Abstract
Behavioral studies suggest a relationship between autobiographical memory, rumination and depression. The objective of this study was to determine whether remitted depressed patients show alterations in connectivity of the posterior cingulate cortex (PCC, a node in the default mode network) with the parahippocampal gyri (PHG, a region associated with autobiographical memory) while intensively recalling negative memories and whether this is related to daily life symptoms and to the further course of depression. Sad mood was induced with keywords of personal negative life events in participants with remitted depression (n = 29) and matched healthy controls (n = 29) during functional magnetic resonance imaging. Additionally, daily life assessments of mood and rumination and a 6-month follow-up were conducted. Remitted depressed participants showed greater connectivity than healthy controls of the PCC with the PHG, which was even stronger in patients with more previous episodes. Furthermore, patients with increased PCC–PHG connectivity showed a sadder mood and more rumination in daily life and a worsening of rumination and depression scores during follow-up. A relationship of negative autobiographical memory processing, rumination, sad mood and depression on a neural level seems likely. The identified increased connectivity probably indicates a ‘scar’ of recurrent depression and may represent a prognostic factor for future depression.
Keywords: depression, parahippocampal gyri, autobiographical memory, default mode network, rumination
INTRODUCTION
Major depressive disorder is a mental disorder characterized by lasting pervasive feelings of low mood, guilt and worthlessness. One factor that has been linked to cognitive vulnerability in depression is rumination—repeatedly thinking about one’s negative emotional state and its possible causes and consequences (Nolen-Hoeksema et al., 2008; Huffziger et al., 2009). According to the perseverative cognition hypothesis by Brosschot and colleagues (Brosschot et al., 2005), rumination is a mediator between stressors and prolonged stress-related processes by entailing an ongoing mental representation of the stressor (cf. Huffziger et al., 2013). Such stressors could be negative life events, that is, autobiographical experiences and related attention processes, which play an important role in depression (Sumner et al., 2010; Whalley et al., 2012) and rumination (Watson et al., 2012).
Emotions play a major formative role in episodic memory (see Dere et al., 2010 for an overview) and particularly in autobiographical memory (Williams and Broadbent, 1986), the part of episodic memory which is highly self-referential. Therefore, it is not surprising that emotional memories are generally remembered more often than neutral memories (Howes et al., 1993; Moritz et al., 2005). Positive autobiographical memories contain more sensory and contextual details than neutral or negative memories (D'Argembeau et al., 2003), whereas negative autobiographical memories, which seem to be ‘more central to identity’ in depressive patients (Watson, et al., 2012), appear to be more salient and remembered in a more general way, possibly sustained and boosted by rumination.
Rumination has also been linked to an increased activity in the brain’s default mode network (DMN; Berman et al., 2011; Hamilton et al., 2011). The DMN is an activity pattern of the brain, presumably representing self-referential processes (Whitfield-Gabrieli et al., 2011). Major brain regions involved are the posterior cingulate cortex (PCC), the medial prefrontal cortex and the angular gyri. Increased connectivity and activity in the DMN have been reported in depressed individuals (Greicius et al., 2007; Sheline et al., 2009, 2010; Berman et al., 2011; Li et al., 2013). Recently, Zhu and colleagues (2012) described two dissociated DMNs in depressive patients in comparison with healthy controls, one of increased functional connectivity between the medial prefrontal cortex and anterior cingulate cortex and the other one posterior, including PCC and precuneus, showing decreased connectivity. Although these findings are partially inconsistent, reporting both increased and decreased connectivity, the overall conclusion is that altered DMN connectivity and activity seem to occur in depression and may, for example, predict future relapses (Farb et al., 2011) or help to classify participants with resting state functional connectivity data as depressed or non-depressed (Zeng et al., 2012).
Interestingly, one of the four best regions for discriminating between depressed and healthy participants in the aforementioned analyses by Zeng and colleagues (2012) was the parahippocampal gyrus (PHG) which showed, among others, altered connectivity to the PCC and the anterior cingulate cortex in patients when compared with controls.
Furthermore, Cooney and colleagues found greater activity in the PHG during rumination versus abstract distraction in depressive patients in contrast to healthy controls (Cooney et al., 2010). Moreover, the posterior default mode network seems to recruit among others the parahippocampal gyri during episodic memory retrieval (Sestieri et al., 2011). These findings could implicate a connection between DMN aberrations in depressed patients, rumination and autobiographical memory processes. In general, autobiographical memory pathways in the brain seem to include the PHG. For example, patients with transient epileptic amnesia, displaying specific autobiographical memory problems, show decreased activity in the posterior PHG (Milton et al., 2012). Furthermore, a recent meta-analysis showed that the left posterior parahippocampal gyrus was more likely to be recruited during recall or imagination of personal events compared with recognition tasks (Viard et al., 2012). In a further quantitative meta-analysis, Spreng and colleagues demonstrated a strong overlap of the autobiographical brain network and the DMN, particularly in the PHG (Spreng et al., 2009). Interestingly, increased gray matter volume in the parahippocampal gyri was found in meditators (Leung et al., 2013), whereas the cortex was thinner in this area in late life depressive patients who did not respond to psychotherapy (Mackin et al., 2012).
