Abstract
The social brain hypothesis proposes that the large size of the primate neocortex evolved to support complex and demanding social interactions. Accordingly, recent studies have reported correlations between the size of an individual’s social network and the density of gray matter (GM) in regions of the brain implicated in social cognition. However, the reported relationships between GM density and social group size are somewhat inconsistent with studies reporting correlations in different brain regions. One factor that might account for these discrepancies is the use of different measures of social network size (SNS). This study used several measures of SNS to assess the relationships SNS and GM density. The second goal of this study was to test the relationship between social network measures and functional brain activity. Participants performed a social closeness task using photos of their friends and unknown people. Across the VBM and functional magnetic resonance imaging analyses, individual differences in SNS were consistently related to structural and functional differences in three regions: the left amygdala, right amygdala and the right entorhinal/ventral anterior temporal cortex.
Keywords: Facebook, social networks, amygdala, orbitofrontal cortex, Dunbar’s number
Primates, compared to other vertebrates, have a disproportionately large brain to body ratio that is monotonically related to the size of their social groups (Jerison, 1973). This uneven proportion can primarily be accounted for by an increased size of the neocortex (Finlay and Darlington, 1995), which according to the social brain hypothesis, evolved in order for primates to sustain more complex and demanding social interactions (Dunbar, 1993, 1998). Individual differences in brain size as they relate to social group size can also be found within species. For example, recent studies of humans provide evidence of quantitative relationships between individual differences in social group size and regions of the brain that play a major role in social cognition. Particularly prominent have been studies of humans that have reported positive correlations between social function (e.g. social competence), social network size (SNS) and complexity, and volumetric changes in gray matter (GM) regions implicated in social cognition, such as the amygdala, orbitofrontal cortex (OFC), superior temporal sulcus, middle temporal sulcus and entorhinal cortex.
The fact that previous studies have reported diverse loci of volumetric changes is particularly relevant to the goals of the current paper. Bickart et al. (2010) reported that amygdala volume correlated with the size and complexity of social networks, whereas Kanai et al. (2012) tested a larger sample size and reported that the density of GM in the right superior temporal sulcus, amygdala, left middle temporal gyrus and entorhinal cortex correlated with differences in the size of social networks. In contrast, Lewis et al. (2011) emphasized that portions of the frontal lobe including OFC correlated best with social network measures.
One factor that could be responsible for the discrepancies reported in previous studies is the disparate set of social network measures used by different research groups such as Dunbar’s number (Dunbar, 1993), the number of Facebook friends, the Social Network Index (SNI) (Cohen et al., 1997) or the Norbeck Social Support Network Score (Norbeck et al., 1981). Although, presumably, there is a correlation between these social network measures, the correlations are not as strong as one might expect and different measures of SNS should not be considered interchangeable. The number of ‘friends’ a person has on Facebook, for example, is often significantly larger than the size of a person’s real-world social network (Kanai et al., 2012). This difference in size is partially attributable to the greater ease with which online vs real-world social connections can be initiated and maintained, but other factors such as differences in the purpose of maintaining online vs real-world social networks could also play a role (Lewis et al., 2008; Wellman, 2012). There are also inconsistencies between various measures of real-world SNS. Dunbar’s number, for example, measures the number of individuals a person can maintain in their active social circle, which averages ∼150 people for most individuals (Dunbar, 1993). In contrast, the SNI (Cohen et al., 1997) measures SNS as the number of individuals a person has had regular contact with at least once every 2 weeks and typically ranges between 0 and 60 people for each individual. Although each of these reportedly measures real-world SNS, they assess it in distinctly different ways. Understanding the meaning of brain structure–function relationships is critically dependent on the underlying measurement tools. In the case of social networks, we do not yet understand the relationship between various measures of SNS and volumetric changes in distinct brain regions.
In this study, we begin by examining the correlations between one online measure of SNS, the number of Facebook friends and two real-world measures, Dunbar’s number [sympathy group], and Norbeck Social Support Group. The first two measures were chosen to attempt replication of two prior studies of SNS (Lewis et al., 2011; Kanai et al., 2012). The latter measure was chosen as a second measure of real-world SNS operationalized as the number of important people in an individual’s social support network (Norbeck et al., 1981).
Compared to real-world relationships, building strong and emotionally close relationships via online media is limited and provides an opportunity for humans to circumvent the cognitive limitations associated with real-world social relationships (Dunbar, 2012). Depending on the social forum, it can be far less cognitively expensive to maintain connections with others online than in person (e.g. maintaining someone as a friend on Facebook takes far less cognitive effort than maintaining a friendship in the real world). Despite these differences, there is evidence to suggest that the number of social contacts one maintains online might correlate to some degree with the size of the real-world social networks (Goncalves et al., 2011; Kanai et al., 2012). Thus, we predicted that there would be significant correlations between our online and real-world measures of SNS.
The second goal of this study was to examine the relationship between SNS and volumetric changes in GM in regions of the brain implicated in social cognition. As noted earlier, the regions of the brain associated with SNS are troublingly inconsistent (Table 4). One factor that might be responsible for these discrepancies is the use of distinctly different measures of SNS across studies. This study uses three measures of SNS to investigate the relationship between SNS and volumetric changes in GM. We were particularly interested in finding brain regions in which correlations with SNS were evident across all social network measures, thus providing a robust triangulation of evidence.
Table 4.
A list of neuroimaging studies, including ours, of SNS
| Correlations with GM density |
||||||
|---|---|---|---|---|---|---|
| Previous studies |
Present study |
|||||
| ROI |
Bickart et al. (2010) |
Kanai et al. (2012) |
Lewis et al. (2011) |
|||
| SNI | FB | D[SG] | FB | D[SG] | NSSN | |
| L amygdale* | √ | √ | – | √ | √ | √ |
| R amygdala* | √ | √ | – | √ | √ | √ |
| R subgenual ACC | √ | – | – | – | – | – |
| L posterior ITS | √ | – | – | – | – | √ |
| L posterior SFG (premotor) | √ | – | – | √ | √ | – |
| R posterior STS | – | √ | – | – | – | – |
| L MTG | – | √ | – | – | – | – |
| R MTG | – | – | – | – | – | – |
| L entorhinal | – | – | – | √ | – | – |
| R entorhinal | – | √ | – | – | √ | √ |
| L OFC* | – | – | – | √ | √ | √ |
| R OFC* | – | – | √ | √ | √ | √ |
The leftmost column lists neuroanatomical regions found to positively correlate with various social network measures. A check indicates that study reported a significant correlation between a particular measure of SNS and GM density in that brain region. Bold text indicates that our study replicated a prior finding. An asterisk indicates that a finding from a previous study was replicated across all of our SNS measures. Results listed from Kanai et al. (2012) are from Experiments 1 and 2.
Finally, we were interested in gaining some insight into why some people might have larger social networks than others? It is plausible that individuals with larger and richer social networks simply find social information more interesting, salient, rewarding and memorable. Social animals have evolved neural mechanisms that cause them to preferentially orient toward stimuli with social value, cultivate social networks through ‘social grooming’ behaviors and find social interactions rewarding. For instance, we tend to look in the direction indicated by another’s eye gaze (Frischen et al., 2007). We use gossip and flattery of other people, as well as trade favors, to enhance our social networks (Burt and Knez, 1996; Dunbar, 2004). And we tend to find certain social signals, such as a smile, sexually attractive (Otta et al., 1996) and rewarding to the degree that primitive neural structures that process basic rewards such as food also respond to human smiles (Tsukiura and Cabeza, 2008). It should be assumed that there are individual differences in social interest and motivation across the population (Chevallier et al., 2012). Thus, we hypothesized that individuals who maintain larger ‘real world’ social networks would find socially important stimuli more interesting, salient and rewarding compared to individuals who maintain smaller social networks. We predicted that SNS would be significantly and positively correlated with blood oxygenation level-dependent (BOLD) activity in response to social stimuli in the same regions of the brain that are implicated in social network maintenance.
