Abstract
When facing strangers, one of the first evaluations people perform is to implicitly assess their trustworthiness. However, the underlying processes supporting trustworthiness appraisal are poorly understood. We hypothesized that visual working memory (VWM) maintains online face representations that are sensitive to physical cues of trustworthiness, and that differences among individuals in representing untrustworthy faces are associated with individual differences in anxiety. Participants performed a change detection task that required encoding and maintaining for a short interval the identity of one face parametrically manipulated to be either trustworthy or untrustworthy. The sustained posterior contralateral negativity (SPCN), an event-related component (ERP) time-locked to the onset of the face, was used to index the resolution of face representations in VWM. Results revealed greater SPCN amplitudes for trustworthy faces when compared with untrustworthy faces, indicating that VWM is sensitive to physical cues of trustworthiness, even in the absence of explicit trustworthiness appraisal. In addition, differences in SPCN amplitude between trustworthy and untrustworthy faces correlated with participants’ anxiety, indicating that healthy college students with sub-clinical high anxiety levels represented untrustworthy faces in greater detail compared with students with sub-clinical low anxiety levels. This pattern of findings is discussed in terms of the high flexibility of aversive/avoidance and appetitive/approach motivational systems.
Keywords: trustworthiness, visual working memory, anxiety, event-related potentials, faces
INTRODUCTION
The ability to adequately read and react to social cues conveyed by others’ faces is a foundation of social interaction. Among the disparate evaluations people constantly perform when facing strangers, one critical evaluation related to the first impression of others is their perceived trustworthiness (e.g. Adolphs, 2002; Willis and Todorov, 2006; Engell et al., 2007; Oosterhof and Todorov, 2008; Todorov, 2008; Todorov et al., 2008a; Stirrat and Perrett, 2010; Baron et al., 2011). This is not surprising, especially from an evolutionary perspective, since assessing faces’ trustworthiness is a critical social tool in order to avoid untrustworthy individuals (and consequent risky social interactions), and to approach trustworthy individuals for immediate or future cooperation (e.g. Trivers, 1971; Boone and Buck, 2003; Zebrowitz and Montepare, 2005; Oosterhof and Todorov, 2008). This evolutionary view is supported by empirical evidence linking trustworthiness and approachability appraisals (Santos and Young, 2008a,b; Todorov, 2008). Nevertheless, people may notice, remember or interpret the same social information quite differently, in particular when facing ambiguous social cues. This observation suggests that individuals may differ in their perception of a stranger as innocuous or, rather, as a potential offender. Notably, compelling evidence suggests that anxiety may play a crucial role in this context since individual differences in anxiety levels are associated with differences in the distribution of cognitive resources (e.g. Hirsch and Clark, 2004; Moser et al., 2008; Holmes et al., 2009; Rossignol et al., 2012) such that high-anxious individuals tend to allocate excess attention and working memory resources to threat-related cues and to misinterpret emotionally ambiguous stimuli as more negative compared with non-anxious individuals (see Mathews and MacLeod, 1994, 2005 for reviews; Calvo et al., 1997; Mathews and Mackintosh, 1998; Yoon and Zinbarg, 2007; Klumpp et al., 2010; Stout et al., 2013).
On the basis of these findings, we hypothesized that individual differences in anxiety (either general or social anxiety) would predict the resolution (i.e. precision) of visual working memory (VWM) representations of moderately untrustworthy faces. To investigate this question we examined event-related potentials while participants performed a change detection task that required encoding and maintaining for a short interval the identity of standardized either trustworthy and untrustworthy faces. The sustained posterior contralateral negativity (SPCN), an event-related component (ERP) time-locked to the onset of the face, was used to index the resolution of face representations in VWM.
A conspicuous body of behavioral research examined the physical characteristics that guide people in trustworthiness evaluation (e.g. Knutson, 1996; Montepare and Dobish, 2003; Oosterhof and Todorov, 2008; Todorov et al., 2008b). To this end, Oosterhof and Todorov (2008) developed a data-driven statistical model individuating facial features related to judgments of trustworthiness: high inner eyebrows, pronounced cheekbones, wide chins and shallow nose sellion, characterize faces appearing trustworthy while faces evaluated as untrustworthy are characterized by low inner eyebrows, shallow cheekbones and thin chins and deep nose sellion. While this behavioral model provides a clear indication of which facial cues are involved in trustworthiness appraisal, researchers have more recently begun to investigate the neural underpinnings of such facial evaluation. Thus far these investigations have primarily utilized functional magnetic resonance imaging (fMRI; e.g. Adolphs et al., 1998; Adolphs, 2002; Winston et al., 2002; Engell et al., 2007; Said et al., 2008). Two recent meta-analyses (Bzdok et al., 2011; Mende-Siedlecki et al., 2012) summarized these findings as revealing differential roles of brain circuitries in processing trustworthy and untrustworthy faces, with the former class of faces engaging reward-associated brain regions (including the nucleus accumbens) and untrustworthy faces engaging a brain region responding to potential threat, i.e. the ventral portion of amygdala. In general, these favor a key role of approach and avoidance motivation systems in reacting to faces characterized by different levels of trustworthiness as mentioned above (Chen and Bargh, 1999; Cosmides and Tooby 2000; Said et al. 2008; Todorov, 2008). Incidentally, high levels of anxiety are closely linked to avoidance motivation (e.g. Gable et al., 2003). Taken together, the aforementioned findings converge upon the novel hypothesis that high levels of anxiety may be related to deeper processing of untrustworthy faces, potentially indexed by more detailed VWM representations of untrustworthy faces in high-anxious individuals than in low-anxious individuals.
Blood-oxygen-level-dependent (BOLD) fMRI signal is characterized by a particularly low temporal resolution and is not suitable to investigate the time-course of trustworthiness appraisal. Event-related brain potentials (ERPs) provide instead high-resolution measures of the time-course of neural activity patterns associated with perceptual and cognitive processes. In the context of trustworthiness appraisal, only a few recent ERP studies have explored which stages of processing are sensitive to physical cues of faces’ trustworthiness (Rudoy and Paller, 2009; Yang et al., 2011; Dzhelyova et al., 2012; Marzi et al., 2012). Modulations of face processing driven by explicit trustworthiness appraisal occur as early as the C1 and P1 components time-locked to face onset (Yang et al., 2011; Marzi et al., 2012). Faces’ trustworthiness continues to modulate cascading neural activity during early selection of visual stimuli with affective and motivational significance (as reflected in early posterior negativity modulations, i.e. EPN; Dzhelyova et al., 2012; Marzi et al., 2012), later structural encoding (as reflected in N170 modulations; Dzhelyova et al., 2012) and higher-order stages of processing as reflected in modulations of a fronto-central positivity (Rudoy and Paller, 2009; Marzi et al., 2012) and late positive component (LPC; Yang et al., 2011; Marzi et al., 2012). However, these studies did not illuminate whether the trustworthiness of a face may be implicitly appraised when individuals are simply exposed to such face and, at the present, it remains unknown whether processing of facial trustworthiness may be linked to individual differences in anxiety.
