Abstract
Over the past 60 years, human intracranial electrophysiology (HIE) has been used to characterize seizures in patients with epilepsy. Secondary to the clinical objectives, electrodes implanted intracranially have been used to investigate mechanisms of human cognition. In addition to studies of memory and language, HIE methods have been used to investigate emotions. The aim of this review is to outline the contribution of HIE (electrocorticography, single-unit recording and electrical brain stimulation) to our understanding of the neural representations of emotions. We identified 64 papers dating back to the mid-1950s which used HIE techniques to study emotional states. Evidence from HIE studies supports the existence of widely distributed networks in the neocortex, limbic/paralimbic regions and subcortical nuclei which contribute to the representation of emotional states. In addition, evidence from HIE supports hemispheric dominance for emotional valence. Furthermore, evidence from HIE supports the existence of overlapping neural areas for emotion perception, experience and expression. Lastly, HIE provides unique insights into the temporal dynamics of neural activation during perception, experience and expression of emotional states. In conclusion, we propose that HIE techniques offer important evidence which must be incorporated into our current models of emotion representation in the human brain.
Keywords: intracranial, EEG, epilepsy, emotion, social, stimulation, laughter, mirth, electrophysiology, microelectrodes
INTRODUCTION
The study of the neural representation of emotions is one of the cornerstones of cognitive neuroscience research. One encompassing definition sees emotions as helping to coordinate, adapt and reinforce sets of changes to the brain and body towards the triggering event (Adolphs et al., 2010). Despite enormous progress over the past 15 years in our understanding of the neural representation of emotions in the human brain thanks to functional neuroimaging, several recent meta-analyses point out continued controversy over many central concepts (e.g. Murphy et al., 2003; Phan et al., 2002; Kober et al., 2008; Lindquist et al., 2012). For instance, despite general agreement that emotional states causes wide-spread brain activation, some investigators postulate that the data support the existence of discrete emotional processing centers in the brain while others see the data as supporting less discrete, more network-based processing. The more locationist view argues that a small set of discrete emotions (e.g. anger) are represented by discrete, evolutionarily conserved anatomical systems in the brain (e.g. amygdala) (Adolphs et al., 1994; Bechara et al., 1995; Calder et al., 1996; Scott et al., 1997; Adolphs et al.1999; Schmolck and Squire, 2001; Vytal and Hamann, 2010; Lench et al., 2011). The contrary position is a distributionist view which argues that no single macro-anatomical structure uniquely specializes for individual emotion categories and proposes a dimensional view (i.e. positive and negative valence, high and low arousal) of emotions. In certain views, emotion categories are composed of even more basic psychological units called ‘psychological primitives’ (Cunningham and Zelazo, 2007; Barrett, 2011, 2012; LeDoux, 2012; Lindquist and Barrett, 2012; Lindquist et al., 2012; Russell, 2012; Barrett and Satpute, 2013; Lindquist et al., 2013; Hamann, 2012). While these distributionist theories either focus on some combination of the psychological and the neural levels, the ones we will focus on will primarily be of the neural level.
While to some extent the dispute over what the evidence shows may stem from presuppositions of the investigators, it is possible that limitations in methods used to investigate human emotions are fueling this dispute. Techniques typically used in the neuroscience of emotion depend on the organisms under study. Human studies primarily use some form of neuroimaging (e.g. fMRI, EEG, MEG) and either temporary (e.g. TMS) or permanent lesion participants. One limitation could be the spatiotemporal resolution of functional neuroimaging. Perhaps the processing units of interest can be better dissociated at a level smaller and faster than conventional neuroimaging can detect? A second limitation stems from caveats of inferring causality from lesion studies. Lesion map data primarily come from stroke patients with the possibility of wide lesions that effect cortical, subcortical and white matter regions (Duffau, 2012). Even focal lesions that are localized to a single region have been shown to produce different behavioral results in different participants (Feinstein, 2013). The individual variability and lack of control leads to many third-variable confounds that hurt causal inferences.
To alleviate some of these issues, studying animal models opens up an array of different invasive techniques with the best spatiotemporal resolution possible. Research with non-human models typically uses invasive forms of electrophysiology [e.g. single neuron recordings, local field potentials (LEPs), etc.] and lesion techniques. While these studies in rodents and non-human primates have contributed to our understanding of emotion and pro-social behavior (LeDoux, 2000; Burgdorf and Panksepp, 2006), there are numerous, complex behaviors unique to humans that are not possible to study in animals yet are critical to the understanding of hypothesized neural emotion models.
Human Intracranial Electrophysiology
Human intracranial electrophysiology (HIE) techniques have been used since the 1950’s for characterization of epilepsy in people who experience seizures despite optimal treatment with medications. The clinical goal of HIE is to establish which regions of the brain generate seizures and to determine if surgical removal of this tissue is possible. Following the implantation of intracranial electrodes, regions of the brain that are epileptogenic are identified. In addition, areas around epileptogenic tissue are tested for cognitive and sensorimotor function by electric current stimulation via the implanted electrodes.
Secondary to the clinical goals, HIE techniques can be used for research by allowing measurement of localized brain activity during the performance of cognitive tasks (Engel et al. 2005; Adolphs. 2007). The type of brain activation seen using HIE techniques depends mainly on the type of electrode used for recording. If macroelectrode contacts (2–5 mm discs) are used, then LFPs can be recorded. If microelectrodes (20–40 µ wires) are used, then activity of single neurons is seen and referred to as single-unit activity (SUA). In addition to measurements of brain activation, electrical brain stimulation (EBS) via macroelectrode contacts can be used to determine if temporary neural suppression or activation leads to an effect on cognitive function.
Advantages of HIE Methods
HIE methods allow for the measurement of electrical activity similar to scalp EEG but with greater spatial resolution, better gamma frequency resolution (30–150 Hz activity) and higher signal-to-noise ratio (Mukamel and Fried, 2012). Compared to functional MRI, HIE techniques offer comparable spatial resolution but with microsecond temporal resolution which is on the same order of magnitude as the speed of cognitive processing. Furthermore, HIE recordings are somewhat immune to muscle and eye movement artifact (Kovach et al., 2011). This allows for the study of phenomena involving motor actions which is difficult when using functional MRI, such as laughter.
An additional major advantage of HIE methods includes the ability to deliver targeted EBS which is useful for making causal inferences. Methods like EEG, fMRI and intracranial recordings can establish covariance between brain and behavior but temporal precedence and third-variable exclusion is necessary to make a causal inference. HIE stimulation techniques have the advantage of the stimulation having temporary effects (vs lesion) and having the ability to record from the same electrodes used for stimulation (vs TMS). Intraoperative stimulation procedures can also afford the opportunity to stimulate subcortical and white matter tracts (Duffau, 2010). The induction of distinct behaviors with EBS offers compelling evidence that this brain region is in some way involved in the neural representation of that emotional state.
Signal analysis techniques used in HIE are similar to techniques for scalp EEG (Makeig et al., 2004). If the question is focused on looking at the neural signal during a specific event, there are time-locking methods for looking at the evoked potentials or the frequency distribution. If the question is focused on looking at the similarity of processing across brain areas, then techniques that are similar to fMRI network analysis can be used (Lachaux et al., 2003).
Limitations of HIE techniques
Several significant limitations of HIE techniques exist. First, the recordings are performed in patients with epilepsy, not neurotypical subjects. While it is true that parts of their brains are impaired, it is not correct to assume that the entire brain is pathological. A historical example of how data from intracranial patients can inform healthy brain activity is Wilder Penfield’s work on the somatotopic organization of primary sensory and motor cortex. Intracranial electrodes are generally implanted to cover wide areas of the brain with the goal of understanding which brain regions are pathological and which are healthy. Electrodes which record from pathological brain areas are excluded from analysis.
Second limitation of HIE research is the issue of limited spatial coverage in a single patient. In fact, as many as 10 000 intracranial recording sites would be necessary to get the same whole-brain spatial coverage as fMRI (Lachaux et al., 2003). This limitation can be partially circumvented by hypothesis-driven study designs which ask questions about focal networks. For instance, a typical intracranial electrode placement for suspected temporal lobe epilepsy may cover the amygdala, hippocampus, temporal pole, temporal neocortex and occipital-temporal cortex. This implant strategy may allow for the testing of hypotheses regarding visual processing of fear-inducing stimuli. However, this implant strategy does not allow for the understanding of how the rest of the brain functions during the task.
Third limitation of HIE research involves inferences which can be drawn from EBS. EBS can either activate a brain region (e.g. causing hand movements during stimulation of the motor cortex), inhibit a brain region (e.g. causing language arrest during stimulation of Broca’s area), or activate a whole sub-network distant to the site of stimulation (David et al., 2010; Mandonnet et al., 2010). Therefore, the experience of fear during stimulation of the amygdala may result from activation of the amygdala itself, may result from activation of a distant brain region via neural connections, or conversely may result from disinhibition of a distant brain region by inhibition of the amygdala. Furthermore, it is difficult to assess from stimulation experiments the extent of the brain volume which is influenced by the stimulation. Some of these uncertainties have been addressed by reports of good motor outcomes in surgical resections up to 1 cm of cortical distance from eloquent regions mapped by stimulation (Gregorie and Goldring, 1984). In addition, during EBS it is often noted that adjacent electrode sites are not affected by the stimulation.
