Abstract
Both children and adults with bipolar disorder (BD) exhibit face emotion labeling deficits and neural circuitry dysfunction in response to emotional faces. However, few studies have compared these groups directly to distinguish effects of age and diagnosis. Such studies are important to begin to elucidate the developmental trajectory of BD and facilitate its diagnosis, prevention and treatment. This functional magnetic resonance imaging study compares 41 individuals with BD (19 children; 22 adults) and 44 age-matched healthy individuals (25 children; 19 adults) when making explicit or implicit judgments about angry or happy face morphs across a range of emotion intensity. Linear trend analyses revealed that BD patients, irrespective of age, failed to recruit the amygdala in response to increasing angry face. This finding was no longer significant when the group was restricted to euthymic youth or those without comorbid attention deficit hyperactivity disorder although this may reflect low statistical power. Deficits in subgenual anterior cingulate modulation were observed in both patient groups but were related to implicit processing for child patients and explicit processing for adult patients. Abnormalities in face emotion labeling and the circuitry mediating it may be biomarkers of BD that are present across development.
INTRODUCTION
Abnormal face emotion processing is a candidate endophenotype for bipolar disorder (BD) given extensive evidence for face emotion labeling deficits in pediatric and adult BD populations (Guyer et al., 2007; Kohler et al., 2011) and unaffected individuals at-risk for the illness (Brotman et al., 2008a,b). Such difficulties may contribute to the interpersonal deficits observed in BD (Coryell et al., 1993; Geller et al., 2000; MacQueen et al., 2001; Goldstein et al., 2009), and therefore with poor outcome and quality of life (Keenan-Miller and Miklowitz, 2011). An important, but often overlooked, question regarding the neural correlates of dysfunctional face processing in BD is the role of developmental differences (Blumberg et al., 2004). A better understanding of developmental differences in BD would contribute to better prevention and to developmentally appropriate treatments, while also addressing questions about phenotypic differences between pediatric and adult forms of BD (National Institute of Mental Health, 2008). We used a parametric face emotion processing task to probe how children (child BD) and adults with BD (adult BD) differ from each other and from age-matched healthy comparison populations in neural activity in response to increasing emotion intensity on a face.
A number of functional magnetic resonance imaging (fMRI) studies have examined brain activation while either child BD or adult BD complete face emotion processing tasks. These studies find abnormal recruitment of the amygdala (Lawrence et al., 2004; Blumberg et al., 2005; Chen et al., 2006; Pavuluri et al., 2007, 2009; Kalmar et al., 2009; Surguladze et al., 2010; Garrett et al., 2012), medial and lateral prefrontal cortices (Lawrence et al., 2004; Lennox et al., 2004; Pavuluri et al., 2007; Ladouceur et al., 2011), and posterior regions (Lennox et al., 2004; Marchand et al., 2011; Garrett et al., 2012; Kim et al., 2012b) among BD populations.
However, only two published neuroimaging studies compare child BD and adult BD directly on face emotion tasks (Kim et al., 2012b; Adleman et al., 2013). In the first (Kim et al., 2012b), relative to adult BD and healthy children, child BD exhibited amygdala hyperactivation across a greater number of emotion types. Also, relative to healthy youths, child BD showed hypoactivation in the posterior cingulate cortex (PCC) in response to angry faces. In contrast, adult BD patients did not exhibit PCC abnormalities. Such findings suggest more generalized amygdala dysfunction in youths vs adults with BD (Kim et al., 2012b), consistent with more robust evidence of structural abnormalities in the amygdala among youths vs adults with BD (Chen et al., 2006; Pfeifer et al., 2008). The second study identified fusiform and middle frontal gyrus abnormalities in BD populations during a face emotion memory task, but did not find differences between child BD and adult BD (Adleman et al., 2013). Additional studies directly contrasting child BD and adult BD are needed to replicate these findings and clarify the nature of developmental differences and similarities.
This study examined age- and diagnosis-related differences in neural activity during implicit and explicit processing of happy and angry faces with varying emotional intensity. We used a parametric design involving the systematic variation of face emotion intensity levels because of its ecological validity, its robustness to amygdala habituation (Phillips et al., 2010), its ability to distinguish non-psychiatric and psychiatric populations (Surguladze et al., 2005) and between related psychiatric conditions (Blair et al., 2008), as well as its prior successful differentiation between youths and adults with BD (Kim et al., 2012b). Specifically, we examined which regions of the brain respond to increasing emotionality in a face.
In a prior study using the current task design and a partially overlapping sample (Thomas et al., 2012), we found that, in contrast to healthy youths, child BD failed to increase amygdala activity in response to increasing anger intensity on a face. This study examined whether this deficient amygdala modulation is specific to child BD or is also present among adults with the illness. In addition, we conducted whole-brain analyses to examine other regions with distinct diagnostic and developmental recruitment.
Based on the existing literature, we hypothesized age-related differences in BD in the amygdala and PCC. Specifically, we hypothesized that although both BD groups would exhibit reduced amygdala modulation in response to increasing anger intensity, such deficits will be more marked in the pediatric BD sample. This hypothesis was based on prior work documenting amygdala abnormalities in child BD and adult BD (Kalmar et al., 2009; Surguladze et al., 2010), deficient modulation in a parametric study using an overlapping sample of child BD (Thomas et al., 2012) and more generalized amygdala dysfunction among child BD vs adults with the illness (Kim et al., 2012b). We also hypothesized that youths, but not adults, with BD would exhibit stronger negative modulation of the PCC relative to healthy comparison children (child HC). This hypothesis builds on prior evidence documenting abnormal PCC activation in child BD but not adults with the illness (Kim et al., 2012b) and negative PCC modulation among an overlapping sample of child BD (Thomas et al., 2012).
Finally a robust literature suggests that child BD and adult BD exhibit abnormal prefrontal cortex (PFC) activation, including ventromedial and ventrolateral PFC, during face processing tasks (Delvecchio et al., 2012), with a recent meta-analysis suggesting that adult BD populations exhibit PFC hypoactivation during emotional tasks (Chen et al., 2011). Therefore, we expected abnormal recruitment of the PFC (especially ventromedial and ventrolateral regions) in response to increasing emotion intensity in angry and happy faces among child BD and adult BD.
