Abstract
Numerous studies have investigated the neural substrates supporting cognitive reappraisal, identifying the importance of cognitive control processes implemented by prefrontal cortex (PFC). This study examined how valence and attention affect the processes used for cognitive reappraisal by asking participants to passively view or to cognitively reappraise positive and negative images with full or divided attention. When participants simply viewed these images, results revealed few effects of valence or attention. However, when participants engaged in reappraisal, there was a robust effect of valence, with the reappraisal of negative relative to positive images associated with more widespread activation, including within regions of medial and lateral PFC. There also was an effect of attention, with more lateral PFC recruitment when regulating with full attention and more medial PFC recruitment when regulating with divided attention. Within two regions of medial PFC and one region of ventrolateral PFC, there was an interaction between valence and attention: in these regions, divided attention reduced activity during reappraisal of positive but not negative images. Critically, participants continued to report reappraisal success even during the Divided Attention condition. These results suggest multiple routes to successful cognitive reappraisal, depending upon image valence and the availability of attentional resources.
Keywords: affect, cognition, emotion, prefrontal, regulation, fMRI
INTRODUCTION
What are the building blocks of a life that flourishes and is subjectively ‘happy’? Happiness and emotional well-being are certainly high on most individuals’ lists of essential life goals (see Suh et al., 1998), and research has shown that intentional activity, such as consciously regulating emotions to make ourselves feel better (Lyubomirsky et al., 2005a), contribute to our happiness. Indeed, as suggested by Lyubomirsky et al. (2005b), ‘intentional activities [may] offer the best potential route to higher and sustainable levels of happiness' (p. 115).
Emotion regulation is one cognitive process that is an important part of psychological health and well-being (for a review, see Nyklíček et al., 2011), allowing individuals to flexibly assess and respond to situations in ways that permit them to minimize negative or maximize positive affect (Gross, 1998). Regulatory processes of emotion regulation can help attenuate inappropriate, unchecked or extreme emotional reactions that could be seen or felt as personally, socially or behaviorally unwanted or abnormal (Parrott, 1993; Cicchetti et al., 1995; Gross and Munoz, 1995; Mayer and Salovey, 1995; Paulhus et al., 1997).
There are a number of strategies that lead to successful emotion regulation via the up-regulation or down-regulation of an emotional response to a stimulus. Specifically, antecedent-focused strategies are those that are evoked as input is initially being processed, and response-focused strategies are those that act on the output from the system rather than its inputs (Gross, 1998). In this experiment, we focus on the antecedent-focused strategy of reappraisal, which has been defined as ‘changing the way we think in order to change the way we feel’ (Ochsner and Gross, 2004, p. 2). Individuals can use cognitive reappraisal to up-regulate or to down-regulate the intensity of an emotional response, and to influence the resultant physiological (e.g. Mauss et al., 2007), cognitive (e.g. Gross, 2003) or social outcomes (e.g. Gross, 2003) associated with the emotional reaction. It is one of the most effective means for emotion regulation (Gross and John, 2003), and it has been widely studied using behavioral and cognitive neuroscientific approaches (reviewed by Gross and Thompson, 2007).
Reappraisal may rely on many of the same cognitive control processes that are used to modify or inhibit other behavioral responses (Davidson and Irwin, 1999; Ochsner and Feldman Barrett, 2001; Kanske et al., 2011). Studies examining the effect of cognitive reappraisal on simultaneous or subsequent task performance have provided behavioral evidence for a role of cognitive control in reappraisal: actively reappraising emotions can disrupt performance on cognitively demanding tasks performed simultaneously or shortly after the reappraisal (Richards, 2004; Baumeister et al., 2007), suggesting that reappraisal may utilize, and thereby deplete, cognitive resources. Neuroimaging studies have further linked reappraisal to cognitive control processes. Cognitive reappraisal has been tied to activation in various regions of the prefrontal cortex (PFC), including the dorsolateral PFC, ventrolateral PFC, ventromedial PFC (often extending into the cingulate gyrus) and dorsomedial PFC. These regions have been implicated in working memory and selective attention, response selection and inhibition, monitoring processes and the reflection on affective states, respectively (e.g. Beauregard et al., 2001; Levesque et al., 2004; Ochsner et al., 2004; Kalisch et al., 2005, 2006; Phan et al., 2005; Urry et al., 2006; Eippert et al., 2007; Kim and Hamann, 2007). Not only are these regions recruited during cognitive reappraisal, but their recruitment is also correlated with participants’ success in regulating their emotions (e.g. Ochsner et al., 2002; Goldin et al., 2008; McRae et al., 2010).
Although much research points to a role for cognitive control in the reappraisal process, it is less clear how individuals implement these reappraisal processes when cognitive resources are taxed. Despite the fact that cognitive reappraisal processes are typically attention-demanding, there is evidence for successful reappraisal even in populations with depletion of cognitive resources (e.g. older adults; Urry and Gross, 2010) and in conditions where distractions are present (e.g. Leroy et al., 2012). This finding raises the question of whether depletion of cognitive resources leads to a potential emotion regulation ‘workaround’, so that effective reappraisal can still be realized in depleted conditions. This workaround could reflect a shift from a more demanding to a less demanding reappraisal strategy. For instance, evidence suggests that a distancing approach (detached reappraisal) is impaired in old age, but a re-framing approach (positive reappraisal) is enhanced (Shiota and Levenson, 2009). More generally, it could reflect multiple neural routes to the same reappraisal outcome, although evidence for such routes has not yet been demonstrated. These possibilities raise the question of whether or how the depletion of cognitive resources in younger adults affects the engagement of cognitive reappraisal processes. We address this question by asking participants either to devote their full attention to a cognitive reappraisal task or to divide their attention between a reappraisal task and an auditory-discrimination task while undergoing an functional magnetic resonance imaging (fMRI) scan.
We also address the possibility that the effect of divided attention on cognitive reappraisal processes may differ depending upon the valence of the information reappraised. Though most neuroimaging research has examined the reappraisal of responses to negative stimuli (e.g. Ochsner et al., 2002, 2004; Eippert et al., 2007; Goldin et al., 2008; Hayes et al., 2010; McRae et al., 2010), a few studies have also examined the cognitive reappraisal of positive stimuli (Beauregard et al., 2001; Kim and Hamann, 2007; Mak et al., 2009). The extant data suggest that regulation of negative pictures may recruit a more widespread network of frontal regions (including medial, lateral, orbitofrontal and cingulate cortex), whereas the regulation of positive stimuli may recruit a more concise network of frontal regions (including medial PFC). For example, Kim and Hamann (2007)—when comparing the up- and down-regulation of images to passively viewing them—found that the regulation of negative images activated the dorsomedial PFC, lateral PFC, anterior cingulate gyrus and orbitofrontal cortex, whereas the regulation of positive pictures activated the dorsomedial and orbital PFC. Similarly, Mak et al. (2009)—when comparing hedonic regulation to viewing of images—found that the regulation of negative pictures activated orbitofrontal cortex, anterior cingulate, lateral PFC, precuneus and middle occipital gyrus, whereas the regulation of positive images activated dorsomedial and dorsolateral PFC.