Summing up, autobiographical memory seems to be linked to the DMN, the PHG and to rumination. However, the role of autobiographical memory processes, their neurobiological underpinning and interaction with the DMN and their relation to daily life experiences in depression are not yet fully understood. Recently, Hamilton and colleagues proposed that dysfunctional interactions between different neural networks could be an important aspect in understanding depressive pathology (Hamilton et al., 2013). They argue that an altered inter-connection between DMN and task-positive networks like the executive or the salience network might contribute to the pathophysiology of depression. Following this argument, it is an interesting issue whether in depression the DMN shows altered connections to structures associated with autobiographical memory. Therefore, in this study, we address the question whether the PHG are more strongly connected with the PCC as a core region of the DMN during a negative affective state induced by recalling and immersing negative autobiographical life events in patients with remitted depression compared with healthy controls. This induction procedure should increase self-referential processes and therefore increase DMN connectivity.
Remitted patients were chosen as we expect the altered connectivity to be vulnerability-related and therefore it should be present even when the criteria of a major depressive episode are currently not fulfilled. If a neural signature of autobiographical memory processing is indicative of vulnerability, it should be related to both present and future depressive symptoms and rumination. We, therefore, examined whether connectivity between PCC and PHG is related to daily life measures of mood and rumination and predicts changes in depression and rumination scores in a 6-month follow-up.
METHODS
Participants
Participants were 29 remitted depressed patients (rMDD) with at least two previous major depressive episodes (n = 27) or a previous chronic major depressive episode of at least 2 years duration (n = 2) according to DSM-IV and 29 healthy controls (HC), who were individually matched to the patients by age, sex and education level. All patients had to be in a state of partial or full remission, that is they, did not fulfill the criteria of a major depressive episode according to DSM-IV for at least the previous 2 months. Participants were recruited using online (e.g. on the homepage of the Central Institute of Mental Health) and newspaper call outs. The study initially included 64 individuals aged between 26 and 55 years; however, six cases (three out of each group) were excluded from analyses due to altered anatomical or other physiological parameters (head motion exclusion criteria: rotation >1.5°, translation >2 mm). One patient fulfilled criteria for a co-morbid current agoraphobia and another patient for a co-morbid current social phobia. A detailed sample description is available in Table 1.
Table 1.
Descriptive and psychometric variables of the depression group (rMDD) and the control group (HC) for the time points t1 and follow-up 6 months later (t2)
| Variable | Mean ± SD |
P-value | |
|---|---|---|---|
| rMDD | HC | ||
| n | 29 | 29 | |
| sex (female/male) | 20/9 | 21/8 | 0.842a |
| age (years) | 45.55 ± 7.45 | 44.24 ± 8.09 | 0.524b |
| education (CSE/high school diploma/A levels) | 4/7/18 | 2/8/19 | 0.684a |
| age of illness onset (years) | 23.14 ± 11.36 | ||
| number of depressive episodes | 3.96 ± 2.22 | ||
| previous inpatient treatment | 72% | ||
| current psychotropic medication | 24% | ||
| current psychotherapy | 28% | ||
| BDI II t1 | 10.14 ± 8.35 | 3.32 ± 4.04 | 0.001b |
| BDI II t2 | 10.83 ± 10.18 | 4.14 ± 6.37 | 0.002b |
| MADRS t1 | 5.83 ± 5.29 | 1.38 ± 2.44 | 0.001b |
| MADRS t2 | 7.62 ± 7.48 | 2.38 ± 5.32 | 0.002b |
| dep-score t1 (z) | 0.51 ± 1.11 | −0.46 ± 0.49 | 0.001b |
| dep-score t2 (z) | 0.35 ± 1.06 | −0.40 ± 0.70 | 0.002b |
| RRS-R t1 | 10.66 ± 3.59 | 8.69 ± 3.40 | 0.037b |
| RRS-R t2 | 10.52 ± 3.09 | 9.00 ± 3.17 | 0.070b |
| RRS-B t1 | 10.97 ± 3.96 | 8.07 ± 2.52 | 0.002b |
| RRS-B t2 | 10.72 ± 4.22 | 8.00 ± 3.01 | 0.006b |
| daily rumination (mean) | 1.36 ± 1.25 | 0.86 ± 0.90 | 0.083b |
| daily sadness (mean) | 0.95 ± 1.23 | 0.41 ± 0.71 | 0.075b |
aχ test
btwo sample t-test
CSE: Certificate of Secondary Education, 8 years
BDI II: Beck Depression Inventory revised, self-rated
MADRS: Montgomery and Asberg Depression Rating Scale, rated by a trained clinical psychologist
dep-score: z-standardized sum score of the BDI II and MADRS scores
RRS, Ruminative Response Scale; R: reflection subscale, B: brooding subscale
Exclusion criteria for both groups were bipolar and psychotic disorders, substance dependence, current substance abuse, generalized anxiety disorder, current obsessive-compulsive, post-traumatic stress and eating disorders according to DSM-IV as well as contraindications for the functional magnetic resonance imaging (fMRI). Psychopathology-related in- and exclusion criteria were assessed by a trained clinical psychologist with the Structured Clinical Interview for DSM-IV axis I (SCID; Wittchen et al., 1997) applied in an individual session.
The study was approved by the local ethics committee of the University of Heidelberg. All participants gave written informed consent after complete description of the study.
Interview-, questionnaire-based and daily life measures
At baseline (t1), depressive symptoms during the previous 2 weeks were assessed with the self-rated Beck Depression Inventory II-Revised (BDI II; Beck et al., 1996; German version: Hautzinger et al., 2006) and the Montgomery and Asberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979; German version: Schmidtke et al., 1988) rated by a trained clinical psychologist. As both measures were highly correlated (r = 0.73) and to increase reliability, a mean score of z-standardized sum scores of the BDI II and MADRS was created. Trait rumination in response to depressed mood was assessed with the two five-item subscales brooding and reflection of the Ruminative Response Scale (RRS; Treynor et al., 2003; German version: Huffziger and Kühner, 2012).