The authors are interested in the use of HIE to study the neural basis of emotions because the methodology has many strengths which complement conventional neuroimaging. The goals of this review are 2-fold. First, we aim to organize the findings from half-a-century of HIE research into human emotions. Second, we aim to draw conclusions on what the evidence from HIE adds to our present understanding of the neural representation of emotions. By the end of the manuscript, we will see how the current HIE evidence gives answers to the following questions: (i) Are emotional responses localized specifically and consistently to specific brain regions or are they widely distributed? (ii) Do experience, expression and perception share any neural machinery? (iii) Is there any (absolute or relative) hemispheric lateralization during emotion?
CRITERIA FOR INCLUSION
A Pubmed and Google Scholar search was performed using the following keywords: emotions, social, face, intracranial, iEEG, EcOG, cortical stimulation, deep brain stimulation, laughter, intraoperative, sad, fear, mirth, dyad. For the purpose of finding as many articles as possible for this review, three delineating terms for different emotion phenomena needs to be defined. We will be defining emotional perception as the cognitive capability to perceive the emotion of another human being. Emotional expression will be defined as the motoric act associated with having an emotion. This is independent from the emotional experience, which is the subjective feeling associated with an emotional event. Several related topics were not included in this review. Studies investigating reward processing, learning, and decision making were omitted (for review, see Oya et al., 2005; and Lega et al., 2011). Second, studies aimed at investigation of DBS in treatment of mood disorders were not included (for review, see Holtzheimer and Mayberg, 2011). Lastly, studies of pain experience were not included (for review, see Selimbeyoglu and Parvizi, 2010). Only English-language articles and chapters were used.
QUALITATIVE FINDINGS
Overview of articles
Sixty-four studies which investigated emotional processing using HIE methods were identified. Year of publication ranged from 1954 to 2012. Twenty-one studies examined neural activity during perception of emotional states in others (Table 1). Twenty-seven studies examined neural activity during the experience of negatively valenced emotional states (Table 2) and 12 papers during the experience of positively valenced emotional states (Table 3). Eleven studies examined neural activity during the motoric expression of emotional states (Table 4). Some papers are listed under more than one table. The investigated brain regions include significant portions of the temporal, frontal, parietal and occipital neocortex, multiple limbic areas including the hippocampus, amygdala, insula and cingulate gyrus, and several subcortical regions including the subthalamic nucleus, substantia nigra, zona incerta and various parts of the internal capsule. To see this same data organized by brain regions, please refer to tables in the Supplementary Data.
Table 1.
Emotion perception
| Study | Year | Type of study | Areas | Results |
|---|---|---|---|---|
| Krolak-Salmon et al. | 2003 | ERP, Stimulation | Anterior insula | Regions with differential potentials for disgust face perception also elicit negative valence experience during stimulation |
| Krolak-Salmon et al. | 2004 | ERP | Amygdala | Amygdala active in fear perception > other emotions |
| Tsuchiya et al. | 2008 | ERP | Fusiform, STS | Fusiform gyrus participates in emotion decoding |
| Pourtois et al. | 2010a | ERP | Fusiform | Early face response and late emotion and eye direction response |
| Pourtois et al. | 2010b | ERP | Amygdala | Different early and late responses in amygdala. Only late modulated by attention. |
| Jung et al. | 2011 | ERP | Lateral orbitofrontal | OFC active in negative emotion processing |
| Rømer-Thomsen et al. | 2011 | ERP | ACC | ACC showed differences between happy and sad faces ∼500 ms |
| Ojemann et al. | 1992 | Single unit | Right lateral temporal | SUA to facial emotion perception. |
| Fried et al. | 1997 | Single unit | Hippocampus, amygdala | SUA to facial emotion encoding and recognition. |
| Fried et al. | 1982 | Stimulation | Lateral temporal | Impaired judgment of emotional facial expressions. |
| Péron et al. | 2010a | Stimulation | STN | Impairment of recognition for sad and fearful facial expressions |
| Péron et al. | 2010b | Stimulation | STN | Impairment in recognizing the emotional prosody in speech stimuli. |
| Mukamel et al. | 2010 | Single Unit | SMA, MTL | Responsive firing for emotional face perception and execution |
| Brück et al. | 2011 | Stimulation | STN | Enhanced processing of highly-conflicting emotional messages. |
| Marinkovic et al. | 2000 | ERP, Stimulation | Anterior inferior PFC | Intracranial recording and stimulation evidence found cortex sensitive for faces (including hallucinations of faces when stimulated). Area was resected and produced deficits in emotional face recognition (especially fear) |
| Sato et al. | 2011 | iEEG | Amygdala | Greater gamma-band activity in response to fearful compared with neutral facial expressions between 50 and 150 ms |
| Biseul et al. | 2005 | Stimulation | STN | Impaired recognition of fear expressions |
| Schroeder et al. | 2004 | Stimulation | STN | Impaired recognition of angry expressions |
| Dujardin et al. | 2004 | Stimulation | STN | Impaired recognition of angry and sad expressions |
| Drapier et al. | 2008 | Stimulation | STN | Impaired recognition of fear and sad expressions. Apathy scores had also worsened after DBS implantation. |
| Le Jeune et al. | 2008 | Stimulation, PET | STN, orbitofrontal | Impaired recognition of fear faces. These results positively correlate with glucose metabolism in the right orbitofrontal cortex. |
Abbreviations: ACC, Anterior cingulate cortex; ERP, Event-related potentials; iEEG, Intracranial electroencephalography; MTL, Medial temporal lobe; PET, Positron emission tomography; PFC, Prefrontal cortex; SMA, Supplementary motor cortex; STN, Subthalamic nucleus; STS, Superior temporal sulcus
Table 2.
Experience of negative emotional states
| Study | Year | Type of study | Areas | Results |
|---|---|---|---|---|
| Oya et al. | 2002 | ERP | Amygdala | Response to negative emotions |
| Naccache et al. | 2005 | ERP | Amygdala | Response to emotional words |
| Kawasaki et al. | 2001 | Single Unit | vmPFC | Response to negative emotional states |
| Penfield | 1958 | Stimulation | ITL | Fear |
| Penfield and Perot | 1963 | Stimulation | STG, TPJ | Fear |
| Bancaud et al. | 1994 | Stimulation | STG | Fear |
| Meletti et al. | 2006 | Stimulation | MTL, Amygdala | Of the 79 emotional responses elicited, 67 were fearful, 9 were happy, and 3 were sad. 12% of these responses were in the amygdala and these were all fear responses. |
| Lanteaume et al. | 2006 | Stimulation | Amygdala | Right amygdala induced negative emotions, especially fear and sadness. Left amygdala was able to induce either pleasant (happiness) or unpleasant (fear, anxiety, sadness) emotions. |
| Halgren et al. | 1978 | Stimulation | Amygdala, Hippocampus | Fear, sadness, anger. |
| Mazzola | 2009 | Stimulation | Insula | Fear, anxiety |
| Feindel and Penfield | 1954 | Stimulation | Insula | Fear |
| Ostrowsky et al. | 2002 | Stimulation | Temporal pole | Anxiety, sadness |
| Gordon et al. | 1996 | Stimulation | Temporal pole | Positive and negative emotions |
| Mullan et. al | 1959 | Stimulation | MTG, STG, Insula | Fear |
| Van Buren | 1961 | Stimulation | MTL | Fear, laughter |
| Fish et al. | 1993 | Stimulation | Amygdala, Hippocampus | Fear |
| Blomstedt et al. | 2008 | Stimulation | STN | Stimulation caused acute transient depression with crying and feeling of not wanting to live. |
| Benedetti et al. | 2004 | Stimulation | STN, zona incerta, substantia nigra pars reticulata | Zona incerta and the dorsal pole of the subthalamic nucleus produced autonomic responses that were constant over time. In contrast, the stimulation of the ventral pole of the subthalamic nucleus and the substantia nigra pars reticulate produced autonomic and emotional responses that were inconstant over time and varied according to the condition. |
| Tommasi et al. | 2008 | Stimulation | STN, substantia nigra, zona incerta, fields of forel | Stimulation caused acute transient depression. |
| Bejjani et. al | 1999 | Stimulation | Left substantia nigra | Stimulation caused acute transient depression with crying and feeling of hopelessness. |
| Okun et al. | 2004 | Stimulation | STN | All leads elicited pathological crying but one lead elicited fear, one elicited anxiety, and the rest had no emotion at all. |
| Brázdil et al. | 2009 | ERP | Medial and lateral temporal, medial and lateral PFC, posterior parietal, precuneus and insula | Unpleasant pictures elicited more activity in temporal and frontal regions. Significant findings to emotional stimuli were found in rarely investigated regions (posterior parietal, precuneus and insula). |
| Krolak-Salmon et al. | 2003 | ERP, Stimulation | anterior insula | Regions with differential potentials for disgust face perception also elicit negative valence experience during stimulation |
| Smith et al. | 2006 | Stimulation | cingulate, OFC, MTL, amygdala and insula | Negative responses were more associated with right-sided stimulation. Positive responses were found in each hemisphere (left ACC, right insula). |
| Vicente et al. | 2009 | Stimulation | STN | Lower levels of differentiating sad and fearful videos and less intense feelings towards negative valence videos. |
| Sabolek et al. | 2009 | Stimulation | STN, substantia nigra | Acute fear induced with right substantia nigra stimulation. Depressive feelings induced with caudal STN stimulation. |
| Burdick et al. | 2011 | Stimulation | STN, Globus pallidus interna, Vim | STN and GPi DBS were associated with higher anger scores. It was not confirmed if this was a lesion or a stimulation effect. |
Abbreviations: ACC, Anterior cingulate cortex; ERP, Event-related potentials; iEEG, Intracranial electroencephalography; ITL, Inferior temporal lobe; MTG, Middle temporal gyrus; MTL, Medial temporal lobe; PET, Positron emission tomography; PFC, Prefrontal cortex; OFC, Orbitofrontal cortex; SMA, Supplementary motor cortex; STG, Superior temporal gyrus; STN, Subthalamic nucleus; TPJ, Temporoparietal junction; Vim, Ventral intermediate nucleus; vmPFC, Ventral medial prefrontal cortex
Table 3.
