz P, Eisenegger C, Fehr E. Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice. Nature Neuroscience. 2011;14(11):1468–74. doi: 10.1038/nn.2933. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Dougherty DM, Moeller FG, Cherek DR, Swann AC. The effects of tryptophan depletion and loading on laboratory aggression in men: time course and a food-restricted control. Psychopharmacology. 1999;142:24–30. doi: 10.1007/s002130050858. [DOI] [PubMed] [Google Scholar]
- Bossaerts P, Preuschoff K, Hsu M. The Neurobiological foundations of valuation in human decision-making under uncertainty. In: Glimcher PW, Fehr E, Poldrack RA, editors. Neuroeconomics: Decision Making and the Brain. 2009. New York, NY: Academic Press, pp. 353–65. [Google Scholar]
- Boyd R, Gintis H, Bowles S, Richerson PJ. The evolution of altruistic punishment. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(6):3531–5. doi: 10.1073/pnas.0630443100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan N, Croson R, Johnson E, Wu G, John M. Gain and loss ultimatums. Advances in Applied Microeconomics. 2005;13:1–23. [Google Scholar]
- Buchanan JM. The constitution of economic policy. The American Economic Review. 1987;77(3):243–50. [Google Scholar]
- Buckholtz JW, Asplund CL, Dux PE, et al. The neural correlates of third-party punishment. Neuron. 2008;60(5):930–40. doi: 10.1016/j.neuron.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Camerer CF. Behavioural studies of strategic thinking in games. Trends in Cognitive Sciences. 2003;7(5):225–31. doi: 10.1016/s1364-6613(03)00094-9. [DOI] [PubMed] [Google Scholar]
- Chang LJ, Sanfey AG. Great expectations: neural computations underlying the use of social norms in decision-making. Social Cognitive and Affective Neuroscience. 2013;8(3):277–84. doi: 10.1093/scan/nsr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhou X. Vision dominates at the preresponse level and audition dominates at the response level in cross-modal interaction: behavioral and neural evidence. Journal of Neuroscience. 2013;33(17):7109–21. doi: 10.1523/JNEUROSCI.1985-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett MJ, Apergis-Schoute A, Herrmann B, et al. Serotonin modulates striatal responses to fairness and retaliation in humans. Journal of Neuroscience. 2013;33(8):3505–13. doi: 10.1523/JNEUROSCI.2761-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Tabibnia G, Lieberman MD, Robbins TW. Serotonin modulates behavioral reactions to unfairness. Science. 2008;320:1739. doi: 10.1126/science.1155577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ-F, Fischbacher U, Treyer V, et al. The neural basis of altruistic punishment. Science. 2004;305(5688):1254–8. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- Dufwenberg M, Kirchsteiger G. A theory of sequential reciprocity. Games and Economic Behavior. 2004;47(2):268–98. [Google Scholar]
- Espín AM, Brañas-Garza P, Herrmann B, Gamella JF. Patient and impatient punishers of free-riders. Proceedings of the Royal Society of London. Series B, Biological Sciences. 2012;279(1749):4923–8. doi: 10.1098/rspb.2012.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr E, Fischbacher U. Social norms and human cooperation. Trends in Cognitive Sciences. 2004;8(4):185–90. doi: 10.1016/j.tics.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Fehr E, Gächter S. Altruistic punishment in humans. Nature. 2002;415(6868):137–40. doi: 10.1038/415137a. [DOI] [PubMed] [Google Scholar]
- Fehr E, Schmidt KM. A theory of fairness, competition, and cooperation. Quarterly Journal of Economics. 1999;114(3):817–68. [Google Scholar]
- Guo X, Zheng L, Zhu L, et al. Increased neural responses to unfairness in a loss context. Neuroimage. 2013;77:246–53. doi: 10.1016/j.neuroimage.2013.03.048. [DOI] [PubMed] [Google Scholar]
- Güroğlu B, van den Bos W, Rombouts SA, Crone EA. Unfair? It depends: neural correlates of fairness in social context. Social Cognitive and Affective Neuroscience. 2010;5(4):414–23. doi: 10.1093/scan/nsq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güth W, Schmittberger R, Schwarze B. An experimental analysis of ultimatum bargaining. Journal of Economic Behavior & Organization. 1982;3(4):367–88. [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich J, McElreath R, Barr A, et al. Costly punishment across human societies. Science. 2006;312(5781):1767–70. doi: 10.1126/science.1127333. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Rieger JW, Baecke S, et al. Predicting decisions in human social interactions using real-time fMRI and pattern classification. PLoS One. 2011;6(10):e25304. doi: 10.1371/journal.pone.0025304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Anen C, Quartz SR. The right and the good: distributive justice and neural encoding of equity and efficiency. Science. 2008;320:1092–5. doi: 10.1126/science.1153651. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Knetsch JL, Thaler R. Fairness as a constraint on profit seeking: entitlements in the market. The American Economic Review. 1986;76:728–41. [Google Scholar]
- Kirk U, Downar J, Montague PR. Interoception drives increased rational decision-making in meditators playing the ultimatum game. Frontiers in Neuroscience. 2011;5:49. doi: 10.3389/fnins.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser D, SteemerS B, Branchi I, Homberg JR. The reciprocal interaction between serotonin and social behaviour. Neuroscience and Biobehavioral Reviews. 2012;36:786–98. doi: 10.1016/j.neubiorev.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Knoch D, Nitsche MA, Fischbacher U, Eisenegger C, Pascual-Leone A, Fehr E. Studying the neurobiology of social interaction with transcranial direct current stimulation—the example of punishing unfairness. Cerebral Cortex. 2008;18(9):1987–90. doi: 10.1093/cercor/bhm237. [DOI] [PubMed] [Google Scholar]
- Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006;314(5800):829–32. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- Krämer UM, Jansma H, Tempelmann C, Münte TF. Tit-for-tat: the neural basis of reactive aggression. Neuroimage. 2007;38(1):203–11. doi: 10.1016/j.neuroimage.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience. 2009;12:535–40. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leliveld MC, Beest Iv, Dijk Ev, Tenbrunsel AE. Understanding the influence of outcome valence in bargaining: a study on fairness accessibility, norms, and behavior. Journal of Experimental Social Psychology. 2009;45(3):505–14. [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messick DM, McClintock CG. Motivational bases of choice in experimental games. Journal of Experimental Social Psychology. 1968;4(1):1–25. [Google Scholar]
- Montague PR, Lohrenz T. To detect and correct: norm violations and their enforcement. Neuron. 2007;56(1):14–8. doi: 10.1016/j.neuron.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Forstmann BU, Wagenmakers E. Nature Neuroscience. 2011;14:1105–7. doi: 10.1038/nn.2886. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Hampton A, Kim H. Model-based fMRI and its application to reward learning and decision making. Annals of the New York Academy of Sciences. 2007;1104:35–53. doi: 10.1196/annals.1390.022. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, et al. The neural system that bridges reward and cognition in humans: an fMRI study. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(8):5669–74. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences. 2006;10(2):59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron. 2011;72:692–7. doi: 10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor D, Williamson RA, de Waal FB, Brosnan SF. Chimpanzees play the ultimatum game. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(6):2070–5. doi: 10.1073/pnas.1220806110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Range F, Horn L, Viranyi Z, Huber L. The absence of reward induces inequity aversion in dogs. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(1):340–5. doi: 10.1073/pnas.0810957105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls J. Justice as fairness. The Philosophical Review. 1958;67(2):164–94. [Google Scholar]
- Rawls J. A Theory of Justice. Cambridge, MA: Harvard University Press; 1971. [Google Scholar]
- Rilling JK, Gutman DA, Zeh TR, Pagnoni G, Berns GS, Kilts CD. A neural basis for social cooperation. Neuron. 2002;35(2):395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Rogers RD. The roles of dopamine and serotonin in decision making: evidence from pharmacological experiments in humans. Neuropsychopharmacology Reviews. 2011;36:114–32. doi: 10.1038/npp.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggeman C, Santens S, Fias W, Verguts T. Stages of nonsymbolic number processing in occipitoparietal cortex disentangled by fMRI adaptation. Journal of Neuroscience. 2011;31(19):7168–73. doi: 10.1523/JNEUROSCI.4503-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau J-J. The Basic Political Writings. Indianapolis, IN: Hackett Publishing Company; 1754/2011. [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the ultimatum game. Science. 2003;300(5626):1755–8. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Purushotham A, Kim S-G, Uğurbil K, Willingham D, Ashe J. Cerebellum activation associated with performance change but not motor learning. Science. 2002;29:2043–6. doi: 10.1126/science.1068524. [DOI] [PubMed] [Google Scholar]
- Seymour B, Singer T, Dolan R. The neurobiology of punishment. Nature Reviews Neuroscicence. 2007;8(4):300–11. doi: 10.1038/nrn2119. [DOI] [PubMed] [Google Scholar]
- Sober E, Wilson DS. Unto Others: The Evolution and Psychology of Unselfish Behavior. Cambridge, MA: Harvard University Press; 1999. [Google Scholar]
- Strobel A, Zimmermann J, Schmitz A, et al. Beyond revenge: neural and genetic bases of altruistic punishment. Neuroimage. 2011;54(1):671–80. doi: 10.1016/j.neuroimage.2010.07.051. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Satpute AB, Lieberman MD. The sunny side of fairness—preference for fairness activates reward circuitry (and disregarding unfairness activates self-control circuitry) Psychological Science. 2008;19(4):339–47. doi: 10.1111/j.1467-9280.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- Tocqueville A. Democracy in America. Indianapolis, IN: Liberty Fund; 1835/2010. [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315(5811):515–8. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Tricomi E, Rangel A, Camerer CF, O’Doherty JP. Neural evidence for inequality-averse social preferences. Nature. 2010;463(7284):1089–91. doi: 10.1038/nature08785. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211(30):453–8. doi: 10.1126/science.7455683. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. Loss aversion in riskless choice: a reference-dependent model. The Quarterly Journal of Economics. 1991;106(4):1039–61. [Google Scholar]
- Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage. 2005;26:99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- White SF, Brislin SJ, Sinclair S, Blair JR. Punishing unfairness: rewarding or the organization of a reactively aggressive response? Human Brain Mapping. 2014;35(5):2137–2147. doi: 10.1002/hbm.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright ND, Symmonds M, Fleming SM, Dolan RJ. Neural segregation of objective and contextual aspects of fairness. Journal of Neuroscience. 2011;31(14):5244–52. doi: 10.1523/JNEUROSCI.3138-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Wu Y. Sharing losses and sharing gains: increased demand for fairness under adversity. Journal of Experimental Social Psychology. 2011;47(3):582–8. [Google Scholar]