Some research suggests that, at least in young adults, negative or threatening stimuli may be processed more automatically (Vuilleumier et al., 2001; Gläscher and Adolphs, 2003; Kimura et al., 2004) or preferentially (Dijksterhuis and Aarts, 2003; Zhao and Li, 2006; De Martino et al., 2009) than neutral and/or positive stimuli. For instance, fearful faces are more likely to capture attention than happy faces (Bradley et al., 2000), and negative stimuli are associated with stronger sensory activity than are positive stimuli (for a review, see Kensinger, 2009), perhaps suggesting that they provide stronger bottom-up input. For further discussion, see Murphy and Isaacowitz (2008) for a meta-analysis of emotional memory and attention. We hypothesized that this prioritized processing of negative stimuli would lead participants to recruit additional cognitive control processes to regulate such stimuli; if participants prioritize negative stimuli, emotional responses may start faster (be more ‘full blown’ within the first 5 s), thus requiring more regulation. Although we expected to find some evidence for additional recruitment of such processes even when full attention could be devoted to the regulatory task, consistent with Kim and Hamann (2007) and Mak et al. (2009), we hypothesized that these valence effects would be exaggerated when participants were placed under cognitive load.
This study addressed these hypotheses by using fMRI to examine how image valence and the attention devoted toward the regulation task affected the neural processes recruited during successful cognitive reappraisal. Participants were presented with positive, negative or neutral images and either (i) passively viewed the stimuli (passive view) or (ii) attempted to increase or decrease their emotional reactions to the images (reappraisal). Half of the participants performed the reappraisal task with full attention, whereas half of the participants performed the task along with an auditory-discrimination task, thereby dividing their attention. We replicate prior findings, showing that reappraisal recruits both lateral and medial PFC regions, with a number of regions activated more for the reappraisal of negative than positive images. We additionally demonstrate a novel effect of attention, with divided attention leading to increased recruitment of medial PFC and reduced recruitment of lateral PFC. We further show that, in a few regions of PFC, the effect of divided attention depends upon the valence of the stimuli being reappraised: divided attention reduces the neural activity engaged during the regulation of positive but increases the activity during the reappraisal of negative stimuli.
METHODS
Participants
Thirty-nine volunteers participated in this study. All participants were native English speakers with normal or corrected-to-normal vision, and they reported no history of psychiatric, neurological or learning disorders. In addition, participants reported no history of psychiatric or psychoactive medication use. Participants received $75 for their participation. The data from five participants were not included in analyses due to excessive head motion in the scanner. The final sample included 34 participants (18 female and 16 male; aged 18–34 years, mean = 22.7 years). Of these, 16 adults (9 female) performed the regulation task with full attention devoted to the task, whereas 18 adults (9 female) completed the task with divided attention. Informed consent was obtained from all participants in a manner approved by Boston College and Massachusetts General Hospital Institutional Review Boards.
Materials
The cognitive reappraisal task was adapted from a previous study by Ochsner et al. (2004). Stimuli were 105 images selected from the International Affective Picture System (IAPS) database (Lang et al., 2008). The 105 images were presented across five scan runs of 21 images. Images in each run were selected to represent three valence categories, with 9 positive, 9 negative and 3 neutral images presented in each run, for a total of 45 positive, 45 negative and 15 neutral images. The negative and positive pictures were equated in normative arousal ratings (P = 0.11) and in absolute valence (i.e. distance from neutral valence; P = 0.61). Valence and arousal ratings from the IAPS database are reported on a scale from 1 to 9, such that 1 equates to a low rating on each dimension (i.e. very negative valence, low arousal), and 9 equates to a high rating on each dimension (i.e. very positive valence, high arousal). Negative images held a mean normative valence rating of 2.99 (s.d. = 0.85) and a mean arousal rating of 5.73 (s.d. = 0.59), and positive images received a mean normative valence rating of 7.15 (s.d. = 0.56) and a mean arousal rating of 5.55 (s.d. = 0.70). Negative and positive images were selected to be significantly more arousing than neutral images; neutral pictures received a mean normative valence rating of 5.26 (s.d. = 0.36) and a mean arousal rating of 3.63 (s.d. = 0.75). Images from each valence category did not differ significantly (all Ps > 0.50) in terms of ratings of visual complexity or general scene content (i.e. images containing people, animals, buildings or landscapes).
Procedure
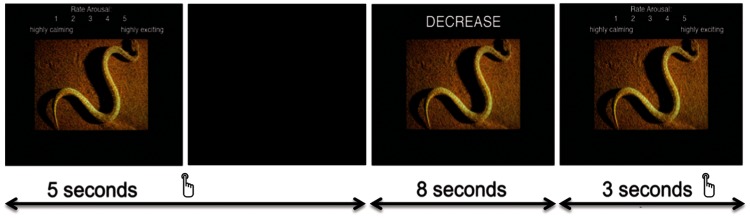
Participants first completed a short battery of cognitive tasks. They were then given instructions for the emotion regulation task (similar to those given by Kim and Hamann, 2007). Participants viewed emotional and neutral images and were instructed to either INCREASE or DECREASE the intensity of their emotional reaction to the image. In an additional control condition, participants were asked to VIEW emotional or neutral images without intentionally changing the intensity of their emotional reaction. For all conditions, the instructions included a computerized practice during which participants were familiarized with the task. This practice included a number of self-paced trials, example regulation strategies (similar to those presented to participants in Ochsner et al., 2004), and an actual-speed practice. Participants had the option to repeat any or all sections of the practice until they understood the instructions and were able to reliably come up with reappraisal strategies within the allotted time period. Each trial began with an image and arousal rating scale. Participants first rated their emotional arousal in response to the image on a scale from 1 (low emotional arousal) to 5 (high emotional arousal). They then had 5 s to enter their response for each image using an MRI-compatible button box. The rating scale remained on the screen until a response was entered, after which a blank black screen replaced it. Participants then viewed the images again for 8 s with one of the three regulation instructions (INCREASE, DECREASE, VIEW; Figure 1). During this period, participants were instructed to reappraise their original emotional reaction to the image in the direction indicated (or to VIEW the image without intentionally altering the intensity of their emotional reaction in the case of VIEW instructions). After the regulation period, participants were presented with the same arousal rating scale for a maximum of 3 s and provided a post-regulation rating of their perceived emotional arousal. Responses from the second presentation were compared to those at the first presentation to calculate a difference score representing the change in emotional reaction to each image as a function of the reappraisal attempt.