Experience of positive emotional states
| Study | Year | Type of study | Areas | Results |
|---|---|---|---|---|
| Satow et al. | 2003 | Stimulation | Left ITL | Mirth or mirth with laughter depending on intensity of stimulation |
| Gordon et al. | 1996 | Stimulation | Temporal pole | Positive and negative emotions |
| Van Buren | 1961 | Stimulation | MTL | Fear, laughter |
| Meletti et al. | 2006 | Stimulation | MTL, Amygdala | Of the 79 emotional responses elicited, 67 were fearful, 9 were happy, and 3 were sad. 12% of these responses were in the amygdala and these were all fear responses. |
| Lanteaume et al. | 2006 | Stimulation | Amygdala | Right amygdala induced negative emotions, especially fear and sadness. Left amygdala was able to induce either pleasant (happiness) or unpleasant (fear, anxiety, sadness) emotions. |
| Smith et al. | 2006 | Stimulation | ACC, OFC, MTL, amygdala, and insula | Negative responses were more associated with right-sided stimulation. Positive responses were found in each hemisphere (left ACC, right insula). |
| Haq et al. | 2011 | Stimulation | ALIC, Nucleus accumbens | After stimulation, patients felt mirth followed by a smile or laugh. For sites with smiling or laughing, the mood was congruent in 28 of 31 conditions. For sites with smiling or laughing, mood positively correlated with voltage. |
| Krack et al. | 2001 | Stimulation | STN | Laughter with mirth was elicited in two Parkinson’s patients. When the stimulation was set to the therapeutic parameters, there is an improvement in akinesia symptoms |
| Stefan et al. | 2004 | Stimulation | Temporal, frontal, parietal | Ictal pleasantness localized mainly to temporal mesiobasal areas, but also found it localized in frontal and parietal in a minority of patients. |
| Greenhouse et al. | 2011 | Stimulation | Ventral STN | Ventral contact stimulation led to an increase of positive emotion. |
| Fernandez-Baca Vaca | 2011 | Stimulation | Left IFG | Mirth and laughter |
| Arroyo et al. | 1993 | Stimulation | ACC, parahippocampal, fusiform | Patient with gelastic seizures without mirth had onset at left ACC. Other patients elicited laughter with mirth when stimulated around the fusiform and parahippocampal gyri. |
Abbreviations: ACC, Anterior cingulate cortex; ALIC, Anterior limb of the internal capsule; ITL, Inferior temporal lobe; MTL, Medial temporal lobe; OFC, Orbitofrontal cortex; STN, Subthalamic nucleus
Table 4.
Isolated motoric expression of emotion
| Study | Year | Type of study | Areas | Results |
|---|---|---|---|---|
| Davis et al. | 2005 | Single unit | Caudal ACC | Attention-demanding stroop task involving emotional content can alter the firing rate |
| Mukamel et al. | 2010 | Single unit | SMA, MTL | Responsive firing for emotional face perception and execution |
| Chassagnon et al. | 2008 | Stimulation | Cingulate motor area | In one patient, the urge to laugh without mirth was elicited |
| Fried et. al | 1998 | Stimulation | SMA | The duration and intensity of the laughter increased with stimulation current. Smiling was induced at lower currents. The patient consistently contributed the laughter to some outside source. |
| Sperli et al. | 2006 | Stimulation | Right cingulate | Stimulation induced smiling and laughing without mirth. |
| Schmitt et al. | 2006 | Stimulation | SMA and pre-motor area | Laughter without mirth was elicited. |
| Bartolomei et al. | 2005 | iEEG | ACC, orbitofrontal, amygdala, temporal pole | Negative motoric expression preceding seizures located in ACC, Orbitofrontal, and Temporal Pole. These expressions also associated with signal decorrelation between orbitofrontal and amygdala. |
| Arroyo et al. | 1993 | Stimulation | ACC, parahippocampal, fusiform | Patient with gelastic seizures without mirth had onset at left ACC. Other patients elicited laughter with mirth when stimulated around the fusiform and parahippocampal gyri. |
| Krolak-Salmon et al. | 2006 | iEEG, Stimulation | SMA | Stimulation of left pre-SMA consistently got a smile or a laugh from the patient when stimulated (needed at least 0.6 mA at 50 Hz). The patient reported the mirth followed the movement. At .8mA, crying followed the laughter. The field potentials in this area responded mainly for happy faces. |
| Wojtecki et al. | 2007 | Stimulation | STN | Pathological crying |
| Low et al. | 2008 | Stimulation | Caudal internal capsule | Pathological crying |
| Hiyoshi et al. | 1989 | iEEG | Lateral and mesial temporal lobe | Disgust expression with mesial temporal focus and happy expression with lateral temporal focus. |
Abbreviations: ACC, Anterior cingulate cortex; iEEG, Intracranial electroencephalography; MTL, Medial temporal lobe; SMA, Supplementary motor cortex; STN, Subthalamic nucleus
The studies reviewed varied in many ways. There was a lack of consistent methodological standardization in the study designs, stimulus presentation, recording techniques, analysis techniques and electrode localization methods. In addition, most authors did not describe the exact type of epilepsy that the patient was suffering from or how trials or electrodes with epileptic activity were excluded from the analysis. For these reasons, quantitative meta-analysis was not possible with this data. In turn, data were organized in a semi-quantitative fashion based on reported neuroanatomy and descriptions of emotional processes.
Perception of emotional states in others
Most investigators used pictures of static faces with emotional expressions as stimuli and recorded neuronal activity in the fusiform gyrus, the amygdala and multiple regions of the temporal and frontal lobes (Table 1). Other tasks included studying emotional prosody either in a specific emotion framework or on a positive/negative gradient scale.
Experience of positively and negatively valenced emotional states
Emotional experience was studies by two distinct methods. The first method involved measuring neural activity during the presentation of emotionally provocative stimuli, such as International Affective Picture System (IAPS) pictures. These pictures were designed to evoke changes in different aspects of emotion experience in general (e.g. valence and arousal) and do not elicit a specific emotion per se. The second method involved subjective reports of emotional states induced by electrical stimulation of various brain regions (Tables 2 and 3). Stimulation findings are primarily incidental in that the emotional experience was spontaneously elicited and self-reported by the patients.
Motoric execution of emotional expression
Studies in this section include reports of elicitation of facial expression of an emotional state with the subject reporting no associated emotional experience. Regions of the brain include areas of the frontal lobe and subcortical structures (Table 4). Many of these expressions elicited were complex phenomena like laughing or crying without the appropriate emotion. The more subtle emotional expressions elicited are taken on face value from the clinician’s reports but future researchers should take care that these motoric acts are specific to emotions and not under voluntary control for communicative purposes (Fridlund, 1991).
DISCUSSION
The study of emotions in the human brain using HIE techniques dates back almost 60 years to the pioneering work of Wilder Penfield. Studies reviewed can be divided into two main types. The first type includes studies which aimed to measure brain activation during viewing of emotionally provoking stimuli. The types of stimuli included presentation of faces with emotional facial expressions (e.g. Ekman faces), presentation of emotionally provoking pictures (e.g. IAPS pictures) and audio presentations of emotionally provoking stories. In these studies, brain activity was measured using one of several methods: time-locked analysis of evoked LFPs, frequency analysis of synchronization or desynchronization, or change in the firing frequency of a neuron if recording SUA. Second type of study involved EBS of specific brain regions and measuring of either self-reports of descriptions of evoked emotional states or measuring the ability of the patient to detect emotional states in others.