METHODS
Participants
Eighty-five participants [19 child BD, 25 child HC, 22 adult BD, 19 healthy comparison adults (adult HC)], ages 10–54, enrolled in an Institutional Review Board approved study at the National Institute of Mental Health and provided informed assent/consent. All participants received $100 for participation. Structured clinical interviews [Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime (Kaufmann et al., 1997) with children and the Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (First et al., 2002) or the Diagnostic Interview for Genetic Studies (Nurnberger et al., 1994) for adults] were used to determine diagnostic status for patients and controls. Child BD participants met criteria for ‘narrow-phenotype’ BD (Leibenluft et al., 2003) and adult BD participants met standard DSM-IV criteria for BD and were included regardless of mood episode type. Child HC and adult HC populations had no lifetime history of psychiatric illness (as determined by diagnostic interview) nor any first-degree relative with a history of mood disorders (as determined by semi-formal clinician assessment). No participants were biological relatives. Sixteen child BD and 20 child HC were reported in Thomas et al. (2012). Data from all of the adult BD and adult HC participants, as well as three child BD and five child HC, have not been reported previously.
Exclusion criteria for all subjects included: IQ < 70, unstable medical illness, substance abuse within the past 2 months, pervasive developmental disorder, active psychotic symptoms, or a neurological disorder.
Stimuli
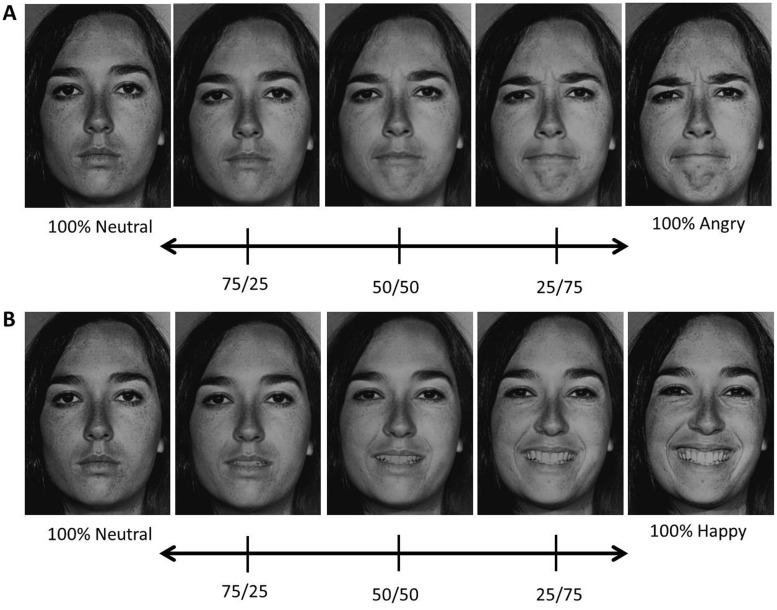
Neutral, angry and happy facial expressions from 10 exemplars (five female; five male) were taken from the Pictures of Facial Affect set (Ekman and Friesen, 1976) and used to create two stimulus sets (Figure 1). Each set consisted of morphs between the emotional face (angry or happy) and the neutral face of the same exemplar created with MorphMan 2000 software (STOIK, Moscow, Russia). Each set included five morph intensities: 100% neutral, 25% emotion/75% neutral, 50% emotion/50% neutral, 75% emotion/25% neutral and 100% emotion (prototypic expression). Separate sets were created for angry and happy facial expressions.
Fig. 1.
Example of the morphed stimuli used in the experiment from a single female exemplar. Stimulus range from 100% neutral to (A) 100% angry and (B) 100% happy in 25% increments.
Given prior evidence that BD and healthy youths may differ in their responses to ambiguous emotions (Rich et al., 2006; Brotman et al., 2010), we included more trials of the ambiguous emotion morphs (25% and 50% emotion). The number of trials/stimulus category were as follows: neutral (40 trials), 25% emotion (48 trials), 50% emotion (48 trials), 75% emotion (24 trials) and 100% emotion stimuli (24 trials).
Task
Details of the task have been described elsewhere (Thomas et al., 2012). Briefly, in each trial participants viewed a facial stimulus (3000 ms) and made an implicit (how wide is the nose?) or explicit (how hostile is the face?) rating using a five-button response device (1 = least wide/hostile and 5 = most wide/hostile). After 3000 ms the stimulus disappeared, a blank black screen was presented for a varied interval (750–1250 ms; average = 1000 ms), and then the next trial began.
Participants completed four blocks of the task. Each block consisted of 41 trials/condition (nose width or hostile ratings) and 12 fixation trials (white crosses presented on a black screen for 3000 ms). Participants saw angry and happy faces within each block and different morph intensities were randomly presented. Scanning time for this task was approximately 25 min (∼6.3 min/block).
fMRI acquisition
Due to the decommissioning of one scanner, neuroimaging data were acquired on two General Electric 3T scanners: a Signa VH/i (16 child BD, 20 child HC, 12 adult BD, 14 adult HC) and a Signa HDx (3 child BD, 5 child HC, 10 adult BD, 5 adult HC). The same GE head coil and scanning parameters were used on each scanner and included: a high-resolution structural scan (T1-weighted axial acquisition, one hundred and twenty-four 1.2 mm slices, 15° flip angle, 256 × 256 matrix, 24 cm field-of-view) and gradient echo-planar imaging images (38 contiguous 2 mm3 slices, repetition time = 2300 ms, echo time = 25 ms, flip angle = 90°, 96 × 96 mm).
The percentage of individuals studied on each scanner did not differ between diagnostic groups (Table 1). Nonetheless, scanner was included as a covariate in the fMRI analyses. In addition, for each of the regions identified in the primary analyses, we evaluated the potential contribution of scanner using two strategies. First, we evaluated whether linear trends differed between participants studied on the first vs the second scanner. No significant differences were observed. Second, we limited the sample to the 62 individuals studied on the first scanner and repeated the primary analyses. All three primary findings remained significant with this subsample of participants. The same analyses were not possible for the 23 individuals studied on the second scanner because of sample size (3 child BD, 5 child HC, 10 adult BD, 5 adult HC).
Table 1.
