Abstract
Childhood emotional maltreatment (CEM) has adverse effects on medial prefrontal cortex (mPFC) morphology, a structure that is crucial for cognitive functioning and (emotional) memory and which modulates the limbic system. In addition, CEM has been linked to amygdala hyperactivity during emotional face processing. However, no study has yet investigated the functional neural correlates of neutral and emotional memory in adults reporting CEM. Using functional magnetic resonance imaging, we investigated CEM-related differential activations in mPFC during the encoding and recognition of positive, negative and neutral words. The sample (N = 194) consisted of patients with depression and/or anxiety disorders and healthy controls (HC) reporting CEM (n = 96) and patients and HC reporting no abuse (n = 98). We found a consistent pattern of mPFC hypoactivation during encoding and recognition of positive, negative and neutral words in individuals reporting CEM. These results were not explained by psychopathology or severity of depression or anxiety symptoms, or by gender, level of neuroticism, parental psychopathology, negative life events, antidepressant use or decreased mPFC volume in the CEM group. These findings indicate mPFC hypoactivity in individuals reporting CEM during emotional and neutral memory encoding and recognition. Our findings suggest that CEM may increase individuals’ risk to the development of psychopathology on differential levels of processing in the brain; blunted mPFC activation during higher order processing and enhanced amygdala activation during automatic/lower order emotion processing. These findings are vital in understanding the long-term consequences of CEM.
Keywords: Anxiety, childhood abuse, depression, emotional maltreatment, magnetic resonance imaging (MRI), medial prefrontal cortex (PFC)
INTRODUCTION
Childhood emotional maltreatment (CEM; emotional abuse and/or emotional neglect) is experienced by one out of 10 children growing up in Western societies every year (Gilbert et al., 2009). CEM is the most prevalent type of child-maltreatment and has a profound negative impact on social, cognitive, behavioral and emotional functioning (Pollak et al., 2009; Egeland, 2009; Gilbert et al., 2009; Hart and Rubia, 2012; Spinhoven et al., 2010; Schechter, 2012). After chronic exposure to CEM, individuals may develop sustained negative self-associations (Van Harmelen et al., 2010a), which may bias attention toward negative information about the self and others. Even as adults, this may result in negative interpretations when engaged in stressful interpersonal situations, or when retrieving memories of such situations (Beck, 2008). In line, individuals with CEM are more prone to develop depressive and anxiety disorders (Spinhoven et al., 2010; Iffland et al., 2012).
Chronic childhood stress is associated with structural and functional changes in the brain, especially within the (medial) prefrontal cortex [(m)PFC], hippocampus and the amygdala [see overviews and mechanisms; (Arnsten, 2009; Lupien et al., 2009; Danese and McEwen, 2012; Hart and Rubia, 2012; McCrory et al., 2012; McEwen et al., 2012)]. In line, we reported CEM-related smaller mPFC volume (Van Harmelen et al., 2010b) and amygdala hyperactivation during the processing of emotional faces in patients and healthy controls (HC) (Van Harmelen et al., 2013); see also Bogdan et al.(2012); Dannlowski et al. (2012a; 2012b) and McCrory et al. (2011). The mPFC is crucial for emotional processing, memory and modulates the stress response (Cardinal et al., 2002; Phillips et al., 2003; Etkin et al., 2011). The dorsal mPFC plays a vital role in the (re-) appraisal of emotional stimuli, whereas the ventral mPFC dampens fear responses through its regulation of the amygdala (Phillips et al., 2003; Etkin et al., 2011). The dorsal and ventral mPFC are functionally inextricably intertwined, therefore abnormalities in either or both may be associated with abnormalities in emotional processing, memory and stress response (Phillips et al., 2003; Etkin et al., 2011). The mPFC is also crucial for understanding other people’s beliefs, feelings and motivations (i.e. mentalizing) (Frith & Frith, 2003; Frith and Frith, 2006; Mitchell et al., 2006; Denny et al., 2012; Meyer et al., 2013). In children, a smaller PFC volume has been found to mediate the link between childhood stress and reduced cognitive functioning (Hanson et al., 2012). However, the neural correlates of cognitive functioning in adults reporting CEM are unknown.
During and immediately after acute interpersonal stress, brain activity shifts from higher cortical (e.g. mPFC) regions to ‘lower’ subcortical regions (e.g. amygdala, hippocampus) (Hermans et al., 2011; Oei et al., 2012). Stress activates the amygdala as part of a ‘salience network’ for vigilant attentional reorienting, strengthening of emotional memory traces and autonomic-neuroendocrine control, facilitating the processing/encoding of emotional information, at the detriment of higher order cognitive functions (Davis and Whalen, 2001; Whalen, 2007; Hermans et al., 2011; Todd et al., 2011; Oei et al., 2012). In HCs, exposure to acute psychosocial stress increases coupling of mPFC and amygdala activations, which persists even some time after the stress has waned (Veer et al., 2011). To investigate whether CEM is related to a reduction in higher order cognitive functioning, the functional neural correlates of CEM during cognitive tasks that are known to engage frontal regions need be examined.
Here, we examined the neural correlates of CEM during the encoding and recognition of (positive, negative and neutral) words in a large sample (N = 194), by comparing patients and HC reporting CEM [n = 96; i.e. patients with major depressive disorder (MDD; n = 20), anxiety disorder (ANX; n = 27), co-morbid depression and anxiety disorder (CDA; n = 40) and HC n = 9)], with those reporting no abuse [n = 98; [i.e. MDD (n = 24), ANX (n = 22), CDA (n = 19) and HC (n = 33)]. We expected that self-reported CEM was associated with a memory bias (i.e. relative enhanced recognition) with respect to negative stimuli and limbic (amygdala and hippocampal) hyperactivations during encoding and recognition of negative words, but not for positive or neutral words. In addition, we expected a general reduction in cognitive functioning in individuals with CEM, associated with overall reduced mPFC activations (across valence).