METHODS
Participants
Forty female participants, both adolescents and adults, were recruited from the greater Philadelphia area via local advertisements. The mean age of the adult sample was 25, range 19–30, and the mean age of the adolescent sample was 15, range 12–18. Thus, the overall mean age was 20, range 12–30. The original sample had eight additional participants who were removed for excessive head motion, failure to comply with instructions, are outlier behavioral results, resulting in a final total sample size of 40 participants. Three of the removed participants were adolescents who reported upwards of 1700 Facebook friends; the most extreme example had 2400+ friends. Removing them from the analysis decreased the skewness to a very reasonable 0.538. Our sample was restricted to female participants to decrease variance due to gender differences in face recognition and social cognition (Cross et al., 1971; Ellis et al., 1973; Killgore and Yurgelun-Todd, 2001; Lewin and Herlitz, 2002; Rehnman and Herlitz, 2006, 2007; McBain et al., 2009; Ino et al., 2010; Megreya et al., 2011). All participants received monetary compensation for their participation and were right-handed, native English speakers, had normal or corrected-to-normal vision, normal hearing and had no history of psychological, developmental or neurological disorders. Informed consent was obtained according to the guidelines of the Institutional Review Board of the Temple University. Portions of this data set (adult sample, select conditions) were published in another manuscript (Von Der Heide et al., 2013).
Estimation of social network size
SNS was estimated in three ways: (i) number of Facebook friends. Participants were asked to look up and report the number of ‘friends’ they had on Facebook. We considered this a measure of the size of an individual’s online social network. (ii) Dunbar’s number [sympathy group]. Using the questionnaire described in Lewis et al. (2011), participants were asked to report the number of individual’s they had social contact with over the last 30 days. This was considered a measure of a person’s real-world SNS and has also been referred to as the number of individuals in a person’s ‘sympathy group’. (iii) Norbeck Social Support Questionnaire (Norbeck et al., 1981). This is a measure of real-world social networks individual’s social support network.
The first two SNS measures were chosen because they were used in prior Voxel based morphometry (VBM) studies of SNS (Lewis et al., 2011; Kanai et al., 2012). The later questionnaire was included as a measure of the size of an individual’s social support network and a second measure of real-world SNS (Norbeck et al., 1981). We considered using the SNI, but ultimately excluded it because the questions would not be suitable for use with an adolescent population.
VBM methods
Neuroimaging sessions [both VBM and functional magnetic resonance imaging (fMRI)] were conducted at the Temple University Hospital on a 3.0 T Siemens Verio scanner (Erlangen, Germany) using a 12-channel Siemens head coil. Immediately prior to collecting the fMRI data, a high-resolution anatomical scan that lasted ∼10 min was collected for each participant. The anatomical image was used to conduct the VBM analyses and was also used to fit the volume of covered brain tissue acquired in the functional scan. The T1-weighted images were acquired using a three-dimensional magnetization-prepared rapid acquisition gradient echo pulse sequence [repetition time (TR), 1900 ms; echo time (TE), 2.94 ms; field of view (FOV) = 188 × 250 mm; inversion time, 900 ms; voxel size, 1 × 0.9766 × 0.9766 mm; matrix size, 188 × 256; flip angle = 9°, 144 contiguous slices of 0.9766 mm thickness].
Images were segmented for GM and white matter (WM) [as well as cerebrospinal fluid (CSF), the skull, soft tissue and extracranial regions] using the segmentation tools in Statistical Parametric Mapping 8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm). Using the GM and WM from segmentation, we performed Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) in SPM8 to generate averaged templates to be used for iterative alignment. Participants’ data were normalized to MNI space in SPM8 using DARTEL generated templates, GM images and Jacobian determinants of the deformation fields. A Gaussian Kernel (full width at half maximum = 8 mm) was used for smoothing. Image intensity was modulated for smoothing and spatial normalization. Total intracranial volume (TIV) for each participant was calculated by summing the volume of GM, WM and CSF in ml (Ashburner and Friston, 2000).
A multiple regression analysis was performed on the smoothed GM images in SPM8 to determine regions in which GM density showed a correlation with our participants’ various behavioral measures. The image intensity of the preprocessed images was set to an absolute threshold of 0.1 for masking. Participants’ age and TIV were included in the design matrix as covariates of no interest to regress out any effects these factors may have. Age and TIV were not significantly related (Pearson’s correlation coefficient = 0.148, P = 0.362). Separate models were created for different behavioral measures. Subjects who did not provide a given behavioral measure were not included in that specific analysis.
Using previously utilized social network measures, as well as our novel behavioral measures as covariates of interest, we performed a region of interest (ROI) analysis using ROIs implicated in social networks and theory of mind (Dunbar, 2004; Bickart et al., 2010; Lewis et al., 2011; Kanai et al., 2012) to observe volumetric differences correlated to those measures. Ten millimeter radius spheres were created around the coordinates listed in Table 1. A mask for the OFC, thought to be implicated in social behavior (Powell et al., 2012) was created using the AAL template provided by Tzourio-Mazoyer et al. (2002) in the WFU Pickatlas toolbox (Maldjian et al., 2003, 2004). Pre-existing masks from the AAL atlas were used to construct a combined a mask for the left (Frontal_Inf_Orb_L, Frontal_Med_Orb_L, Frontal_Sup_Orb_L) and right (Frontal_Inf_Orb_R, Frontal_Med_Orb_R, Frontal_Sup_Orb_R) OFC. This combined mask included anterior, posterior and medial orbital gyri and the gyrus rectus. It did not include the anterior cingulated. The most superior slice was at z = −3.
Table 1.
ROIs were represented using 10 mm spheres centered on the MNI coordinates listed below
| ROI | MNI coordinates |
||
|---|---|---|---|
| x | y | z | |
| L amygdala (Kanai et al., 2012) | −24 | −2 | −22 |
| R amygdala (Kanai et al., 2012) | 26 | 0 | −22 |
| R subgenual anterior cingulate cortex (ACC) (Bickart et al., 2010) | 8 | 29 | −8 |
| L posterior ITS (Bickart et al., 2010) | −59 | −42 | −17 |
| L posterior SFG (Bickart et al., 2010) | −10 | 6 | 67 |
| L posterior STS (Kanai et al., 2012) | −63 | −54 | 10 |
| R posterior STS (Kanai et al., 2012) | 63 | −54 | 10 |
| L MTG (Kanai et al., 2012) | −57 | −54 | −6 |
| R MTG (Kanai et al., 2012) | 57 | −54 | −6 |
| L entorhinal (Kanai et al., 2012) | −30 | −10 | −42 |
| R entorhinal (Kanai et al., 2012) | 30 | −10 | −42 |
| L OFC (AAL toolbox) | |||
| R OFC (AAL toolbox) | |||
These coordinates were chosen based on published findings linking these regions to SNS. The publication associated with the coordinates used to create the ROI is listed after the region. In the case of the OFC, standard anatomical markers were created using the AAL toolbox in the WFU Pickatlas (Tzourio-Mazoyer et al., 2002; Maldjian et al., 2003). This mask included anterior, posterior and medial orbital gyri and the gyrus rectus. The most inferior slice was the inferior surface of the frontal lobe; the most superior slice was at z = −3.
Small volume correction (SVC) was performed on the resulting statistical parametric maps using the aforementioned ROIs to prevent errors of multiple comparisons. All results were significant at P < 0.05, family-wise error corrected for small volumes.
Functional magnetic resonance imaging
Stimuli
Two types of stimuli were used in the fMRI tasks: photographs of friends and photographs of unfamiliar people. Participants brought two photos of five best friends and five close friends to a pre-testing session. The photos of participants’ friends varied in lighting, poses, facial expressions, quality, etc., and the photos of unfamiliar face stimuli were selected to match the approximate age and ethnicities of friends and famous faces. Consistent with the photos of friends and famous people, the unfamiliar faces consisted of a mixture of professional photographs that varied in lighting, facial expression, quality, etc. Visual stimuli were presented to participants through goggles with a resolution of 800 × 600 pixels, purchased from Resonance Technologies, California. Responses were recorded using a four-button fiber optic response pad system. E-prime software (Psychology Software Tools Inc., Pittsburgh, PA, USA) on a Windows laptop located in the scanner room was used to present the stimuli.
fMRI task and design
All participants were provided with standardized computer-based instructions and completed a practice session prior to the scanning session. The stimuli used in the practice session were unique so that participants were naïve to all stimuli presented during the experimental runs. Following the practice session and a 10 min long high-resolution anatomical scan, participants completed a functional localizer task (not discussed here) followed by five fMRI runs of the main task, described next.