Synthesis of reviewed articles
HIE techniques offer evidence in support of six conclusions with regards to the neural representation of emotions:
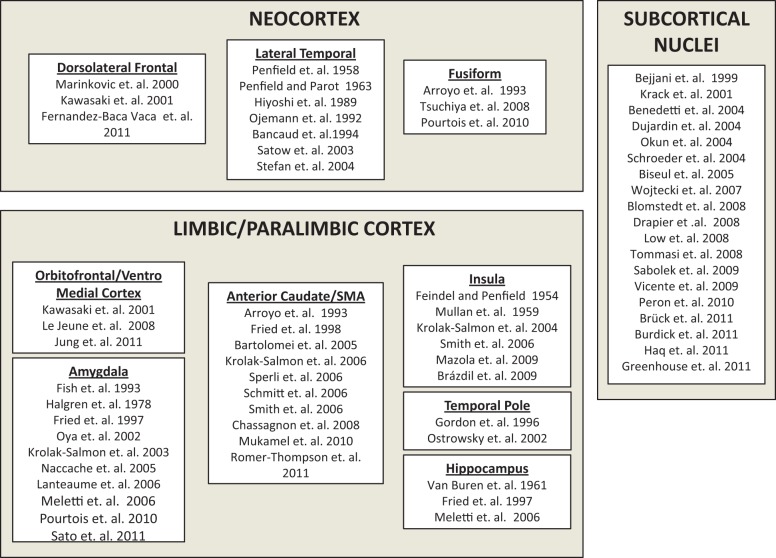
Evidence from HIE methods supports a model of widely distributed representations of emotions spanning the subcortical nuclei, the limbic/paralimbic regionsand the neocortex (Figure 1). Evidence from HIE suggests that subcortical nuclei play an important role in representation of emotional states. Stimulation of subcortical nuclei such as the STN can impair perception of sadness, fear and anger and impair recognition of emotional prosody in speech stimuli (Table 1). Stimulation of STN, zona incerta, substantia nigra pars reticulata, fields of Forel, globus pallidus interna and ventral intermediate nucleus of thalamus can induce experience of sadness and crying (Table 2) while stimulation of the nucleus accumbens, anterior limb of the internal capsule, and ventral STN has been associated with sensation of joy and mirth (Table 3). In addition, recording of LFPs and stimulation of amygdala, hippocampus, insula, orbitofrontal cortex, temporal pole and anterior cingulate cortex clearly points to limbic/paralimbic representation of emotional states (Tables 1, 2 and 3). Furthermore, HIE evidence implicates neocortical regions in representation of emotional states. Stimulation of the basal temporal lobe, superior temporal gyrus, middle temporal gyrus, temporal-parietal junction, inferior frontal gyrus and supplementary motor area has been shown to induce states of fear and mirth (Tables 2 and 3).
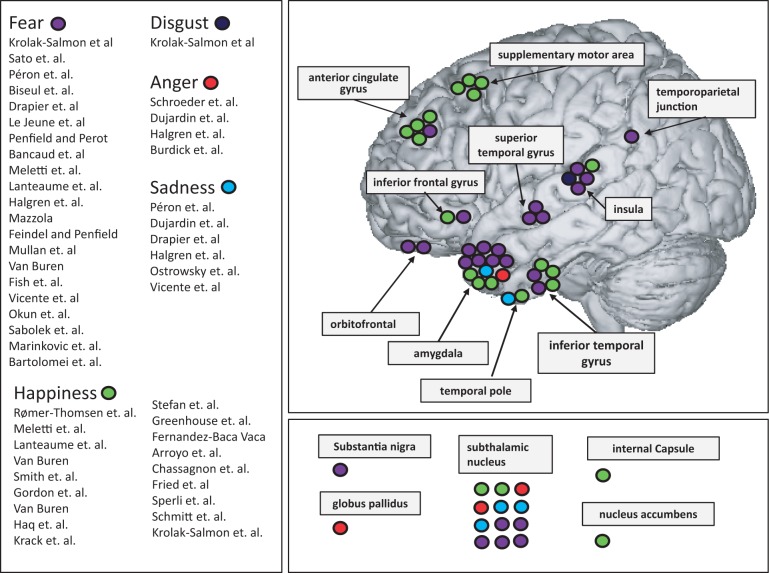
HIE methods supports the idea that emotional state categories (such as fear) are represented in multiple brain areas (Figure 2). Evidence comes especially from EBS studies. For example, self-reported experience of fear has been evoked by EBS of the amygdala, insula, inferior temporal lobe, middle temporal gyrus, superior temporal gyrus, hippocampus, temporo-occipital junction and even substantia nigra (Table 1). Furthermore, experience of mirth has been evoked by EBS of left inferior temporal lobe, left inferior frontal gyrus, supplementary motor area, cingulate gyrus, parahippocampal and fusiform gyri (Table 1). Further evidence comes from ERP studies showing for instance that the hippocampus, amygdala, pre-frontal cortex, insula and precuneus activate during presentation of fearful stimuli (Brázdil et al., 2009).
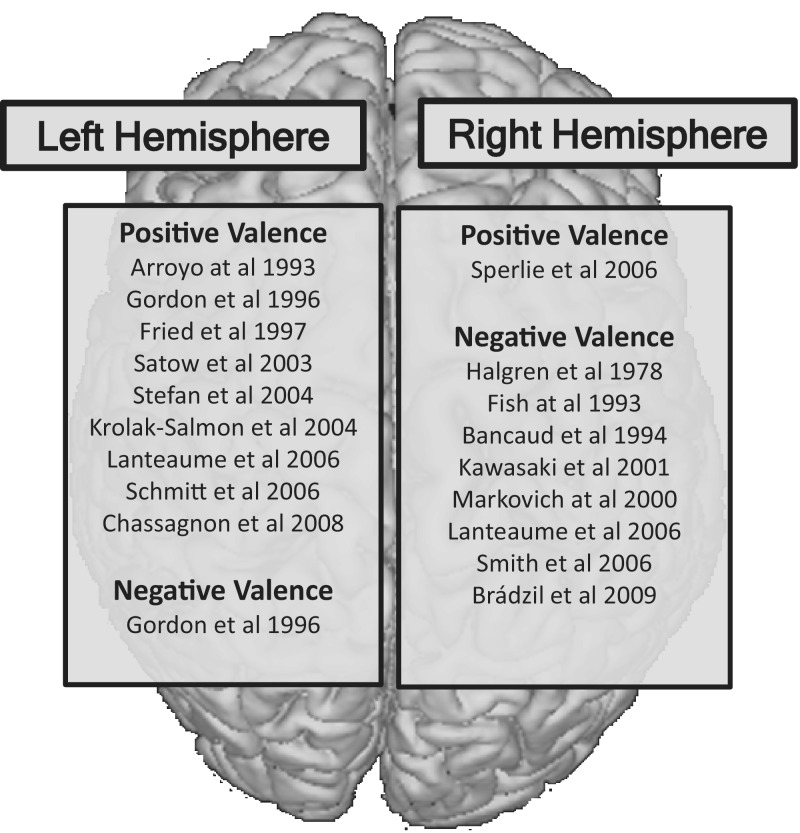
HIE methods support the existence of right hemisphere dominant networks for representation of negatively valenced emotional states (e.g. fear) and left-hemisphere dominant networks for positively valenced emotions states (e.g. mirth) (Figure 3). Evidence for hemispheric dominance for emotional valence comes primarily from qualitative analysis of EBS studies. Multiple investigators report the induction of negatively valenced emotional states (e.g. fear, anger, sadness) with EBS of the right hemisphere and positively valenced emotional states (e.g. mirth) with EBS of the left hemisphere (Tables 2 and 3). However, HIE studies do not suggest absolute separation of emotional valence by hemisphere, only dominance. For example, stimulation of the left amygdala can induce either pleasant (e.g. happiness) or unpleasant (e.g. fear) emotions (Lanteaume et al., 2006).