The present investigation focuses on a privileged stage of face processing, i.e. VWM, since it acts as a hub (‘the hub of cognition’; Haberlandt, 1997) for low-level processes—by which physical cues of trustworthiness are first encoded—and higher-order cognitive processes including decision-making and long-term memory (Luck, 2008). In particular, we aimed to elucidate whether individual anxiety would be predictive of the amount of VWM resources allocated to untrustworthy vs trustworthy faces. Notably, uncovering such a relationship would also imply that VWM is sensitive to physical cues of faces’ trustworthiness under conditions in which this dimension is task-irrelevant. To do so we adopted faces moderately trustworthy and untrustworthy included in the database created according to the method described by Oosterhof and Todorov (2008). Incidentally, the vast majority of strangers an observer continuously comes across are more likely perceived as moderately trustworthy or untrustworthy, therefore, understanding whether these moderately trustworthy/untrustworthy faces are differently represented in VWM has a relevant ecological significance.
The VWM task used in the current study was a modified version of the change detection task (e.g. Vogel and Machizawa, 2004; Sessa et al., 2011, 2012) and required participants to memorize face identities without an explicit trustworthiness evaluation, emphasizing the ecological validity of the task. We monitored an electrophysiological marker time-locked to faces recorded at posterior parietal sites indexing VWM maintenance, namely the sustained posterior contralateral negativity (SPCN; also labeled contralateral delay activity, CDA; Vogel and Machizawa, 2004) component of the ERP (Dell’Acqua et al., 2006; Jolicœur et al., 2006a,b; Sessa et al., 2011, 2012), which is computed as the difference between contralateral and ipsilateral activity time-locked to a lateralized target stimulus. The SPCN amplitude correlates positively with VWM informational load (e.g. Vogel and Machizawa, 2004; Jolicœur et al., 2008; Perron et al., 2009; Robitaille et al., 2009) and it has been shown to increase as the number (Vogel and Machizawa, 2004) and the complexity (Luria et al., 2010) of stimuli to be held in VWM is increased up to the level of VWM saturation. Sessa et al. (2011, 2012) have further demonstrated that SPCN amplitude varies proportionally to the resolution of faces’ representations in VWM, such that high-resolution faces’ representations elicit larger SPCN amplitudes relative to low-resolution faces’ representations.
We predicted that differences in the resolution between untrustworthy and trustworthy faces representations (computed by subtracting SPCN elicited by trustworthy and untrustworthy faces) would have been related to the level of participants’ anxiety, such that higher anxious participants would have maintained higher-resolution representation of untrustworthy faces as compared with lower anxious participants. To this aim, at the end of the ERP recording session, participants were also administered the State Trait Anxiety Inventory (Spielberger, 1983) and the Italian version of the Social Interaction Anxiety Scale and the Social Phobia Scale questionnaires (Sica et al., 2007; for the English version refer to Mattick and Clarke, 1998).
METHODS
Participants
Data were collected from 16 healthy volunteer students (three males) from the University of Padova (mean age: 24.56 years, SD = 1.63) who reported normal or corrected-to-normal vision and no history of neurological disorders. All participants gave their informed consent according to the ethical principles approved by the University of Padova. Data from four participants (all females) were discarded from the analyses because of an excessive rate (>30% of trials) of EEG artifacts.
Stimuli and procedure
Memory task
Prior to the ERP experiment, an independent student sample (N = 30; 12 males, mean age: 23.43 years, SD = 1.86) provided seven-step ratings of facial trustworthiness (trustworthy vs untrustworthy) and emotional expression (happy vs angry) of 110 neutral facial expression identities generated using FaceGen Modeller 3.2 (Singular Inversions, 2007; ±2 and ±3 SD from neutral) according to the methods described by Oosterhof and Todorov (2008). This procedure allowed selecting the most appropriate face stimuli for the present investigation resulting in 10 untrustworthy (−2 and −3 SD) and 10 trustworthy (+2 and +3 SD) bald Caucasian male faces with a non-significant correlation with the emotion scale (consensus neutral expression P > 0.1; for a similar procedure, see Yang et al., 2011).
The face stimuli were scaled using image-processing software so that each face fitted in 3.3° × 4.5° (width × height) rectangle from a viewing distance of ∼70 cm. Face stimuli were randomly selected and memory-display and test-display were composed of two faces—with either trustworthy or untrustworthy facial characteristics—horizontally aligned with fixation. The distance between the center of the face and the fixation cross was 4.9°.
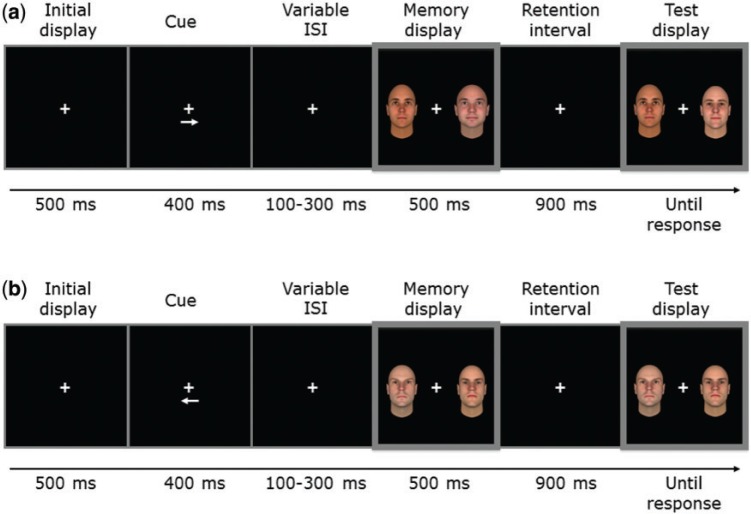
Examples of two different trials are reported in Figure 1a and b and described in detail in the respective captions. The memory-display consisted of two faces presented in each visual hemifield, preceded by an arrow cue pointing to the side of the to-be-memorized face. The face located in the opposite hemifield had to be ignored. Following the memory-display, participants were required to examine the same pre-cued side of the test-display for a possible change in the identity of the face, which occurred unpredictably on 50% of trials. When a change occurred, the face was replaced with a different face of the same level of trustworthiness. The experiment consisted of 192 trials per condition (trustworthy vs untrustworthy; eight blocks of 48 trials each).
Fig. 1.
Examples of change detection task trials when either (a) a trustworthy face (example for the right hemifield) or (b) an untrustworthy face (example for the left hemifield) had to be encoded. ISI: interstimulus interval. At the beginning of each trial, a central arrow (both pointing to the left or to the right) instructed participants to memorize the face in only one visual hemifield of the memory-display, and ignore the face in the opposite hemifield. Following the memory-display, a blank interval elapsed before the onset of the test-display. On each trial, memory and test-displays contained faces of the same level of trustworthiness, and trials with trustworthy and untrustworthy faces were presented intermixed at random in each block. A change in identity of the face between memory and test-displays could occur unpredictably on 50% of the trials. Participants responded without speed pressure by pressing one of two appropriately labeled buttons of the computer keyboard to indicate whether a change in identity between memory and test-displays had occurred or not.