Daily life variables were measured using ambulatory assessment (Trull and Ebner-Priemer, 2013) which was conducted over two consecutive weekdays with 10 pseudo-randomized assessments per day using personal digital assistants (PDAs; Palm Tungsten E2, Palm Inc.). At each subjective assessment, which was indicated by a beep, participants rated momentary mood and ruminative self-focus (Huffziger et al., 2013). Sadness was assessed with the item “At the moment I feel sad”. Item scores ranged from 0 (not at all) to 6 (very much). Momentary ruminative self-focus was assessed with the two items “At the moment, I am thinking about my problems” and “At the moment, I am thinking about my feelings” which were averaged (Moberly and Watkins, 2008). These two items were rated on a scale from 0 (not at all) to 7 (very much). For this analyses, both sadness and ruminative self-focus scores were aggregated per person over 2 days. Compliance with the ambulatory assessment was high in both groups (percentages of responded assessments rMDD: 93%, HC: 94%). Patients responded on average to 18.5 assessments (SD = 1.4, range: 15–20), control participants to 18.8 assessments (SD = 1.6, range 14–20).
After 6 months (t2), a follow-up on depressive symptoms (MADRS, BDI II) and rumination (RRS) was conducted via telephone interviews and questionnaires. None of the participants dropped out between t1 and t2.
Functional magnetic resonance imaging
The fMRI session used built-in goggles and the Presentation software package from Neurobehavioral Systems Inc. The procedure was carried out within 2 weeks after the SCID interview and the ambulatory assessment. Every participant underwent six phases, each of which was 4.5 min: two resting states, two sad mood inductions, one rumination phase and one distraction phase (the order of the rumination and distraction phases were counterbalanced across participants). During the resting state phases, participants were told to keep their eyes open and background pink noise was presented. During the sad mood induction phase, key words to remind participants of personal negative life events and background sad music were presented. The rumination and distraction induction phases had corresponding induction statements selected from Nolen-Hoeksema and Morrow (1993) and did not include pink noise or music.
The individually matched patients and controls were scanned in a timely manner. Positive and negative affect was assessed by the Positive and Negative Affect Scale (PANAS; Watson et al., 1988; German version: Krohne et al., 1996) before and after each phase with a built-in key-pad. Participants also rated how well they could concentrate on the negative life events (presented in the mood induction phase, see below) at the end of the session. An exemplary schematic overview of the fMRI paradigm is given in Figure 1.
Fig. 1.
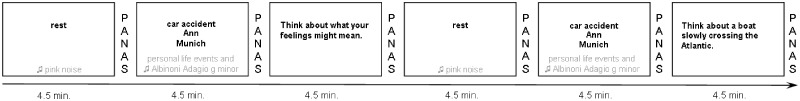
Exemplary scheme of the fMRI paradigm; 2 × 3 phases (overall 6 × 4.5 min): resting state phase (eyes open; with pink noise), sad mood induction phase (with key words of personal negative life events and music, every key word presented for 1.5 min) and either rumination or distraction induction phase (counterbalanced), figure shows rumination induction phase first with corresponding induction statements selected from the original item pool provided by Nolen-Hoeksema and Morrow (1993). PANAS, Positive and Negative Affect Scale.
In the following, we focus mainly on the sad mood induction phases. Sad mood was induced with three negative life events that were individually assessed for every participant immediately before the fMRI session started and were later presented consecutively in the scanner via a keyword (each for 1.5 min; resulting in a 4.5 min phase). In parallel, participants listened to instrumental background music (parts of Adagio in g-minor by Albinoni) during the mood induction phase.
6 × 180 T2* weighted EPI images (TR = 1.5 s, α = 80°, TE = 28 ms) with 24 slices (slice thickness 4 mm, voxel size 3 × 3 × 4 mm3, FOV 192 mm) were recorded with a 3 T Trio TIM Scanner with a 12 channel head coil (Siemens Medical Systems, Erlangen, Germany). In addition, heart rate and respiration rate were sampled at 50 Hz with the scanner built-in equipment. Initially, the first 20 images of each phase were discarded to let participants get into the phase. Data were corrected for physiological artefacts using the Aztec software tool (van Buuren et al., 2009) including a high-pass filter of 1/512 Hz. Pre-processing included motion correction, slice time correction (13th slice as reference), normalization to an EPI template and smoothing with a 9-mm Gaussian kernel.
For the analyses, the mean of both sad mood induction phases was used for every participant. Seed region for the connectivity analysis was the PCC (10 mm sphere around −7, −45, 24) as main posterior part of the DMN (Berman et al., 2011). Pre-processing and statistics were conducted with SPM8 (Wellcome Trust Centre for Neuroimaging, University College London, UK), IBM SPSS20 (SPSS Inc., Chicago, Il, USA) and G*Power 3.1.2 (Faul et al., 2007). First level analyses were conducted for every single subject and second level analysis to compare groups. In the whole brain analyses minimal cluster size was set to 10 adjacent voxels at punc. < 0.001. Using an uncorrected P-value together with a cluster size threshold has been discussed as an appropriate way to control for type I errors but also to avoid an inflation of type II errors (Lieberman and Cunningham, 2009). For further regression analyses, a mask including all connected voxels at punc. = 0.01 during the mood induction phase was overlaid with an anatomical mask for the PHG from the WFU pickatlas (Lancaster et al., 1997; Maldjian et al., 2003). The resulting intersection mask was used to extract the mean connectivity from the PCC to the PHG in each participant during the mood induction phase. The latter were used for regression analyses on the relationship of PCC–PHG connectivity with interview- and questionnaire-based and daily life measures. To account for the baseline scores in longitudinal analyses, stepwise regressions were conducted.