Fig. 1.
Participants were given 5 s to make an initial arousal rating (left frame). An instruction to INCREASE, DECREASE or VIEW was then presented with the image for 8 s (center frame). After this regulation period, participants had a maximum of 3 s to provide a post-regulation rating of emotional arousal (right frame).
After each trial, an inter-trial fixation cross was presented for a variable duration, ranging between 5 and 13 s, to provide jitter (Dale, 1999). Participants completed 105 trials: 30 INCREASE trials (15 negative, 15 positive), 30 DECREASE trials (15 negative, 15 positive) and 45 VIEW trials (15 negative, 15 positive, 15 neutral). Trials from each of these experimental conditions were presented in a pseudorandomized order across scanning runs, with no more than two trials of a given valence or condition presented consecutively. Each participant saw each image only once (i.e. in only one of the conditions).
Once participants had completed the full set of 105 regulation trials (one full attention participant only completed 84 trials) and exited the scanner, they then freely recalled as many of the images as they could remember; however, post-scan data will not be discussed here. Participants were debriefed as a method of ensuring task compliance.
Divided auditory attention paradigm
The divided attention participants underwent identical screening, instruction and scanning as outlined earlier, but with an additional task in the scanner. During each 8 s regulation phase, participants in the Divided Attention condition monitored a sound pattern that played throughout the phase and indicated, by button press, whenever the sound pattern changed. The sounds were pre-selected to ensure that the pattern changes could be detected while in the noisy MRI environment, yet these pattern changes were sufficiently difficult to distinguish that they had previously been shown to interfere with performance of a memory tasks (‘hard’ sounds adapted from Kensinger et al., 2003).
Image acquisition and preprocessing
Participants were scanned on a Siemens Avanto 1.5 T MR scanner at the Martinos Center for Biomedical Imaging in Charlestown, MA. Detailed anatomical data were acquired using a T1*-weighted MP-RAGE sequence, and functional images were acquired using a T2*-weighted echo-planar imaging sequence with the following parameters: TR = 2000 ms, TE = 40 ms, FOV = 200 mm and flip angle = 90°. Twenty-six axial-oblique slices, aligned in the plane along the AC-PC axis (with a 3.125 mm slice thickness and 20% skip between slices), were acquired in an interleaved fashion.
Preprocessing
Preprocessing and data analysis were conducted in SPM8 (Wellcome Department of Cognitive Neurology, London) implemented in MATLAB R2009b (The Mathworks, USA). Standard preprocessing was conducted on the functional data, including slice-timing correction, rigid-body motion correction, normalization to the Montreal Neurological Institute (MNI) template and spatial smoothing (using a 8 mm full-width half-maximum isotropic Gaussian kernel).
Block-design fMRI data analysis
We defined a successful trial as one in which the post-regulation arousal rating value was greater than (for INCREASE trials), less than (for DECREASE trials) or within one point of the pre-regulation arousal rating value (for VIEW trials). Therefore, a successful INCREASE trial was a post-regulation minus pre-regulation score from 1 to 4, a successful DECREASE trial was a score from −1 to −4 and a successful VIEW score was between −1 and 1. Participants were successful on 81.3% (s.e. = 1.9%) of trials in the Full Attention condition, and successful on 75.3% (s.e. = 2.0%) of trials in the Divided Attention condition. The leeway on the VIEW trials allowed for mild changes in experienced intensity in the VIEW condition that may occur even in the absence of any strategic ER strategy. All other trials were binned as unsuccessful. All analyses were additionally conducted with more conservative scoring, with successful VIEW trials defined as a change score of 0. Using this more conservative scoring method, participants were successful on 74.2% (s.e. = 2.7%) of trials in the Full Attention condition and 71.8% (s.e. = 2.9%) of trials in the Divided Attention condition. The far right column of Tables 1–3 reports whether regions remained active using this more conservative scoring. Out of a possible maximum arousal change of four points, the majority of successful reappraisal trials had a change of 1 or 2 points, whereas the majority of successful view trials had a change of 0 points. See Supplementary Figure S1 for a breakdown of the arousal change scores for successful trials.
Table 1.
Regions activating for negative > positive image regulation
| Region | Gyrus | Approximate Brodmann area (BA) | MNI coordinates (x, y, z) | TAL coordinates (x, y, z) | Cluster size (no. voxels) | Region active with more conservative scoring |
|---|---|---|---|---|---|---|
| Frontal lobe | Inferior frontal gyrus | 45 | −36, 28, 12 | −36, 28, 10 | 21 | Yes |
| – | – | 46 | 50, 42, 10 | 50, 41, 9 | 296a | Yes |
| – | Middle frontal gyrus | – | – | – | 296a | Yes |
| – | Middle frontal gyrus | 46 | 42, 20, 24 | 42, 20, 21 | 55 | Yes |
| – | Medial frontal gyrus | 10 | 6, 52, 8 | 8, 51, 5 | 48 | Yes |
| – | – | 8 | 52, 10, 46 | 51, 12, 42 | 66 | Yes |
| – | Precentral gyrus | 4 | 54, −10, 50 | 51, −12, 42 | 154 | Yes |
| – | – | 6 | −54, −4, 16 | −55, −3, 17 | 55 | Yes |
| – | – | 6 | −52, −6, 36 | −51, −4, 33 | 83 | Yes |
| – | Superior frontal gyrus | 9 | 36, 50, 34 | 36, 50, 29 | 41 | Yes |
| – | – | 8 | −22, 38, 46 | −22, 39, 40 | 40 | No |
| Temporal lobe | Superior temporal gyrus | 38 | −44, −10, −14 | −44, 10, −11 | 21 | Yes |
| – | – | 38 | 36, −2, −10 | 36, −2, −8 | 28 | Yes |
| – | Parahippocampal gyrus | 37 | 30, −46, −10 | 30, −45, −6 | 41 | Yes |
| Occipital lobe | Middle occipital gyrus | 19 | −42, −54, −8 | −42, −53, −4 | 30 | Yes |
| – | – | 19 | −26, −96, 20 | −28, −92, 21 | 225 | Yes |
| – | – | 37 | 38, −68, 2 | 38, −66, 5 | 1521a | Yes |
| – | Inferior occipital gyrus | – | – | – | 1521a | Yes |
| Cingulate gyrus | NA | 24/32 | 12, 4, 38 | 12, 6, 35 | 252 | Yes |
| Insula | NA | 13 | −36, 10, 12 | −36, 10, 11 | 40 | Yes |
| – | – | 13 | −26, −22, 14 | −26, −21, 14 | 529 | Yes |
| Putamen | NA | NA | 18, 0, 14 | 18, 1, 13 | 57 | No |
| – | – | – | 30, −20, 4 | 30, −19, 5 | 87 | Yes |
| Thalamus | NA | NA | 16, −20, 8 | 16, −19, 8 | 32 | No |
aCluster extends throughout these regions.