METHOD
Participants
Participants were a subset from the Netherlands Study of Depression and Anxiety [NESDA; N = 2981; (Penninx et al., 2008)], consisting of 233 patients with MDD and/or ANX and 68 HC. Participants underwent magnetic resonance imaging (MRI) scanning in the Leiden University Medical Center (LUMC), Academic Medical Center Amsterdam (AMC) or University Medical Center Groningen (UMCG). Trained interviewers established diagnoses using the structured Composite International Diagnostic Interview (Wittchen et al., 1991). Patients were included when they had a diagnosis <6 months recency of current DSM-IV MDD and/or ANX (panic disorder and/or social anxiety disorder). Patients were excluded if they were taking any psychotropic medication other than stable use of selective serotonin reuptake inhibitors (SSRIs) or infrequent benzodiazepine use (i.e. equivalent to two doses of 10 mg of oxazepam three times per week or use within 48 h prior to scanning). HCs had no lifetime MDD or ANX and were not taking any psychotropic drugs. Ethical Review Boards of each participating center approved this study, and after complete description of the study, written informed consent was obtained.
Childhood maltreatment
Childhood maltreatment was assessed through the NEMESIS trauma interview (De Graaf et al., 2002). Participants were asked whether they had experienced emotional neglect, emotional abuse, physical abuse or sexual abuse before the age of 16 years, and if so, how often it occurred (‘never, once, sometimes, regularly, often, or very often’), and what their relationship with the perpetrator was. Emotional neglect was described as: ‘people at home didn’t listen to you, your problems were ignored, and you felt unable to find any attention or support from the people in your house’. Emotional abuse was described as: ‘you were cursed at, unjustly punished, your brothers and sisters were favored – but no bodily harm was done’. CEM was defined as multiple incidents (more than once) of emotional neglect and/or emotional abuse (in line with our previous studies, e.g. van Harmelen et al., 2010b, 2013). In the final sample (N = 194, Table 1; additional exclusion criteria in Supplementary Data), 96 adults reported CEM (n = 20 MDD, n = 27 ANX, n = 40 CDA, n = 9 HC) and 98 reported no abuse (n = 24 MDD, n = 22 ANX, n = 19 CDA, n = 33 HC). This is largely the same cohort in whom we found CEM-related reduced mPFC volume (Van Harmelen et al., 2010b) and enhanced amygdala responses (Van Harmelen et al., 2013). In the CEM group, participants reported isolated emotional neglect (n = 46, 47.9%), isolated emotional abuse (n = 3, 3.1%) or both emotional neglect and emotional abuse (n = 47, 49.0%) in childhood. In addition, 95 participants (99.0%) reported their biological parents as perpetrators, one person (1.0%) reported a stepfather as perpetrator.
Table 1.
Demographics, clinical characteristics and memory performance of the No Abuse and CEM groups
| Characteristics and performance | No Abuse (N = 98) | CEM (N = 96) | |||
|---|---|---|---|---|---|
| Mean (s.d.) | Mean (s.d.) | χ2 | F-value | P-value | |
| Age | 36.48 (10.56) | 38.11 (9.52) | 1.28 | 0.26 | |
| Gender (male/female) (n) | 32/66 | 37/59 | 0.73 | 0.39 | |
| Education level (attained in years) | 13.16 (2.88) | 12.5 (3.28) | 2.24 | 0.14 | |
| Scan location (A/L/G) (n) | 30/37/31 | 32/38/26 | 0.50 | 0.78 | |
| Diagnosis (yes/no) (n) | 65/33 | 87/9 | 16.88 | <0.001 | |
| Diagnosis (MDD/CDA/ANX/HC) (n) | 24/19/22/33 | 20/40/27/9 | 22.04 | <0.001 | |
| Type of abuse (CEM + S / CEM + P/ CEM + S&P) (n) | 56/16/13/11 | ||||
| Frequency of CEM (Som/Reg/Often/very Often) (n) | 15/27/19/35 | ||||
| SSRI use (yes/no) (n) | 21/77 | 29/67 | 1.95 | 0.16 | |
| Parental psychopathology (yes/no) (n) | 38/25 | 54/18 | 3.37 | 0.07 | |
| Negative life events | 4.06 (1.97) | 5.43 (2.17) | 20.99 | <0.001 | |
| Neuroticism | 34.31 (7.93) | 41.81 (9.34) | 36.31 | <0.001 | |
| MADRS | 8.19 (9.29) | 15.08 (9.99) | 26.81 | <0.001 | |
| BAI | 9.29 (9.62) | 12.82 (9.04) | 6.63 | <0.011 | |
| Anxiety score (VAS) before encoding | 34.12 (24.71) | 34.94 (27.27) | 0.05 | 0.83 | |
| Anxiety score (VAS) after encoding | 29.54 (21.66) | 30.13 (24.75) | 0.03 | 0.86 | |
| Word classification | |||||
| Proportion words classified as positive | 98.94 (24.04) | 98.37 (22.35) | 0.03 | 0.87 | |
| Proportion words classified as negative | 96.97 (5.68) | 96.07 (11.39) | 0.45 | 0.51 | |
| Proportion words classified as neutral | 103.14 (24.52) | 102.77 (25.03) | 0.01 | 0.92 | |
| Memory | |||||
| Proportion correctly recognized positive words | 0.73 (0.13) | 0.73 (0.15) | 0.01 | 0.93 | |
| Proportion correctly recognized negative words | 0.69 (0.13) | 0.69 (0.16) | 0.07 | 0.80 | |
| Proportion correctly recognized neutral words | 0.69 (0.15) | 0.71 (0.17) | 1.41 | 0.24 | |
| Proportion false alarms positive words | 0.12 (0.10) | 0.11 (0.09) | 0.03 | 0.85 | |
| Proportion false alarms negative words | 0.17 (0.11) | 0.15 (0.10) | 1.27 | 0.26 | |
| Proportion false alarms neutral words | 0.06 (0.06) | 0.06 (0.05) | 0.00 | 0.97 | |
| Discriminant sensitivity positive words | 0.61 (0.16) | 0.62 (0.15) | 0.04 | 0.85 | |
| Discriminant sensitivity negative words | 0.52 (0.12) | 0.54 (0.14) | 1.40 | 0.24 | |
| Discriminant sensitivity neutral words | 0.63 (0.16) | 0.65 (0.17) | 1.37 | 0.24 |
Additional assessments
In the NESDA study, we assessed lifetime negative life events with the List of Threatening Events Questionnaire (Brugha et al., 1985) and Neuroticism with the NEO Five-Factor Inventory (Costa and McGrae, 1992). Parental psychopathology was assessed using a family tree approach interview, assessing whether a member of their family had experienced anxiety, depression or other psychopathological problems, and if so, which member of their family. On the day of scanning (∼8 weeks following NESDA baseline assessment), severity of depression and anxiety (last 2 weeks) was assessed using the Beck Anxiety Inventory (BAI; Beck et al., 1988) and the Montgomery Åsberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979).