RESULTS
Mood ratings
Both groups reported sadder mood after mood induction (increased negative affect: rMDD: 12.84 ± 2.61 vs 19.17 ± 7.93, t(28) = 4.57, P < 0.001, dz = 0.85, HC: 12.55 ± 4.23 vs 14.40 ± 5.18, t(28) = 3.52, P = 0.001, dz = 0.65; reduced positive affect: rMDD: 25.55 ± 6.13 vs 22.90 ± 5.88, t(28) = 2.61, P = 0.008, dz = 0.48, small but not statistically significant effect in the HC: 27.76 ± 7.99 vs 26.74 ± 8.56, t(28) = 1.55, P = 0.067, dz = 0.29; rMDD reported a significantly more increased negative affect and a similarly decreased positive affect compared with controls (ANOVA group × time interactions): negative: F(1,57)=9.16, P = 0.004, f = 0.41; positive: F(1,57) = 1.83, P = 0.182, f = 0.18) and comparable concentration ratings on the negative life events (rMDD 73 ± 14%, HC 75 ± 19%; t(56) = 0.65, P = 0.519, d = 0.17).
Brain connectivity during mood induction
To test whether the DMN can be identified during sad mood induction we calculated the main effect for PCC connectivity over all participants after regressing out the mean signal. When doing so, we identified a network including parietal, prefrontal and occipital regions, which is strongly overlapping with the DMN as described by Smith et al. (2009). See supplementary online material for details (Supplementary Figures S1 and S2).
As the pattern of the functional connectivity results remained the same when only the first sad mood induction phase was considered, we averaged across both phases to increase power.
Group comparisons of brain connectivity
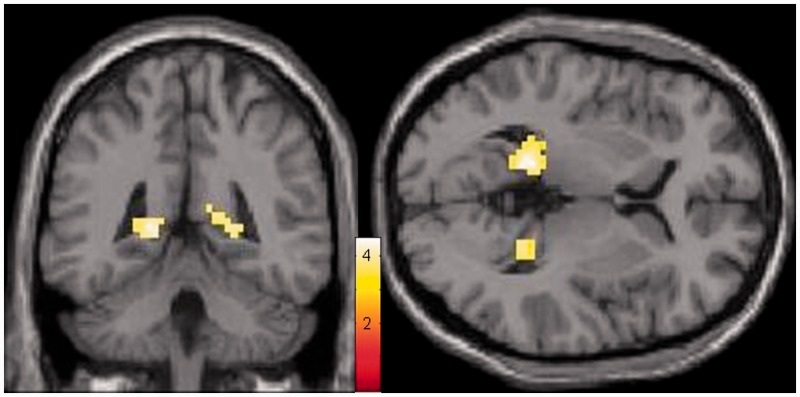
In a whole brain analysis of the sad mood induction phase, remitted depressed patients showed greater connectivity between PCC and PHG compared with healthy controls (Fig. 2 and Table 2). No regions showed greater connectivity to the PCC in the controls compared with patients. When looking only at the resting state, there were no differences between groups at the same threshold (punc. < 0.001).
Fig. 2.
Increased connectivity of the PCC and PHG during the sad mood induction phase in the depression group (n = 29) contrasted to the control group (n = 29); whole-brain main effect, min. k = 10 voxels, punc. < 0.001, colors indicate t-values.
Table 2.
Functional brain imaging results of the sad mood induction for the whole brain main effect of group (rMDD>HC), punc. < .001; rMDD: depression group, HC: control group
| Anatomical region | MNI |
Cluster size | Peak t-value | ||
|---|---|---|---|---|---|
| x | y | Z | |||
| left parahippocampal gyrus/PCC, BA29 | −15 | −43 | 4 | 62 | 4.54 |
| right parahippocampal gyrus | 24 | −43 | 4 | 33 | 3.98 |
| left mid frontal cortex | −33 | 26 | 25 | 20 | 4.19 |
The connectivity between PCC and PHG appeared stronger in the left than right PHG [F(1,56) = 14.30, P < 0.001, f = 0.50] but this did not differ between groups [F(1,56) = 0.13, P = 0.717, f = 0.04].
Relations of PCC–PHG connectivity to number of depressive episodes and sadness and rumination in daily life
In the rMDD, more lifetime episodes of major depression were linked to a stronger connectivity of the PCC and PHG during sad mood induction (R2 = 0.29, B = 0.01, SE < 0.01, t = 3.26, P = 0.003). Furthermore, a stronger connectivity of PCC and PHG during sad mood induction was significantly associated with higher levels of rumination and sadness in daily life in the rMDD, but not in the HC (rumination: rMDD: R2 = 0.28, B = 10.76, SE = 3.37, t = 3.19, P = 0.004, HC: R2 = 0.06, B = −5.44, SE = 4.26, t = −1.28, P = 0.212; sadness: rMDD: R2 = 0.24, B = 10.74, SE = 4.22, t = 2.54, P = 0.019, HC: R2 = 0.02, B = −2.80, SE = 3.42, t = −0.82, P = 0.419). All regression coefficients remained significant when including t1 depression scores into the models.