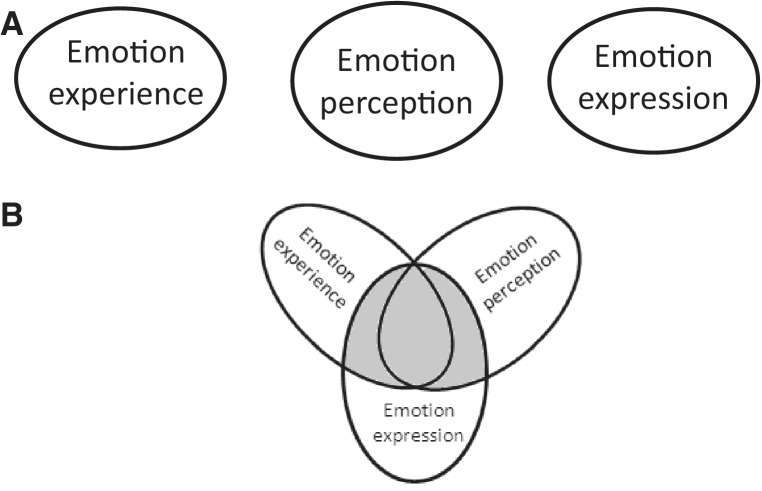
Evidence from HIE methods supports the existence of distinct yet partially overlapping neural systems for emotion perception, emotion experience, and formation of emotional motoric acts (Figure 4). This conclusion is supported by EBS studies, by LFP recordings, and by SUA recordings. EBS of regions in the neocortex, limbic/paralimbic cortex or deep nuclei has been shown to induce motoric expressions of emotional states without the accompanied emotional state as reported by the patient. For example, EBS of the left SMA cortex elicited ‘laughter without mirth’, and stimulation of the STN induced ‘crying without sadness’ (Table 4). An interesting EBS study by Satow et al. found the experience of ‘mirth only’ with low EBS intensities and ‘mirth accompanied by laughter’ at high EBS intensities (Satow et al., 2003). These findings suggest that neural regions for emotion expression and emotion experience are distinct but partially overlapping. Further support for this conclusion comes from LFP recordings and EBS studies of the amygdala. The amygdala activates during perception of negatively valenced emotional faces, during experience of negatively valenced emotional states and EBS of the amygdala can induce the experience of negatively valenced emotional states (Tables 1, 2 and 3). Lastly, this conclusion is supported by single unit recordings in the SMA and basal temporal cortex by Mukamel et al. which revealed that not only the same region, but also the same neuron can fire during perception and during motoric execution of emotional faces (Mukamel et al., 2010). These findings are largely consistent with concepts gleamed from fMRI (Grimm et al., 2006; Schiller and Delgado, 2010; Satpute et al., 2013).
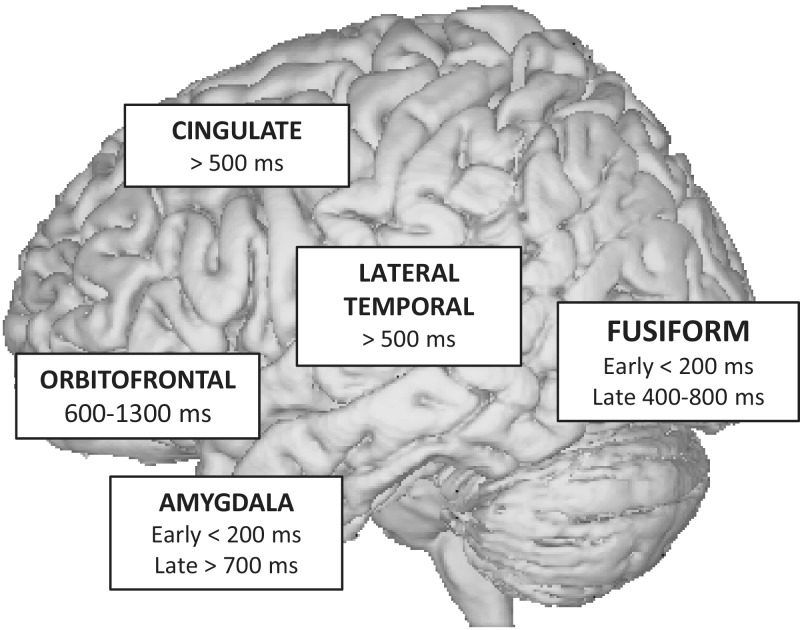
HIE methods elucidate the dynamics of neural activation (Figure 5). The high temporal resolution of HIE methods offers a unique window into the mechanics of human cognition. A fantastic example comes from recording of LFPs during processing of facial expressions as reported by multiple investigators. In these studies, intracranial macroelectrodes are placed over occipital, lateral temporal, amygdalar and orbitofrontal regions. Following the presentation of a photograph depicting a face with emotional expression, initial activation occurs in the primary visual cortex ∼100 ms following stimulus presentation. Next, activation in the fusiform face area (FFA) occurs at 120–200 ms following presentation of facial stimuli but not to non-facial stimuli (e.g. Pourtois et al., 2010a). Next, the FFA and the superior temporal gyrus (STG) activate to morphing emotional faces between 200 and 500 ms (Tsuchiya et al., 2008). Surprisingly, activation of the amygdala to fearful expressions occurs within 200 ms following stimulus presentation, which precedes activation in the FFA, STG and orbitofrontal cortex (Krolak-Salmon et al., 2004). This suggests that the amygdala may modulate the activity of the FFA in a retrograde fashion. Last, activation of the orbitofrontal cortex occurs 500–1000 ms following presentation of fearful stimuli (Jung et al., 2011). Amygdala and temporal lobe findings are consistent with previous EEG/MEG reviews (Pessoa and Adolphs, 2010) but much faster (<200 ms) activity has been observed in frontal scalp EEG findings (Barrett and Bar, 2009). Localizing neural sources from scalp responses is problematic and it cannot be assumed that the electrical activity came from parenchyma adjacent to the cortex. Nonetheless, it is interesting that sub-100 ms activity has been observed in scalp studies and where this activity is localized would be an excellent future direction.
HIE methods suggest that the processing function of brain regions may change with respect to the temporal latency from the stimulus onset. The FFA shows early activation to faces independent of facial emotion at ∼200 ms and distinct late activation based on facial emotion and eye direction at ∼500 ms (Pourtois et al., 2010a). This finding provides evidence to suggest that the role of a certain brain region in cognitive processing may change depending on the latency from stimulus presentation, i.e. the FFA processes all faces early and only later processes facial emotion. The high temporal resolution of HIE suggest a slightly different model of emotion processing as compared to fMRI. HIE model suggests that FFA processes most of the information during perception of static and dynamic facial expressions, the only difference being the latency from stimulus presentation.
Fig. 1.
HIE studies which reported neural representation of emotional states separated by region.
Fig. 2.
HIE studies which reported neural representation of emotion separated by emotion type.
Fig. 3.
Valence-based hemispheric dominance of emotions in HIE studies
Fig. 4.
Graphic conceptual depictions of two distinct models of the neural representation of emotion processing. (A) Emotion experience, perception and expression are distinctly represented in the brain. (B) Emotion experience, perception and expression share partial overlapping representation. HIE studies support this model.
Fig. 5.
HIE provides information on the temporal sequence of activation during perception of emotional faces.
CONCLUSIONS AND COMPARISON TO NON-HIE EVIDENCE
Evidence from HIE methods supports several general conclusions. First, HIE offers strong evidence against the existence of super-specialized macro-anatomical structures for representation of single emotion categories (e.g. amygdala for fear) but rather offers evidence that their representation is more broadly distributed between the subcortical nuclei, the limbic/paralimbic regions, and the neocortex. Although many investigators report clear descriptions of emotional experiences during EBS of neuroanatomically confined areas (i.e. fear with amygdala), induction of same emotional experiences have been reported during EBS of many other areas (e.g. insula, parahippocampal gyrus) with significant variability between studies. Evidence from HIE for broad distribution of emotional representation is largely consistent with neuroimaging and animal studies, especially for subcortical and limbic system involvement. However, it is important to note clear and strong evidence from HIE for neocortical involvement in emotion representation in light of the controversy in this area (Barrett et al., 2007; Panksepp, 2007).
Second, HIE methods offers modest evidence in support of hemispheric specialization for positive or negative emotional valence, with left hemisphere being dominant for positive and right hemisphere dominant for negative emotion valence. The neuroimaging literature has mixed results when it comes to laterality differences. Some work finds left prefrontal as primarily involved with approach mechanisms while right prefrontal as involved with avoidance mechanisms (Spielberg et al., 2013). Other neuroimaging reviews do not find laterality differences (Kringelbach and Rolls, 2004) or found laterality with opposite valences assigned (Wager et al., 2008).
Third, HIE methods offer strong evidence that neural representation of emotion observation, emotion experience, and emotion expression are partially dissociated and partially overlapping, findings which are largely consistent with neuroimaging and animal research. Evidence from HIE offers further support to the theory of embodied cognition which holds that the nature of the human mind is largely determined by the form of the human body (Niedenthal et al., 2005; Barrett, 2006). Further research needs to be done in terms of better outlining the overlap of emotional processes in the brain and this is one topic which is uniquely situated for HIE methods to explore.