At the end of the ERP recording session, participants completed in hardcopy the State Trait Anxiety Inventory (STAI; Spielberger, 1983) and the Italian version of the Social Interaction Anxiety Scale (SIAS) and the Social Phobia Scale (SPS) questionnaires (Sica et al., 2007; for the English version see Mattick and Clarke, 1998).
STAI
The Italian version of the STAI is a 20-item self-report questionnaire (for each form: Y-1, state and Y-2, trait) that measures a temporary or persisting emotional state of generalized anxiety. Participants responded using a 4-point Likert-type scale.
SIAS and SPS
The Italian version of the SIAS is a 19-item self-report questionnaire that measures the fear of social interaction situations. The Italian version of the SPS is a 20-item self-report questionnaire that measures the fear of being evaluated or observed by unknown people during daily activities. Participants responded using a 5-point Likert-type scale.
Electrophysiological recording and analysis
EEG was recorded during the change detection task from 32 active electrodes distributed over the scalp in accordance with the international 10/20 system, placed on an elastic Acti-Cap referenced to the left earlobe. The EEG was re-referenced offline to the average of the left and right earlobes. Trials contaminated by eye blinks, large horizontal eye movements or incorrect responses in the change detection task were discarded from analysis. We computed contralateral waveforms by averaging the activity recorded at right hemisphere electrodes when participants were cued to encode the face stimulus on the left side of the memory-display with the activity recorded from the left hemisphere electrodes when they were cued to encode the face stimulus on the right side of the memory-display. SPCN was quantified at posterior electrodes sites (P7/P8) as the difference in mean amplitude between the ipsilateral and contralateral waveforms in a time window of 500–1100 ms relative to the onset of the memory array (i.e. the SPCN mean amplitude was quantified in a time-window following the disappearance of the memory-display).
We computed, for each participant and condition, an SPCN trustworthiness score reflecting differential resolution of VWM representations of trustworthy and untrustworthy faces based on the following equation:
SPCN is a negative-going ERP response. Positive SPCN trustworthiness scores (SPCN differences) thus indicated that more information was encoded in VWM from untrustworthy faces compared with trustworthy faces (i.e. VWM advantage for representations of untrustworthy faces) and negative SPCN trustworthiness scores indicated the opposite (i.e. VWM advantage for representations of trustworthy faces).
RESULTS
Behavior
Memory task
VWM performance was quantified using a standard index of sensitivity (d′; Green and Swets, 1974). This measure allowed estimating how sensitive the participants were to changes between the memory and test-displays and whether this sensitivity differed as a function of faces’ trustworthiness. These values were submitted to paired-sample t-test considering the independent variable face trustworthiness (trustworthy faces, i.e. including faces +2 and +3 SD from neutral, vs untrustworthy faces, i.e. including faces −2 and −3 SD from neutral). Analysis revealed that participants were equally accurate in responding to trustworthy (mean d′ = 2.43, SD = 0.57) and untrustworthy (mean d′ = 2.38, SD = .64) faces, t < 1. To better characterize these findings, we performed a backward stepwise regression analysis in which variables were sequentially removed from a full model (including STAI Y-1, STAI Y-2, SPS and SIAS as predictors, and d′ values, for trustworthy and untrustworthy faces separately, as dependent variables). When d′ for trustworthy faces was predicted, it was found that STAI Y-1 (β = 0.711, P < 0.005) and SPS (β = −0.711, P < 0.005) were significant predictors (adjusted R2 = 0.654), but affected behavioral performance in opposite directions. For untrustworthy faces, SPS was a marginally significant predictor (β = −0.503, P = 0.075; adjusted R2 = 0.211). In general, these findings suggest that high levels of social phobia may deteriorate behavioral performance for both trustworthy and untrustworthy faces.
STAI
The mean rating scores was 37.13 (SD = 11.94) for state anxiety (form Y-1) and 41.19 (SD = 8.45) for trait anxiety (form Y-2).
SIAS and SPS
The mean rating scores was 17.06 (SD = 7.96) for SIAS and 13.44 (SD = 9.27) for SPS.
SPCN
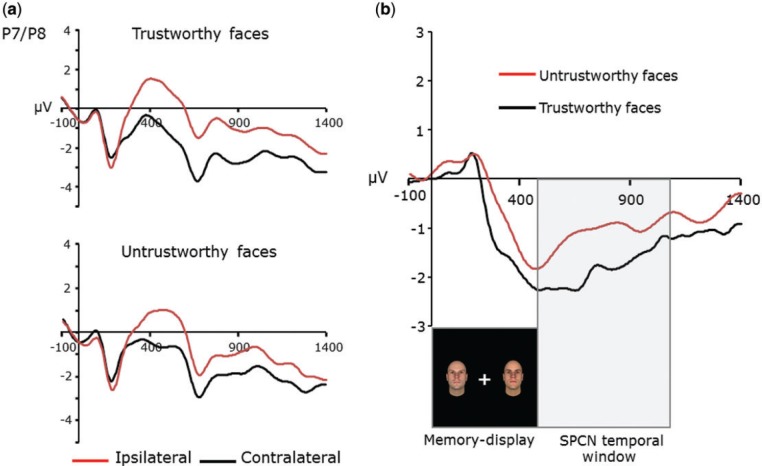
Figure 2a illustrates contralateral and ipsilateral waveforms recorded at electrode sites P7/P8 time-locked to the memory-display for trustworthy and untrustworthy faces, separately. Figure 2b shows SPCN (contralateral minus ipsilateral) waveforms. An informal observation of waveforms indicates that SPCN was modulated by faces’ trustworthiness.
Fig. 2.
(a) Contralateral and ipsilateral waveforms recorded at P7/P8 electrode sites time-locked to the onset of the memory-display for trustworthy and untrustworthy faces separately. (b) SPCN (contralateral-minus-ipsilateral) for trustworthy and untrustworthy faces, separately. The shaded area indicates the SPCN (contralateral-minus-ipsilateral waveforms) temporal window selected for statistical analyses (500–1100 ms).