To investigate whether the relations between neural connectivity and behavioral ratings were largely due to their associations with number of previous episodes, a series of stepwise regressions were conducted with daily life rumination and daily sadness as dependent variables. In these models, depression score at t1 was entered in a first step, followed by number of episodes and PCC–PHG connectivity score in a stepwise manner, depending on their effect size. In all models PCC–PHG connectivity yielded a significant effect, while number of episodes did not (daily life rumination: PHG–PCC connectivity: 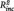 = 0.16, B = 8.76, SE = 2.66, t = 3.28, P = 0.003, n of episodes:
= 0.16, B = 8.76, SE = 2.66, t = 3.28, P = 0.003, n of episodes: 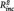 < 0.01, B = −0.04, SE = 0.08, t = −0.50, P = 0.621; daily life sadness: PCC–PHG connectivity:
< 0.01, B = −0.04, SE = 0.08, t = −0.50, P = 0.621; daily life sadness: PCC–PHG connectivity: 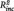 = 0.09, B = 6.81, SE = 2.43, t = 2.80, P = 0.011, n of episodes:
= 0.09, B = 6.81, SE = 2.43, t = 2.80, P = 0.011, n of episodes: 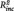 = 0.02, B = 0.091, SE = 0.065, t=1.40, p=.181).
= 0.02, B = 0.091, SE = 0.065, t=1.40, p=.181).
Predictive effects of PCC–PHG connectivity on the course of depressive symptoms and rumination
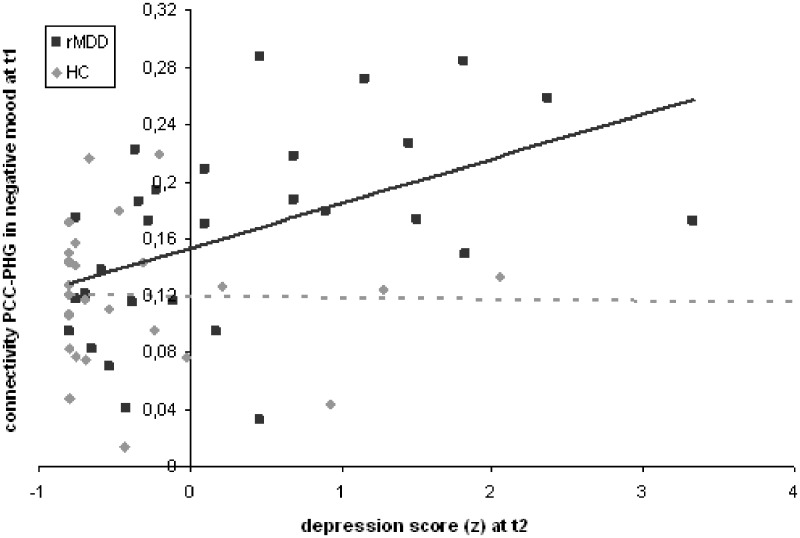
The stepwise regression analysis revealed a significant prediction of t2 depression scores by t1 depression scores in both groups (rMDD: R2 = 0.37, B = 0.58, SE = 0.15, t = 3.95, P = 0.001; HC: R2 = 0.32, B = 0.80, SE = 0.23, t = 3.53, P = 0.002). However, adding the PCC–PHG connectivity to the model revealed that the latter significantly predicted a significant worsening of depressive symptoms in the rMDD (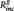 = 0.13, B = 6.66, SE = 2.54, t = 2.62, P = 0.015; Fig. 3) but not in the HC (t = 1.18, P = 0.249).
= 0.13, B = 6.66, SE = 2.54, t = 2.62, P = 0.015; Fig. 3) but not in the HC (t = 1.18, P = 0.249).
Fig. 3.
Prediction of t2 depression scores with t1 connectivity of PCC and PHG during sad mood induction; rMDD: depression group (n = 29); HC: control group (n = 29).
Furthermore, baseline habitual rumination predicted rumination scores 6 months later in both groups (brooding: rMDD: R2 = 0.52, B = 0.77, SE = 0.14, t = 5.37, P < 0.001; HC: R2 = 0.35, B = 0.70, SE = 0.19, t = 3.78, P = 0.001; reflection: rMDD: R2 = 0.52, B = 0.62, SE = 0.12, t = 5.43, P < 0.001; HC: R2 = 0.32, B = 0.53, SE = 0.14, t = 3.59, P = 0.001). Adding the PCC–PHG connectivity to the stepwise regression model revealed that the latter predicted significant higher brooding scores in the rMDD but not in the HC (brooding: rMDD: 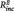 = 0.07, B = 18.71, SE = 8.99, t = 2.08, P = 0.047; HC: t = 0.05, P = 0.957; reflection: rMDD: t = 1.69, P = 0.104; HC: t = −1.25, P = 0.224).
= 0.07, B = 18.71, SE = 8.99, t = 2.08, P = 0.047; HC: t = 0.05, P = 0.957; reflection: rMDD: t = 1.69, P = 0.104; HC: t = −1.25, P = 0.224).
The overall pattern of the reported results remained the same, for both connectivity and predictions, when excluding all patients with psychotropic medication and their matched controls (n = 14). However, due to reduced power the results did not reach significance for the prediction of daily life sadness and brooding after 6 months.
DISCUSSION
The study highlights the potential role of connectivity between the PCC (a node in DMN) and PHG (a node in the autobiographical network) during negative autobiographical recollection in remitted depression, its association with daily life mood and rumination and its prognostic value for the further course of illness. To this end, we induced sad mood in remitted recurrently or chronic depressed patients and healthy controls by presenting key words of personal negative life events in the scanner. Within this context, we investigated whether the remitted depressed group would show a stronger connection between the PCC and structures of the autobiographical memory network during recollection of negative life events and whether this connectivity would be related to concurrent daily life assessments of mood and rumination and to the prospective 6 months course of depressive symptoms and habitual rumination.