Task paradigm
The word-encoding and -recognition task was event-related, subject-paced (max 5 s) (Daselaar et al., 2003) (Supplementary Data). During encoding, participants were asked to classify 40 positive, 40 negative and 40 neutral words according to their valence. During a baseline control condition, participants viewed the words ‘left’, ‘middle’ or ‘right’ and were instructed to press the corresponding key. After a 10 min retention interval, participants indicated whether they had ‘seen’ (i.e. remembered), ‘probably had seen’ (i.e. know), or ‘hadn’t seen’ (i.e. rejection) 120 old encoding target words, 120 new distracter words and 40 baseline control trials. Trial presentation was pseudo-randomized. We recorded response accuracy and times (RT). Anxiety levels were recorded before and after word encoding and recognition using a Visual Analogue Scale (0–100; Huskisson, 1993).
Image acquisition
Imaging data were acquired using Philips 3-Tesla MRI-systems (Best, The Netherlands) located at the LUMC, AMC and UMCG, equipped with SENSE-8 (LUMC, UMCG) and SENSE-6 (AMC) channel head coils. Echo-planar images were obtained using a T2*-weighted gradient echo sequence [repetition time (TR) = 2300 ms; echo time (TE) = 30 ms (UMCG: 28 ms), matrix size: 96 × 96 (UMCG: 64 × 64), 35 axial slices (UMCG: 39), interleaved acquisition, 2.29 × 2.29 mm in-plane resolution (UMCG: 3 × 3 mm), 3 mm slice thickness]. Anatomical imaging included a sagittal three-dimensional gradient-echo T1-weighted sequence (TR = 9ms, TE = 3.5 ms; matrix 256 × 256; voxel size: 1 × 1 × 1 mm; 170 slices).
Imaging data
Functional imaging data were pre-processed in Statistical Parametric Mapping software (SPM5) in Matlab7.1 (www.mathworks.co.uk) and analyzed using SPM8 in Matlab7.8. Pre-processing of the imaging data included reorientation of the functional images to the anterior commissure, slice time correction, image realignment, registration of the T1 scan to the mean image, warping to Montreal Neurological Institute (MNI)-space as defined by the SPM5 T1-template, reslicing to 3 × 3 × 3 mm voxels and spatial smoothing using an 8 mm FWHM Gaussian kernel. Next, data were analyzed in the context of the General Linear Model. Hemodynamic responses to each stimulus were modeled with a delta function convolved with a synthetic hemodynamic response function and modulated using RT. The model included regressors for encoding1 and recognition2 parameters. In addition, filler words, error- and no-response trials were included as a regressor of no interest. Low-frequency noise was removed by applying a high-pass filter (cut-off: 128 s) to the fMRI time-series at each voxel. Owing to the small proportion of ‘know responses’ on the recognition trials, these responses were treated as ‘remembered’ and added to either correct recognition (CREC) or false alarms (FA).
Contrast images for subsequently correctly recognized (SCR) words during encoding (SCR_pos > baseline, SCR_neg > baseline, SCR_neu > baseline) and CREC words during recognition (CREC_pos > baseline, CREC_neg > baseline and CREC_neu > baseline) were calculated per subject on a voxel-by-voxel basis and entered into second-level analyses for between-group comparisons.
We next set up CEM (No Abuse, CEM) × Words (Positive, Negative, Neutral) RM ANCOVAs for the encoding and recognition task separately. Age, gender and education level were specified as covariates (Iidaka et al., 2002; Hart and Rubia, 2012) and two dummy variables were added as covariates to control for variation caused by the different scanning locations. To examine if CEM-related word encoding and recognition was confounded by individual’s psychiatric status, we also added a dummy for current MDD, ANX (yes/no), demeaned within the CEM and No Abuse group to control for variation caused by psychopathology. As only nine HC reported CEM, we were unable to perform group (MDD, ANX, CDA, HC) × CEM (No Abuse, CEM) RM ANOVAs, as these analyses would be seriously underpowered. For the specific effects of MDD, ANX and HC on word encoding and recognition in largely the same sample, see van Tol et al. (2012).
We defined the following ROIs: hippocampus, amygdala, and mPFC. Because the anatomical location of the mPFC is less well defined than that of the hippocampus and amygdala, we focused on the mPFC in the broadest sense (i.e. dorsal mPFC (Brodmann area (BA) 8 and 9), ventral mPFC (BA 10), dorsolateral mPFC (BA 8, 9, and 46), and the dorsal and pregenual ACC (BA 32,24), using the AAL toolbox implemented in the Wake Forest University (WFU)-Pickatlas (Maldjian et al., 2003). The main effects of task are reported at P < 0.05, Family Wise Error (FWE) (voxel level). Activations outside our ROIs were examined using whole-brain analyses at P < 0.05 FWE corrected, while masking for the main effect of task (P < 0.05 uncorrected). All results are reported in MNI space.