SPCN mean amplitude values recorded at electrode sites P7/P8 were submitted to a paired-sample t-test considering the independent variable face trustworthiness (trustworthy vs untrustworthy). Trustworthy faces elicited larger SPCN amplitude (−1.83 µV; SD = 1.05) than untrustworthy faces (−1.05 µV; SD = 1.36), t(11) = 2.984, P < 0.012, 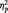 = 0.447, indicating that implicit evaluation of trustworthiness modulated VWM processing; in particular, under conditions of exposure to faces in the middle of the trustworthiness dimension, trustworthy faces were overall maintained as higher-resolution representations compared with untrustworthy faces. For completeness, we also analyzed mean SPCN amplitude values recorded at electrode sites P3/P4 and O1/O2. The pattern for P3/P4 was similar to that observed for P7/P8 [i.e. a larger SPCN amplitude for trustworthy faces than untrustworthy faces, t(11) = 2.386, P < .036,
= 0.447, indicating that implicit evaluation of trustworthiness modulated VWM processing; in particular, under conditions of exposure to faces in the middle of the trustworthiness dimension, trustworthy faces were overall maintained as higher-resolution representations compared with untrustworthy faces. For completeness, we also analyzed mean SPCN amplitude values recorded at electrode sites P3/P4 and O1/O2. The pattern for P3/P4 was similar to that observed for P7/P8 [i.e. a larger SPCN amplitude for trustworthy faces than untrustworthy faces, t(11) = 2.386, P < .036, 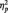 = 0.341]. The trend was analogous for SPCN mean amplitudes at electrode sites O1/O2, but statistically not significant (t < 1).
= 0.341]. The trend was analogous for SPCN mean amplitudes at electrode sites O1/O2, but statistically not significant (t < 1).
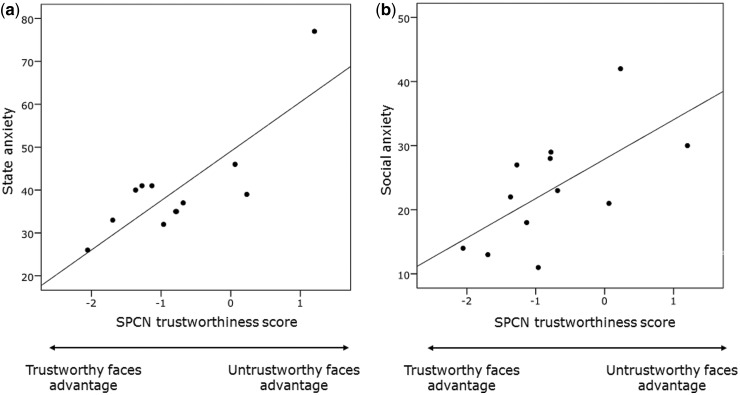
Notably, SPCN trustworthiness scores were highly correlated with the level of both participants’ state anxiety (i.e. STAI Y-1 scores), r = 0.812, P < 0.005 and social anxiety (i.e. SIAS scores), r = 0.631, P < 0.05 (Figure 3a and b).
Fig. 3.
Correlations between SPCN trustworthiness scores and the level of participants’ (a) state anxiety (r = 0.812, P < 0.005) and (b) social anxiety (r = 0.631, P < 0.05).
The bar graph depicted in Figure 4 is presented only for illustrative purposes and illustrates mean SPCN amplitudes for trustworthy (blue bars) and untrustworthy (red bars) faces in participants with high and low levels of state and social anxiety, separately. The graph noticeably shows that trustworthy faces elicited similar SPCN amplitudes irrespective of the level of participants’ anxiety; on the other hand untrustworthy faces elicited increased SPCN amplitudes in high-anxious participants compared with low-anxious individuals.
Fig. 4.
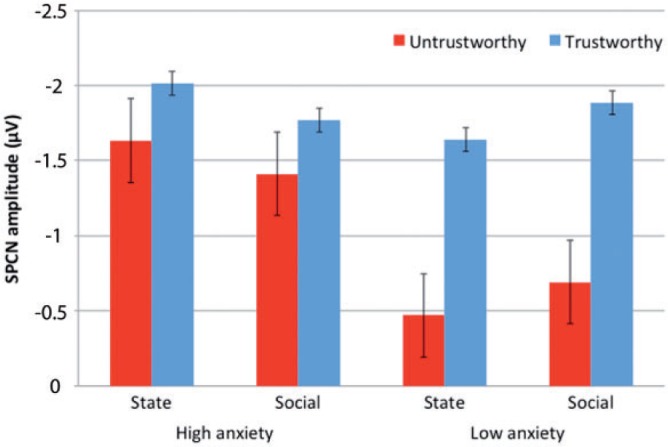
Bar graph of mean SPCN amplitudes, with standard error bars, for trustworthy (blue bars) and untrustworthy (red bars) faces for high and low levels of state and social anxiety, separately.
Based on the known properties of SPCN, these findings support the conclusion that the higher the anxiety self-reported by participants (in particular state and social anxiety) the higher was the resolution of VWM representations of untrustworthy faces (compared with lower-anxious participants). Finally, since the unbalance between the number of male (i.e. three) and female (i.e. nine) participants in this study might prevent interpreting our results as indicative of the general population, we analyzed separately the effect of trustworthiness on the SPCN amplitude in male and female participants. Although not significant, both males and females showed a comparable tendency in showing increased SPCN amplitudes when trustworthy faces (vs untrustworthy faces) had to be encoded (males: t = −3.541 P = 0.072; females: t = −1.912 P = 0.092), suggesting that, at least under the present experimental conditions, both males and females encoded trustworthy faces as higher resolution representations compared with untrustworthy faces.
DISCUSSION
The purpose of this study was to investigate whether individuals’ level of general/social anxiety is related to the resolution with which faces characterized by features of trustworthiness and untrustworthiness (Oosterhof and Todorov, 2008) as represented in VWM. To this aim, we monitored a neural correlate of VWM processing, namely the SPCN (e.g. Vogel and Machizawa, 2004; Dell’Acqua et al., 2006; Jolicœur et al., 2006a,b; Luria et al., 2010; Sessa et al., 2011, 2012), during maintenance of representations of parametrically manipulated trustworthy and untrustworthy faces that differed slightly from faces that are neutral on the trustworthiness dimension (Oosterhof and Todorov, 2008). Importantly, the memory task did not require explicit evaluation of faces’ trustworthiness. On the basis of known properties of the SPCN, modulations of SPCN amplitude as a function of faces’ trustworthiness denote differences in the resolution of those faces representations in VWM such that larger SPCN amplitudes reveal maintenance of higher-resolution representations (Sessa et al., 2011, 2012).
SPCN amplitude, on average, was increased for trustworthy faces compared with untrustworthy faces. This finding suggests that participants maintained in VWM trustworthy faces in higher-resolution representations as compared with untrustworthy faces. Notably, individual estimates of the difference in SPCN amplitude elicited by trustworthy vs untrustworthy faces (i.e. SPCN trustworthiness scores) strongly correlated with individual estimates of state and social anxiety (i.e. STAI Y-1 scores and SIAS scores, r = 0.812 and 0.631, respectively), indicating that untrustworthy faces elicited larger SPCN amplitudes in high-anxious individuals than in low-anxious individuals. To our knowledge, this is the first evidence showing that high-anxious individuals encode and retain untrustworthy faces’ representations in VWM with a higher degree of resolution compared with low-anxious individuals.