Fourth, HIE reveals that processing of emotional information has complex dynamics which may be largely imperceptible to fMRI. The temporal sequence of activation gleaned during processing of facial emotion observation can be extended to other emotional processes. In addition, HIE evidence that same brain area processes different information depending on the temporal latency from stimulus onset (i.e. temporally dependent processing) cannot be adequately investigated using fMRI. Temporal latency dependence for function has been briefly hypothesized in previous fMRI work (Lindquist et al., 2012) but it is still currently untested in the imaging modality.
Future directions
HIE methods have been largely underutilized in the study of emotional phenomena in humans. For instance, the approach successfully utilized to study temporal dynamic of amygdalar activation during perception of fearful stimuli has not been utilized in the study of other emotion categories. In addition, despite being well suited to clarify long-standing dispute over the existence of basic emotions, core affect or psychological primitives HIE methods have never been used to investigate these concepts. For instance, SUA methods have the necessary spatial-temporal resolution to uncover if individual emotion categories can be further broken down into more basic component parts. Furthermore, while the HIE results do not show single localization for an emotional process, there has yet to be reported evidence of functional connectivity between involved brain regions the same way that it’s been show in fMRI research. It is entirely possible to do this with HIE techniques, in fact, there is the added benefit of being able to stimulate across electrodes to causally test connectivity but it simply has not been done.
In future studies, we encourage HIE researchers to obtain exact stereotactic coordinates for electrode placement to make meta-analyses a feasible option. In addition, we encourage HIE researchers to utilize standardized scales of emotional assessment as self-reported measures are imprecise.
The main indication for HIE is to define the borders between pathological and functionally necessary brain regions. Unfortunately, the only cognitive domains typically mapped during routine clinical practice are motor and language while mapping for social and emotional phenomena has not reached clinical threshold. In our opinion, the goals of HIE should be elevated to encompass mapping cognitive functions that are specific to the patient’s individual personality, occupation, hobbies and social life. With as long as HIE methods have been around, mapping for social and emotional phenomena has not reached established methods as with sensorimotor and language mapping. To make this mission a reality will take more basic research (invasive and non-invasive) that is aimed at these goals. There are many types of studies that have not been run with HIE and the technology is steadily improving to allow for more ‘wireless’ studies of brain activity so one could study how real life situations and contexts may influence emotional processing (which could answer long standing debates on how emotions are represented in the brain). With the combination of noninvasive and HIE methods, the only thing to limit finding out about emotional phenomena are the questions asked and the experiments commenced.
Supplementary Material
Acknowledgments
This work was supported by a National Science Foundation Graduate Research Fellowship (S.A.G.).
REFERENCES
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–72. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Hamann S, et al. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37:1111–7. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Investigating human emotion with lesions and intracranial recording. In: Allen J, Coan J, editors. Handbook of Emotion Elicitation and Assessment. New York: Oxford University Press; 2007. pp. 426–39. [Google Scholar]
- Adolphs R. Emotion. Current Biology. 2010;20(13):549–52. doi: 10.1016/j.cub.2010.05.046. [DOI] [PubMed] [Google Scholar]
- Arroyo S, Lesser RP, Gordon B, et al. Mirth, laughter and gelastic seizures. Brain. 1993;116:757–80. doi: 10.1093/brain/116.4.757. [DOI] [PubMed] [Google Scholar]
- Bancaud J, Brunet-Bourgin F, Chauvel P, Halgren E. Anatomical origin of deja vu and vivid ‘memories’ in human temporal lobe epilepsy. Brain. 1994;117:71–90. doi: 10.1093/brain/117.1.71. [DOI] [PubMed] [Google Scholar]
- Barrett LF. Emotions as natural kinds? Perspectives on Psychological Science. 2006;1:28–58. doi: 10.1111/j.1745-6916.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Barrett LF. Bridging token identity theory and supervenience theory through psychological construction. Psychological Inquiry. 2011;22:115–27. doi: 10.1080/1047840X.2011.555216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF. Emotions are real. Emotion. 2012;12:413–29. doi: 10.1037/a0027555. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Bar M. See it with feeling: affective predictions in the human brain. Royal Society Phil Trans B. 2009;364:1325–34. doi: 10.1098/rstb.2008.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Lindquist K, Bliss-Moreau E, et al. Of mice and men: natural kinds of emotion in the mammalian brain? Perspectives on Psychological Science. 2007;2:297–312. doi: 10.1111/j.1745-6916.2007.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Satpute A. Large-scale brain networks in affective and social neuroscience: towards an integrative architecture of the human brain. Current Opinion in Neurobiology. 2013;23:361–72. doi: 10.1016/j.conb.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei F, Trebuchon A, Gavaret M, Regis J, Wendling F, Chauvel P. Acute alteration of emotional behaviour in epileptic seizures is related to transient desynchrony in emotion-regulation networks. Clinical Neurophysiology. 2005;116:2473–9. doi: 10.1016/j.clinph.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio A. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–8. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Bejjani BP, Damier P, Arnulf I, et al. Transient acute depression induced by high-frequency deep-brain stimulation. New England Journal of Medicine. 1999;340:1476–80. doi: 10.1056/NEJM199905133401905. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Colloca L, Lanotte M, Bergamasco B, Torre E, Lopiano L. Autonomic and emotional responses to open and hidden stimulations of the human subthalamic region. Brain Research Bulletin. 2004;63:203–11. doi: 10.1016/j.brainresbull.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Biseul I, Sauleau P, Haegelen C, et al. Fear recognition is impaired by subthalamic nucleus stimulation in Parkinson’s disease. Neuropsychologia. 2005;43:1054–9. doi: 10.1016/j.neuropsychologia.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Blomstedt P, Hariz MI, Lees A, et al. Acute severe depression induced by intraoperative stimulation of the substantia nigra: a case report. Parkinsonism Related Disorders. 2008;14:253–6. doi: 10.1016/j.parkreldis.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Brázdil M, Roman R, Urbánek T, et al. Neural correlates of affective picture processing–a depth ERP study. NeuroImage. 2009;47:376–83. doi: 10.1016/j.neuroimage.2009.03.081. [DOI] [PubMed] [Google Scholar]
- Brück C, Wildgruber D, Kreifelts B, Krüger R, Wächter T. Effects of subthalamic nucleus stimulation on emotional prosody comprehension in Parkinson's disease. PLoS One. 2011;6:19140. doi: 10.1371/journal.pone.0019140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick AP, Foote KD, Wu S, et al. Do patient's get angrier following STN, GPi, and thalamic deep brain stimulation. NeuroImage. 2011;54:S227–32. doi: 10.1016/j.neuroimage.2010.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. The neurobiology of positive emotions. Neuroscience & Biobehavioral Reviews. 2006;30(2):173–87. doi: 10.1016/j.neubiorev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Young AW, Rowland D, Perrett DI, Hodges JR, Etcoff NL. Facial emotion recognition after bilateral amygdala damage: differentially severe impairment of fear. Cognitive Neuropsychology. 1996;13:699–745. [Google Scholar]
- Chassagnon S, Minotti L, Kremer S, Hoffmann D, Kahane P. Somatosensory, motor, and reaching/grasping responses to direct electrical stimulation of the human cingulate motor areas. Journal of Neurosurgery. 2008;109:593–604. doi: 10.3171/JNS/2008/109/10/0593. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Zelazo PD. Attitudes and evaluations: a social cognitive neuroscience perspective. Trends in Cognitive Sciences. 2007;11:97–104. doi: 10.1016/j.tics.2006.12.005. [DOI] [PubMed] [Google Scholar]
- David O, Bastin J, Chabardès S, Minotti L, Kahane P. Studying network mechanisms using intracranial stimulation in epileptic patients. Frontier System Neuroscience. 2010;4:148. doi: 10.3389/fnsys.2010.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Taylor KS, Hutchison WD, et al. Human anterior cingulate cortex neurons encode cognitive and emotional demands. Journal of Neuroscience. 2005;25:8402–6. doi: 10.1523/JNEUROSCI.2315-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier D, Peron J, Leray E, et al. Emotion recognition impairment and apathy after subthalamic nucleus stimulation in Parkinson’s disease have separate neural substrates. Neuropsychologia. 2008;46:2796–801. doi: 10.1016/j.neuropsychologia.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Duffau H. Awake surgery for non-language mapping. Neurosurgery. 2010;66:523–9. doi: 10.1227/01.NEU.0000364996.97762.73. [DOI] [PubMed] [Google Scholar]