Bilateral Amygdala (131 voxels) and hippocampal (536 voxels) activations were examined by extracting their activations for the main effect of task (F) to SPSS using Marsbar (Brett et al., 2002) and binary masks using WFU-Pickatlas. MPFC activations were examined using CEM vs No Abuse (F) analysis at P < 0.005, uncorrected and post hoc t-tests had to meet P < 0.05 FWE corrected for the spatial extend of the activated region with an initial height threshold of Z > 3.09, and K > 5 voxels, while masking for the main effect of task (P < 0.05 uncorrected). For this small volume correction (PSVC) we used the WFU-pickatlas and to extract significant mPFC activations for the main effect of task to SPSS we used the Marsbar Toolbox.
Behavioral analyses
Psychometric and performance data were analyzed with SPSS-19. Proportions (p) Correctly Recognized words (pCREC), False Alarms (pFA) and old/new discriminant accuracy (d′ = pCREC − pFA) were calculated for positive, negative and neutral words. For all tests, significance was set at P < 0.05 two-tailed, Bonferroni-corrected.
RESULTS
CEM vs No Abuse group characteristics and memory performance
The CEM vs No Abuse groups did not differ in age, education, gender, SSRI-use, scan location and anxiety levels before and after the task. The CEM group included more patients, reported higher depressive and anxious symptomatology, higher neuroticism scores, more lifetime negative life events and slightly more parental psychopathology (Table 1). RM ANOVAs revealed no differences in valence classification,3 memory performance or RTs, between the CEM and No Abuse groups (Table 1 and Supplementary Table S1).
Imaging results
Main effect of task during word encoding
The main effect of task during encoding was associated with bilateral amygdala [K = 6, x = −18, y = −6, z = −18, Z-score (Z) = 6.73) and (K = 1, x = 24, y = −9, z = −15, Z = 5.38)], hippocampal, (K = 174, x = −21, y = −15, z = −18, Z > 8, K = 60, x = −21, y = −15, z = −21, Z = 6.97), (K = 31, x = 21, y = −12, z = −18, Z = 6.93) and mPFC activations (K = 740, x = −6, y = 60, z = 30, Z > 8); (K = 57, x = −27, y = 0, z = 57, Z = 7.67) and (K = 38, x = −39, y = 36, z = 30, Z = 6.45). Supplementary Table S2 depicts main effect of task activations outside our ROIs.
CEM and word encoding: amygdala and hippocampus
Extracted amygdala and hippocampal activations for the main effect of task (SCR_pos > baseline, SCR_neg > baseline and SCR_neu > baseline) were analyzed in a CEM (No Abuse, CEM) × Words (Positive, Negative, Neutral) × Lateralization (Left, Right) RM ANCOVA, with psychiatric status (demeaned within group), age, gender, education level and dummies for location as covariates. Contrary to our expectations, there were no significant main or interaction effects of CEM [amygdala (F-values < 1.41, all P-values > 0.24) and hippocampus (F-values < 2.69, P-values > 0.10), details in Supplementary Data].
CEM and word encoding: mPFC
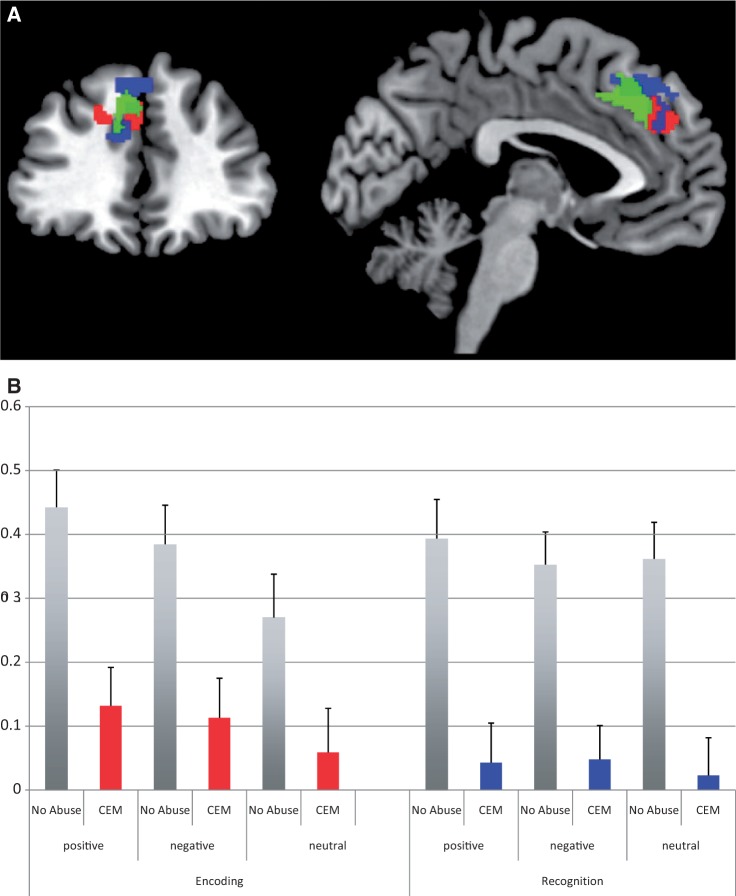
A CEM vs No Abuse analysis showed CEM-related mPFC hypoactivation during the encoding of positive, negative and neutral words (K = 26, x = −3 y = 45 z = 33, Z = 3.91, Psvc = 0.024; Figure 1).4 No other clusters were found in or outside our ROIs (Table 2).
Fig. 1.
Medial prefrontal cortex activations during encoding and recognition of positive, negative and neutral words in adults reporting CEM (N = 96) vs No Abuse (N = 98).
(A) Depicts the main effect of CEM on medial prefrontal cortex activation during encoding (red) and recognition (blue) at P < 0.005 K > 5 uncorrected. The green blob depicts the region that has been found to be smaller in adults reporting CEM (van Harmelen et al., 2010b). (B) Depicts the medial prefrontal cortex activations (BOLD signal change) during encoding (red) and recognition (blue) of positive, negative and neutral words in adults reporting CEM vs No Abuse.