Demographic and clinical characteristics of child BD, adult BD, child HC and adult HC
| Characteristic | Child BD (N = 19) |
Adult BD (N = 22) |
Child HC (N = 25) |
Adult HC (N = 19) |
||||
|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | |
| Age | 15.7 | 2.3 | 35.54 | 11.2 | 14.8 | 2.0 | 32. 8 | 11.4 |
| IQ | 104.4 | 14.9 | 113.59 | 10.4 | 108.0 | 14.0 | 113.2 | 15.4 |
| YMRSa | 6.1 | 5.0 | 3.4 | 3.2 | — | — | — | — |
| CDRSa | 28.9 | 8.0 | — | — | — | — | — | — |
| SIGH-SADa | — | — | 14.7 | 13.3 | — | — | — | — |
| Age of onseta | 10.5 | 2.9 | 18.5 | 6.8 | — | — | — | — |
| Number of medicationsa | 2.9 | 1.6 | 2.6 | 1.6 | — | — | — | — |
| N | % | N | % | N | % | N | % | |
| GE Signa VH/ib | 16 | 84.2 | 12 | 54.5 | 20 | 80.0 | 14 | 73.7 |
| Male | 5 | 26.3 | 7 | 31.8 | 12 | 48.0 | 7 | 36.8 |
| Bipolar type | ||||||||
| Bipolar I | 12 | 63.2 | 14 | 63.6 | — | — | — | — |
| Bipolar II | 7 | 36.8 | 8 | 36.4 | — | — | — | — |
| Mood statea,c | ||||||||
| Euthymic | 15 | 83.3 | 13 | 68.4 | — | — | — | — |
| Depressed | 1 | 5.6 | 6 | 31.6 | — | — | — | — |
| Hypomanic/Manic | 1 | 5.6 | 0 | 0 | — | — | — | — |
| Mixed | 1 | 5.6 | 0 | 0 | — | — | — | — |
| Comorbid conditions | ||||||||
| ADHD | 10 | 52.6 | 3 | 13.6 | — | — | — | — |
| ODD | 5 | 26.3 | — | — | — | — | — | — |
| Anxiety disorderd | 9 | 47.4 | 9 | 40.9 | — | — | — | — |
| Medicationa | ||||||||
| Unmedicated | 2 | 10.5 | 2 | 12.5 | 25 | 100 | 19 | 100 |
| Atypical antipsychotic | 8 | 42.1 | 9 | 56.3 | — | — | — | — |
| Lithium | 10 | 52.6 | 2 | 12.5 | — | — | — | — |
| Antiepileptic | 11 | 57.9 | 9 | 56.3 | — | — | — | — |
| Antidepressant | 9 | 47.4 | 11 | 68.8 | — | — | — | — |
| Stimulants | 7 | 36.8 | 0 | 0.0 | — | — | — | — |
ADHD, Attention Deficit Hyperactivity Disorder; IQ, WASI Full-Scale IQ; YMRS, the Young Mania Rating Scale; CDRS, Children’s Depression Rating Scale; SIGH-SAD, Structural Clinical interview for the Hamilton depression scale - seasonal affective disorder version; ODD, oppositional defiant disorder. No group differences were observed for age, IQ, sex distribution and total psychotropic medications. Relative to adult BD, child BD had an earlier onset of BD [t(36) = −4.60, P < 0.001] and higher ratio of comorbid ADHD [χ2(41) = 7.2, P < 0.03]. A trend emerged for child BD to have higher YMRS scores vs adult BD [t(36) = 1.97, P < 0.06]. aData were not available for all participants for these variables. The following subsample of data was included for child BD participants: CDRS (n = 18); age of onset (n = 18); mood state (n = 18). For adult BD: YMRS (n = 19); SIGH-SAD (n = 19); medication status (n = 16); mood state (n = 19); age of onset (n = 20). For variables with missing data, percentages are calculated relative to the number of participants with data for this variable. bParticipants completed the task on two GE Scanners. There was no significant difference between the proportion of individuals on the different scanners. cEuthymia was defined as: CDRS ≤ 40 and YMRS ≤ 12 in child BD and YMRS ≤ 12, SIGH-SAD ≤ 20 in adult BD. Depression was defined as: CDRS > 40 and YMRS ≤ 12 in child BD; SIGH-SAD > 20 and YMRS ≤ 12 in adult BD; hypomania/mania as: CDRS ≤ 40 and YMRS > 12 in child BD; SIGH-SAD ≤ 20 and YMRS > 12 in adult BD; and mixed state as: CDRS > 40 and YMRS > 12 in child BD SIGH-SAD > 20 and YMRS > 12 in adult BD; dIncludes generalized anxiety disorder, separation anxiety disorder, social phobia, panic disorder, post-traumatic stress disorder and obsessive compulsive disorder.
Data analyses
Participant demographics
Unpaired t-tests or Chi-square analysis were used to compare age, IQ, sex distribution, and scanner between patients and their age-matched HC group. Univariate analyses of variance (ANOVAs) and Chi square analyses were used to compare IQ, sex distribution and scanner across all four participant groups.
Behavioral data
The linear trends for rating and response time for each stimulus set (neutral → 100% angry, neutral → 100% happy) were extracted and compared across groups using repeated measures ANOVAs with Diagnosis (BD, HC) × Age (child, adult) × Condition (hostile, nose width) as between and within-subjects factors. Separate analyses were conducted for angry and happy faces and for each behavioral variable (rating and response time).
Imaging analyses
Data were analyzed using Analysis of Functional Neuroimages (AFNI) (Cox, 1996) using standard preprocessing methods including slice timing correction, a 6 mm full width at half maximum blur, and scaling to a percentage of the voxel-wise mean. Time points with motion above 1.5 mm were censored from the regression (Thomas et al., 2012). Event types included all possible combinations of morph (100% neutral, 25%, 50%, 75% and 100% emotion), condition (nose width or hostility rating) and emotion category (happy, angry). Regressors were created by convolving a gamma-variate hemodynamic response function with stimulus times for each event type. Individual linear regressions included each event type regressor, six motion parameters and baseline drift for each of the four experimental blocks. β coefficients and t-statistics were calculated for each regressor at each voxel. Blank fixation trials and the inter trial intervals provided a baseline.