Persons with remitted recurrent or chronic depression in comparison with healthy controls showed a stronger connectivity of the PCC with the bilateral PHG which was further increased in patients with more previous depressive episodes. Furthermore, in the patient group a stronger connectivity of the PCC and PHG was related to a sadder mood and more ruminative thoughts in daily life and a worsening of rumination and depression scores over the 6-months period, even when controlling for current depressive symptoms. Although former studies of depression have casually reported data suggesting a potentially important role of the PHG, this region has not previously been a major focus of discussion. Our own results strengthen the assumption that the PHG may have a key role in depression, especially in recurrent and chronic depression.
Our findings are interesting in several respects. First, the stronger correlation between PCC and PHG during mood induction in remitted depressed patients suggests that the recollection of negative events in patients was associated with a more intensive recruitment of autobiographical memories. This could be a vulnerability mechanism for the re-occurrence of depression. We assume that whenever these vulnerable individuals are reminded of personally relevant negative events, a broad associative network is being activated, thereby reinstating negative thoughts and rumination. This interpretation is supported by the significant association of PCC–PHG connectivity with sad mood and rumination in daily life. This might indicate that increased involvement of the autobiographical memory network is linked to negative mood and rumination. Furthermore, the observed correlation between PHG involvement during mood induction and the number of anamnestic depressive episodes supports the idea that this mechanism not only reflects vulnerability but also a neural signature of the disorder. This signature could represent a pre-existing, possibly genetic, risk factor but could also be acquired over the course of illness. Although our design does not allow clear conclusions, we speculate that the greater connectivity between PCC and PHG in patients compared with controls might represent a kind of neural ‘scar’ (cf. Marchetti et al., 2012) of former episodes in recurrent and chronic depression which leads even in remission to enhanced reactions in demanding situations such as recalling personal negative events. Furthermore, in our study, this responsiveness of the PHG was not only related to illness history and daily life emotional and cognitive experiences but also predictive for the worsening of rumination and depressive symptoms during a 6-month period. Importantly, this relation cannot be explained by the association between PHG involvement and current depressive symptoms as we controlled for t1 depression scores. This finding further emphasizes the importance of this system for the course of illness.
In addition, our result of a stronger PCC–PHG connectivity in the remitted depressed group during sad mood induction but not in the resting state matches with findings from non-fMRI studies showing that remitted depressed people perform similarly on measures of maladaptive cognitions and information processing as never-depressed individuals. However, if primed by the activation of maladaptive schemata or sad mood induction, these patients show differences from healthy controls in various types of information processing. This phenomenon, labeled as cognitive reactivity, was shown by self-reports, memory, attention and interpretive biases and found to be connected with a poorer longitudinal course of the disorder (e.g. Scher et al., 2005; Segal et al., 2006). In this context, our work is the first to study DMN activity in relation to cognitive reactivity in remitted depressed individuals, as suggested also by Marchetti et al. (2012). We identified increased PCC–PHG connectivity specifically during sad mood induction, which was furthermore linked to daily life rumination and to a poorer symptom course over the next months. So far, only Farb et al. (2011) used sad mood induction to perform a neuroimaging study on risk of relapse. Using an experimental design different from ours, these authors found greater medial prefrontal cortex reactivity to emotional challenges which predicted rumination and seems to be a marker for relapse risk during the following 18 months. Together, these two studies are the first to demonstrate neuroimaging phenotypes of cognitive reactivity in remitted depressed individuals and lend further support for the diathesis-stress hypothesis of depression (Beck, 2008).
Depressed individuals have problems with remembering specific personal past events and instead recall more general events (over general memory, OGM; Williams and Broadbent, 1986). Studies indicate that OGM predicts higher follow-up depressive symptoms (Sumner, et al., 2010) as well as rumination and avoidance (Watson et al., 2013). Furthermore, memory specificity was reduced after an induction of a self-discrepancy focus in persons with high trait rumination (Raes et al., 2012). Therefore, OGM is possibly modulated via self-discrepancy (Raes et al., 2012; Schoofs et al., 2013) and related to rumination. We can only speculate that the association between higher PHG involvement during negative mood induction and a poorer course of depressive symptoms and rumination is mediated by OGM for negative events, which might be associated with stronger autobiographical involvement during mood induction in patients. However, this stronger involvement might not result from a more specific memory for negative experiences but from repeatedly thinking of them with a ruminative focus that generally takes place at a rather abstract level of thinking (Watkins et al., 2009). This cognitive bias is apparently not state-dependent but can be observed in remission. Unfortunately, we did not measure OGM directly in this study. Future studies on the relation between neural correlates of negative mood, rumination and outcome should include this construct to further elucidate possible connections.
In conclusion, our results suggest a vicious circle in which remembering and ruminating about negative events sensitizes a neural network involved in self-reference and autobiographical memory, which again facilitates the recollection of negative life events and rumination, thereby leading to a poor outcome. Interestingly, this interpretation is to a large extent compatible with a neurobiological model of cognitive vulnerability recently presented by Marchetti and colleagues (2012). Based on a review of the literature on DMN alterations in depression, they argue that an altered DMN can be seen as a neural mechanism involved in the cognitive risk for the recurrence of depressive episodes leading to increased rumination, cognitive reactivity and impaired attentional control.
A drawback of our study might be seen in the fact that the fMRI results were not corrected for multiple testing. However, as mentioned before, this procedure has been discussed as an appropriate way to control for type I errors but also avoiding an inflation of type II errors (Lieberman and Cunningham, 2009). Particularly for patient studies, applying a whole head corrected significance level and therefore reaching an incredibly high probability to overlook true effects is also, from an ethical point of view as then patients would have been exposed in vain to a potentially stressful situation, a disputable approach. Furthermore, when analyzing group differences, we observed only three significant clusters for the whole brain, two of them located in the PHG, which further validates our findings. However, replication of these findings is clearly warranted to confirm our conclusions.