P = 0.005, k = 15.
Table 2.
Regions showing a Group × Valence interaction
| Region | Gyrus | Approximate BA | MNI coordinates (x, y, z) | TAL coordinates (x, y, z) | Cluster size (no. voxels) | Region active with more conservative scoring |
|---|---|---|---|---|---|---|
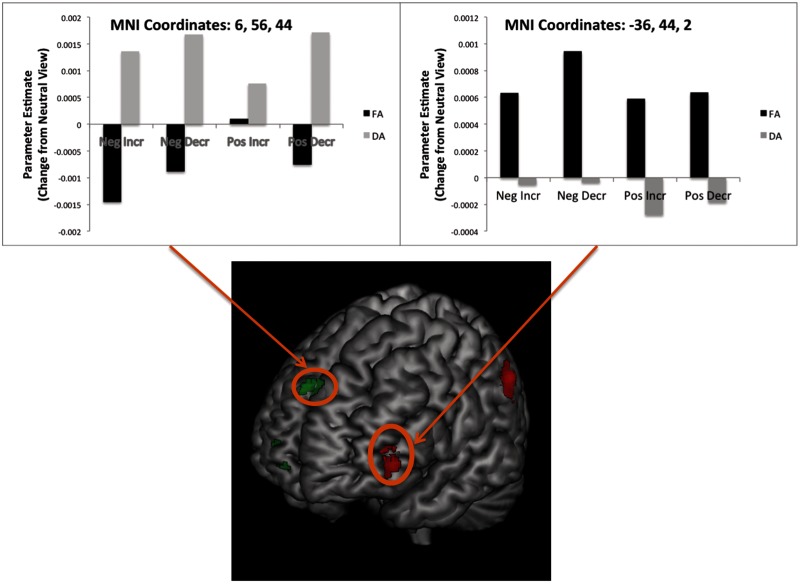
| Frontal lobe | Medial frontal gyrus | 10 | −14, 64, 8 | −14, 62, 4 | 40 | Yes |
| – | – | – | 12, 56, 6 | 12, 55, 3 | 35 | Yes |
| – | Inferior frontal gyrus | 47/11 | −26, 30, −6 | −26, 29, −6 | 53 | Yes |
| Temporal lobe | Fusiform gyrus | 36 | −44, −20, −14 | −44, −20, −11 | 17 | Yes |
| – | Middle temporal gyrus | 39 | 32, −62, 18 | 32, −59, 20 | 29 | Yes |
| Caudate | NA | NA | −2, 10, 2 | −2, 10, 1 | 18 | No |
P = 0.005, k = 15.
Table 3.
Regions showing a main effect of Group
| Region | Gyrus | Approximate BA | MNI coordinates (x, y, z) | TAL coordinates (x, y, z) | Cluster size (no. voxels) | Region active with more conservative scoring |
|---|---|---|---|---|---|---|
| Full Attention > Divided Attention | ||||||
| Frontal lobe | Inferior frontal gyrus | 10 | −36, 44, 2 | −36, 43, 0 | 274a | Yes |
| Middle frontal gyrus | 46 | 274a | Yes | |||
| Middle frontal gyrus | 6 | 24, −4, 46 | 24, −2, 42 | 51 | Yes | |
| Parietal lobe | Inferior parietal lobe | 40 | −52, −44, 32 | −51, −41, 32 | 320 | Yes |
| Caudate | NA | NA | −10, −8, 24 | −10, −7, 22 | 118 | Yes |
| Putamen | NA | NA | 22, −2, 18 | 22, −1, 17 | 134 | Yes |
| Thalamus | NA | NA | 8, −10, 20 | 8, −9, 19 | 29 | No |
| Insula | NA | 13 | −50, −24, 20 | −50, −22, 20 | 16 | No |
| Divided Attention > Full Attention | ||||||
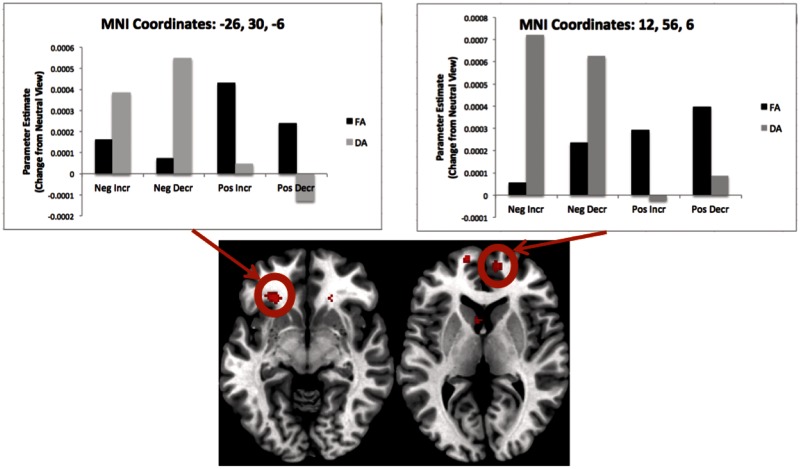
| Frontal lobe | Medial frontal gyrus | 9 | 6, 56, 44 | 6, 56, 38 | 56 | Yes |
| – | Paracentral lobule | 6 | 8, −30, 50 | 8, −27, 47 | 55a | Yes |
| – | Medial frontal gyrus | 55a | Yes | |||
| – | Superior frontal gyrus | 10 | 14, 66, −4 | 14, 64, −7 | 30 | Yes |
| – | – | 8 | 24, 26, 50 | 24, 27, 45 | 17 | No |
| Parietal lobe | Inferior parietal lobule | 7 | 28, −62, 32 | 28, −60, 32 | 823 | Yes |
| – | – | 13 | 44, −40, 24 | 44, −38, 24 | 31 | Yes |
| – | – | 40 | 44, −38, 36 | 44, −35, 35 | 32 | Yes |
| Temporal lobe | Middle temporal gyrus | 19 | 38, −62, 8 | 38, −60, 10 | 25 | No |
| – | Parahippocampal gyrus | 34 | −12, −4, −16 | −12, −5, −13 | 31 | Yes |
| – | – | 19 | −18, −50, −8 | −18, −49, −4 | 49 | Yes |
| – | – | 36 | 22, −40, −10 | 22, −39, −6 | 79a | Yes |
| – | Fusiform gyrus | 37 | 79a | Yes | ||
| Occipital lobe | Cuneus | 17 | −18, −68, 8 | −18, −66, 11 | 71 | Yes |
| – | – | 19 | −24, −88, 40 | −24, −83, 41 | 135 | Yes |
| – | – | 31 | 30, −74, 16 | 30, −71, 18 | 15 | Yes |
| – | – | 31 | 24, −80, 26 | 24, −76, 28 | 26 | Yes |
| – | – | 30 | 4, −76, 4 | 4, −73, 7 | 326a | Yes |
| – | Lingual gyrus | 17 | 326a | Yes | ||
| Hippocampus | NA | NA | 36, −10, −12 | 36, −10, −10 | 33 | Nob |
| Cingulate gyrus | NA | 30 | 8, −52, 14 | 8, −50, 15 | 33 | Yes |
| – | – | 31 | −18, −54, 36 | −18, −51, 36 | 73 | Yes |
| – | – | 24 | −8, 30, −8 | −8, 29, −8 | 50 | No |
| – | – | 32 | −16, 32, 18 | −16, 32, 15 | 17 | Yes |
| Thalamus | NA | NA | 16, −14, −2 | 16, −14, −1 | 27 | Yes |
aCluster extends throughout these regions.