The task was a social closeness task using photos of friends and unknown individuals. On each trial, one photo was presented left of center, and the second photo was presented right of center. One photo in each pair was always presented at a discriminable location above the center x-axis of the screen, and the other photo was presented at a counter location below the x-axis. During the social closeness condition, participants indicated by button press whether the friend shown on the left or on the right of the screen was socially closest to them. During baseline blocks, participants were asked to decide which photograph of an unknown person was presented in a higher position on the screen by pressing the left or right button with their index finger. Location was counterbalanced and not included in the analysis. Each run was comprised of 14 blocks that were 15 s long each. Each block consisted of a 3 s instruction screen, followed by a series of five trials consisting of 2.5 s presentations of two facial photographs separated by a 0.5 s inter-trial interval. A 9 s rest period consisting of a fixation cross followed each block. Each condition was presented the same amount of times in each run.
fMRI imaging procedure
Functional T2*-weighted images sensitive to BOLD contrasts were acquired using a gradient-echo echo-planar pulse sequence: TR = 3 s; TE = 20 ms; FOV = 240 × 240; voxel size = 3 × 3 × 2.5 mm; matrix size = 80 × 80; flip angle = 90° and automatic shimming. This pulse sequence was chosen because it optimizes coverage of the anterior temporal lobes (ATL) and the OFC.
Thirty-eight interleaved axial slices with 2.5 mm thickness were acquired to cover the temporal lobes. On the basis of the anatomical information of the structural scan, the lowest slice was individually fitted to cover the most inferior aspect of the inferior temporal lobes.
fMRI image analysis
fMRI data were preprocessed and analyzed using Brain Voyager software (Kriegeskorte et al., 2007). The preprocessing of the functional data included a correction for head motion (trilinear/sinc interpolation), the removal of linear trends and frequency temporal filtering using a fast fourier transform and a cut off of three cycles or sine waves. The data were coregistered with their respective anatomical data and transformed into Talairach space (Waiter et al., 2004). The resulting volumetric time course data were smoothed using an 8 mm Gaussian kernel.
For all blocks, a canonical hemodynamic response function (HRF) was modeled spanning the 15 s for each block. A z-transform was applied to normalize the time course. Predictors were built by convolving the boxcar waveform for each condition with a double-gamma HRF (onset = 0, response undershoot ratio = 6, time to response peak = 5 s, time to undershoot peak = 15 s, response dispersion = 1, undershoot dispersion = 1). Motion parameters were not included as covariates in the regression, because motion was corrected for in preprocessing. Including them as covariates in the regression has been shown to have a deleterious effect on the mean contrast estimates in block design studies (Chevallier et al., 2012). The 3 s instruction screen at the start of each block and the 9 s rest period following each block were modeled out and were also not included in the HRF. Inferior portions of the frontal and temporal lobe are prone to susceptibility artifacts. An examination of individual temporal signal to noise ratio maps were examined for each subject and indicated that the quality of the signal in these regions was excellent. Visual inspection of the co-registered functional image confirmed excellent signal coverage in the anterior temporal lobes in all participants. Signal loss was observed in the ventral-most slice of the OFC, particularly in the gyrus rectus, in some participants.
fMRI data analysis
Peak MNI coordinates from ROIs in the VBM analyses (Table 5) were transformed to Talairach coordinates to create corresponding anatomical ROI for analysis of the fMRI data using the Brainvoyager software. All ROIs were restricted to GM and drawn on a Talairach aligned standard brain. We performed random-effects GLM of the data obtained from all runs of the main experiment using each anatomical ROI. Mean beta weights from this analysis were extracted for three conditions: friends faces, unfamiliar faces and the rest condition. Age was not treated as a variable of interest in the fMRI analyses.
Table 5.
Partial correlations between measures of SNS and BOLD activity, in the friends and unfamiliar faces conditions, controlling for age and activity during fixation (rest period)
| ROI | Friend’s faces |
Unfamiliar faces |
||||
|---|---|---|---|---|---|---|
| FB | D[SG] | NSSN | FB | D[SG] | NSSN | |
| L amygdala | – | 0.44** | 0.36* | – | 0.46** | 0.34* |
| R amygdala | 0.49** | 0.35* | – | 0.55** | 0.38* | 0.31‡ |
| R subgenual ACC | – | – | – | – | – | – |
| L posterior ITS | – | –† | – | – | –† | – |
| L posterior SFG (premotor) | – | – | – | – | – | – |
| R posterior STS | – | –† | – | – | –† | – |
| L MTG | – | 0.30‡ | – | – | 0.42* | – |
| R MTG | – | –† | – | –† | –† | – |
| L entorhinal | – | – | – | – | – | – |
| R entorhinal | 0.35* | –† | – | –† | –† | – |
| L OFC | – | – | – | – | – | – |
| R OFC | – | – | – | – | – | – |
Bold text indicates that the VBM correlations were also significant in this brain region. The ‘†’ symbol indicates that there was a significant correlation when the fixation condition was not included as a covariate, potentially because a degree of freedom was lost during this process. †Approaching significance at P < 0.10; *significant at P < 0.05; **significant at P < 0.01.
Data were inspected to ensure that the assumptions of multiple regression were met: variables were normally distributed, there was a linear relationship existed between the independent and dependent variables, variables met the assumption of homoscedasticity, and outliers >3 standard deviations were removed from the analyses. Regression analyses were performed using social network measures as predictors, beta weights as the dependent variables and by covarying out the effects of age in each analysis. Separate models were created for different behavioral measures. Subjects who did not have a behavioral measure were not included in that specific analysis.
RESULTS
Behavioral results
Summary statistics for social network measures are listed in Table 2. Controlling for age, there were significant correlations between all of the social network measures (Table 3). However, the size of the three types of social networks (Facebook friends, Norbeck SSN, Dunbar’s number [sympathy group]) was all significantly different from each other with the size of participant’s online network (Facebook friends) being the largest (mean = 477.61, s.d. = 252.65), the size of the number of individuals in their sympathy group (Dunbar’s number [sympathy group]) being the second largest (mean = 41.29, s.d. = 18.32) and the number of important individuals in their social support group being the smallest (mean = 12.23, s.d. = 5.33).
Table 2.
Summary statistics of social network measures
| FB | NSSN | D[SG] | |
|---|---|---|---|
| Adults | Mean = 511.14 (275.98) | Mean = 14.29 (5.84) | Mean = 45.50 (18.07) |
| r = −0.56 | r = 0.32 | r = 0.04 | |
| Adolescents | Mean = 430.67 (216.18) | Mean = 9.95 (1.13) | Mean = 36.61 (4.25) |
| r = 0.38 | r = 0.11 | r = 0.20 | |
| All | Mean = 477.61 (252.65) | Mean = 12.23 (5.33) | Mean = 41.29 (18.32) |
| r = −0.09 | r = 0.48 | r = 0.25 |
Means are followed by standard deviations. Note that higher numbers for all social network measures indicate larger social networks. There was a significant difference between adults and adolescents in only one measure, NSSN (P < 0.01). The correlation between SNS and age is listed below the means. In a small number of cases, participants were excluded from specific analyses for the following reasons: their scores were considered outliers (scores >3 standard deviations from the mean), they did not maintain an online Facebook account and/or their SNS could not be calculated because they did not follow instructions. FB, number of Facebook friends; D[SG], Dunbar’s [sympathy group]; NSSN, Norbeck Social Support Network.
Table 3.
Partial correlations between measures of SNS, controlling for age
| FB | NSSN | D[SG] | |
|---|---|---|---|
| FB | r = 1.00 | – | – |
| NSSN | r = 0.37* | r = 1.00 | – |
| Dunbar’s number | r = 0.42* | r = 0.50** | r = 1.00 |
Asterisks indicate that the correlation was significant (*P < 0.05; **P < 0.01).