A variety of explicative models of anxiety have been proposed over the past years (see, for instance, Cisler and Koster, 2010 and Hofmann et al., 2012, for recent reviews), leading to divergent predictions. However one broadly accepted theory is that high-anxiety individuals show preferential processing of threatening stimuli, including negative facial expressions, as demonstrated by a number of behavioral and ERP studies (Gilboa-Schechtman et al., 1999; Mogg and Bradley, 2002; Amir et al., 2003; Mogg et al., 2004; Eastwood et al., 2005; Kolassa and Miltner, 2006; Bar-Haim et al., 2007; Kolassa et al., 2009; Rossignol et al., 2012). In this vein, a recent ERP work using a similar task to that implemented here monitored the SPCN component and demonstrated that anxiety is associated with inefficient gating of threat-related faces (i.e. fearful faces) from VWM even when task-irrelevant (Stout et al., 2013). Furthermore, even when stimuli are not characterized by a clear negative valence, high-anxious individuals tend to misinterpret these emotionally ambiguous stimuli as more negative compared with non-anxious individuals (Calvo and Castillo, 2001; Mathews and Mackintosh, 1998; Yoon and Zinbarg, 2007; Klumpp et al., 2010). To note, the memory task implemented in the present investigation required participants to encode faces’ identities that did not express an emotion, although it has been proposed that trustworthiness appraisal approximates the detection of emotional facial expressions (Oosterhof and Todorov, 2008; Todorov et al., 2008a; Zebrowitz and Montepare, 2008), in particular for those faces at the extremes of the trustworthiness dimension, such that highly trustworthy and untrustworthy faces are perceived as happy and angry, respectively (Todorov, 2008). These observations suggest that high anxiety individuals tended to perceive moderately untrustworthy faces as more untrustworthy compared with low-anxiety individuals, leading to increased resolution of those VWM representations. One attractive neuroanatomical hypothesis is that activity in the ventral amygdala critically contributes to this increased VWM processing of moderately untrustworthy faces in high-anxious individuals compared with low-anxious individuals (Mende-Siedlecki et al., 2012). The amygdala is a central brain node for processing threatening stimuli (e.g. threatening facial expressions, such as anger or fear; e.g. Whalen et al., 2001; see Adolphs, 2003; Adolphs and Spezio 2006; LeDoux, 1998, for reviews) as well as appraisal of faces as untrustworthy (e.g. Winston et al., 2002; Engell et al., 2007; Todorov et al., 2008a). Furthermore, compelling evidence indicates increased amygdala reactivity in high-anxious individuals when exposed to potential threat, including emotional faces (Mogg and Bradley, 2002; Schwartz et al., 2003; Etkin and Wager, 2007; Ball et al., 2012). In the context of the present work, we may speculate that individual differences in amygdala reactivity would bias processing within regions of the prefrontal cortex involved in VWM maintenance (e.g. LoPresti et al., 2008), and, in turn, the prefrontal cortex would modulate SPCN activity (e.g. Voytek and Knight, 2010).
One additional facet of our finding is that levels of anxiety predicted neural measures of VWM. Although recent studies demonstrated dissociable effects between general anxiety (referred to a temporary, i.e. state anxiety, or persisting, i.e. trait anxiety, intrusive worry about a broad array of everyday life circumstances) and social anxiety (i.e. referred to worries of being in social situations or feeling scrutinized by the others) on behavioral tasks involving face processing (Bourne and Vladeanu, 2011; Davis et al., 2011), in the present investigation we did not observe convincing dissociable effects of general anxiety and social anxiety in modulating face processing, at least during the stage of VWM maintenance of faces’ representations. To note, generalized anxiety disorder and social anxiety disorder are both classified as anxiety disorders in DSM-IV-TR (American Psychiatric Association, 2000), but this last appears to be confined to social situations/interactions. The present findings suggest that the improved VWM processing of untrustworthy faces in high-anxious individuals (compared with low-anxious individuals) is related to a general oversensitivity towards potential threat regardless of the nature (either social or not) of the threat.
We also observed overall larger SPCN amplitude for trustworthy faces relative to untrustworthy faces. As noted above, it has been suggested that trustworthiness appraisal, in particular for those faces at the extremes of the trustworthiness dimension, approximates the detection of emotional facial expressions (e.g. Oosterhof and Todorov, 2008). Congruent with these observations, ERP modulations for extreme trustworthy and untrustworthy faces nearly mimics ERP modulations for the corresponding facial emotional expressions (e.g. Marzi et al., 2012), indicating increased processing for highly untrustworthy faces compared with highly trustworthy faces. To our knowledge, only two recent studies reported findings of memory tasks for trustworthy and untrustworthy faces (Todorov et al., 2011; Rule et al., 2012), providing contrasting evidence on which class of faces (trustworthy vs untrustworthy) benefit from prioritized memory processing. Rule et al. (2012) have found a long-term memory advantage in terms of behavioral accuracy for untrustworthy faces compared with trustworthy faces. On the contrary, Todorov et al. (2011) reported a higher hit rate for trustworthy than untrustworthy faces in the context of a one-back recognition task. Notably, face stimuli used in those two investigations differed such that Rule et al. (2012) selected faces composing the trustworthy and untrustworthy face sets from faces that obtained highest and lowest trustworthiness scores on participants’ ratings along a 7-point scale; conversely, and similarly to the present investigation, Todorov et al. (2011) used face stimuli in the middle of the trustworthiness dimension (i.e. ±1 SD and ±3 SD) generated on the basis of the approach of Oosterhof and Todorov (2008). The findings of the present investigation, in demonstrating a neural VWM advantage for moderately trustworthy faces relative to moderately untrustworthy faces, appear to complement previous evidence of prioritized processing of extreme untrustworthy faces (compared with trustworthy faces). Taken together, these findings seem to reveal high flexibility of aversive/avoidance and appetitive/approach motivational systems (Chen and Bargh, 1999; Cosmides and Tooby 2000; Todorov, 2008) in reacting to social stimuli and consequently biasing downstream cognition, such as memory. This flexibility may rely on the notion of value-prediction code linked to a stimulus (e.g. Raymond and O’Brien, 2009; Anderson et al., 2011a,b) that combines information of both intensity/magnitude and valence of that stimulus and potential outcome in terms of gains or losses (i.e. here opportunities of cooperation with trustworthy individuals vs risky interactions with untrustworthy individuals). Within this theoretical framework it appears reasonable to hypothesize that when exposed to very untrustworthy faces, which also may appear as expressing anger, the high intensity and negative valence conveyed by those faces increases the likelihood of reaching a threshold of threat detection such that the value-prediction code assigned by individuals is weighted more highly than value assigned to trustworthy faces. This will then bias processing (including VWM maintenance) in favor of potential threat in the environment. Incidentally, this explanation fits nicely with previous behavioral and ERP work showing a VWM advantage for negative facial expressions (i.e. angry and fearful faces) compared with neutral and/or positive facial expressions (i.e. happy faces; Jackson et al., 2008, 2009; Sessa et al., 2011). On the other hand, when perceived threat from untrustworthy faces is low, people may tend to assign a low value to them, and to assign a higher value and to allocate a larger proportion of cognitive resources to/on stimuli with rewarding characteristics (e.g. Raymond and O’Brien, 2009), such as trustworthy faces (e.g. Todorov, 2008), according to an approach behavior. Along this theoretical perspective, our findings also advocate that value-prediction codes assigned to untrustworthy faces strongly depend on the levels of individuals’ anxiety, such that higher levels of anxiety are associated with higher-resolution representations of untrustworthy faces (compared with low-anxious individuals).