Table 2.
Main effect of CEM at P < 0.005, K > 5
| Main effects CEM | K-values | F-values | Z-values | P(unc) | x, y, z (mm) |
|---|---|---|---|---|---|
| Encoding | |||||
| Medial frontal gyrus | 28 | 15.31 | 3.71 | <0.001 | −3, 45, 33 |
| Superior temporal gyrus | 22 | 13.53 | 3.47 | <0.001 | 57, −51, 9 |
| Inferior frontal gyrus | 10 | 13.26 | 3.43 | <0.001 | −51, 30, 0 |
| Insula | 12 | 12.14 | 3.27 | 0.001 | 39, −27, 6 |
| 10.36 | 3 | 0.001 | 39, −27, 18 | ||
| Middle temporal gyrus | 5 | 10.73 | 3.06 | 0.001 | −54, −9, −15 |
| 6 | 9.78 | 2.9 | 0.002 | 54, 3, 39 | |
| Recognition | |||||
| Medial frontal gyrus | 129 | 17.76 | 4.02 | <0.001 | −6, 48, 39 |
| 15.49 | 3.74 | <0.001 | −3, 33, 45 | ||
| 12.04 | 3.26 | 0.001 | −12, 39, 24 | ||
| Putamen | 5 | 12.41 | 3.31 | <0.001 | −30,−3, −6 |
| Inferior parietal lobe | 8 | 10.52 | 3.02 | 0.001 | −24, 57, 15 |
A CEM (No Abuse, CEM) × Words (positive, negative, neutral) RM ANCOVA on extracted mPFC activations in this cluster, with psychiatric status (demeaned within group), age, gender, education level and dummies for location as covariates showed, besides the main effect of CEM [F(1, 186) = 11.26, P = 0.001], a marginal main effect of Words [F(2, 372) = 2.78, P = 0.06]. Positive words elicited more mPFC activation (mean = 0.28, s.e. = 0.04) compared with neutral (mean = 0.16, s.e. = 0.05; P < 0.01), but not negative words (mean = 0.25, s.e. = 0.04, P = 0.70). No other differences were found (P-values > 0.11). There was no Words × CEM interaction neither other significant main nor interaction effects (F-values < 2.19, P-values > 0.13). Current psychiatric status had a main effect on mPFC activation [F(1, 186) = 7.93, P = 0.01); HC had more mPFC activations than patients (t-values > 2.75, P-values < 0.007).
Additional covariance analyses showed that the main effect of CEM remained significant when we co-varied for depression or anxiety severity, neuroticism scores, parental psychopathology, negative life events, concurrent physical and/or sexual abuse, antidepressant medication use or mPFC volume in the CEM group (see Supplementary Data).
Finally, to investigate the functional connectivity of this mPFC cluster (x = −3 y = 45 z = 33) in individuals with CEM (compared with No Abuse), we performed a psycho-physiological interaction (PPI) analysis (specifics in Supplementary Data; Friston et al., 1997).5 Across participants, the PPI showed positive connectivity with the right amygdala (K = 9, x = 21, y = 0, z = −15, Z = 3.87, Psvc < 0.004) and left hippocampus (K = 17, x = −24, y = −12, z = −18, Z = 3.97, Psvc < 0.02). No negative connectivity was found with our ROIs. However, no differential connectivity was found for the CEM vs No Abuse groups within our ROIs (Supplementary Data and Supplementary Table S3).
Recognition
Main effect of task during word recognition
The main effect of task during recognition was associated with mPFC activations (K = 129, x = −3, y = 27, z = 48, Z = 6.85); (K = 54, x = −30, y = −3, z = 57, Z = 6.71); (K = 45, x = 3, y = 63, z = 3, Z = 6.57); (K = 51, x = 33, y = 48, z = 30, Z = 6.46), (K = 5, x = 0, y = 9, z = 39, Z = 4.79), but neither with amygdala nor hippocampal activations. Supplementary Table S2 displays task activations outside our ROIs.
Impact of CEM on word recognition in the mPFC
A CEM vs No Abuse analysis showed CEM-related mPFC hypoactivation during the correct recognition of positive, negative and neutral words (K = 152, x = −6 y = 48 z = 39, Z = 4.18, PSVC = 0.007, Figure 1). No other significant clusters were found in or outside our ROIs (Table 2).
Next, we performed a CEM (CEM vs No Abuse) × Words (Positive, Negative, Neutral) RM ANCOVA on extracted mPFC activations, with psychiatric status (demeaned within group), age, gender, education level and dummies for location as covariates. Besides the main effect of CEM [F(1, 186) = 18.34, P < 0.001], there was no main effect of Words [F(2, 372) = 0.04, P = 0.96]. Psychiatric status did have a main effect [F(1, 186) = 9.25, P = 0.003], with HCs having higher mPFC activations than patients (t-values > 3.54, P-values < 0.001). Furthermore, gender had a marginal main effect [F(1, 186) = 3.53, P = 0.06], with males having marginally more mPFC activation than females for positive words (t = 1.74, P = 0.08), but not for negative or neutral words (t-values < 1.48, P-values > 0.14). Location had a significant main effect {i.e. AMC = [F(1, 186) = 5.24, P = 0.02] and LUMC = [F(1, 186) = 3.62, P = 0.06]}. Participants scanned at the AMC had marginally more mPFC activation for negative words (t = 1.90, P = 0.06), but not for positive or neutral words (ts > 1.14, P-values > 0.26). Post hoc t-tests showed that participants scanned in Leiden did not have more mPFC activation (all ts > 1.40, all P-values > 0.16). There was no Words × CEM interaction, neither other main nor interaction effects (Fs < 1.82, P-values > 0.16).