We conducted trend analyses which measured the degree to which a linear trend explained the neural activity between 0% (neutral), 25%, 50%, 75% and 100% emotion intensity levels. Separate trend analyses were conducted for each condition (implicit, explicit) and each emotion (angry, happy). Happy and angry faces were not considered in the same analysis for two reasons. First, the same neutral stimuli could serve as ‘anchor points’ on the linear trends for both analyses, resulting in an inappropriate duplication of neutral data if considered within the same analysis. Second, including happy and angry faces on a single emotional continuum implies that these emotions represent polar opposites. Although both emotions are exemplars of pleasant and unpleasant emotions, we believe that treating them like opposites assumes a relationship between the two emotions that is not truthful. Finally, analyzing the emotions separately allows our findings to compare with previously published data (Thomas et al., 2012, p. 370). The resulting four β coefficients were used in two group analysis approaches
First, given prior findings of amygdala abnormalities in adult BD and child BD, we conducted an anatomical region of interest (ROI) analysis using the bilateral amygdala defined by the Talairach–Tournoux Daemon. A single linear trend value in the amygdala was extracted for each condition and emotion combination for the left and right amygdala separately. These values were submitted to a Diagnosis (BD, HC) × Age (child, adult) × Condition (implicit, explicit) × Hemisphere (left, right) analysis of covariance (ANCOVA) (Scanner was entered as a covariate) in SPSS. Separate analyses were computed for happy and angry emotions for the reasons described earlier. Because a single mean value was used for each condition, traditional significance levels (P < 0.05) were used to evaluate the results from this analysis.
Next, we computed a whole-brain analysis using a Diagnosis × Age × Condition ANCOVA (Scanner was entered as a covariate) with Subject as a random effects factor. The group analysis was constrained to voxels present in grey matter and present in all participants. Separate analyses were computed for happy and angry emotions. Using criteria outlined by Lieberman and Cunningham (2009) that provide a balance between correcting for Type I and Type II errors and those used in a prior study in our lab with these developmental groups (Kim et al., 2012b), significant clusters consisted of regions with a cluster-extent threshold of k ≥ 20 at P < 0.005. For clusters meeting identified thresholds, average β coefficients for the linear trends were extracted and post hoc ANOVAs were performed in SPSS. Greenhouse–Geisser correction was used when analyses violated sphericity assumptions. Given our a priori interests in findings related to the BD groups, we limit the results reported in the manuscript to findings relating to Diagnosis or Diagnosis × Age.
Exploratory post hoc analyses examined the effects of comorbid attention deficit hyperactivity disorder (ADHD) or mood state on neural activation patterns by testing whether the group differences identified in the primary analysis remained when the BD population was limited to individuals without co-occurring ADHD (9 child BD, 19 adult BD) or to euthymic individuals (15 child BD, 13 adult BD). Given the severity of BD, all but four patients (two child BD and two adult BD) were taking psychotropic medications at the time of the scan. We explored the potential impact of medication on our findings by performing a Pearson correlation between number of psychotropic medications and neural activation in each BD group.
RESULTS
Participants
A total of 131 individuals completed the scanning session, resulting in 85 useable scans. Forty-six individuals (32%) were excluded from the analyses: five (4%) due to excessive movement (two child BD, three child HC), 27 (21.6%) for poor behavioral performance (defined as <85% of trials with a behavioral response; 11 child BD, 9 child HC, 5 adult BD, 2 adult HC), eight (6.4%) for technical difficulties (two child HC, two adult BD, four adult HC) and six for not completing the scan (three child BD, two child HC, one adult BD). A trend (P < 0.06) for differences in the exclusion reason between participant groups appeared to be driven by more technical difficulties among the adult HC. The final sample included 19 child BD, 25 child HC, 22 adult BD and 19 adult HC (Table 1). IQ, sex distribution and percentage of individuals completing the task on each scanner did not differ among groups and age did not differ between the two child groups or the two adult groups (Table 1).
Behavioral results
Angry faces
No significant effect of Diagnosis or interaction between Diagnosis × Age was observed.
Happy faces
A Diagnosis × Age × Condition interaction, F(1,81) = 4.08, P < 0.05 emerged for ratings of happy faces. Post hoc analyses indicated that a negative linear trend between explicit hostility ratings and increasing happy intensity was stronger among child HC vs child BD and between child HC and adult HC.
fMRI trend analyses
In the results below, the term ‘positive linear trend’ indicates that activation in a region increased with increasing face emotion intensity (i.e. positive slope value). The term ‘negative linear trend’ indicates that activation in a region decreased with increasing face emotion intensity (i.e. negative slope value).
Amygdala ROI results
Angry faces
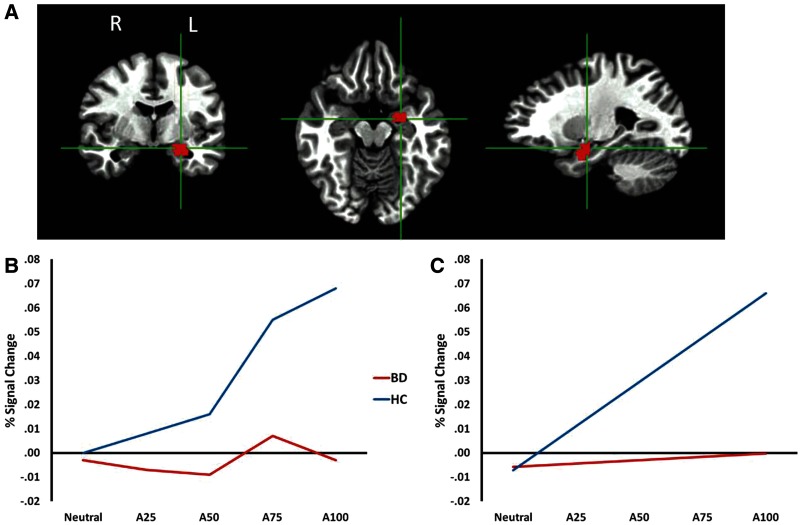
A main effect of Diagnosis, F(1,80) = 4.07, P < 0.05 indicated a strong positive linear trend between increasing anger on a face and increasing amygdala activity in the HC participants that was absent in the BD populations (Figure 2). This finding extends our prior finding (Thomas et al., 2012) by suggesting that the failure to modulate amygdala activity with increasing anger intensity in a face is characteristic of individuals with BD, regardless of age. No Diagnosis × Age interactions were observed.
