Abstract
Human context conditioning studies have focused on acquisition and extinction. Subsequent long-term changes in fear behaviors not only depend on associative learning processes during those phases but also on memory consolidation processes and the later ability to retrieve and express fear and extinction memories. Clinical theories explain relapse after successful exposure-based treatment with return of fear memories and remission with stable extinction memory expression. We probed contextual fear and extinction memories 1 week (Day8) after conditioning (Day1) and subsequent extinction (Day2) by presenting conditioned contexts before (Test1) and after (Test2) a reinstatement manipulation. We find consistent activation patterns in two independent samples: activation of a subgenual part of the ventromedial prefrontal cortex before reinstatement (Test1) and (albeit with different temporal profiles between samples) of the amygdala after reinstatement (Test2) as well as up-regulation of anterior hippocampus activity after reinstatement (Test2 > Test1). These areas have earlier been implicated in the expression of cued extinction and fear memories. The present results suggest a general role for these structures in defining the balance between fear and extinction memories, independent of the conditioning mode. The results are discussed in the light of hypotheses implicating the anterior hippocampus in the processing of situational ambiguity.
Keywords: reinstatement, amygdala, return of fear, anterior hippocampus, phasic and sustained fear
INTRODUCTION
Learning to predict danger is critical for adaptive behavior in changing environments. In fear conditioning, repeated pairing of a neutral conditioned stimulus (CS) with an aversive event (unconditioned stimulus, US) evokes a conditioned response (CR) to the CS as the predictor of the US. Conditioning to environmental contexts induces sustained CRs, as opposed to phasic CRs in cue conditioning, and both modes of conditioning have been proposed to model diverse features of anxiety disorders (Grillon et al., 2006). In addition, learning to disregard a CS that no longer predicts the US (extinction learning) serves successful adaptation and is an important mechanism underlying resilience to stress or trauma (Lommen et al., 2013) and the behavioral treatment of pathological fears (Rachman, 1989). Extinction, in most circumstances, does not erase conditioned fear memories, but generates competing, fear-inhibitory extinction memories (Bouton, 2004; Myers and Davis, 2007). Insufficient expression of extinction memories upon re-confrontation with a CS allows the return of fear though dominancy of the fear memory trace and represents a likely basis for relapse after successful extinction-based therapy (Rachman, 1989; Bouton, 2004; Craske et al., 2008). Dominance of the fear memory trace can be facilitated through contextual changes between extinction and test (renewal) (Bouton and Bolles, 1979a,b), the mere passage of time (spontaneous recovery) (Rescorla, 2004) or unsignaled presentations of the US alone before testing (reinstatement) (Rescorla and Heth, 1975; Bouton and Bolles, 1979a,b). In this study, we focus on reinstatement as one avenue toward return of fear as well as on extinction expression after a longer delay. Separating experimental phase in time allows for the passage of time and thereby consolidation between the experimental phases, which has a major impact on the results (e.g. Huff et al., 2009; Golkar and Öhman, 2012).
A clinical example of reinstatement is the case of an individual who develops a driving phobia following serious injuries (=US) in a car accident (=CS). After successful cognitive-behavioral treatment of this phobia, the association between driving a car and injury may be reinstated when the same individual is injured when doing sports. As a consequence, there may be a relapse of the individuals’ previous driving phobia.
In human laboratory experiments, reinstatement has so far only been investigated using cue conditioning paradigms. The above example, however, highlights that situation-bound fears or configurations of cues (driving a car) may be reinstated as well, motivating an investigating reinstatement of context CRs. Therefore, in this study, we used a combined cue and context conditioning paradigm (see also Haaker et al., 2013). Still, as previous research has nearly exclusively used cued paradigms, our hypothesis has to build on results from cue conditioning and extinction.