During the fMRI task, social closeness judgments participants made of their friends were 90% consistent with their previously reported rank orderings. Response consistency was judged by comparing the behavioral data during the scanner task to the rank orderings of friends on social closeness that participants made during the preliminary testing session. Participants were 98% accurate performing the baseline task with photos of unknown people. Adolescents and adults did not differ in their accuracy.
Voxel-based morphometry results
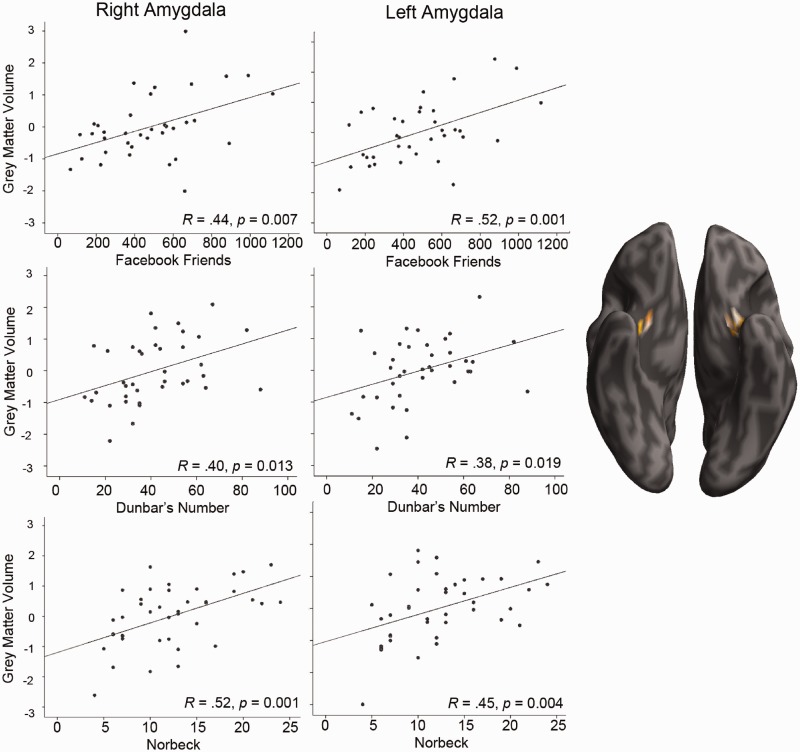
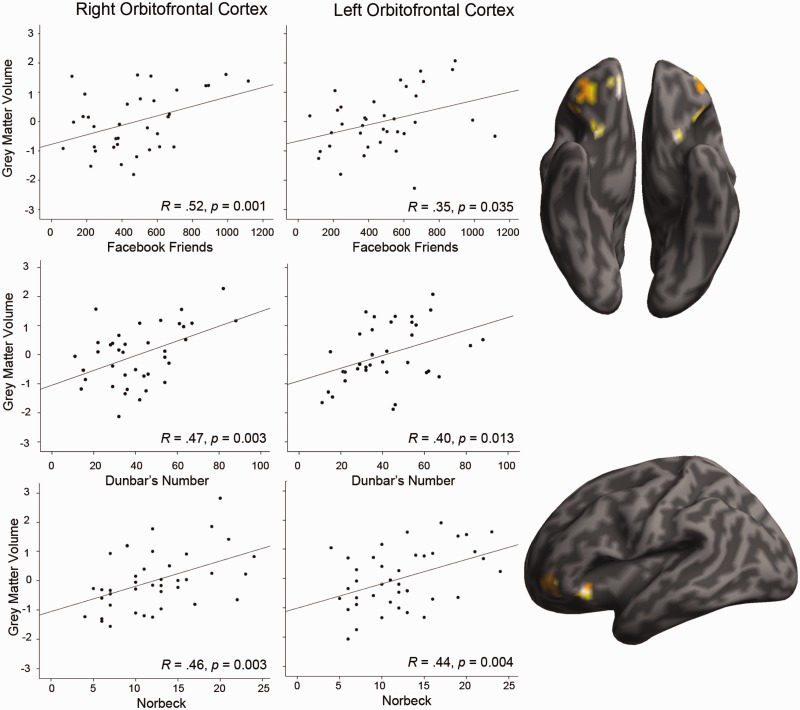
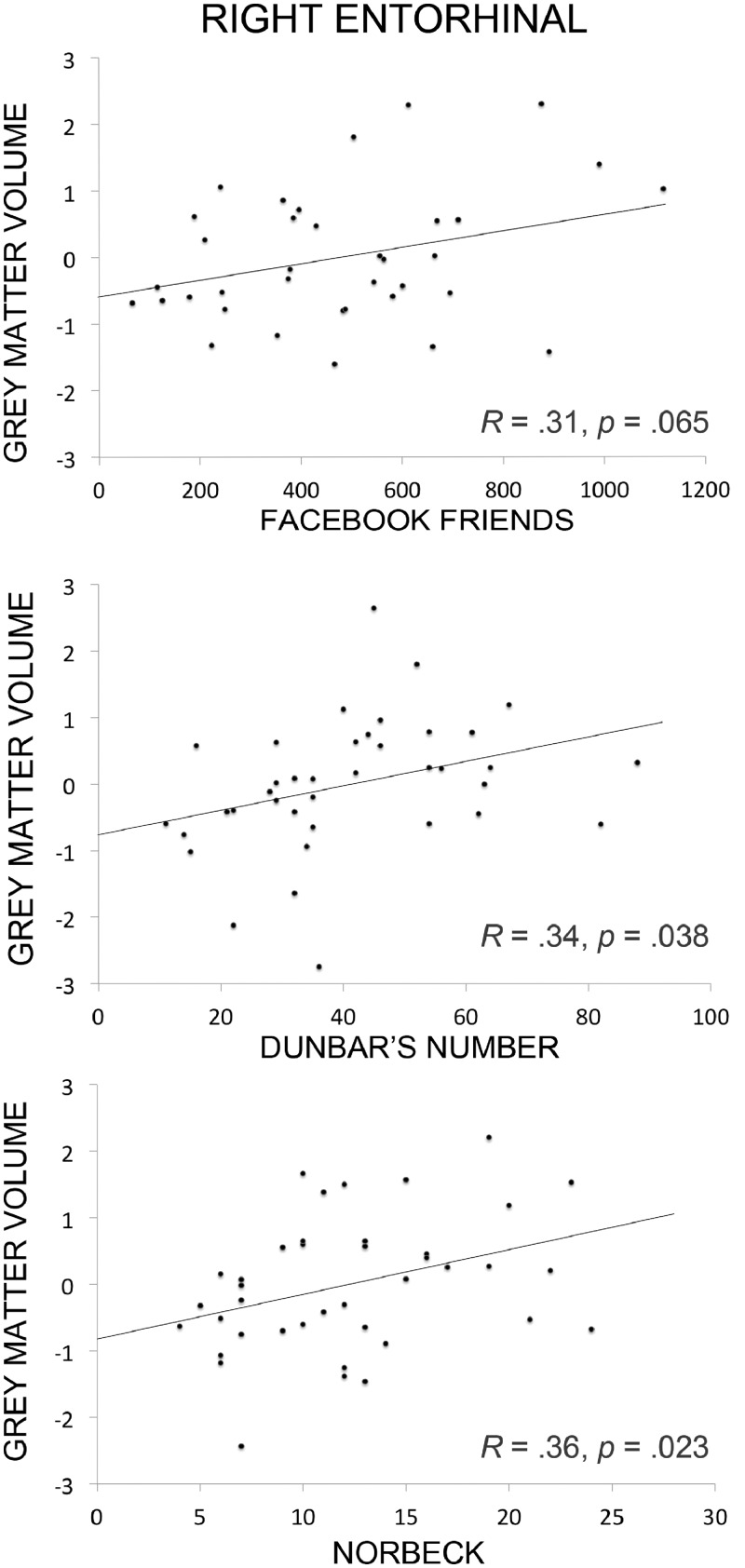
In the first analysis, we examined whether individual differences in the number of social relationships as indexed by our three measures of SNS predicted variability in GM volume in predefined brain regions, based on prior findings. There were statistically significant positive correlations across several regions, however, different social network measures gave rise to different correlations as can be seen in Table 4. Nevertheless, two findings stood out because of their high degree of consistency across social network measures: the bilateral amygdala and portions of the OFC correlated with all three social network measures (Figures 1 and 2), as well as right entorhinal cortex which correlated with real-world social network measures (Figure 3). Several regions identified by prior investigators, such as the posterior superior temporal sulcus and medial temporal gyrus, failed to replicate across any of our social network measures.