Fig. 2.
Amygdala ROI from the angry face analysis. (A) Left anatomical ROI used in the analysis; a homologous region from the right hemisphere was also used. (B) BOLD signal at each intensity level for each diagnostic group. (C) Linear trend between BOLD signal and intensity level for each diagnostic group. Note: A25 = 25% angry/75% neutral; A50 = 50% angry/50% neutral; A75 = 75% angry/25% neutral. BD = bipolar disorder and HC = healthy comparison.
Happy faces
No significant effects involving Diagnosis or Diagnosis × Age were observed in the amygdala.
Whole-brain results
Angry faces
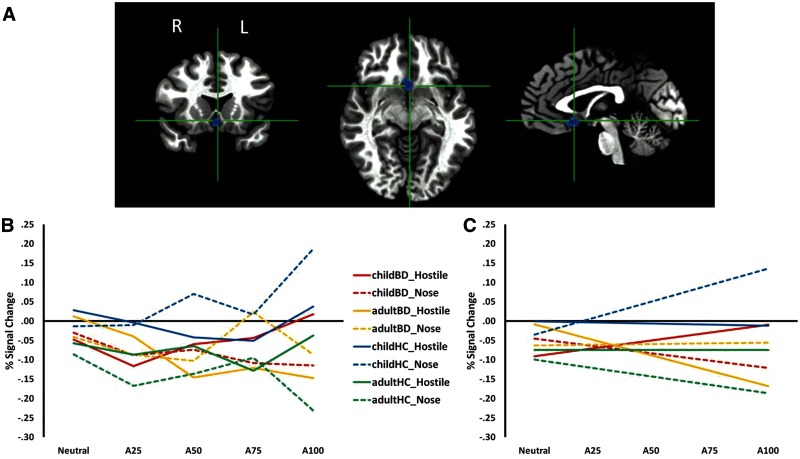
The whole-brain analysis revealed a significant Diagnosis × Age × Condition [F(1,81) = 14.58, P < 0.001] interaction in a single 71 voxel cluster in the bilateral subgenual anterior cingulate cortex (sgACC) and subcallosal gyrus (BA25) (Table 2; Figure 3). Post hoc analyses revealed that deficits in sgACC recruitment in response to increasing anger intensity in a face were related to the implicit condition for child BD and the explicit condition for adult BD. Specifically, during the implicit condition, child HC showed a more positive linear trend than did child BD (P < 0.01). In contrast, during the explicit condition, adult BD exhibited a more negative linear trend than did adult HC (P = 0.05). The two patient groups also differed in their recruitment of the sgACC, with a more negative linear trend among adult BD than child BD in the explicit condition (P < 0.005).
Table 2.
Significant findings from the Diagnosis × Age × Condition interaction (neutral to angry) and the Diagnosis × Condition (neutral to happy) observed in the whole-brain fMRI analyses
| Talairach coordinatesa |
Analysisb |
|||||||
|---|---|---|---|---|---|---|---|---|
| Area of cortical activation | Side | Cluster sizec | x | y | z | BA | F | P |
| Neutral to angry | ||||||||
| Subgenual anterior cingulate | R/L | 71 | −1 | 13 | −6 | 25 | 14.58 | <0.001 |
| Neutral to happy | ||||||||
| Cuneus | L | 48 | −21 | −71 | 8 | 30 | 8.42 | <0.01 |
aCoordinates refer to the voxel with maximum signal intensity. bStatistics refer to the analysis of the extracted clusters in SPSS; df = 1,81. cCluster size was determined using a significance threshold of P < 0.005, with a minimum of k > 20.
Fig. 3.
SgACC cluster identified by a Diagnosis × Age × Condition interaction from the whole-brain analysis of angry faces. (A) Cluster identified from the whole-brain analysis (P < 0.005, k ≥ 20). (B) BOLD signal at each intensity level for each participant group and each condition. (C) Linear trend between BOLD signal and intensity level for each participant group and each condition. Solid lines reflect the explicit rating condition (hostility ratings) and dashed lines reflect the implicit rating condition (nose width). Note: A25 = 25% angry/75% neutral; A50 = 50% angry/50% neutral; A75 = 75% angry/25% neutral; child BD = children with bipolar disorder; adult BD = adults with bipolar disorder.
Additional within-subjects findings in the sgACC cluster for angry faces included (i) among child HC, a more positive linear trend in the implicit vs explicit condition (P < 0.005); and (ii) among adult BD, a more negative linear trend in the explicit vs implicit condition (P < 0.03).
Happy faces
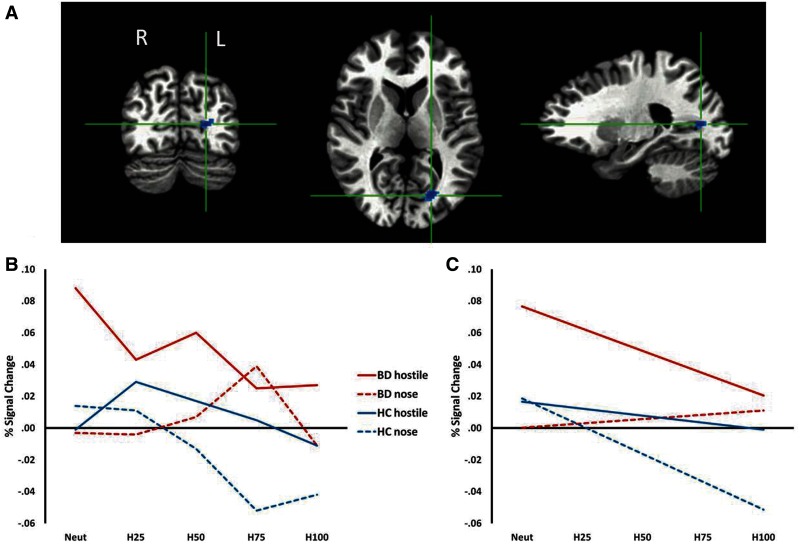
The whole-brain analysis revealed a significant Diagnosis × Condition interaction in the left cuneus [F(1,81) = 8.42, P < 0.01; Table 2; Figure 4]. There was no interaction with age group. In the implicit condition, HC participants demonstrated a negative linear trend that was absent in the BD patients (P < 0.01). The only significant within-subjects finding was a larger negative linear trend in the explicit vs implicit condition among BD patients (P < 0.03).