In short, remitted patients with a recurrent or chronic depression precourse showed a stronger connectivity between PCC and PHG when they were cued to think about own negative life events. This finding suggests that depression is associated with a greater salience of negative autobiographical memories. This connectivity was associated with the number of previous episodes, with daily life measures of depression-related features such as more rumination and sadder mood and with a worsening of depression and rumination over 6 months. These results highlight the importance of the autobiographical memory network and dysfunctional cognitions related to it and emphasize current interventions that target rumination in the context of negative autobiographical memories on a neurobiological level. Further research on this specific pathway could be a possible opportunity to ameliorate interventions in depression. A promising candidate for such interventions might be mindfulness-based training which has not only been found to reduce rumination (Jain et al., 2007; van Vugt et al., 2012), but also to particularly reduce those neural connections with the PHG under resting conditions (Taylor et al., 2013) that we identified to be involved in mood, rumination and the risk of recurrence of depression.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
The project was supported by grants of the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG; KI 576/12-1 to P.K., KU 1464/4-1 to C.K.).
REFERENCES
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. American Journal of Psychiatry. 2008;165(8):969–77. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Social Cognitive and Affective Neuroscience. 2011;6(5):548–55. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot JF, Pieper S, Thayer JF. Expanding stress theory: prolonged activation and perseverative cognition. Psychoneuroendocrinology. 2005;30(10):1043–49. doi: 10.1016/j.psyneuen.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Joormann J, Eugene F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cognitive Affective & Behavioral Neuroscience. 2010;10(4):470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argembeau A, Comblain C, Van der Linden M. Phenomenal characteristics of autobiographical memories for positive, negative, and neutral events. Applied Cognitive Psychology. 2003;17(3):281–294. [Google Scholar]
- Dere E, Pause BM, Pietrowsky R. Emotion and episodic memory in neuropsychiatric disorders. Behavioural Brain Research. 2010;215(2):162–71. doi: 10.1016/j.bbr.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Bloch RT, Segal ZV. Mood-linked responses in medial prefrontal cortex predict relapse in patients with recurrent unipolar depression. Biological Psychiatry. 2011;70(4):366–72. doi: 10.1016/j.biopsych.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Chen MC, Gotlib IH. Neural systems approaches to understanding major depressive disorder: an intrinsic functional organization perspective. Neurobiology of Disease. 2013;52:4–11. doi: 10.1016/j.nbd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biological Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautzinger M, Keller F, Kuehner C, Beck AT. Das Beck Depressionsinventar II. Deutsche Bearbeitung und Handbuch zum BDI II. Frankfurt a.M.: Harcout Test Services; 2006. [Google Scholar]
- Howes M, Siegel M, Brown F. Early childhood memories: accuracy and affect. Cognition. 1993;47(2):95–119. doi: 10.1016/0010-0277(93)90001-c. [DOI] [PubMed] [Google Scholar]
- Huffziger S, Ebner-Priemer U, Zamoscik V, Reinhard I, Kirsch P, Kuehner C. Effects of mood and rumination on cortisol levels in daily life: an ambulatory assessment study in remitted depressed patients and healthy controls. Psychoneuroendocrinology. 2013;38(10):2258–67. doi: 10.1016/j.psyneuen.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Huffziger S, Kühner C. Die Ruminationsfacetten Brooding und Reflection. Zeitschrift Fur Klinische Psychologie Psychiatrie Und Psychotherapie. 2012;41(1):38–46. [Google Scholar]
- Huffziger S, Reinhard I, Kuehner C. A longitudinal study of rumination and distraction in formerly depressed inpatients and community controls. Journal of Abnormal Psychology. 2009;118(4):746–56. doi: 10.1037/a0016946. [DOI] [PubMed] [Google Scholar]
- Jain S, Shapiro SL, Swanick S, et al. A randomized controlled trial of mindfulness meditation versus relaxation training: effects on distress, positive states of mind, rumination, and distraction. Annals of Behavioral Medicine. 2007;33(1):11–21. doi: 10.1207/s15324796abm3301_2. [DOI] [PubMed] [Google Scholar]
- Krohne HW, Egloff B, Kohlmann CW, Tausch A. Investigations with a German version of the positive and negative affect schedule (PANAS) Diagnostica. 1996;42(2):139–156. [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, et al. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Human Brain Mapping. 1997;5(4):238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung MK, Chan CC, Yin J, Lee CF, So KF, Lee TM. Increased gray matter volume in the right angular and posterior parahippocampal gyri in loving-kindness meditators. Social Cognitive and Affective Neuroscience. 2013;8(1):34–9. doi: 10.1093/scan/nss076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Liu L, Friston KJ, et al. A treatment-resistant default mode subnetwork in major depression. Biological Psychiatry. 2013;74(1):48–54. doi: 10.1016/j.biopsych.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4(4):423–28. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackin RS, Tosun D, Mueller SG, et al. Patterns of reduced cortical thickness in late-life depression and relationship to psychotherapeutic response. American Journal of Geriatric Psychiatry. 2012;21(8):794–802. doi: 10.1016/j.jagp.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Marchetti I, Koster EH, Sonuga-Barke EJ, De Raedt R. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychology Review. 2012;22(3):229–51. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- Milton F, Butler CR, Benattayallah A, Zeman AZ. The neural basis of autobiographical memory deficits in transient epileptic amnesia. Neuropsychologia. 2012;50(14):3528–41. doi: 10.1016/j.neuropsychologia.2012.09.027. [DOI] [PubMed] [Google Scholar]