Fig. 1.
The relationship between GM density (arbitrary units) of the left and right amygdala and measures of SNS. From top to bottom: Facebook friends (n = 36), Dunbar’s number (n = 38) and Norbeck’s Social Support Network (n = 40). GM density was computed by regressing out age and TIV, and normalized to z-scores across participants. Portions of the amygdala that correlated with SNS are displayed on an inflated brain at P < 0.05 (uncorrected) for illustrative purposes.
Fig. 2.
The relationship between GM density (arbitrary units) in the left and right OFC and measures of SNS. From top to bottom: Facebook friends (n = 36), Dunbar’s number (n = 38) and Norbeck’s Social Support Network (n = 40). GM density was computed by regressing out age and TIV, and normalized to z-scores across participants. Areas within the OFC ROI that correlated with SNS are displayed on an inflated brain at P < 0.05 (uncorrected) for illustrative purposes.
Fig. 3.
The relationship between GM density (arbitrary units) in the right entorhinal cortex and measures of SNS. From top to bottom: Facebook friends [n = 36, result was not significant at P < 0.05 (SVC)], Dunbar’s number (n = 38) and Norbeck’s Social Support Network (n = 40). Gray matter density was computed by regressing out age and TIV, and normalized to z-scores across participants.
BOLD friends vs unfamiliar faces
We extracted beta weights from the friends and unfamiliar faces conditions in each ROI. Controlling for age, we used a RM ANOVA to test whether there were differential activations to friends. We found that there were significantly greater activations to friends vs unfamiliar individuals in the right amygdala [F(1,38) = 4.91, P = 0.033] and right orbital frontal cortex [F(1,38) = 11.91, P = 0.002]. There were also differences approaching significance for the left middle temporal gyrus (MTG) [F(1,38) = 2.978, P = 0.093] and right MTG [F(1,38) = 3.095, P = .087].
BOLD correlations with SNS
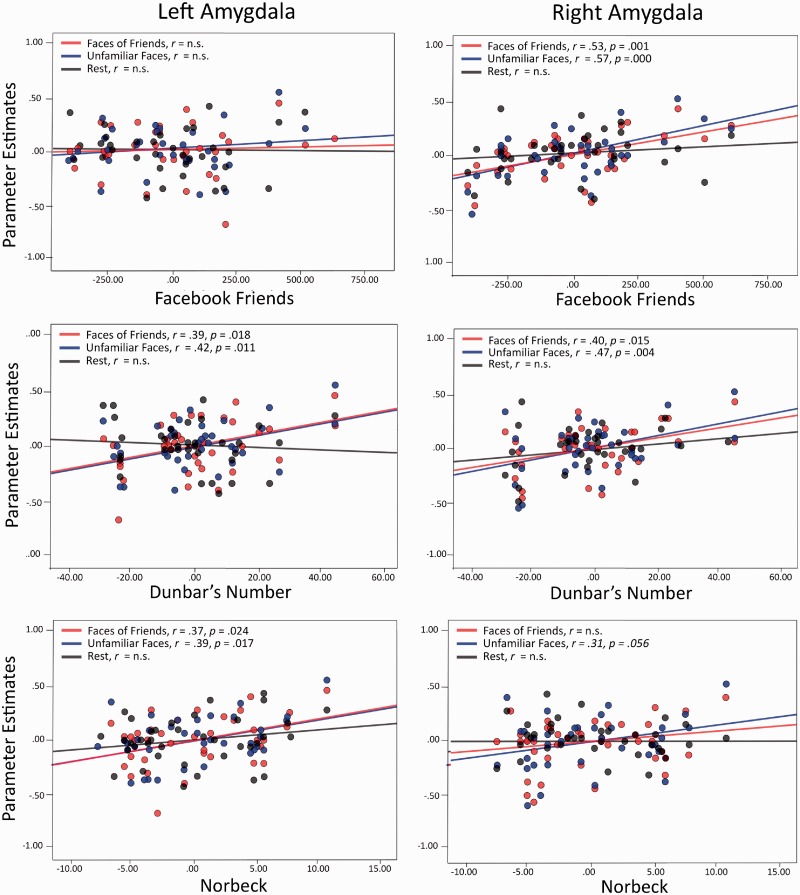
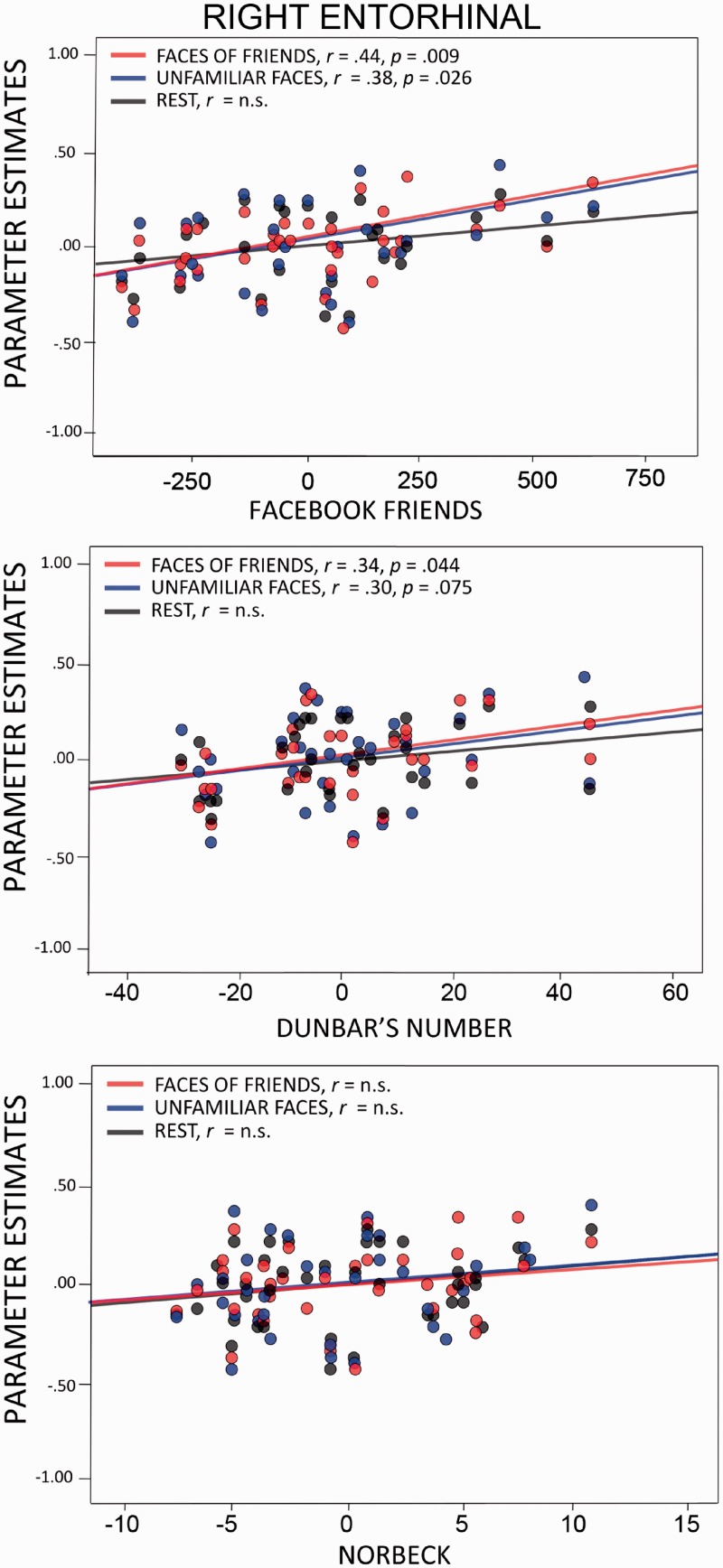
We conducted separate regressions to assess the relationship between measures of SNS and BOLD activity in our pre-defined ROIs while controlling for age and fixation baseline activity (Figures 4 and 5). As predicted, there were significant correlations between online and real-world measures of SNS and BOLD activity in several regions when participants viewed photos of their friends and photographs of unknown people (see Table 5 for a list of brain regions and correlations). Three regions stood out as they showed significant correlations in the VBM analysis as well as the BOLD analysis: left amygdala, right amygdala and the right entorhinal cortex. Surprisingly, there were similar correlations between SNS and the beta weights extracted for unfamiliar faces. Overall, these results suggest that individuals with larger social networks process facial social signals differently.