Fig. 4.
Cuneus cluster identified by a Diagnosis × Condition interaction from the whole-brain analysis of happy faces. (A) Cluster identified from the whole-brain analysis (P < 0.005, k ≥ 20). (B) BOLD signal at each intensity level for each diagnostic group and each condition. (C) Linear trend between BOLD signal and intensity level for each diagnostic group and each condition. Solid lines reflect the explicit rating condition (hostility ratings) and dashed lines reflect the implicit rating condition (nose width). Note: A25 = 25% angry/75% neutral; A50 = 50% angry/50% neutral; A75 = 75% angry/25% neutral.
Exploratory analyses: ADHD comorbidity, mood state and medications
The four differences between BD and HC groups identified in the primary analyses were in the modulation of the (i) amygdala in response to angry faces regardless of condition (all BD vs all HC), (ii) sgACC to angry faces processed implicitly (child BD vs child HC), (iii) sgACC to angry faces processed explicitly (adult BD vs adult HC) and (iv) cuneus to happy faces processed implicitly (all BD vs all HC). When we limited the sample to BD individuals without comorbid ADHD (9 child BD, 19 adult BD), the following between-group differences remained significant: sgACC activity to angry faces processed explicitly (adult BD vs adult HC) and cuneus activity to happy faces in the implicit condition (all BD vs HC) (ts > |2.04|, P < 0.05). The group difference in amygdala modulation to angry faces was significant at the level of a trend (P = 0.09). When the sample was limited to euthymic BD individuals (15 child BD, 13 adult BD), both sgACC findings and the cuneus difference remained significant (ts > |2.16|, P < 0.04). Pearson correlations between number of medications and linear trends in each of the regions were non-significant for child BD and adult BD groups (rs < |0.47|, P > 0.06).
DISCUSSION
This parametric face processing study revealed abnormal recruitment of regions associated with emotional (amygdala, sgACC) and visual (cuneus) processing among patients with BD in response to increasing face emotion intensity. The findings point to shared dysfunction among adult BD and child BD in the amygdala and cuneus as candidate biomarkers of BD. The findings also highlight abnormal recruitment of the sgACC in both adult BD and child BD. However, in this region, adult and child patients differed in the task condition that elicited between-group differences, possibly reflecting the developmental trajectory of the pathophysiology of BD.
Although amygdala abnormalities during face emotion processing tasks have been well-documented in both child BD and adult BD (Pavuluri and Passarotti, 2008; Chen et al., 2011; Delvecchio et al., 2012), only two studies directly compare the two age groups (Kim et al., 2012b; Adleman et al., 2013). This study extends this prior work by suggesting that failure to modulate amygdala activity in response to increasing anger is characteristic of both child BD and adult BD and is independent of task demands.
In addition to amygdala dysfunction, BD populations also exhibited abnormal sgACC modulation relative to age-matched controls, suggesting that deficits in BD extend to emotion-regulation regions with connections to the amygdala (Drevets et al., 2008). We found that healthy children engaged the sgACC in response to increasing anger during implicit processing, perhaps reflecting the need to recruit emotion-regulation regions that mediate ignoring the prepotent face emotion and making a non-emotional rating (nose width). Although child BD did not recruit the sgACC during implicit face emotion processing, adult BD did, perhaps reflecting the development of compensatory mechanisms. However, in adult patients, sgACC dysfunction was still evident in the form of abnormal disengagement during the explicit condition. However, additional developmental work in healthy populations is necessary to better understand the function of this region in the context of face-viewing tasks.
Together, the amygdala and sgACC findings point to a general deficit in BD in the recruitment of emotion regulation circuitry. Such findings are consistent with independent neuroimaging studies of child BD and adult BD (Drevets et al., 2008; Passarotti et al., 2011), and with a study reporting aberrant functional connectivity between the amygdala and sgACC during face processing in adult BD (Wang et al., 2009). Our findings extend this literature to suggest that deficits in amygdala recruitment during a face processing task are comparable across age groups, but sgACC modulation abnormalities may differ with age and task demands. The failure to engage the amygdala and sgACC appropriately with increasing face emotion intensity may lead to the abnormal coordination of downstream cognitive and behavioral responses to the stimuli—including the face emotion labeling deficits which have been documented extensively in BD in behavioral studies (Kohler et al., 2011). However, because this study did not test emotion labeling ability and groups did not differ in hostility or nose width ratings of angry faces, the implications of these aberrant recruitment patterns on behavior are unclear. Ongoing studies in our lab are testing associations between neural modulation and face labeling deficits in BD populations.
Based on prior work using an implicit face processing task, we hypothesized that amygdala deficits would be greater among children relative to adult BD (Kim et al., 2012b). However, this study did not reveal an interaction with age. Indeed, visual inspection of Figure 2 suggests that the BD group did not show the amygdala hyperactivity as is often reported in the literature (Pavuluri and Passarotti, 2008; Chen et al., 2011; Ladouceur et al., 2011; Delvecchio et al., 2012). The exact reasons for the discrepancies are not clear and warrant further investigation, however, we highlight a few possibilities here. First, our fMRI analysis technique differs from those in the existing literature documenting amygdala hyperactivation in BD patients. Those studies compare mean BOLD signal activation to emotional faces, whereas this study modeled the degree to which amygdala activation increased in a linear fashion with increasing face emotion. A second possible explanation for our somewhat surprising finding is that our use of implicit and explicit labeling conditions hindered our ability to detect amygdala hyperactivation. Amygdala hyperactivation among patients with BD has been documented consistently in implicit face processing tasks, but less so in explicit face processing tasks (Lennox et al., 2004; Hassel et al., 2009; Versace et al., 2010; Foland-Ross et al., 2012). Our use of both conditions may have introduced noise that reduced our ability to detect group differences. A third possibility is that the study design resulted in amygdala habituation, as has been seen previously (Phillips et al., 2010). Although the parametric design employed in this study reflected an effort to minimize this risk by including different face intensities, it is possible that these subtle differences between stimuli were not enough to overcome amygdala habituation tendencies, resulting in an inability to detect the typical amygdala hyperactivity observed among patients with BD. Future research exploring these possible explanations is necessary.