- Moberly NJ, Watkins ER. Ruminative self-focus and negative affect: an experience sampling study. Journal of Abnormal Psychology. 2008;117(2):314–23. doi: 10.1037/0021-843X.117.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Moritz S, Glascher J, Brassen S. Investigation of mood-congruent false and true memory recognition in depression. Depression and Anxiety. 2005;21(1):9–17. doi: 10.1002/da.20054. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. Effects of rumination and distraction on naturally occurring depressed mood. Cognition & Emotion. 1993;7(6):561–70. [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3(5):400–24. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Raes F, Schoofs H, Griffith JW, Hermans D. Rumination relates to reduced autobiographical memory specificity in formerly depressed patients following a self-discrepancy challenge: the case of autobiographical memory specificity reactivity. Journal of Behavior Therapy and Experimental Psychiatry. 2012;43(4):1002–7. doi: 10.1016/j.jbtep.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Scher CD, Ingram RE, Segal ZV. Cognitive reactivity and vulnerability: empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clinical Psychology Review. 2005;25(4):487–510. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Schmidtke A, Fleckenstein P, Moises W, Beckmann H. Studies of the reliability and validity of the German version of the Montgomery-Asberg Depression Rating Scale (MADRS) Schweizer Archiv fur Neurologie und Psychiatrie. 1988;139(2):51–65. [PubMed] [Google Scholar]
- Schoofs H, Hermans D, Griffith JW, Raes F. Self-discrepancy and reduced autobiographical memory specificity in ruminating students and depressed patients. Cognition & Emotion. 2013;27(2):245–62. doi: 10.1080/02699931.2012.703640. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Kennedy S, Gemar M, Hood K, Pedersen R, Buis T. Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Archives of General Psychiatry. 2006;63(7):749–55. doi: 10.1001/archpsyc.63.7.749. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. Journal of Neuroscience. 2011;31(12):4407–20. doi: 10.1523/JNEUROSCI.3335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences USA. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences USA. 2010;107(24):11020–5. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences USA. 2009;106(31):13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Sumner JA, Griffith JW, Mineka S. Overgeneral autobiographical memory as a predictor of the course of depression: a meta-analysis. Behaviour Research and Therapy. 2010;48(7):614–625. doi: 10.1016/j.brat.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VA, Daneault V, Grant J, et al. Impact of meditation training on the default mode network during a restful state. Social Cognitive and Affective Neuroscience. 2013;8(1):4–14. doi: 10.1093/scan/nsr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: a psychometric analysis. Cognitive Therapy and Research. 2003;27(3):247–259. [Google Scholar]
- Trull TJ, Ebner-Priemer UW. Ambulatory assessment. Annual Review of Clinical Psychology. 2013;9:4.1–4.27. doi: 10.1146/annurev-clinpsy-050212-185510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren M, Gladwin TE, Zandbelt BB, et al. Cardiorespiratory effects on default-mode network activity as measured with fMRI. Human Brain Mapping. 2009;30(9):3031–42. doi: 10.1002/hbm.20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vugt MK, Hitchcock P, Shahar B, Britton W. The effects of mindfulness-based cognitive therapy on affective memory recall dynamics in depression: a mechanistic model of rumination. Frontiers in Human Neuroscience. 2012;6:257. doi: 10.3389/fnhum.2012.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard A, Desgranges B, Eustache F, Piolino P. Factors affecting medial temporal lobe engagement for past and future episodic events: an ALE meta-analysis of neuroimaging studies. Brain Cognition. 2012;80(1):111–125. doi: 10.1016/j.bandc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Watkins ER, Baeyens CB, Read R. Concreteness training reduces dysphoria: proof-of-principle for repeated cognitive bias modification in depression. Journal of Abnormal Psychology. 2009;118(1):55–64. doi: 10.1037/a0013642. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson LA, Berntsen D, Kuyken W, Watkins ER. The characteristics of involuntary and voluntary autobiographical memories in depressed and never depressed individuals. Consciousness and Cognition. 2012;21(3):1382–92. doi: 10.1016/j.concog.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Watson LA, Berntsen D, Kuyken W, Watkins ER. Involuntary and voluntary autobiographical memory specificity as a function of depression. Journal of Behavior Therapy and Experimental Psychiatry. 2013;44(1):7–13. doi: 10.1016/j.jbtep.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Whalley MG, Rugg MD, Brewin CR. Autobiographical memory in depression: an fMRI study. Psychiatry Research. 2012;201(2):98–106. doi: 10.1016/j.pscychresns.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Moran JM, Nieto-Castanon A, Triantafyllou C, Saxe R, Gabrieli JD. Associations and dissociations between default and self-reference networks in the human brain. Neuroimage. 2011;55(1):225–232. doi: 10.1016/j.neuroimage.2010.11.048. [DOI] [PubMed] [Google Scholar]
- Williams JM, Broadbent K. Autobiographical memory in suicide attempters. Journal of Abnormal Psychology. 1986;95(2):144–9. doi: 10.1037//0021-843x.95.2.144. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Wunderlich U, Gruschwitz S, Zaudig M. SCID: Structured Clinical Interview for DSM-IV axis I disorders. Goettingen: Hogrefe; 1997. [Google Scholar]
- Zeng LL, Shen H, Liu L, et al. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain. 2012;135(Pt 5):1498–507. doi: 10.1093/brain/aws059. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang X, Xiao J, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71(7):611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.