A potential limitation of the present findings is that modulations of the SPCN as a function of trustworthiness were not accompanied by analogous behavioral effects. However, this dissociation between neural and behavioral measures in this context should not alarm for at least two classes of considerations. First, SPCN is a pure measure of VWM representation; on the contrary the overt response required in the change detection task reflects not only the quality of current VWM representation, but also additional processes allowing to compare the face presented in the test-display with current VWM representation (see, for instance, Awh et al., 2007). In this regard anxiety may have different effects on these diverse stages involved in the change detection task. This observation is in line with the finding of deteriorating effect of social phobia selectively on behavioral performance, for both trustworthy and untrustworthy faces. Convincing evidence suggests that anxiety may deteriorate behavioral performance, in both matching tasks (i.e. Attwood et al., 2013) and VWM tasks (e.g. Lucas et al., 1991; Eysenck, 1998; Hayes et al., 2008; Moriya and Sugiura, 2012; Lapointe et al., 2013). This all may suggest that anxiety tend to boost the online VWM representation of potential threat (i.e. untrustworthy faces, compared with low-anxious individuals) but lowers the general level of response accuracy, independently of the valence of the memorized materials. A second type of considerations refers to the observation that brain responses are often more sensitive to subtle processing differences than behavioral measures (e.g. Luck et al., 1996; Heil et al., 2004; Wilkinson and Halligan, 2004) and this dissociation may be particularly evident when the behavioral task requires a dichotomous response such that was required in the change detection task implemented in this study (Sessa et al., 2011, 2012).
To conclude, our findings provide evidence that physical cues of faces’ trustworthiness modulate the resolution of faces’ representations in VWM even under conditions in which trustworthiness is implicitly appraised, and given the privileged position of VWM within the stream of processing, this finding may be particularly relevant for models of approach/avoidance motivational systems. Importantly, high levels of individuals’ anxiety modulate VWM maintenance of those faces’ representations, further strengthening the conclusion that VWM is sensitive to facial cues of trustworthiness on the basis of individuals’ approach/avoidance tendencies.
Conflict of Interest
None declared.
Acknowledgments
The authors acknowledge Dr. Micah Allen and two anonymous reviewers for their valuable comments.
REFERENCES
- Adolphs R. Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behavioral and Cognitive Neuroscience Reviews. 2002;1:21–62. doi: 10.1177/1534582302001001003. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience. 2003;4:165–78. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Spezio M. Role of the amygdala in processing visual social stimuli. Progress in Brain Research. 2006;156:363–78. doi: 10.1016/S0079-6123(06)56020-0. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–4. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. 2000. text revision. Washington, DC: American Psychiatric Association. [Google Scholar]
- Amir N, Elias J, Klumpp H, Przeworski A. Attentional bias to threat in social phobia: facilitated processing of threat or difficulty disengaging attention from threat? Behaviour Research and Therapy. 2003;41:1325–35. doi: 10.1016/s0005-7967(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Learned value magnifies salience-based attentional capture. PloS One. 2011a;6:e27926. doi: 10.1371/journal.pone.0027926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, Yantis S. Value-driven attentional capture. Proceedings of the National Academy of Sciences of the United States of America. 2011b;108:10367–71. doi: 10.1073/pnas.1104047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood AS, Penton-Voak IS, Burton AM, Munafò MR. Acute anxiety impairs accuracy in identifying photographed faces. Psychological Science. 2013;24:1591–4. doi: 10.1177/0956797612474021. [DOI] [PubMed] [Google Scholar]
- Awh E, Barton B, Vogel EK. Visual working memory represents a fixed number of items regardless of complexity. Psychological Science. 2007;18:622–8. doi: 10.1111/j.1467-9280.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- Ball TM, Sullivan S, Flagan T, et al. Selective effects of social anxiety, anxiety sensitivity, and negative affectivity on the neural bases of emotional face processing. Neuroimage. 2012;59:1879–87. doi: 10.1016/j.neuroimage.2011.08.074. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Dan O, Eshel Y, Sagi-Schwartz A. Predicting children's anxiety from early attachment relationships. Journal of Anxiety Disorders. 2007;21:1061–8. doi: 10.1016/j.janxdis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Baron SG, Gobbini MI, Engell AD, Todorov A. Amygdala and dorsomedial prefrontal cortex responses to appearance-based and behavior-based person impressions. Social Cognitive and Affective Neuroscience. 2011;6:572–81. doi: 10.1093/scan/nsq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone R, Buck R. Emotional expressivity and trustworthiness: the role of nonverbal behavior in the evolution of cooperation. Journal of Nonverbal Behavior. 2003;27:163–82. [Google Scholar]
- Bourne VJ, Vladeanu M. Lateralisation for processing facial emotion and anxiety: contrasting state, trait and social anxiety. Neuropsychologia. 2011;49:1343–9. doi: 10.1016/j.neuropsychologia.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Caspers S, et al. ALE meta-analysis on facial judgments of trustworthiness and attractiveness. Brain Structure & Function. 2011;215:209–23. doi: 10.1007/s00429-010-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo MG, Dolores Castillo M. Selective interpretation in anxiety: Uncertainty for threatening events. Cognition & Emotion. 2001;15:299–320. [Google Scholar]
- Calvo MG, Eysenck MW, Castillo MD. Interpretation bias in test anxiety: the time course of predictive inferences. Cognition & Emotion. 1997;11:43–64. [Google Scholar]