Deficient modulation of the cuneus in response to increasing happy face intensity was characteristic of both BD groups and was related to task condition. Specifically, although healthy participants disengaged the cuneus in response to increasing happiness during the implicit condition, BD subjects did not. Instead, BD participants disengaged this region during the explicit condition. Our prior study with an overlapping sample of children (Thomas et al., 2012) identified a region in the right middle occipital gyrus and cuneus that was disengaged in child BD relative to healthy children when processing happy faces, regardless of task condition. Other studies have documented abnormal activation in visual regions during the processing of happy faces among BD populations (Ladouceur et al., 2011; Mourao-Miranda et al., 2012). Relatedly, we did not observe the PCC abnormalities that we hypothesized would occur. Null results are difficult to interpret, and may represent Type II error rather than an absence of group differences. In sum, further study is needed to clarify the nature of posterior abnormalities in BD populations, including their relation to age group and task demands.
Limitations
Ethical considerations precluded us from withdrawing patients from their medications for non-therapeutic reasons. Therefore, almost all of the patients were taking psychotropic medications at the time of the scan. Correlations between total number of psychotropic medications and neural activation in regions identified in the primary analyses were not significant. Furthermore, to the extent that psychotropic medications reduce symptoms and normalize neural activity, as suggested by a recent systematic review (Hafeman et al., 2012), the inclusion of medicated patients should bias our findings to Type II rather than Type I errors. However, future studies evaluating the impact of medication on modulation of neural activity are important.
Similarly, future studies should examine clinical factors that might play a role in the present findings. For example, we cannot rule out the contribution of comorbid ADHD to the differences observed between child BD and their age-matched controls. Regarding mood state, group differences in the sgACC and cuneus were maintained when the BD group was limited to euthymic participants, but the amygdala finding was no longer significant. Further study is necessary to clarify whether the lack of significant findings in these subgroups is related to the reduced statistical power of eliminating participants or whether they reflect neural activation differences related to comorbid ADHD and mood state.
Finally, adult and child BD subjects differed not only in current age but also in age of onset of BD. Therefore, the sgACC abnormalities that differentiated child BD and adult BD may reflect differences in an early vs later onset illness or differences in participant age at the time of task completion. Although longitudinal studies are the gold standard for answering developmental questions, cross-sectional studies such as this represent a first step toward understanding the developmental trajectory of neural abnormalities in BD. Such cross-sectional studies are helpful in identifying paradigms for investment-intensive longitudinal designs.
Conclusion
This study uses a parametric design and linear trend analyses to examine the degree to which BD children and adults exhibit abnormal neural modulation in response to increasing anger and happiness in faces. The findings reveal aberrant recruitment of emotion-related circuitry and visual processing regions in BD, irrespective of age group, and thus suggest potential biomarkers of BD. Future work linking neural abnormalities to emotion labeling deficits may contribute to the field’s understanding of psychosocial deficits in this population and suggest treatment targets. These findings join a small but increasing literature examining developmental differences in BD, including a prior study of implicit face processing (Kim et al., 2012b), a face emotion memory study (Adleman et al., 2013), two fMRI studies of cognitive flexibility and motor inhibition (Weathers et al., 2012, 2013), and a diffusion tensor imaging study (Lu et al., 2012). Together, such studies have the potential to make significant contributions to the prevention, treatment and diagnosis of BD.
Acknowledgments
This research was supported by the Intramural Research Program at the National Institute of Mental Health, part of the National Institutes of Health (ZIA MH002786 12). C.A.Z. is listed as a co-inventor on a patent application for the use of ketamine in major depression; he has assigned his rights in the patent to the US government but will share a percentage of any royalties that may be received by the government. All other authors report no financial relationships with commercial interests. The authors gratefully acknowledge the efforts of members of the Emotion and Development Branch, and the participation of the patients and families in their research endeavors.
REFERENCES
- Adleman NE, Kayser RR, Olsavsky AK, et al. Abnormal fusiform activation during emotional-face encoding assessed with functional magnetic resonance imaging. Psychiatry Research. 2013;212(2):161–3. doi: 10.1016/j.pscychresns.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K, Shaywitz J, Smith BW, et al. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. American Journal of Psychiatry. 2008;165(9):1193–202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Donegan NH, Sanislow CA, et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berlin) 2005;183(3):308–13. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Martin A, Charney DS, Krystal JH, Peterson BS. Significance of adolescent neurodevelopment for the neural circuitry of bipolar disorder. Annals of the New York Academy of Sciences. 2004;1021:376–83. doi: 10.1196/annals.1308.048. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Guyer AE, Lawson ES, et al. Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. American Journal of Psychiatry. 2008a;165(3):385–9. doi: 10.1176/appi.ajp.2007.06122050. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Rich BA, Guyer AE, et al. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. American Journal of Psychiatry. 2010;167(1):61–9. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman MA, Skup M, Rich BA, et al. Risk for bipolar disorder is associated with face-processing deficits across emotions. Journal of the American Academy of Child and Adolescent Psychiatry. 2008b;47(12):1455–61. doi: 10.1097/CHI.0b013e318188832e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Lennox B, Jacob R, et al. Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: a functional magnetic resonance imaging study. Biological Psychiatry. 2006;59(1):31–9. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disorders. 2011;13(1):1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- Coryell W, Scheftner W, Keller M, Endicott J, Maser J, Klerman GL. The enduring psychosocial consequences of mania and depression. American Journal of Psychiatry. 1993;150(5):720–7. doi: 10.1176/ajp.150.5.720. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Delvecchio G, Fossati P, Boyer P, et al. Common and distinct neural correlates of emotional processing in bipolar disorder and major depressive disorder: a voxel-based meta-analysis of functional magnetic resonance imaging studies. European Neuropsychopharmacology. 2012;22(2):100–13. doi: 10.1016/j.euroneuro.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. 2008;13(8):663–81. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen W. Pictures of Facial Affect. Palot Alto, CA: Consulting Psychologists; 1976. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for the DSM-IV TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: New York State Psychiatric Insistute, Biometrics Research; 2002. [Google Scholar]