bBut new hippocampal cluster at 38, −24, −16.
P = 0.005, k = 15.
The following seven successful trial types were modeled as regressors of interest: negative increase, negative decrease, negative view, positive increase, positive decrease, positive view and neutral view. Each of these trial types was modeled as an event with an 8 s time epoch, anchored to the onset of the reappraisal or maintenance phase (i.e. Figure 1, middle panel). An additional regressor of no interest modeled the trials during which participants were unsuccessful. Trials were concatenated across the five scan runs. To account for the breaks in scanning, separate linear trend regressors were included for each of the five scans.
To confirm that the divided attention task resulted in the additional recruitment of cognitive control regions, we conducted a contrast analysis comparing successful regulation to baseline in the full attention and divided attention participants. We then examined used a one-way analysis of variance (ANOVA) to examine the effect of attention group on this activity.
Our main interest was in contrast analyses comparing the regulation conditions to the neutral view condition (e.g. negative increase > neutral view); these contrasts controlled for activation due to image viewing and, in the case of the divided attention group, for activation related to performance of the auditory monitoring task. These contrast analyses generated image files that were used in a second-level ANOVA. At this second level, the analysis collapsed across the Direction of regulation (increase or decrease) and included the within-subject factor of Valence (positive regulation or negative regulation) and the between-subject factor of Group (full or divided attention). Including the additional factor of Direction of regulation did not change the regions that showed main or interactive effects of Valence or Group. We report as significant those regions revealed by this ANOVA to consist of at least 15 contiguous voxels that were all active at a threshold of P < 0.005; this voxel extent and probability was shown by Monte Carlo simulations to correct for multiple comparisons at P < 0.05 (Slotnick et al., 2003). Coordinates were converted from MNI to Talairach space using the Brett (mni2tal) transform (Brett et al., 2001). Within active clusters, beta weights for each condition were extracted using the REX ROI toolbox implemented within SPM8. These parameter estimates are plotted in graphs for select clusters.
RESULTS
MRI analysis results
Confirmation of cognitive control engagement with auditory discrimination task
A comparison of activity in the full and divided attention groups confirmed that the inclusion of a secondary task was sufficiently taxing to result in engagement of cognitive control regions. Numerous regions of the PFC, including the inferior frontal gyrus (MNI coordinates: 30, 28, −4; k = 663*; −32, 28, 6; k = 45), middle frontal gyrus (40, 28, 16; sub-cluster of inferior frontal gyrus cluster), anterior cingulate gyrus (−10, 16, 48; k = 21) and medial frontal gyrus (8, 28, 48; k = 40) were more active in the divided attention participants than in the full attention participants.
Replication of previous reappraisal findings
We sought to replicate prior findings regarding the engagement of medial and lateral PFC during successful reappraisal. Because most studies have examined the ability to decrease responses to negative stimuli, the first contrast compared decrease-negative to view-negative conditions in the full attention group. Consistent with prior research, this contrast revealed widespread activity in the medial PFC (MNI coordinates: 12, 26, 42; 6, 12, 56) and lateral PFC (MNI coordinates: 32, 56, −10; 38, 48, −12); all regions depicted in Figure 2A.
Fig. 2.
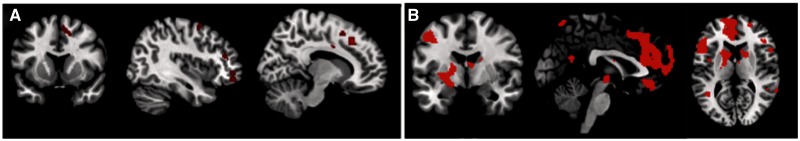
(A) Activated brain regions for the contrast of Decrease Negative > View Negative in the full attention condition. (B) Main effect of regulation, collapsed across valence and regulation direction in both full and divided attention.
The next analysis used an ANOVA to more generally examine the effect of reappraisal, collapsing across valence (Positive, Negative) and group (Full, Divided Attention). This and all further MRI analyses discussed used view-neutral as comparison conditions (e.g. decrease-negative > view-neutral). This analysis revealed activity in medial (MNI coordinates: −8, 20, 50; −8, 30, 38) and lateral (MNI coordinates: 48, 38, −6; 38, 54, 24; −38, 50, 6) PFC; see Figure 2B for all regions.
Effect of divided attention and valence on cognitive reappraisal processes
The main focus of this investigation was to identify how divided attention and valence would affect the processes engaged during successful cognitive reappraisal. Therefore, a 2 (Positive or Negative Valence) × 2 (Full or Divided Attention) ANOVA was conducted.
Effect of attention
A number of regions showed an effect of Group (Full or Divided Attention) in the 2 × 2 ANOVA discussed earlier (Figure 5). Results revealed greater activity in the lateral PFC in the Full Attention compared to the Divided Attention condition, as well as activity in the striatum and insula (see top panel of Table 3 for all regions). In the Divided Attention condition (Table 3; Figure 5), there was increased activity in the dorsomedial PFC (MNI: 6, 56, 44), anterior cingulate gyrus (MNI: −8, 30, −8) and orbitofrontal cortex (MNI: 14, 66, −4; see lower panel of Table 3 for all regions).
Fig. 5.
Regions showing activity for the contrast of Divided > Full (green) and Full > Divided (red). Graphs depict activity in demarcated clusters. These graphs reveal that lateral regions were preferentially engaged during the regulation of all images with full attention, but medial regions were preferentially engaged during the regulation of all images with divided attention.