- Chen M, Bargh JA. Consequences of automatic evaluation: immediate behavioral predispositions to approach or avoid the stimulus. Personality and Social Psychology Bulletin. 1999;25:215–24. [Google Scholar]
- Cisler JM, Koster EH. Mechanisms of attentional biases towards threat in anxiety disorders: an integrative review. Clinical Psychology Review. 2010;30:203–16. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmides L, Tooby J. Evolutionary psychology and the emotions. Handbook of emotions. 2000;2:91–115. [Google Scholar]
- Davis JM, McKone E, Dennett H, O'Connor KB, O'Kearney R, Palermo R. Individual differences in the ability to recognise facial identity are associated with social anxiety. PloS One. 2011;6:e28800. doi: 10.1371/journal.pone.0028800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Acqua R, Sessa P, Jolicoeur P, Robitaille N. Spatial attention freezes during the attention blink. Psychophysiology. 2006;43:394–400. doi: 10.1111/j.1469-8986.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- Dzhelyova M, Perrett DI, Jentzsch I. Temporal dynamics of trustworthiness perception. Brain Research. 2012;1435:81–90. doi: 10.1016/j.brainres.2011.11.043. [DOI] [PubMed] [Google Scholar]
- Eastwood J, Smilek D, Oakman J, Farvolden P, van Ameringen M, Mancini C, et al. Individuals with social phobia are biased to become aware of negative faces. Visual Cognition. 2005;12:159–79. [Google Scholar]
- Engell AD, Haxby JV, Todorov A. Implicit trustworthiness decisions: automatic coding of face properties in the human amygdala. Journal of Cognitive Neuroscience. 2007;19:1508–19. doi: 10.1162/jocn.2007.19.9.1508. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW. Working memory capacity in high trait-anxious and repressor groups. Cognition & Emotion. 1998;12:697–713. [Google Scholar]
- Gable SL, Reis HT, Downey G. He said, she said: a quasi-signal detection analysis of daily interactions between close relationship partners. Psychological Science. 2003;14:100–5. doi: 10.1111/1467-9280.t01-1-01426. [DOI] [PubMed] [Google Scholar]
- Gilboa-Schechtman E, Foa E, Amir N. Attentional biases for facial expressions in social phobia: the face-in-the-crowd paradigm. Cognition & Emotion. 1999;13:305–18. [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. Huntington, NY: Krieger; 1974. [Google Scholar]
- Haberlandt K. Cognitive Psychology. 2nd edn. Boston: Allyn & Bacon; 1997. [Google Scholar]
- Hayes S, Hirsch C, Mathews A. Restriction of working memory capacity during worry. Journal of Abnormal Psychology. 2008;117:712. doi: 10.1037/a0012908. [DOI] [PubMed] [Google Scholar]
- Heil M, Rolke B, Pecchinenda A. Automatic semantic activation is no myth: semantic context effects on the N400 in the letter-search task in the absence of response time effects. Psychological Science. 2004;15:852–7. doi: 10.1111/j.0956-7976.2004.00766.x. [DOI] [PubMed] [Google Scholar]
- Hirsch CR, Clark DM. Information-processing bias in social phobia. Clinical Psychology Review. 2004;24:799–825. doi: 10.1016/j.cpr.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Ellard KK, Siegle GJ. Neurobiological correlates of cognitions in fear and anxiety: a cognitive-neurobiological information-processing model. Cognition & Emotion. 2012;26:282–99. doi: 10.1080/02699931.2011.579414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Nielsen MK, Tipper S, Green S. An electrophysiological investigation into the automaticity of emotional face processing in high versus low trait anxious individuals. Cognitive, Affective & Behavioral Neuroscience. 2009;9:323–34. doi: 10.3758/CABN.9.3.323. [DOI] [PubMed] [Google Scholar]
- Jackson MC, Wolf C, Johnston SJ, Raymond JE, Linden DE. Neural correlates of enhanced visual short-term memory for angry faces: an FMRI study. PloS One. 2008;3:e3536. doi: 10.1371/journal.pone.0003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MC, Wu CY, Linden DE, Raymond JE. Enhanced visual short-term memory for angry faces. Journal of Experimental Psychology: Human Perception and Performance. 2009;35:363–74. doi: 10.1037/a0013895. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P, Sessa P, Dell'Acqua R, Robitaille N. On the control of visual spatial attention: evidence from human electrophysiology. Psychological Research. 2006;70:414–24. doi: 10.1007/s00426-005-0008-4. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Angstadt M, Nathan PJ, Phan KL. Amygdala reactivity to faces at varying intensities of threat in generalized social phobia: an event-related functional MRI study. Psychiatry Research. 2010;183:167–9. doi: 10.1016/j.pscychresns.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B. Facial expressions of emotion influence interpersonal trait inferences. Journal of Nonverbal Behavior. 1996;20:165–82. [Google Scholar]
- Kolassa I, Kolassa S, Bergmann S, et al. Interpretive bias in social phobia: an ERP study with morphed emotional schematic faces. Cognition & Emotion. 2009;23:69–95. [Google Scholar]
- Kolassa IT, Miltner WH. Psychophysiological correlates of face processing in social phobia. Brain Research. 2006;1118:130–41. doi: 10.1016/j.brainres.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Lapointe MLB, Blanchette I, Duclos M, Langlois F, Provencher MD, Tremblay S. Attentional bias, distractibility and short-term memory in anxiety. Anxiety, Stress & Coping. 2013;26:293–313. doi: 10.1080/10615806.2012.687722. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Fear and the brain: where have we been, and where are we going? Biological Psychiatry. 1998;44:1229–38. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- LoPresti ML, Schon K, Tricarico MD, Swisher JD, Celone KA, Stern CE. Working memory for social cues recruits orbitofrontal cortex and amygdala: an fMRI study of delayed matching to sample for emotional expressions. Journal of Neuroscience. 2008;28:3718–28. doi: 10.1523/JNEUROSCI.0464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JA, Telch MJ, Bigler ED. Memory functioning in panic disorder: a neuropsychological perspective. Journal of Anxiety Disorders. 1991;5:1–20. [Google Scholar]
- Luck, S.J. (2008). Visual short-term memory. In Luck, S.J., Hollingworth, A., editors. Visual Memory. New York: Oxford University Press, pp. 43–86.