- Duffau H. The ‘Frontal Syndrome’ revisited: lessons from electrostimulation mapping studies. Cortex. 2012;48(1):120–31. doi: 10.1016/j.cortex.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Dujardin K, Blairy S, Defebvre L, et al. Subthalamic nucleus stimulation induces deficits in decoding emotional facial expressions in Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75:202–8. [PMC free article] [PubMed] [Google Scholar]
- Engel A, Moll C, Fried I, Ojemann G. Invasive recordings from the human brain: clinical insights and beyond. Neuroscience. 2005;6:35–47. doi: 10.1038/nrn1585. [DOI] [PubMed] [Google Scholar]
- Feindel W, Penfield W. Localization of discharge in temporal lobe automatism. Archives of Neurology & Psychiatry. 1954;72:603–30. [PubMed] [Google Scholar]
- Feinstein JS. Lesion studies of human emotion and feeling. Current Opinion in Neurobiology. 2013;23:304–9. doi: 10.1016/j.conb.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Fernández-Baca VG, Lüders HO, Basha MM, Miller JP. Mirth and laughter elicited during brain stimulation. Epileptic Disorders. 2011;13:435–40. doi: 10.1684/epd.2011.0480. [DOI] [PubMed] [Google Scholar]
- Fish DR, Gloor P, Quesney FL, Olivier A. Clinical responses to electrical brain stimulation of the temporal and frontal lobes in patients with epilepsy. Pathophysiological implications. Brain. 1993;116:397–414. doi: 10.1093/brain/116.2.397. [DOI] [PubMed] [Google Scholar]
- Fridlund AJ. The sociality of solitary smiles: effects of an implicit audience. Journal of Personality and Social Psychology. 1991;60:229–40. [Google Scholar]
- Fried I, MacDonald K, Wilson C. Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron. 1997;18:753–65. doi: 10.1016/s0896-6273(00)80315-3. [DOI] [PubMed] [Google Scholar]
- Fried I, Mateer C, Ojemann G, Wohns R, Fedio P. Organization of visuospatial functions in human cortex: evidence from electrical stimulation. Brain. 1982;105:349–71. doi: 10.1093/brain/105.2.349. [DOI] [PubMed] [Google Scholar]
- Fried I, Wilson CL, MacDonald KA, Behnke EJ. Electric current stimulates laughter. Nature. 1998;391:650. doi: 10.1038/35536. [DOI] [PubMed] [Google Scholar]
- Gordon B, Hart J, Lesser RP, Arroyo S. Mapping cerebral sites for emotion and emotional expression with direct cortical electrical stimulation and seizure discharges. Progress in Brain Research. 1996;107:61722. doi: 10.1016/s0079-6123(08)61891-9. [DOI] [PubMed] [Google Scholar]
- Greenhouse I, Gould S, Houser M, Hicks G, Gross JJ, Aron AR. Stimulation at dorsal and ventral electrode contacts targeted at the subthalamic nucleus has different effects on motor and emotion functions in Parkinson’s disease. Neuropsychologia. 2011;49:528–34. doi: 10.1016/j.neuropsychologia.2010.12.030. [DOI] [PubMed] [Google Scholar]
- Gregorie EM, Goldring S. Localization of function in the excision of lesions from the sensorimotor region. Journal of Neurosurgery. 1984;61:1047–54. doi: 10.3171/jns.1984.61.6.1047. [DOI] [PubMed] [Google Scholar]
- Grimm S, Schmidt CF, Bermpohl F, et al. Segregated neural representation of distinct emotion dimensions in the prefrontal cortex—an fMRI study. NeuroImage. 2006;30:325–40. doi: 10.1016/j.neuroimage.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Halgren E, Walter RD, Cherlow DG, Crandall PH. Mental phenomena evoked by electrical stimulation of the human hippocampal formation and amygdala. Brain. 1978;101:83–117. doi: 10.1093/brain/101.1.83. [DOI] [PubMed] [Google Scholar]
- Hamann S. Mapping discrete and dimensional emotions onto the brain: controversies and consensus. Trends in Cognitive Sciences. 2012;16:458–66. doi: 10.1016/j.tics.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Haq IU, Foote KD, Goodman WG, et al. Smile and laughter induction and intraoperative predictors of response to deep brain stimulation for obsessive-compulsive disorder. NeuroImage. 2011;54:S247–55. doi: 10.1016/j.neuroimage.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzheimer PE, Mayberg HS. Deep brain stimulation for psychiatric disorders. Annual Review Neuroscience. 2011;34:289–307. doi: 10.1146/annurev-neuro-061010-113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Bayle D, Jerbi K, et al. Intracerebral gamma modulations reveal interaction between emotional processing and action outcome evaluation in the human orbitofrontal cortex. International Journal of Psychophysiology. 2011;79:64–72. doi: 10.1016/j.ijpsycho.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Kaufman O, Damasio H, et al. Single-neuron responses to emotional visual stimuli recorded in human ventral prefrontal cortex. Nature Neuroscience. 2001;4:15–6. doi: 10.1038/82850. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph JJ, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach CK, Tsuchiya N, Kawasaki H, Oya H, Howard MA, Adolphs R. Manifestation of ocular-muscle EMG contamination in human intracranial recordings. Neuroimage. 2011;54:213–33. doi: 10.1016/j.neuroimage.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krack P, Kumar R, Ardouin C, et al. Mirthful laughter induced by subthalamic nucleus stimulation. Movement Disorders. 2001;16:867–75. doi: 10.1002/mds.1174. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Krolak-Salmon P, Henaff MA, Isnard J, et al. An attention modulated response to disgust in human ventral anterior insula. Annals of Neurology. 2003;53:446–53. doi: 10.1002/ana.10502. [DOI] [PubMed] [Google Scholar]
- Krolak-Salmon P, Hénaff MA, Vighetto A, Bertrand O, Mauguière F. Early amygdala reaction to fear spreading in occipital, temporal, and frontal cortex: a depth electrode ERP study in human. Neuron. 2004;42:665–76. doi: 10.1016/s0896-6273(04)00264-8. [DOI] [PubMed] [Google Scholar]
- Krolak-Salmon P, Hénaff MA, Vighetto A, et al. Experiencing and detecting happiness in humans: the role of the supplementary motor area. Annals of Neurology. 2006;59:196–9. doi: 10.1002/ana.20706. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Rudrauf D, Kahane P. Intracranial EEG and human brain mapping. Journal of Physiology Paris. 2003;97:613–28. doi: 10.1016/j.jphysparis.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Lanteaume L, Khalfa S, Regis J, Marquis P, Chauvel P, Bartolomei F. Emotion induction after direct intracerebral stimulations of human amygdala. Cerebral Cortex. 2006;17:1307–13. doi: 10.1093/cercor/bhl041. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Rethinking the emotional brain. Neuron. 2012;73:653–76. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lega BC, Kahana MJ, Jaggi JL, Baltuch GH, Zaghloul KA. Neuronal and oscillatory activity during reward processing in the human ventral striatum. NeuroReport. 2011;22:795–800. doi: 10.1097/WNR.0b013e32834b2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Jeune F, Peron J, Biseul I, et al. Subthalamic nucleus stimulation affects orbitofrontal cortex in facial emotion recognition: a PET study. Brain. 2008;131:1599–608. doi: 10.1093/brain/awn084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lench HC, Flores SA, Bench SW. Discrete emotions predict changes in cognition, judgment, experience, behavior, and physiology: a meta-analysis of experimental emotion elicitations. Psychological Bulletin. 2011;137:834–55. doi: 10.1037/a0024244. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Barrett LF. A functional architecture of the human brain: insights from Emotion. Trends in Cognitive Sciences. 2012;16:533–40. doi: 10.1016/j.tics.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Siegel EH, Quigley K, Barrett LF. The hundred years emotion war: are emotions natural kinds or psychological constructions? Comment on Lench, Flores, & Bench (2011) Psychological Bulletin. 2013;139:255–63. doi: 10.1037/a0029038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. Behavioral and Brain Sciences. 2012;35:121–43. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low HL, Sayer FT, Honey CR. Pathological crying caused by high-frequency stimulation in the region of the caudal internal capsule. Archives of Neurology. 2008;65:264–6. doi: 10.1001/archneurol.2007.53. [DOI] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends in Cognitive Science. 2004;8:204–10. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Winkler PA, Duffau H. Direct electrical stimulation as an input gate into brain functional networks: principles, advantages and limitations. Acta Neurochirurgica (Wien) 2010;152:185–93. doi: 10.1007/s00701-009-0469-0. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Trebon P, Chauvel P, Halgren E. Localized face-processing by the human prefrontal cortex: face-selective intracerebral potentials and post-lesion deficits. Cognitive Neuropsychology. 2000;17:187–99. doi: 10.1080/026432900380562. [DOI] [PubMed] [Google Scholar]