Follow-up covariance analyses showed that CEM-related hypoactivation could not be explained by more depression or anxiety severity, neuroticism scores, parental psychopathology, negative life events, concurrent physical and/or sexual abuse, antidepressant medication use or mPFC volume (Supplementary Data).
Finally, a PPI analysis in this mPFC cluster (x = −6, y = 48, z = 39), revealed positive connectivity with the left amygdala (K = 11, x = −27, y = 0, z = −18, Z = 3.64, Psvc < 0.009) and left hippocampus (K = 22, x = −21, y = −12, z = −24, Z = 4.98, Psvc < 0.005), but no negative connectivity with the mPFC, across participants. Finally, no CEM-related differential connectivity was found within our ROIs (Supplementary Data and Supplementary Table S4).
DISCUSSION
We show consistent CEM-related mPFC hypoactivation during the encoding and recognition positive, negative and neutral words, a task that requires higher order cognitive processing. Our findings cannot be explained by CEM-related higher levels of neuroticism, parental psychopathology, negative life events, concurrent physical and/or sexual abuse, antidepressant medication use or smaller mPFC volume (Van Harmelen et al., 2010b). In addition, the mPFC hypoactivations were not accounted for by psychiatric status, or by higher depressive or anxiety symptoms, despite the fact that the CEM group contained more patients and those patients showed mPFC hypoactivation compared with HC.
Contrary to our predictions, limbic activations were not enhanced and PPI analyses showed no CEM-related differential mPFC–amygdala coupling either. Therefore, and together with findings of CEM-related amygdala hyperactivity to facial expressions (McCrory et al., 2011, 2013; Bogdan et al., 2012; Dannlowski, et al. 2012a,b; Van Harmelen et al., 2013), these findings suggest that individuals reporting CEM show hypoactive mPFC activation during cognitive processing/evaluation for meaning/content (subserved by the mPFC) and hyperactive amygdala activation in response to emotionally demanding tasks or contexts, which require amygdala processing. Interestingly, this pattern of findings resembles those of studies on the impact of acute stress exposure, showing that stress exposure induces a shift from higher cognitive to more habitual/emotional processes and related neural systems (PFC vs limbic regions) (Hermans et al., 2011; Oei et al., 2012).
Individuals reporting CEM showed similar response accuracy and RTs for positive, negative and neutral words. Thus, although enhanced negative stimuli processing and related brain activations have been reported in depressed individuals (see for an overview: Groenewold et al., 2013), and in post-traumatic stress disorder (PTSD) (see for an overview: Brown and Morey, 2012), we did not find support for CEM-related biased processing of negative stimuli. It is unclear whether this reflects a lack of biased processing, or whether the task at hand was not sensitive enough to detect biases. The classification task did not assess appraisal of the words; hence, even though participants know how to accurately categorize the words they may still appraise them as more negative. In addition, recognition was assessed after a short (10 min) retention interval, making our task prone to performance ceiling effects that may obscure performance biases.
We found CEM-related mPFC hypoactivation across valence, however, on a behavioral level, we did not find similarly reduced cognitive processing. The CEM group was as accurate and fast in categorizing words as the No Abuse group. Hence, mPFC hypoactivation in individuals reporting CEM may resemble a more general blunting of cognitive processing in these individuals; individuals reporting CEM may require less cognitive and related mPFC processing in order to correctly recognize words later on. It is unknown whether this overall blunting of mPFC activation translates to other cognitive domains, which one might expect given that the mPFC is also implicated in self-referential processing and mentalizing (Frith et al., 2003; Frith and Frith, 2006; Mitchell et al., 2006; Denny et al., 2012; Meyer et al., 2013). Future studies are needed to investigate whether CEM-related mPFC hypoactivation is related to dysfunctions in these forms of social cognitive processing, as this may have important clinical implications.
Some limitations need to be taken into account. First, retrospective self-reported CEM is innately subjective and patients may over-report CEM histories. However, maltreatment history is more likely to be under- than over-reported (Hardt and Rutter, 2004; Brewin, 2007), and in the NESDA sample (N = 2981), CEM recall was not affected by current mood state (Spinhoven et al., 2010). Moreover, a history of maltreatment (including emotional abuse and emotional neglect) based on the NEMESIS trauma interview has been associated with an increased incidence and prevalence of psychiatric disorders, suggesting that the NEMESIS trauma interview has good construct validity (e.g. de Graaf et al., 2002; 2004; Wiersma et al., 2009; Hovens et al., 2010; Spinhoven et al., 2010; van Harmelen et al., 2010a). Furthermore, in a confirmatory factor analysis, type of abuse on the NEMESIS trauma interview showed loadings on latent constructs for abuse type comparable with the loading of analogous subscales of the childhood trauma questionnaire (CTQ, Thombs et al., 2009), which is a well validated and reliable questionnaire on childhood trauma (Thombs et al., 2009) (Spinhoven et al., submitted for publication). In addition, compared with the CTQ, CEM is more likely to be under-reported than over-reported in the NEMESIS trauma interview and patients were shown to be somewhat more consistent in their reports than individuals without psychopathology (Spinhoven et al., submitted for publication).