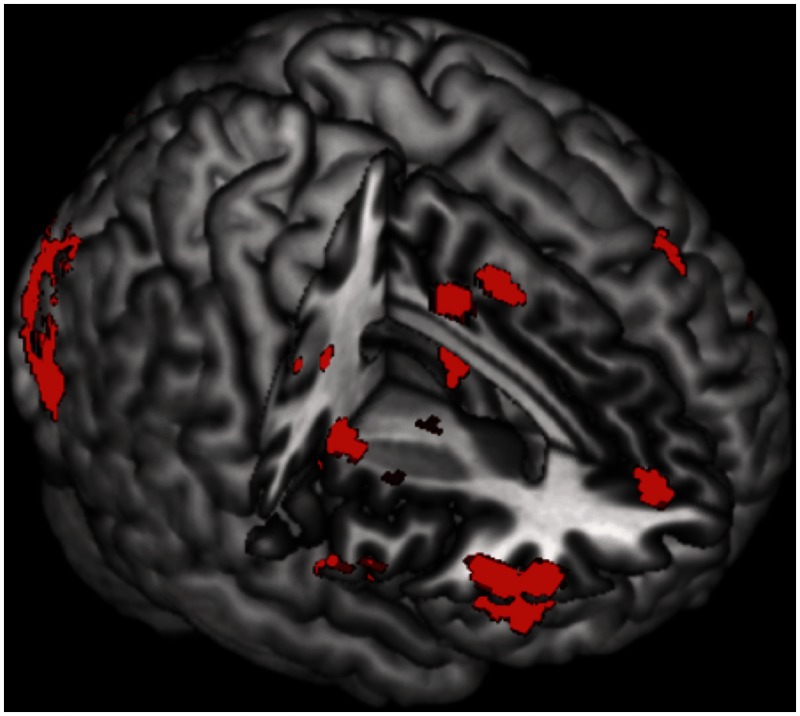
Effect of image valence
A number of regions showed an effect of Image Valence. T-contrasts revealed that almost all the regions that showed a main effect of valence, including widespread activity within the PFC and occipital cortex, showed stronger activity during the regulation of negative images than during the regulation of positive images (Table 1; Figure 3). Only a single region, within the posterior cingulate gyrus (MNI coordinates: 26, −46, 28; BA: 31; k = 35), showed greater activation during the regulation of positive images compared with negative images.
Fig. 3.
Regions showing greater activity during the reappraisal of negative images than during the reappraisal of positive images.
Interaction between attention and image valence
A few regions within the frontal and temporal lobes showed an interaction between Valence and Group. T-contrasts demonstrated that nearly all of this activity reflected a tendency for divided attention to be associated with less activity (relative to full attention) during the regulation of positive images but with more activity during the regulation of negative images (Table 2; Figure 4). Only a single region of activity, within the middle temporal gyrus (MNI coordinates: 32, −62, 17; BA: 39; k = 40), showed the opposite pattern.
Fig. 4.
Regions showing a Group × Valence interaction. Graphs depict activity in demarcated PFC clusters. These graphs reveal that these regions showed greater activity when regulating negative images in the Divided Attention condition, but greater activity when regulating positive images in the Full Attention condition.
Effect of divided attention and valence on viewing emotional images
To rule out the potential confound that some of the aforementioned effects may have been due to differences in viewing images of different valences (rather than to differences in reappraisal processes per se), we conducted an additional second-level ANOVA, also with the factors of Valence (positive, negative) and Attention (full, divided). However, the first-level contrast analyses used for this ANOVA compared the viewing of a valenced image to the viewing of a neutral image (e.g. negative view > neutral view). At the standard threshold, this ANOVA revealed no main effect of valence, and the three clusters of activity that showed an interaction between valence and attention (MNI: 28, −10, 26; 26, 14, 12; 28, −2, 10) did not overlap with the regions revealed in the previous ANOVA. Only one cluster was more active for the main effect of attention, specifically in the Full Attention > Divided Attention contrast (MNI: 26, −52, 34; BA: 31; k = 18).
DISCUSSION
Cognitive reappraisal is one of the most effective antecedent-focused cognitive regulation strategies. In this study, we demonstrated that the neural mechanisms supporting reappraisal differ depending upon the attention granted to the reappraisal task and the valence of the stimuli. Because analyses focus on trials with reported reappraisal success, and because both the full and divided attention participants reported success on the majority of trials, these results suggest multiple neural routes to successful cognitive reappraisal, depending upon image valence and the availability of attentional resources.
Across all conditions, we replicate prior findings regarding the role of lateral and medial PFC in the cognitive reappraisal process. Participants consistently engaged these regions during successful reappraisal. These results are consistent with prior proposals (Davidson and Irwin, 1999; Ochsner and Feldman Barrett, 2001; McRae et al., 2012) that cognitive reappraisal relies on many of the same cognitive processes used to control other types of thoughts and behaviors.
When attention was divided, however, this affected the particular PFC processes that were engaged. Most notably, while participants in the Full Attention condition disproportionately recruited lateral PFC in the service of cognitive reappraisal, participants in the Divided Attention condition disproportionately recruited medial PFC regions. In many of the PFC regions, this shift occurred for both positive and negative images (i.e. many of the regions that showed a main effect of Attention showed no Attention × Valence interaction). The reduced engagement of lateral PFC during divided attention suggests that these processes require cognitive resources not available when attention is divided. In contrast, individuals may implement the medial PFC processes effectively even when cognitive resources are taxed. This suggestion would be consistent with evidence suggesting that older adults, a population known to have impaired cognitive control (e.g. in tasks requiring the inhibition of irrelevant information; Prull et al., 2000; Salthouse et al., 2003; Hedden and Gabrieli, 2004), may rely more on medial PFC than lateral PFC processes for some forms of emotion processing and emotion regulation (Urry et al., 2006; Sakaki et al., 2013).
The lateral and medial PFC engagement in the full and divided attention groups, respectively, is particularly interesting given that all analyses focused on successful reappraisal trials. Thus, the two groups used different processes to impact subjective arousal ratings in the desired direction. These results suggest that there is more than one way to successfully reappraise an emotional response, and that the cognitive resources available may influence the mechanism participants use.
Because reappraisal is defined by the ability to reframe a situation to alter its affective meaning, there could be multiple routes to effect that change, and the availability of cognitive resources may alter the route that is dominant. This change in dominant route could reflect the fact that multiple neural routes could influence an affective response. The change in dominant route could also reflect changes in the strategy used to elicit reappraisal. When participants are placed under cognitive load, they may use different, less cognitively demanding strategies, to change their affective response. For instance, they may use detached reappraisal rather than positive reappraisal to decrease their negative affect, as the former is thought to be less cognitively demanding (Shiota and Levenson, 2009). The different neural correlates could therefore reflect engagement of different strategies to evoke the same regulatory outcome. Future research, asking participants to report their strategy use on a trial-by-trial basis, could examine whether different reappraisal strategies are associated with different neural signatures.