- Luck SJ, Vogel EK, Shapiro KL. Word meanings are accessed but cannot be reported during the attentional blink. Nature. 1996;383:616–8. doi: 10.1038/383616a0. [DOI] [PubMed] [Google Scholar]
- Luria R, Sessa P, Gotler A, Jolicoeur P, Dell'Acqua R. Visual short-term memory capacity for simple and complex objects. Journal of Cognitive Neuroscience. 2010;22:496–512. doi: 10.1162/jocn.2009.21214. [DOI] [PubMed] [Google Scholar]
- Marzi T, Righi S, Ottonello S, Cincotta M, Viggiano MP. Trust at first sight: evidence from ERPs. Social Cognitive and Affective Neuroscience. 2012;9:63–72. doi: 10.1093/scan/nss102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive approaches to emotion and emotional disorders. Annual Review of Psychology. 1994;45:25–50. doi: 10.1146/annurev.ps.45.020194.000325. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology. 2005;1:167–95. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Mathews A, Mackintosh B. A cognitive model of selective processing in anxiety. Cognitive Therapy and Research. 1998;22:539–60. [Google Scholar]
- Mattick RP, Clarke JC. Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behaviour Research and Therapy. 1998;36:455–70. doi: 10.1016/s0005-7967(97)10031-6. [DOI] [PubMed] [Google Scholar]
- Mende-Siedlecki P, Said CP, Todorov A. The social evaluation of faces: a meta-analysis of functional neuroimaging studies. Social Cognitive and Affective Neuroscience. 2012;8:285–99. doi: 10.1093/scan/nsr090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behaviour Research and Therapy. 2002;40:1403–14. doi: 10.1016/s0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113:160–5. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Montepare JM, Dobish H. The contribution of emotion perceptions and their overgeneralizations to trait impressions. Journal of Nonverbal Behavior. 2003;27:237–54. [Google Scholar]
- Moriya J, Sugiura Y. High visual working memory capacity in trait social anxiety. PloS One. 2012;7:e34244. doi: 10.1371/journal.pone.0034244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Huppert JD, Duval E, Simons RF. Face processing biases in social anxiety: an electrophysiological study. Biological Psychology. 2008;78:93–103. doi: 10.1016/j.biopsycho.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Oosterhof NN, Todorov A. The functional basis of face evaluation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11087–92. doi: 10.1073/pnas.0805664105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron R, Lefebvre C, Robitaille N, Brisson B, Gosselin F, Arguin M, Jolicoeur P. Attentional and anatomical considerations for the representation of simple stimuli in visual short-term memory: evidence from human electrophysiology. Psychological Research. 2009;73:222–32. doi: 10.1007/s00426-008-0214-y. [DOI] [PubMed] [Google Scholar]
- Raymond JE, O'Brien JL. Selective visual attention and motivation: the consequences of value learning in an attentional blink task. Psychological Science. 2009;20:981–8. doi: 10.1111/j.1467-9280.2009.02391.x. [DOI] [PubMed] [Google Scholar]
- Robitaille N, Grimault S, Jolicoeur P. Bilateral parietal and contralateral responses during maintenance of unilaterally encoded objects in visual short-term memory: evidence from magnetoencephalography. Psychophysiology. 2009;46:1090–9. doi: 10.1111/j.1469-8986.2009.00837.x. [DOI] [PubMed] [Google Scholar]
- Rossignol M, Philippot P, Bissot C, Rigoulot S, Campanella S. Electrophysiological correlates of enhanced perceptual processes and attentional capture by emotional faces in social anxiety. Brain Research. 2012;1460:50–62. doi: 10.1016/j.brainres.2012.04.034. [DOI] [PubMed] [Google Scholar]
- Rudoy JD, Paller KA. Who can you trust? behavioral and neural differences between perceptual and memory-based influences. Frontiers in Human Neuroscience. 2009;3:16. doi: 10.3389/neuro.09.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule NO, Slepian ML, Ambady N. A memory advantage for untrustworthy faces. Cognition. 2012;125:207–18. doi: 10.1016/j.cognition.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Said CP, Baron SG, Todorov A. Nonlinear amygdala response to face trustworthiness: contributions of high and low spatial frequency information. Journal of Cognitive Neuroscience. 2009;21:519–28. doi: 10.1162/jocn.2009.21041. [DOI] [PubMed] [Google Scholar]
- Santos IM, Young AW. Effects of inversion and negation on social inferences from faces. Perception. 2008a;37:1061–78. doi: 10.1068/p5278. [DOI] [PubMed] [Google Scholar]
- Santos IM, Young AW. Exploring the perception of social characteristics in faces using the isolation effect. Visual Cognition. 2008b;12:213–47. [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagen J, Rauch SL. Inhibited and uninhibited infants “Grown up”: adult amygdalar response to novelty. Science. 2003;300:1952–3. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Sessa P, Luria R, Gotler A, Jolicoeur P, Dell'acqua R. Interhemispheric ERP asymmetries over inferior parietal cortex reveal differential visual working memory maintenance for fearful versus neutral facial identities. Psychophysiology. 2010;48:187–97. doi: 10.1111/j.1469-8986.2010.01046.x. [DOI] [PubMed] [Google Scholar]
- Sessa P, Tomelleri S, Luria R, Castelli L, Reynolds M, Dell'Acqua R. Look out for strangers! Sustained neural activity during visual working memory maintenance of other-race faces is modulated by implicit racial prejudice. Social Cognitive and Affective Neuroscience. 2012;7:314–21. doi: 10.1093/scan/nsr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica C, Musoni I, Chiri LR, Bisi B, Lolli V, Sighinolfi C. Social Phobia Scale (SPS) e Social Interaction Anxiety Scale (SIAS): traduzione ed adattamento italiano. Bollettino di Psicologia Applicata. 2007;252:59. [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory: STAI (Form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stirrat M, Perrett DI. Valid facial cues to cooperation and trust: male facial width and trustworthiness. Psychological Science. 2010;21:349–54. doi: 10.1177/0956797610362647. [DOI] [PubMed] [Google Scholar]
- Stout DM, Shackman AJ, Larson CL. Failure to filter: Anxious individuals show inefficient gating of threat from working memory. Frontiers in Human Neuroscience. 2013;7:1–9. doi: 10.3389/fnhum.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers RL. The evolution of reciprocal altruism. Quarterly Review of Biology. 1971;46:35–57. [Google Scholar]
- Todorov A. Evaluating faces on trustworthiness: An extension of systems for recognition of emotions signaling approach/avoidance behaviors. Annals of the New York Academy of Sciences. 2008;1124:208–24. doi: 10.1196/annals.1440.012. [DOI] [PubMed] [Google Scholar]
- Todorov A, Baron SG, Oosterhof NN. Evaluating face trustworthiness: a model based approach. Social Cognitive and Affective Neuroscience. 2008a;3:119–27. doi: 10.1093/scan/nsn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, Oosterhof NN. Modeling Social Perception of Faces [Social Sciences] Signal Processing Magazine, IEEE. 2011;28:117–22. [Google Scholar]
- Todorov A, Said CP, Engell AD, Oosterhof NN. Understanding evaluation of faces on social dimensions. Trends in Cognitive Sciences. 2008b;12:455–60. doi: 10.1016/j.tics.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Todorov A, Said CP, Oosterhof NN, Engell AD. Task-invariant brain responses to the social value of faces. Journal of Cognitive Neuroscience. 2011;23:2766–81. doi: 10.1162/jocn.2011.21616. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–51. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Voytek B, Knight RT. Prefrontal cortex and basal ganglia contributions to visual working memory. Proceedings of the National Academy of Sciences. 2010;107:18167–72. doi: 10.1073/pnas.1007277107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Wilkinson DT, Halligan PW. The relevance of behavioural measures for functional imaging studies of cognition. Nature Reviews Neuroscience. 2004;5:67–73. doi: 10.1038/nrn1302. [DOI] [PubMed] [Google Scholar]
- Willis J, Todorov A. First impressions: making up your mind after a 100-ms exposure to a face. Psychological Science. 2006;17:592–8. doi: 10.1111/j.1467-9280.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O'Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience. 2002;5:277–83. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Yang D, Qi S, Ding C, Song Y. An ERP study on the time course of facial trustworthiness appraisal. Neuroscience Letters. 2011;496:147–51. doi: 10.1016/j.neulet.2011.03.066. [DOI] [PubMed] [Google Scholar]
- Yoon KL, Zinbarg RE. Threat is in the eye of the beholder: Social anxiety and the interpretation of ambiguous facial expressions. Behaviour Research and Therapy. 2007;45:839–47. doi: 10.1016/j.brat.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA, Montepare JM. Psychology. Appearance DOES matter. Science (New York, N.Y.) 2005;308:1565–6. doi: 10.1126/science.1114170. [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA, Montepare JM. Social psychological face perception: why appearance matters. Social and Personality Psychology Compass. 2008;2:1497. doi: 10.1111/j.1751-9004.2008.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]