- Mazzola L, Isnard J, Peyron R, Guenot M, Mauguiere F. Somatotopic organization of pain responses to direct electrical stimulation of the human insular cortex. Pain. 2009;146:99–104. doi: 10.1016/j.pain.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Meletti S, Tassi L, Mai R, Fini N, Tassinari CA, Russo GL. Emotions induced by intracerebral electrical stimulation of the temporal lobe. Epilepsia. 2006;47:47–51. doi: 10.1111/j.1528-1167.2006.00877.x. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Ekstrom A, Kaplan J, Iacoboni M, Fried I. Single-neuron responses in humans during execution and observation of actions. Current Biology. 2010;20:1–7. doi: 10.1016/j.cub.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamel R, Fried I. Human intracranial recordings and cognitive neuroscience. Annual Review Psychology. 2012;63:16.1–16.27. doi: 10.1146/annurev-psych-120709-145401. [DOI] [PubMed] [Google Scholar]
- Mullan S, Penfield W. Illusions of comparative interpretation and emotion; production by epileptic discharge and by electrical stimulation in the temporal cortex. Archives of Neurology and Psychiatry. 1959;81:269–84. [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cognitive, Affective & Behavioral Neuroscience. 2003;3:207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Naccache L, Gaillard R, Adam C, et al. A direct intracranial record of emotions evoked by subliminal words. Proceedings of the National Academy of Sciences. 2005;102:7713–7. doi: 10.1073/pnas.0500542102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedenthal PM, Barsalou LW, Ric F, Krauth-Gruber S. Embodiment in the acquisition and use of emotion knowledge. In: Feldman Barrett L, Niedenthal PM, Winkielman P, editors. Emotion and consciousness. New York: Guilford; 2005. pp. 21–50. [Google Scholar]
- Ojemann JG, Ojemann GA, Lettich E. Neuronal activity related to faces and matching in human right nondominant temporal cortex. Brain. 1992;115:1–13. doi: 10.1093/brain/115.1.1. [DOI] [PubMed] [Google Scholar]
- Okun MS, Raju DV, Walter BL, et al. Pseudobulbar crying induced by stimulation in the region of the subthalamic nucleus. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75:921–3. doi: 10.1136/jnnp.2003.016485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: An integrative review of ERP findings. Biological Psychology. 2008;77:247–65. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowsky K, Desestret V, Ryvlin P, Coste S, Mauguiere F. Direct electrical stimulations of the temporal pole in human. Epileptic Disorders. 2002;4:S23–7. [PubMed] [Google Scholar]
- Oya H, Kawasaki H, Howard MA, Adolphs R. Electrophysiological responses in the human amygdala discriminate emotion categories of complex visual stimuli. Journal of Neuroscience. 2002;22:9502–12. doi: 10.1523/JNEUROSCI.22-21-09502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya H, Adolphs R, Kawasaki H, Bechara A, Damasio A, Howard MA. Electrophysiological correlates of reward prediction error recorded in the human prefrontal cortex. Proceedings of the National Academy of Sciences. 2005;102:8351–6. doi: 10.1073/pnas.0500899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. Neurologizing the psychology of affects: how appraisal-based constructivism and basic emotion theory can coexist. Perspectives in Psychological Science. 2007;2:281–96. doi: 10.1111/j.1745-6916.2007.00045.x. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci. 2010;11(11):773–83. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W. Some mechanisms of consciousness discovered during electrical stimulation of the brain. Proceedings of the National Academy of Sciences. 1958;44:51–66. doi: 10.1073/pnas.44.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Perot P. The brain’s record of auditory and visual experience. A Final Summary and Discussion. Brain. 1963;86:595–696. doi: 10.1093/brain/86.4.595. [DOI] [PubMed] [Google Scholar]
- Péron J, Biseul I, Leray E, et al. Subthalamic nucleus stimulation affects fear and sadness recognition in Parkinson's disease. Neuropsychology. 2010a;24:1–8. doi: 10.1037/a0017433. [DOI] [PubMed] [Google Scholar]
- Péron J, Grandjean D, Le Jeune F, et al. Recognition of emotional prosody is altered after subthalamic nucleus deep brain stimulation in Parkinson's disease. Neuropsychologia. 2010b;48:1053–62. doi: 10.1016/j.neuropsychologia.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Spinelli L, Seeck M, Vuilleumier P. Modulation of face processing by emotional expression and gaze direction during intracranial recordings in right fusiform cortex. Journal of Cognitive Neuroscience. 2010a;22:2086–107. doi: 10.1162/jocn.2009.21404. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Spinelli L, Seeck M, Vuilleumier P. Temporal precedence of emotion over attention modulations in the lateral amygdala: intracranial ERP evidence from a patient with temporal lobe epilepsy. Cognitive, Affective, & Behavioral Neuroscience. 2010b;10:83–93. doi: 10.3758/CABN.10.1.83. [DOI] [PubMed] [Google Scholar]
- Rømer Thomsen K, Lou HC, Joensson M, et al. Impact of emotion on consciousness: positive stimuli enhance conscious reportability. PLoS One. 2011;6:e18686. doi: 10.1371/journal.pone.0018686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA. From a psychological constructionist perspective. In: Zachar P, Ellis R, editors. Categorical versus Dimensional Models of Affect: A Seminar on the Theories of Panksepp and Russell. Amsterdam: John Benjamins Publishing; 2012. [Google Scholar]
- Sabolek M, Uttner I, Seitz K, Kraft E, Storch A. Stimulation dependent induction of fear and depression in deep brain stimulation: a case report. Journal of Medical Case Reports. 2009;3:9136. [Google Scholar]
- Satpute AB, Shu J, Weber J, Roy M, Ochsner K. The functional neural architecture of self-reports of affective experience. Biological Psychiatry. 2013;73(7):631–8. doi: 10.1016/j.biopsych.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, et al. Rapid amygdala gamma oscillations in response to fearful facial expressions. Neuropsychologia. 2011;49:612–7. doi: 10.1016/j.neuropsychologia.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Satow T, Usui K, Matsuhashi M, et al. Mirth and laughter arising from human temporal cortex. Journal of Neurology, Neurosurgery & Psychiatry. 2003;74:1004–5. doi: 10.1136/jnnp.74.7.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Delgado MR. Overlapping neural systems mediating extinction reversal and regulation of fear. Trends in Cognitive Sciences. 2010;14:268–76. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JJ, Janszky J, Woermann F, Tuxhorn I, Ebner A. Laughter and the mesial and lateral premotor cortex. Epilepsy & Behavior. 2006;8:773–5. doi: 10.1016/j.yebeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Schmolck H, Squire LR. Impaired perception of facial emotions following bilateral damage to the anterior temporal lobe. Neuropsychology. 2001;15:30–38. [PubMed] [Google Scholar]
- Schroeder U, Kuehler A, Hennenlotter A, et al. Facial expression recognition and subthalamic nucleus stimulation. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75:648–50. doi: 10.1136/jnnp.2003.019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Young AW, Calder AJ, Hellawell DJ, Aggleton JP, Johnson M. Impaired auditory recognition of fear and anger following bilateral amygdala lesions. Nature. 1997;385:254–57. doi: 10.1038/385254a0. [DOI] [PubMed] [Google Scholar]
- Selimbeyoglu A, Parvizi J. Electrical stimulation of the human brain: perceptual and behavioral phenomena reported in the old and new literature. Frontiers in Human Neuroscience. 2010;4:46. doi: 10.3389/fnhum.2010.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Lee GP, Fountas K, King DW, Jenkins PD. Intracranial stimulation study of lateralization of affect. Epilepsy & Behavior. 2006;8:534–41. doi: 10.1016/j.yebeh.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Sperli F, Spinelli L, Pollo C, Seeck M. Contralateral smile and laughter, but no mirth, induced by electrical stimulation of the cingulate cortex. Epilepsia. 2006;47:440–3. doi: 10.1111/j.1528-1167.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- Spielberg JM, Heller W, Miller GA. Hierarchical brain networks active in approach and avoidance goal pursuit. Frontiers in Human Neuroscience. 2013;7:Article 284, 1–15. doi: 10.3389/fnhum.2013.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan H, Schulze-Bonhage A, Pauli E, et al. Ictal pleasant sensations: cerebral localization and lateralization. Epilepsia. 2004;45:35–40. doi: 10.1111/j.0013-9580.2004.09303.x. [DOI] [PubMed] [Google Scholar]
- Tommasi G, Lanotte M, Albert U, et al. Transient acute depressive state induced by subthalamic region stimulation. Journal of the Neurological Sciences. 2008;273:135–8. doi: 10.1016/j.jns.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Kawasaki H, Oya H, Howard MA, Adolphs R. Decoding face information in time, frequency and space from direct intracranial recordings of the human brain. PLoS One. 2008;3:e3892. doi: 10.1371/journal.pone.0003892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buren JM. Sensory, motor, and autonomic effects of mesial temporal stimulation in man. Journal of Neurosurgery. 1961;18:273–88. doi: 10.3171/jns.1961.18.3.0273. [DOI] [PubMed] [Google Scholar]
- Vytal K, Hamann S. Neuroimaging support for discrete neural correlates of basic emotions: a voxel based meta-analysis. Journal of Cognitive Neuroscience. 2010;22:2864–85. doi: 10.1162/jocn.2009.21366. [DOI] [PubMed] [Google Scholar]
- Wager TD, Barrett LF, Bliss-Moreau E, et al. The Neuroimaging of Emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of Emotion. New York: The Guilford Press; 2008. pp. 249–71. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.