Second, IQ was not assessed as a potential confound in our analyses. However, education level, which is highly correlated with IQ (r = 0.88; Gottfredson, 1997), did not explain our findings. Third, although the effects of CEM on brain functioning remain after regressing out important potential confounds such as psychopathology, parental psychopathology and neuroticism, comparing the CEM and No Abuse groups is intrinsically confounded by these factors and in the context of GLM, only linear components of such effects are addressed this way. Regressing out confounders cannot fully solve this problem and future studies may have to address this issue by directly comparing, for example, individuals with CEM and high levels of psychopathology vs individuals with CEM and no psychopathology. Fourth, contrary to our expectations, we did not find significant hippocampal or amygdala activations related to CEM during word encoding and retrieval. And although hippocampal and amygdala activations during word encoding and recognition in largely the same sample have been linked to psychopathology (van Tol et al., 2012), we cannot rule out the possibility that our null findings regarding the impact of CEM in these regions may be due to the design of our study, namely a multi-site MRI collaboration. A multi-site MRI study may increase between-subject variability due to different scanner specifications and may therefore decrease sensitivity in detecting small effects. However, previous work on largely the same (multi-site) sample (see van Harmelen et al., 2013) found CEM-related increased activation in the amygdala during emotional face processing. This suggests that our multi-site design is sensitive enough to identify overall group differences, and hence cannot fully explain the lack of effects in the amygdala and hippocampus in the context of word encoding. Fifth, our cross-sectional design obscures causality inferences; mPFC hypoactivation may have been present before CEM and may even been a pre-disposing factor that enhances parental risk to emotionally maltreat their children. However, continuing this line of reasoning, it might be expected that parental psychopathology is related to our findings, and it was not. Theoretically, only longitudinal studies can disentangle the impact of CEM from its pre-disposing factors. However, these studies are highly problematic from an ethical point of view, hence, our cross-sectional study with a large sample of patients and HCs and control of many potential confounds is a good alternative.
CONCLUSION
We found that CEM is related to mPFC hypoactivation during the encoding and recognition of positive, negative and neutral words. This was not explained by higher depression or anxiety symptoms, neuroticism, parental psychopathology, negative life events, antidepressant use or by mPFC volume. Together with previous findings of CEM-related smaller mPFC volume (Van Harmelen et al., 2010b) and amygdala hyperactivity to facial expressions (McCrory et al., 2011, 2013; Bogdan et al., 2012; Dannlowski et al., 2012a,b, submitted for publishing; Van Harmelen et al., 2012), these findings suggest that CEM increases individuals’ risk to the development of psychopathology (Spinhoven et al., 2010; Iffland et al., 2012) on differential levels of processing in the brain; mPFC hypoactivation during cognitive processing or more basal amygdala hyperactivation during emotion processing. Therefore, our findings add substantively to the understanding of the long-term impact of CEM.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The infrastructure for the NESDA study (www.nesda.nl) is funded through the Geestkracht program of the Netherlands Organisation for Health Research and Development (ZonMw, grant number 10-000-1002) and is supported by participating universities and mental health care organizations (VU University Medical Center, GGZ inGeest, Arkin, Leiden University Medical Center, GGZ Rivierduinen, University Medical Center Groningen, University of Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Scientific Institute for Quality of Care (IQ Healthcare), Netherlands Institute for Health Services Research (NIVEL) and Netherlands Institute of Mental Health and Addiction (Trimbos). The authors A.-L. vH. and B.M.E. were funded by a VIDI grant (grant number 016.085.353), awarded by NWO to B.M.E. A.A. received an investigator-initiated unrestricted research grant from Brystol-Myers Squibb and speakers bureau honoraria from AstraZeneca, Brystol-Myers Squibb, GlaxoSmithKline and Janssen. N.J.A.vdW. received speaking fees from Eli Lilly and Wyeth and served on advisory panels of Eli Lilly, Pfizer, Wyeth and Servier.
Footnotes
1SCR_pos, SCR_neg, SCR_neu, SMISS_pos, SMISS_neg, SMISS_neu, BL. (SCR = subsequently correct; SMISS = subsequently missed).
2CREC_pos, CREC_neg, CREC_neu, CREJ_pos, CREJ_neg, CREJ_neu, FA_pos, FA_neg, FA_neu, MISS_pos, BL. (CREC = Correct recognition; CREJ = correct rejections; MISS = misses).
3For the word classification task, data from 16 individuals were missing (six reported No Abuse).
4The mPFC activations for encoding and recognition were small-volume corrected using a mask based on the left superior frontal medial cortex, 584 voxels, region based on AAL toolbox.
5Due to technical problems with fMRI data of three participants (one reported CEM), we could not include these participants in the PPI analyses.
REFERENCES
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10(6):410–22. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. American Journal of Psychiatry. 2008;165(8):969–77. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56(6):893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor Iso/Val (r5522) genotye moderates the association between previous childhood emotional neglect and amygdala reactivity. American Journal Of Psychiatry. 2012;169:515–22. doi: 10.1176/appi.ajp.2011.11060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox [abstract]. In Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan.2002. [Google Scholar]
- Brewin CR. Autobiographical memory for trauma: update on four controversies. Memory. 2007;15(3):227–48. doi: 10.1080/09658210701256423. [DOI] [PubMed] [Google Scholar]
- Brown VM, Morey RA. Neural systems for cognitive and emotional processing in posttraumatic stress disorder. Frontiers in Psychology. 2012;3:449. doi: 10.3389/fpsyg.2012.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugha T, Bebbington P, Tennant C, Hurry J. The list of threatening experiences: a subset of 12 life event categories with considerable long-term contextual threat. Psychological Medicine. 1985;15(1):189–94. doi: 10.1017/s003329170002105x. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience and Biobehavioral Reviews. 2002;26(3):321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr., McGrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) manual. Odessa, FL: Psychological Assessments Resources; 1992. [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Kugel H, Huber F, et al. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Human Brain Mapping. 2012a;34(11):2899–909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012b;71(4):286–93. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SARB, Raaijmakers JGW, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126(Pt 1):43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- De Graaf R, Bijl RV, ten Have M, Beekman AT, Vollebergh WA. Rapid onset of comorbidity of common mental disorders: findings from the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Acta Psychiatrica Scandinavia. 2004;109:55–63. doi: 10.1046/j.0001-690x.2003.00222.x. [DOI] [PubMed] [Google Scholar]