- Foland-Ross LC, Bookheimer SY, Lieberman MD, et al. Normal amygdala activation but deficient ventrolateral prefrontal activation in adults with bipolar disorder during euthymia. Neuroimage. 2012;59(1):738–44. doi: 10.1016/j.neuroimage.2011.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett AS, Reiss AL, Howe ME, et al. Abnormal amygdala and prefrontal cortex activation to facial expressions in pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(8):821–31. doi: 10.1016/j.jaac.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller B, Bolhofner K, Craney JL, Williams M, DelBello MP, Gundersen K. Psychosocial functioning in a prepubertal and early adolescent bipolar disorder phenotype. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(12):1543–8. doi: 10.1097/00004583-200012000-00018. [DOI] [PubMed] [Google Scholar]
- Goldstein TR, Birmaher B, Axelson D, et al. Psychosocial functioning among bipolar youth. Journal of Affective Disorders. 2009;114(1–3):174–83. doi: 10.1016/j.jad.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure EB, Adler AD, et al. Specificity of facial expression labeling deficits in childhood psychopathology. The Journal of Child Psychology and Psychiatry. 2007;48(9):863–71. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disorders. 2012;14(4):375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- Hassel S, Almeida JR, Frank E, et al. Prefrontal cortical and striatal activity to happy and fear faces in bipolar disorder is associated with comorbid substance abuse and eating disorder. Journal of Affective Disorders. 2009;118(1–3):19–27. doi: 10.1016/j.jad.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmar JH, Wang F, Chepenik LG, et al. Relation between amygdala structure and function in adolescents with bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(6):636–42. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children: Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keenan-Miller D, Miklowitz DJ. Interpersonal functioning in pediatric bipolar disorder. Clinical Psychology: Science and Practice. 2011;18:342–56. [Google Scholar]
- Kim P, Thomas LA, Rosen BH, et al. Differing amygdala responses to facial expressions in children and adults with bipolar disorder. American Journal of Psychiatry. 2012b;169(6):642–9. doi: 10.1176/appi.ajp.2012.11081245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CG, Hoffman LJ, Eastman LB, Healey K, Moberg PJ. Facial emotion perception in depression and bipolar disorder: a quantitative review. Psychiatry Research. 2011;188(3):303–9. doi: 10.1016/j.psychres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Farchione T, Diwadkar V, et al. Differential patterns of abnormal activity and connectivity in the amygdala-prefrontal circuitry in bipolar-I and bipolar-NOS youth. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(12):1275–89.e2. doi: 10.1016/j.jaac.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry. 2004;55(6):578–87. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. American Journal of Psychiatry. 2003;160(3):430–7. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- Lennox BR, Jacob R, Calder AJ, Lupson V, Bullmore ET. Behavioural and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychological Medicine. 2004;34(5):795–802. doi: 10.1017/s0033291704002557. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4(4):423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LH, Zhou XJ, Fitzgerald J, et al. Microstructural abnormalities of white matter differentiate pediatric and adult-onset bipolar disorder. Bipolar Disorders. 2012;14(6):597–606. doi: 10.1111/j.1399-5618.2012.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Young LT, Joffe RT. A review of psychosocial outcome in patients with bipolar disorder. Acta Psychiatrica Scandinavica. 2001;103(3):163–70. doi: 10.1034/j.1600-0447.2001.00059.x. [DOI] [PubMed] [Google Scholar]
- Marchand WR, Lee JN, Garn C, et al. Aberrant emotional processing in posterior cortical midline structures in bipolar II depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2011;35(7):1729–37. doi: 10.1016/j.pnpbp.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Mourao-Miranda J, Almeida JR, Hassel S, et al. Pattern recognition analyses of brain activation elicited by happy and neutral faces in unipolar and bipolar depression. Bipolar Disorders. 2012;14(4):451–60. doi: 10.1111/j.1399-5618.2012.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Mental Health, Health and Human Services. National Institute of Mental Health Strategic Plan. Bethesda, MD: 2008. http://www.nimh.nih.gov/about/strategic-planning-reports/index.shtml#strategic-objective2 (17 October 2013, date last accessed) [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of General Psychiatry. 1994;51(11):849–59. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology (Berlin) 2011;216(4):485–99. doi: 10.1007/s00213-011-2243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, O’Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biological Psychiatry. 2007;62(2):158–67. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti A. Neural bases of emotional processing in pediatric bipolar disorder. Expert Review of Neurotherapeutics. 2008;8(9):1381–7. doi: 10.1586/14737175.8.9.1381. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(3):308–19. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JC, Welge J, Strakowski SM, Adler CM, DelBello MP. Meta-analysis of amygdala volumes in children and adolescents with bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(11):1289–98. doi: 10.1097/CHI.0b013e318185d299. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Medford N, Young AW, et al. Time courses of left and right amygdalar responses to fearful facial expressions. Human Brain Mapping. 2001;12(4):193–202. doi: 10.1002/1097-0193(200104)12:4<193::AID-HBM1015>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(23):8900–5. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry. 2005;57(3):201–9. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Marshall N, Schulze K, et al. Exaggerated neural response to emotional faces in patients with bipolar disorder and their first-degree relatives. Neuroimage. 2010;53(1):58–64. doi: 10.1016/j.neuroimage.2010.05.069. [DOI] [PubMed] [Google Scholar]
- Thomas LA, Brotman MA, Muhrer EJ, et al. Parametric modulation of neural activity by emotion in youth with bipolar disorder, youth with severe mood dysregulation, and healthy volunteers. Archives of General Psychiatry. 2012;69(12):1257–66. doi: 10.1001/archgenpsychiatry.2012.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A, Thompson WK, Zhou D, et al. Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biological Psychiatry. 2010;67(5):422–31. doi: 10.1016/j.biopsych.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, He Y, et al. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biological Psychiatry. 2009;66(5):516–21. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers JD, Brotman MA, Deveney CM, et al. A developmental study on the neural circuitry mediating response flexibility in bipolar disorder. Psychiatry Research: Neuroimaging. 2013;214:56–65. doi: 10.1016/j.pscychresns.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers JD, Stringaris A, Deveney CM, et al. A developmental study of the neural circuitry mediating motor inhibition in bipolar disorder. American Journal of Psychiatry. 2012;169(6):633–41. doi: 10.1176/appi.ajp.2012.11081244. [DOI] [PMC free article] [PubMed] [Google Scholar]