The present results also reveal an interesting effect of valence on the processes engaged by reappraisal, and further suggest that the effect of valence may depend upon the cognitive resources available. Participants in both the Full and Divided Attention conditions recruited more PFC and occipital processes during the reappraisal of negative images than during the reappraisal of positive images. This enhanced neural engagement during the reappraisal of negative images occurred when participants devoted full attention toward the reappraisal of the images, consistent with two prior studies (Kim and Hamann, 2007; Mak et al., 2009). One possible explanation for this finding is that, because the processing of negative stimuli is often prioritized by young adults (e.g. Öhman et al., 2001; Charles et al., 2003), and because negative stimuli are often processed with more sensory detail than positive stimuli (e.g. Kensinger et al., 2007; Mickley and Kensinger, 2008), negative stimuli may create stronger representations than positive stimuli, thus requiring additional processes for the successful cognitive reappraisal of those representations. This imbalance between the processes required to reappraise negative and positive stimuli may increase with task demands, as evidenced by the larger effects of valence in the participants in the divided attention condition.
In young adults, emotion regulation relies on PFC processes engaged during tasks that rely on regions implicated in cognitive control. This study replicates those findings but reveals additional complexity in the way PFC regions are recruited. Specifically, this study demonstrates three different patterns of emotion-regulation response within the PFC. Within many lateral PFC and medial PFC/ACC regions (Figure 5), there was only an effect of attention, with lateral PFC recruited when full attention could be granted toward the regulation task and dorsomedial PFC and ACC regions recruited when resources were taxed by divided attention. These regions showed the same pattern for negative and positive stimuli. Within other lateral PFC and medial PFC/ACC regions (Figure 3), however, there was only a main effect of valence, with stronger activity for negative than positive stimuli. Within a small set of PFC regions, two medial and one lateral (Figure 4; Table 2), there was an interaction between attention and valence, such that divided attention was associated with increased activity during the reappraisal of negative images but with decreased activity during the reappraisal of positive images.
Two limitations of this study are noteworthy. First, we cannot rule out that, rather than using a different mechanism to achieve cognitive reappraisal, the DA group may have used another strategy. Although one such strategy would be distraction, their reliance on this strategy seems unlikely for a few reasons. First, the literature on distraction (i.e. Anderson et al., 2004; Kalisch et al., 2006) suggests that it recruits left-lateralized PFC regions, not the medial PFC regions disproportionately recruited by the DA group. Second, upon debriefing, all participants reported that they were able to obey task instructions a high percentage of the time, suggesting that they were not regularly engaging in a non-instructed strategy. Finally, while it is clear how distraction would help to decrease emotional reactions, it is less clear how it would similarly enable successful performance on INCREASE trials. Because this study combines instances of increasing and decreasing affective responses, distraction seems an unlikely strategy to be consistently effective. A more tenable alternative is that participants in the DA group may have relied more on shifts of attention during scene viewing than participants in the FA group. For instance, when asked to downregulate their emotions, participants in the DA group may have shifted their attention away from the emotional portions of the scenes (see Van Reekum et al., 2007 for evidence of such gaze shifts with instructions to reappraise) rather than remaining focused on those portions and using cognitive processes to change the way they feel. This possibility could be examined in future research by simultaneously measuring eye gaze and neural activity during both Full and Divided Attention conditions, or by comparing the neural activity engaged when participants are instructed to use cognitive reappraisal or instructed to use selective attention to regulate their emotions.
A second limitation is that the present behavioral results could be susceptible to demand characteristics. Because participants were aware they were being instructed to regulate emotion, they could have selected ascending or descending numbers on the scale to fit with the regulation instructions. Because it would take some effort to hold the initial ratings in mind for the 8 s duration of the regulatory period, because accuracy rates were below ceiling, and because no participants reported inflating their success on the regulation trials, demand characteristics may have played a relatively minor role. However, we cannot rule out that our analyses included some trials that were binned as successful not because regulation was achieved but because demand characteristics influenced participants’ ratings.
Despite these limitations, these findings reveal the complexity of the PFC processes implemented in the service of emotion regulation and emphasize that not only are lateral and medial distinctions important, but that even among lateral or medial regions, the specific roles of the regions can depend upon both the valence of the stimuli reappraised and the attentional resources available to implement the reappraisal. It will be interesting for future research to assess whether the reappraisal processes engaged by young adults in the Divided Attention condition parallel those used by older adults, who often experience relative depletion of cognitive resources as compared with younger adults. Future research could also examine how dividing attention in older adults, who already have depleted resources, may further affect the behavioral and neural signatures of reappraisal.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
We thank Eunice Lee, Kristin Hricko, Keely Muscatell and Stephanie Carpenter for assistance with data collection and data management. This research was carried out in whole or in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Regional Resource supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health. This work was supported by grants from the National Institute of Mental Health (MH080833 to E.A.K.) and the Dana Foundation (to E.A.K.).