- De Graaf R, Bijl RV, Smit F, Vollebergh WA, Spijker J. Risk factors for 12-month comorbidity of mood, anxiety, and substance use disorders: findings from the Netherlands Mental Health Survey and Incidence Study. American Journal of Psychiatry. 2002;159(4):620–9. doi: 10.1176/appi.ajp.159.4.620. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience. 2012;24(8):1742–52. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland B. Taking stock: childhood emotional maltreatment and developmental psychopathology. Child Abuse & Neglect. 2009;33(1):22–6. doi: 10.1016/j.chiabu.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50(4):531–4. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society B: Biological Sciences. 2003;358(1431):459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373(9657):68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- Gottfredson LS. Why g matters: The complexity of everyday life. Intelligence. 1997;24(1):79–132. [Google Scholar]
- Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neuroscience and Biobehavioral Reviews. 2013;37(2):152–63. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, et al. Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. Journal of Neuroscience. 2012;32(23):7917–25. doi: 10.1523/JNEUROSCI.0307-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2004;45(2):260–73. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Frontiers in Human Neuroscience. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJF, Ossewaarde L, et al. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334(6059):1151–3. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Hovens JGFM, Wiersma JE, Giltay EJ, et al. Childhood life events and childhood trauma in adult patients with depressive, anxiety and comorbid disorders vs. controls. Acta Psychiatrica Scandinavica. 2010;122(1):66–74. doi: 10.1111/j.1600-0447.2009.01491.x. [DOI] [PubMed] [Google Scholar]
- Huskisson EC. Visual analogue scales. In: Melzack R, editor. Pain Measurement and Assessment. New York: New York Raven Press; 1993. pp. 33–37. [Google Scholar]
- Iffland B, Sansen LM, Catani C, Neuner F. Emotional but not physical maltreatment is independently related to psychopathology in subjects with various degrees of social anxiety: a web-based internet survey. BMC Psychiatry. 2012;12(1):49. doi: 10.1186/1471-244X-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka T, Okada T, Murata T, et al. Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus. 2002;12(3):352–62. doi: 10.1002/hipo.1113. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Kelly PA, et al. Amygdala activation in maltreated children during pre-attentive emotional processing. British Journal of Psychiatry. 2013;202(4):269–76. doi: 10.1192/bjp.bp.112.116624. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Sebastian CL, et al. Heightened neural reactivity to threat in child victims of family violence. Current Biology. 2011;21(23):R947–8. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. The link between child abuse and psychopathology: a review of neurobiological and genetic research. Journal of the Royal Society of Medicine. 2012;105(4):151–6. doi: 10.1258/jrsm.2011.110222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62(1):3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer ML, Masten CL, Ma Y, et al. Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Social Cognitive and Affective Neuroscience. 2013;8(4):446–54. doi: 10.1093/scan/nss019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50(4):655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Oei NYL, Veer IM, Wolf OT, Spinhoven P, Rombouts SA, Elzinga BM. Stress shifts brain activation towards ventral “affective” areas during emotional distraction. Social Cognitive and Affective Neuroscience. 2012;7(4):403–12. doi: 10.1093/scan/nsr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BWJH, Beekman ATF, Smit JH, et al. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. International Journal of Methods in Psychiatric Research. 2008;17(3):121–40. doi: 10.1002/mpr.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry. 2003;54(5):504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Messner M, Cohn JF. Development of perceptual expertise in emotion recognition. Cognition. 2009;110(2):242–47. doi: 10.1016/j.cognition.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter DS. The developmental neuroscience of emotional neglect, its consequences, and the psychosocial interventions that can reverse them. American Journal of Psychiatry. 2012;169(5):452–4. doi: 10.1176/appi.ajp.2012.12020174. [DOI] [PubMed] [Google Scholar]
- Spinhoven P, Elzinga BM, Hovens JGFM, et al. The specificity of childhood adversities and negative life events across the life span to anxiety and depressive disorders. Journal of Affective Disorders. 2010;126(1–2):103–12. doi: 10.1016/j.jad.2010.02.132. [DOI] [PubMed] [Google Scholar]
- Thombs BD, Bernstein DP, Lobbestael J, Arntz A. A validation study of the Dutch Childhood Trauma Questionnaire-Short Form: factor structure, reliability, and known-groups validity. Child Abuse & Neglect. 2009;33(8):518–23. doi: 10.1016/j.chiabu.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Todd RM, Evans JW, Morris D, Lewis MD, Taylor MJ. The changing face of emotion: age-related patterns of amygdala activation to salient faces. Social Cognitive and Affective Neuroscience. 2011;6(1):12–23. doi: 10.1093/scan/nsq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harmelen A-L, De Jong PJ, Glashouwer KA, Spinhoven P, Penninx BWJH, Elzinga BM. Child abuse and negative explicit and automatic self-associations: the cognitive scars of emotional maltreatment. Behaviour Research and Therapy. 2010a;48(6):486–94. doi: 10.1016/j.brat.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Van Harmelen A-L, Van Tol M-J, Demenescu LR, et al. Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Social Cognitive and Affective Neuroscience. 2013;8:362–9. doi: 10.1093/scan/nss007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harmelen A-L, Van Tol M-J, Van Der Wee NJA, et al. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biological Psychiatry. 2010b;68(9):832–8. doi: 10.1016/j.biopsych.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Van Tol M-J, Demenescu LR, van der Wee NJ, et al. Functional magnetic resonance imaging correlates of emotional word encoding and recognition in depression and anxiety disorders. Biological Psychiatry. 2012;71(7):593–602. doi: 10.1016/j.biopsych.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Veer IM, Oei NYL, Spinhoven P, et al. Beyond acute social stress: increased functional connectivity between amygdala and cortical midline structures. NeuroImage. 2011;57(4):1534–41. doi: 10.1016/j.neuroimage.2011.05.074. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. The uncertainty of it all. Trends in Cognitive Sciences. 2007;11(12):499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Wiersma J, Hovens JE, van Oppen P, et al. Childhood trauma as a risk factor for chronicity of depression. Journal of Clinical Psychiatry. 2009;70(7):983–9. doi: 10.4088/jcp.08m04521. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Robins LN, Cottler LB, Sartorius N, Burke JD, Regier D. Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI). The Multicentre WHO/ADAMHA Field Trials. British Journal of Psychiatry. 1991;159:645–58. doi: 10.1192/bjp.159.5.645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.