Fig. 4.
Partial correlation scatterplots illustrating the relationship between social network measures and BOLD activity in the left and right amygdalae controlling for age (n.s. = not significant). The x-axis of each scatterplot represents the standardized residuals of each social network measure, and the y-axis represents the standardized residuals of BOLD activity.
Fig. 5.
Partial correlation scatterplots illustrating the relationship between social network measures and BOLD activity in the right entorhinal cortex controlling for age (n.s. = not significant). The x-axis of each scatterplot represents the standardized residuals of each social network measure, and the y-axis represents the standardized residuals of BOLD activity.
DISCUSSION
The goal of this study was to investigate three questions about SNS and its neural correlates: (i) Do measures of online and real-world SNS correlate with each other? (ii) Is the relationship between SNS and differences in GM volume similar for all measures of SNS? (iii) Can individual differences in social networks be related to differences in task-related BOLD activity in these same regions?
To answer the first question, we used three measures of SNS: an online measure, number of Facebook friends and two real-world measures, Dunbar’s number [sympathy group] and Norbeck Social Support Group. We found significant positive correlations between all three measures after controlling for age. This is consistent with a previous study that reported significant positive correlations between online (Facebook friends) and real-world measures of SNS (Kanai, 2012). It is inconsistent, however, with the findings of a study out of the Dunbar laboratory (Pollet et al., 2011), which reported no significant correlation between individuals in a person’s online networks (i.e. number of friends on Facebook, Hyves or Netlog) and real-world social networks (i.e. friends in sympathy group, friends in support group or outer layer of friends). One possible explanation for the discrepancies between studies is differences in the way online social networks were measured between studies. Whereas this study and the study by Kanai et al. (2012) used number of Facebook friends as a measure of online network size, the study by Pollet et al. (2011) used a hybrid of three online social networks.
In addition to significant correlations between social network measures, this study also found significant differences in size of the three social networks measured within subjects in this study. As expected, online social networks were larger than their real-world counterparts. Recent work suggests the size of an individual’s real-world social network is an index of his or her underlying social cognitive abilities (e.g. mentalizing ability, memory capacity) as well as a product of the time an individual has to devote to the quantity and quality of social relationships (Dunbar, 1993, 1998, 2012; Stiller and Dunbar, 2007). Several key constraints on the size of real-world social networks do not pose the same restrictions on the size of online social networks. For example, it easier to stay apprised of the activities of a large number of connections in online social networks than it is to stay apprised of the activities of individuals in real-world social networks. It is also much more efficient and less time consuming to broadcast one’s own activities to a large group of social connections online than it is to broadcast the same news to a large group of connections in person (Dunbar, 2012). We also found significant differences between the sizes of the two real-world social networks with a greater number of people reported in an individual’s sympathy group than in their social support network. With respect to the differences in size between real-world social networks, the sympathy group was defined as individuals that participants had regular social contact with over the last 30 days, which often included the more select group of individuals that provided regular social support to the participants.
To answer our second and third questions of interest, participants underwent MRI/fMRI. There is an emerging literature demonstrating that there are focal regions of the brain that vary in size based on a person’s online and real-world SNS (Bickart et al., 2010; Lewis et al., 2011; Kanai et al., 2012). However, the small number of neuroimaging studies in this field have all used different social network measures and have reported different findings (Table 4). In this study, we used three social network measures, both online and real world, in an effort to adjudicate the disparate findings reported in previous studies. ROIs were chosen by using all regions reported in prior studies of SNS (Table 4). We were able to successfully replicate some findings but not others. It is possible that the failures to replicate were due to limited power or due to a true absence of a correlation.
Social networks and brain structure
The results of our VBM analysis showed a consistent and robust correlation in the GM density of the bilateral amygdala with increasing number of all social network measures. Our findings replicate and extend that of Bickart et al. (2010) who reported an increase in amygdala volume with larger and more complex social networks using a distinct measure called the SNI. Similarly, Kanai et al. (2012) reported a correlation between real-world SNS (SNI) and online SNS (Facebook friends) with GM volume in the amygdala. Thus, our VBM findings add support to the idea that the amygdala plays a key role in supporting the acquisition and maintenance of social networks.
The OFC is anatomically proximal and has monosynaptic connections with the basolateral and basomedial nuclei of the amygdala (Ghashghaei and Barbas, 2002; Ghashghaei et al., 2007). All our social network measures predicted variability in GM volume in bilateral OFC. Our findings extends a prior work (Lewis et al., 2011) showing that volume in portions of the OFC correlated with real-world SNS as measured by Dunbar’s sympathy group number and one’s score on a theory of mind task. It is worth noting that our measure of online SNS (FBF) showed a cluster located most closely to Lewis’s finding in the medial portion of the OFC; our real-world SN measures showed a correlation with lateral portions of the OFC.
The GM density of a number of other regions correlated with some, but not all measures of SNS (Table 4). For instance, the volume of the left posterior superior frontal gyrus (SFG) correlated with the number of Facebook friends and Dunbar’s number but not the Norbeck Social Support Network. Bickart et al. (2010) also identified this region while cautioning that further investigation was required. Indeed, this region is commonly thought to be part of premotor cortex, and thus is a puzzling structure to correlate with SNS. We note that a VBM study of adolescents with autism spectrum disorder, who would lack the social competency of a neurotypical, also showed differences in this area compared with control subjects (Waiter et al., 2004). The interpretation of this finding requires further investigation.
One finding that deserves attention was that volume in the right entorhinal cortex increased as real-world SNS measures (Dunbar’s sympathy group and Norbeck Social Support Group) increased. An exploratory analysis of the left entorhinal cortex showed that the volume of this region correlated with the number of Facebook friends. These findings extend those of Kanai et al. (2012) who found that the right entorhinal cortex correlated with the number of Facebook friends. Although we term this region ‘entorhinal cortex’ following Kanai’s lead, this region could also be called perirhinal cortex or medial aspects of the anterior temporal lobe. Entorhinal cortex proper is a small region that provides most of the input to the hippocampus thus it is closely aligned with episodic memory functions. Instead, we suspect that this region is analogous to the ventral anterior temporal face patch. Several recent studies in both humans and monkeys have shown that this face patch is sensitive to all faces, but especially faces that are personally salient, such as friends and famous individuals (reviewed by Von Der Heide et al., 2013; J.A. Collins and I.R. Olson, submitted for publication). Kriegeskorte et al. (2007) reported that this face patch, more so than any other neural region, was sensitive to individual faces. The coordinates provided by Kanai to social network measures are nearly identical to the coordinates of peak activations in response to perceiving familiar faces reported in our recent study [Talairach coordinates of Kanai et al. (2012) = 29, −5 −31; Von Der Heide et al. (2013) = 29, −13, −33]. Thus, it is possible that the volumetric changes observed in the entorhinal ROI reflect one’s ability to discriminate between, and retrieve biographical information about different individuals.
Social networks and functional brain activity
In addition to significant correlations between SNS and GM density, there were significant correlations between one’s SNS and BOLD activity in the bilateral amygdala, right entorhinal cortex and left middle temple gyrus. Regions that significantly correlated with SNS across the VBM and BOLD analysis were the left and right amygdala and right entorhinal cortex.