REFERENCES
- Anderson MC, Ochsner KN, Kuhl B, et al. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–5. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Vohs KD, Tice DM. The strength model of self-control. Current Directions in Psychological Science. 2007;16(6):351–5. [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21:1–6. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Millar NH. Covert and overt orienting of attention to emotional faces in anxiety. Cognition & Emotion. 2000;14(6):789–808. [Google Scholar]
- Brett M, Christoff K, Cusack R, Lancaster J. Using the talairach atlas with the MNI template. NeuroImage. 2001;13:S85. [Google Scholar]
- Charles ST, Mather M, Carstensen LL. Aging and emotional memory: the forgettable nature of negative images for older adults. Journal of Experimental Psychology: General. 2003;132:310–24. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Ackerman BP, Izard CE. Emotions and emotion regulation in developmental psychopathology. Development and Psychopathology. 1995;7:1–10. [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–14. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- De Martino B, Kalisch R, Rees G, Dolan RJ. Enhanced processing of threat stimuli under limited attentional resources. Cerebral Cortex. 2009;19(1):127–33. doi: 10.1093/cercor/bhn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijksterhuis A, Aarts H. On wildebeests and humans: the preferential detection of negative stimuli. Psychological Science. 2003;14(1):14–8. doi: 10.1111/1467-9280.t01-1-01412. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28(5):409–23. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. The Journal of Neuroscience. 2003;23(32):10274–82. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63(6):577. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: an integrative review. Review of General Psychology. 1998;2(3):271–99. [Google Scholar]
- Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2003;39(3):281–91. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John O. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–62. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Munoz RF. Emotion regulation and mental health. Clinical Psychology: Science and Practice. 1995;2:151–64. [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: conceptual foundations. Handbook of Emotion Regulation. 2007;3:24. [Google Scholar]
- Hayes JP, Morey RA, Petty CM, et al. Staying cool when things get hot: emotion regulation modulates neural mechanisms of memory encoding. Frontiers in Human Neuroscience. 2010;4:230. doi: 10.3389/fnhum.2010.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nature Reviews Neuroscience. 2004;5(2):87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, Dolan RJ. Levels of appraisal: a medial prefrontal role in high-level appraisal of emotional material. NeuroImage. 2006;30(4):1458–66. doi: 10.1016/j.neuroimage.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, et al. Anxiety reduction through detachment: subjective, physiological, and neural effects. Journal of Cognitive Neuroscience. 2005;17(6):874–83. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Herrmann K, Dolan RJ. Neural correlates of self-distraction from anxiety and a process model of cognitive emotion regulation. Journal of Cognitive Neuroscience. 2006;18(8):1266–76. doi: 10.1162/jocn.2006.18.8.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Bongers A, Wessa M. How to regulate emotion? neural networks for reappraisal and distraction. Cerebral Cortex. 2011;21(6):1379–88. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Remembering the details: effects of emotion. Emotion Review. 2009;1(2):99–113. doi: 10.1177/1754073908100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Clarke RJ, Corkin S. What neural correlates underlie successful encoding and retrieval? A functional magnetic resonance imaging study using a divided attention paradigm. The Journal of Neuroscience. 2003;23(6):2407–15. doi: 10.1523/JNEUROSCI.23-06-02407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity in young and older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2007;62(4):208–15. doi: 10.1093/geronb/62.4.p208. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19:776–98. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Yoshino A, Takahashi Y, Nomura S. Interhemispheric difference in emotional response without awareness. Physiology & Behavior. 2004;82(4):727–31. doi: 10.1016/j.physbeh.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. 2008. Technical Report A-8. University of Florida, Gainesville, FL. [Google Scholar]
- Leroy V, Grégoire J, Magen E, Gross JJ, Mikolajczak M. Lead me not into temptation: using cognitive reappraisal to reduce goal inconsistent behavior. PloS One. 2012;7(7):e39493. doi: 10.1371/journal.pone.0039493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque J, Joanette Y, Mensour B, et al. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–9. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, King L, Diener E. The benefits of frequent positive affect: does happiness lead to success? Psychological Bulletin. 2005a;131(6):803. doi: 10.1037/0033-2909.131.6.803. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, Sheldon KM, Schkade D. Pursuing happiness: the architecture of sustainable change. Review of General Psychology. 2005b;9(2):111. [Google Scholar]
- Mak AK, Hu ZG, Zhang JX, Xiao ZW, Lee T. Neural correlates of regulation of positive and negative emotions: an fMRI study. Neuroscience Letters. 2009;457(2):101–6. doi: 10.1016/j.neulet.2009.03.094. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Cook CL, Cheng JY, Gross JJ. Individual differences in cognitive reappraisal: experiential and physiological responses to an anger provocation. International Journal of Psychophysiology. 2007;66(2):116–24. doi: 10.1016/j.ijpsycho.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Salovey P. Emotional intelligence and the construction and regulation of feelings. Applied and Preventive Psychology. 1995;4:197–208. [Google Scholar]
- McRae K, Gross JJ, Weber J, et al. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience. 2010;22(2):248–62. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley KR, Kensinger EA. Emotional valence influences the neural correlates associated with remembering and knowing. Cognitive, Affective, & Behavioral Neuroscience. 2008;8(2):143–52. doi: 10.3758/cabn.8.2.143. [DOI] [PubMed] [Google Scholar]
- Murphy NA, Isaacowitz DM. Preferences for emotional information in older and younger adults: a meta-analysis of memory and attention tasks. Psychology and Aging. 2008;23(2):263–86. doi: 10.1037/0882-7974.23.2.263. [DOI] [PubMed] [Google Scholar]
- Nyklíček I, Vingerhoets A, Zeelenberg M. Emotion regulation and well-being: a view from different angles. In Emotion Regulation and Well-Being. New York: Springer; 2011. pp. 1–9. [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Feldman Barrett L. A multiprocess perspective on the neuroscience of emotion. In: Mayne TJ, Bonanno GA, editors. Emotions: Current Issues and Future Directions. New York, NY: The Guilford Press; 2001. pp. 38–81. [Google Scholar]
- Ochsner KN, Gross JJ. Think makes it so: a social cognitive neuroscience approach to emotion regulation. In: Baumeister R, Vohs K, editors. The Handbook of Self-regulation. NY: Guilford Press; 2004. pp. 221–5. [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130(3):466. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Parrott WG. Beyond hedonism: motives for inhibiting good moods and for maintaining bad moods. In: Wegner DM, Pennebaker JW, editors. Handbook of Mental Control. Englewood Cliffs, NJ: Prentice Hall; 1993. pp. 278–308. [Google Scholar]
- Paulhus DL, Fridhandler B, Hayes S. Psychological defense: contemporary theory and research. In: Hogan R, Johnson J, Briggs S, editors. Handbook of Personality Psychology. San Diego, CA: Academic Press; 1997. pp. 543–79. [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57(3):210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Prull MW, Gabrieli JDE, Bunge SA. Age-related changes in memory: a cognitive neuroscience perspective. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 2nd edn. Mahwah, NJ: Erlbaum; 2000. pp. 91–153. [Google Scholar]
- Richards JM. The cognitive consequences of concealing feelings. Current Directions in Psychological Science. 2004;13(4):131–4. [Google Scholar]
- Sakaki M, Nga L, Mather M. Amygdala functional connectivity with medial prefrontal cortex at rest predicts the positivity effect in older adults' memory. Journal of Cognitive Neuroscience. 2013;25(8):1206–24. doi: 10.1162/jocn_a_00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. Journal of Experimental Psychology: General. 2003;132(4):566. doi: 10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- Shiota MN, Levenson RW. Effects of aging on experimentally instructed detached reappraisal, positive reappraisal, and emotional behavior suppression. Psychology and Aging. 2009;24(4):890. doi: 10.1037/a0017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Suh E, Diener E, Oishi S, Triandis HC. The shifting basis of life satisfaction: judgments across cultures: emotions versus norms. Journal of Personality and Social Psychology. 1998;74:482–93. [Google Scholar]
- Urry HL, Gross JJ. Emotion regulation in older age. Current Directions in Psychological Science. 2010;19(6):352–7. [Google Scholar]
- Urry HL, Van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience. 2006;26(16):4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reekum CM, Johnstone T, Urry HL, et al. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. NeuroImage. 2007;36(3):1041–55. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30(3):829–41. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Zhao L, Li J. Visual mismatch negativity elicited by facial expressions under non-attentional condition. Neuroscience Letters. 2006;410(2):126. doi: 10.1016/j.neulet.2006.09.081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.