Recent work has reported correlations between the intrinsic resting state functional connectivity of the amygdala and a network of brain regions implicated in social perception and affiliation (Bickart et al., 2012). Although bilateral amygdala activations have traditionally been associated with negative emotions, activations in this region have also been reported with the presence of pleasurable or rewarding stimuli. This suggests that bilateral amygdala activations might actually relate to how arousing or salient the stimuli are to individuals viewing them (McClure et al., 2004). Based on this, one interpretation of the results of this study is that individuals with larger social networks tend to find social stimuli such as personally familiar and unfamiliar faces increasing more interesting and rewarding and that the greater arousal to these stimuli is reflected (or due to) in the increased amygdala activation. Given the close relationship of the amygdala to the hippocampal memory system, it is plausible that individuals with greater amygdala activations to faces would have an easier time remembering these faces, which may also be an important factor in building and maintaining social networks.
The amygdala is tightly interconnected with other limbic and paralimbic regions such as the entorhinal cortex/ventromedial anterior temporal lobe. We also found a significant correlation between BOLD activity and SNS in the entorhinal cortex. As noted earlier, this region has been implicated in individual face perception and recognition. Damage to this region and surrounding anterior temporal lobe tissue often results in an associative prosopagnosia in which one is unable to form or retrieve associations between a face and other information, such as a name (reviewed by Olson et al., 2013). It is also interesting that these findings are right lateralized; the right ventral ATL appears to be more sensitive to perceptual aspects of faces than the left (Kriegeskorte et al., 2007) which is more sensitive to faces coupled with semantic/linguistic information.
Other regions implicated in the BOLD analysis, such as the right middle temporal gyrus (Pourtois et al., 2005; Todorov and Engell, 2008) and the posterior inferior temporal sulcus (ITS) (Homola et al., 2012) have also been implicated in the evaluation and recognition of face stimuli, although it should be noted that these are large regions and the precise anatomical correspondence across studies remains unclear.
We did not find any significant correlations between SNS and BOLD activations to judgments of unfamiliar or familiar faces in the OFC. Several variables presumably predict one’s SNS, such as memory capacity, social motivation and interest, person perception and personality characteristics such as extroversion and social astuteness (Stiller and Dunbar, 2007; Totterdell et al., 2008; Pollet et al., 2011; Becker et al., 2012). From what we know of patients with OFC lesions, the OFC plays a role in specific personality characteristics (Berlin et al., 2004; Barbey et al., 2014), reversal learning (Berlin et al., 2004), impulsivity (Berlin et al., 2004; Berlin et al., 2005), judgments of the approachability of emotional faces (Willis et al., 2010) and aspects of theory of mind (Shamay-Tsoory et al., 2010). None of the aforementioned variables were measured or manipulated in this study, and it is possible that SNS would have correlated with BOLD activations in the OFC if we had used an experimental task that manipulated variables more closely in line with the functions of the OFC. Perhaps, future studies will find relationships between SNS and BOLD activations in the OFC using tasks that measure and manipulate more targeted variables.
It should also be noted that there were similar correlations between SNS and the beta weights for familiar and unfamiliar faces. It is possible that individuals who have higher social interest and motivation find faces more rewarding and informationally rich, ultimately leading to more meaningful interactions with people, and a larger social network.
Limitations of the current study
Where our findings diverge from the existing literature (Table 4) might be explained by methodological restrictions. First, our sample size was relatively small for a VBM study, and as a result, we did not have the statistical power to perform an exploratory analysis at an acceptably stringent threshold. It is possible that an exploratory, whole-brain analysis of our data would have revealed other intriguing findings. Our fMRI task is superficially similar to the ‘Social Distance’ task used by Yamakawa et al. (2009). Yamakawa et al. found that comparing BOLD activation from a social distance task and a physical distance task invoked activity in the posterior parietal cortex. This overlap may reflect the fact that social and physical distance are both represented by an egocentric distance metric, or it may reflect the metaphorical use of language to describe social relationships (e.g. ‘I am close to her’; ‘I feel distant from you’), which may be embodied in the brain’s algorithms that perform distance calculations. Our focus was on brain structures used to sustain social networks; further research may investigate the egocentric representations of members of our social network based on social closeness.
Our sample size was also too small to address the construct validity of social network measures (see work by Goncalves et al., 2011). However, this was not the goal of our study so it is a minor shortcoming.
Second, our sample was entirely female, and the mean age of participants was considerably lower than that tested in previous studies (Bickart et al., 2010; Lewis et al., 2011; Kanai et al., 2012; Powell et al., 2012). Despite controlling for age and TIV, some of our subjects are undoubtedly not finished in their cortical development, nor are their social networks totally matured. A 13-year-old’s social sphere may be dominated by their middle school, whereas a 30-year-old may have obligations—and contacts—in a variety of social settings. At the same time, younger adults, and especially adolescents, are extremely sensitive to social signals and are overly sensitive to the feelings and decisions of their peers (Steinberg and Morris, 2001), making adolescents an ideal population to study the neural basis of social networks and social motivation.
Third, our neural ROI were chosen by using regions reported to vary with SNS in prior studies. It could be argued that Dunbar’s social brain hypothesis is particularly about the frontal lobes and because of this, we should have restricted our ROIs to portions of the frontal lobes. We would argue that there is no reason to think that the frontal lobes evolved to uniquely serve social and only social functions and also, that social functions are served by a network of cortical and subcortical regions, depending on the species. Social behavior is found throughout the animal kingdom in animals that lack frontal lobes, or even in a centralized nervous system. In chordates, primitive structures such as the amygdala clearly play a critical role in sustaining aspects of social behavior [case in point: destruction of the amygdala can lead to gross changes in social behavior such as that observed in Kluver Bucy disorder (Olson et al., 2007)] and other subcortical regions, such as the periaqueductal GM, may also serve important functions. Other, non-frontal cortical regions, such as lateral temporal cortices and inferior parietal cortex, are expanded in humans, most likely due to the evolution of language. Many researchers believe that language should be considered a key social behavior as its function among humans is chiefly to communicate social information (Dunbar, 2004). Thus, we believe that focusing on the frontal lobes would be ad hoc and would not reflect the state of research in social neuroscience.
CONCLUSIONS
Our findings show that volumetric differences, as well as neural activity differences, predict SNS across a variety of different measures. Our study provides the best support for the left and right amygdala’s involvement in this process, as well as providing support for the OFC and entorhinal/ventromedial anterior temporal lobe in processes required for the maintenance of robust social networks. Future studies that corroborate findings from this and other studies will help refine and possibly dissect the functional role of each region in this mysterious social network-network.
Acknowledgments
We would like to thank Laura Skipper and Feroze Mohammed for assistance with imaging. This work was supported by a National Institute of Health grant to I.R.O. (NIH RO1 MH901113). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
REFERENCES
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11(6):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Colom R, Grafman J. Distributed neural system for emotional intelligence revealed by lesion mapping. Social Cognitive and Affective Neuroscience. 2014;9(3):265–72. doi: 10.1093/scan/nss124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B, Mihov Y, Scheele D, et al. Fear processing and social networking in the absence of a functional amygdala. Biological Psychiatry. 2012;72(1):70–7. doi: 10.1016/j.biopsych.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Berlin H, Rolls E, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127(5):1108–26. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, Iversen SD. Borderline personality disorder, impulsivity, and the orbitofrontal cortex. American Journal of Psychiatry. 2005;162(12):2360–73. doi: 10.1176/appi.ajp.162.12.2360. [DOI] [PubMed] [Google Scholar]
- Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC. Intrinsic amygdala—cortical functional connectivity predicts social network size in humans. The Journal of Neuroscience. 2012;32(42):14729–41. doi: 10.1523/JNEUROSCI.1599-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nature Neuroscience. 2010;14(2):163–4. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt RS, Knez M. Trust and third-party gossip. Trust in Organizations: Frontiers of Theory and Research. 1996;68:89. [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012;16(4):231–9. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Social ties and susceptibility to the common cold. JAMA: The Journal of the American Medical Association. 1997;277(24):1940–4. [PubMed] [Google Scholar]
- Cross JF, Cross J, Daly J. Sex, race, age, and beauty as factors in recognition of faces. Perception & Psychophysics. 1971;10(6):393–6. [Google Scholar]
- Dunbar R. Coevolution of neocortical size, group size and language in humans. Behavioral and Brain Sciences. 1993;16(4):681–93. [Google Scholar]
- Dunbar R. The social brain hypothesis. Brain. 1998;9:10. [Google Scholar]
- Dunbar R. Gossip in evolutionary perspective. Review of General Psychology. 2004;8:100–10. [Google Scholar]
- Dunbar R. Social cognition on the internet: testing constraints on social network size. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367(1599):2192–201. doi: 10.1098/rstb.2012.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis H, Shepherd J, Bruce A. The effects of age and sex upon adolescents' recognition of faces. The Journal of Genetic Psychology. 1973;123(1):173–4. doi: 10.1080/00221325.1973.10533202. [DOI] [PubMed] [Google Scholar]
- Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268(5217):1578–84. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- Frischen A, Bayliss AP, Tipper SP. Gaze cueing of attention: visual attention, social cognition, and individual differences. Psychological Bulletin. 2007;133(4):694. doi: 10.1037/0033-2909.133.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei H, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–80. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H, Hilgetag C, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34(3):905–23. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves B, Perra N, Vespignani A. Modeling users' activity on twitter networks: validation of dunbar's number. PloS One. 2011;6(8):e22656. doi: 10.1371/journal.pone.0022656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homola G, Jbabdi S, Beckmann CF, Bartsch AJ. A brain network processing the age of faces. PloS One. 2012;7(11):e49451. doi: 10.1371/journal.pone.0049451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino T, Nakai R, Azuma T, Kimura T, Fukuyama H. Gender differences in brain activation during encoding and recognition of male and female faces. Brain Imaging and Behavior. 2010;4(1):55–67. doi: 10.1007/s11682-009-9085-0. [DOI] [PubMed] [Google Scholar]
- Jerison HJ. Evolution of the Brain and Intelligence. New York: Academic Press; 1973. [Google Scholar]
- Kanai R, Bahrami B, Roylance R, Rees G. Online social network size is reflected in human brain structure. Proceedings of the Royal Society B: Biological Sciences. 2012;279(1732):1327–34. doi: 10.1098/rspb.2011.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Sex differences in amygdala activation during the perception of facial affect. Neuroreport. 2001;12(11):2543–7. doi: 10.1097/00001756-200108080-00050. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Formisano E, Sorger B, Goebel R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(51):20600–5. doi: 10.1073/pnas.0705654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin C, Herlitz A. Sex differences in face recognition—women's faces make the difference. Brain and Cognition. 2002;50(1):121–8. doi: 10.1016/s0278-2626(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Lewis K, Kaufman J, Gonzalez M, Wimmer A, Christakis N. Tastes, ties, and time: a new social network dataset using facebook.Com. Social Networks. 2008;30(4):330–42. [Google Scholar]
- Lewis P, Rezaie R, Brown R, Roberts N, Dunbar R. Ventromedial prefrontal volume predicts understanding of others and social network size. Neuroimage. 2011;57(4):1624–9. doi: 10.1016/j.neuroimage.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the talairach atlas. Neuroimage. 2004;21(1):450–5. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fmri data sets. Neuroimage. 2003;19(3):1233. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McBain R, Norton D, Chen Y. Females excel at basic face perception. Acta Psychologica. 2009;130(2):168. doi: 10.1016/j.actpsy.2008.12.005. [DOI] [PubMed] [Google Scholar]
- McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: the modern role of fMRI. The Neuroscientist. 2004;10(3):260–8. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- Megreya AM, Bindemann M, Havard C. Sex differences in unfamiliar face identification: evidence from matching tasks. Acta Psychologica. 2011;137(1):83–9. doi: 10.1016/j.actpsy.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Norbeck JS, Lindsey AM, Carrieri VL. The development of an instrument to measure social support. Nursing Research. 1981;30(5):264–9. [PubMed] [Google Scholar]
- Olson IR, McCoy D, Klobusicky E, Ross LA. Social cognition and the anterior temporal lobes: a review and theoretical framework. Social Cognitive and Affective Neuroscience. 2013;8(2):123–33. doi: 10.1093/scan/nss119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130(7):1718–31. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Otta E, Abrosio FFE, Hoshino RL. Reading a smiling face: messages conveyed by various forms of smiling. Perceptual and Motor Skills. 1996;82(3c):1111–21. doi: 10.2466/pms.1996.82.3c.1111. [DOI] [PubMed] [Google Scholar]
- Pollet TV, Roberts SG, Dunbar RI. Use of social network sites and instant messaging does not lead to increased offline social network size, or to emotionally closer relationships with offline network members. Cyberpsychology, Behavior, and Social Networking. 2011;14(4):253–8. doi: 10.1089/cyber.2010.0161. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Schwartz S, Seghier ML, Lazeyras FO, Vuilleumier P. View-independent coding of face identity in frontal and temporal cortices is modulated by familiarity: an event-related fMRI study. Neuroimage. 2005;24(4):1214–24. doi: 10.1016/j.neuroimage.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Powell J, Lewis PA, Roberts N, Garcia-Finana M, Dunbar R. Orbital prefrontal cortex volume predicts social network size: an imaging study of individual differences in humans. Proceedings of the Royal Society B: Biological Sciences. 2012;279(1736):2157–62. doi: 10.1098/rspb.2011.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehnman J, Herlitz A. Higher face recognition ability in girls: magnified by own-sex and own-ethnicity bias. Memory. 2006;14(3):289–96. doi: 10.1080/09658210500233581. [DOI] [PubMed] [Google Scholar]
- Rehnman J, Herlitz A. Women remember more faces than men do. Acta Psychologica. 2007;124(3):344–55. doi: 10.1016/j.actpsy.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Harari H, Aharon-Peretz J, Levkovitz Y. The role of the orbitofrontal cortex in affective theory of mind deficits in criminal offenders with psychopathic tendencies. Cortex. 2010;46(5):668–77. doi: 10.1016/j.cortex.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Journal of Cognitive Education and Psychology. 2001;2(1):55–87. [Google Scholar]
- Stiller J, Dunbar RI. Perspective-taking and memory capacity predict social network size. Social Networks. 2007;29(1):93–104. [Google Scholar]
- Todorov A, Engell AD. The role of the amygdala in implicit evaluation of emotionally neutral faces. Social Cognitive and Affective Neuroscience. 2008;3(4):303–12. doi: 10.1093/scan/nsn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totterdell P, Holman D, Hukin A. Social networkers: measuring and examining individual differences in propensity to connect with others. Social Networks. 2008;30(4):283–96. [Google Scholar]
- Tsukiura T, Cabeza R. Orbitofrontal and hippocampal contributions to memory for face-name associations: the rewarding power of a smile. Neuropsychologia. 2008;46(9):2310. doi: 10.1016/j.neuropsychologia.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Olson IR. Anterior temporal face patches: a meta-analysis and empirical study. Frontiers in Human Neuroscience. 2013;7:17. doi: 10.3389/fnhum.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage. 2004;22(2):619–25. doi: 10.1016/j.neuroimage.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Wellman B. Is dunbar's number up? British Journal of Psychology. 2012;103(2):174–6. doi: 10.1111/j.2044-8295.2011.02075.x. [DOI] [PubMed] [Google Scholar]
- Willis ML, Palermo R, Burke D, McGrillen K, Miller L. Orbitofrontal cortex lesions result in abnormal social judgements to emotional faces. Neuropsychologia. 2010;48(7):2182–7. doi: 10.1016/j.neuropsychologia.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Yamakawa Y, Kanai R, Matsumara M, Naito E. Social distance evaulation in human parietal cortex. PLoS One. 2009;4(2):1–10. doi: 10.1371/journal.pone.0004360. [DOI] [PMC free article] [PubMed] [Google Scholar]