For phasic cue CSs, successful long-term extinction expression depends on the ventromedial prefrontal cortex (vmPFC) (Morgan and LeDoux, 1995; Milad and Quirk, 2002, 2012) which inhibits the generation of CRs in the amygdala (Rosenkranz et al., 2003; Quirk et al., 2003; Milad and Quirk, 2012). Human imaging studies have also observed vmPFC activation during cued extinction recall (Phelps et al., 2004; Kalisch et al., 2006; Milad et al., 2007). In addition, the hippocampus, in particular in its more anterior aspects (Kalisch et al., 2006; Milad et al., 2007, 2009), may contribute to extinction memory expression for cue CSs. Return of fear for cue CSs has implicated the hippocampus and the amygdala in animal (Corcoran and Maren, 2004; Ji and Maren, 2007) and human studies (LaBar and Phelps, 2005; Kalisch et al., 2006, 2009; Agren et al., 2012; Lonsdorf et al., 2013), in particular posterior aspects of the hippocampus (Kalisch et al., 2006, 2009).
In contrast, the neural networks underlying extinction expression and return of fear in humans and animals for contextual CSs remain unstudied to date. In animals, the acquisition and expression of contextual fear (Rudy, 2009; Fanselow, 2010; Maren, 2011) as well as its extinction (Tronson et al., 2009) are hippocampus-dependent, and human imaging studies have largely confirmed this picture (Hasler et al., 2007; Alvarez et al., 2008; Marschner et al., 2008; Lang et al., 2009; Indovina et al., 2011). Hence, it is tempting to hypothesize that the hippocampus is also involved in contextual extinction expression and return of fear. An additional source for hypotheses generation is the above neural results for cued extinction expression and returning fear.
Reinstatement in rodents involves activation of the amygdala (Laurent and Westbrook, 2010; Lin et al., 2011), and also the human amygdala has been implicated in returning fear (Kalisch et al., 2006, 2009; Agren et al., 2012; Lonsdorf et al., 2013).
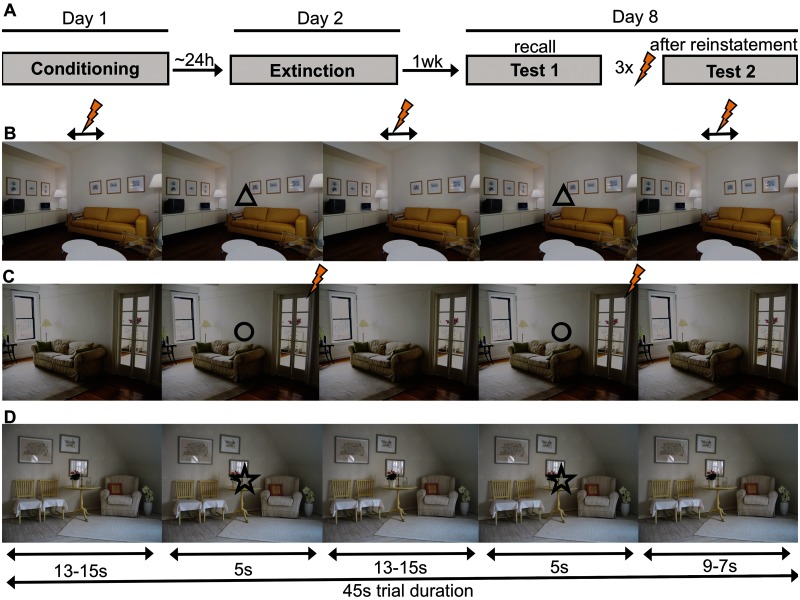
We here employed a multiple-day paradigm [see Figure 1A with conditioning (Day1), extinction (Day2), memory expression and reinstatement (Day8)] of combined cue and context conditioning based on earlier animal work (Fanselow, 1980; Grillon et al., 2006; Marschner et al., 2008) to tap long-term extinction recall as well as reinstatement to different CS modalities (discrete cues and contextual). We investigated (i) whether the vmPFC and the anterior hippocampus activate during long-term expression of contextual extinction and (ii) whether the (posterior) hippocampus and the amygdala are involved in return of contextual fear. As there is a call for greater focus on replication studies in functional magnetic resonance imaging (fMRI) (Lieberman and Cunningham, 2009; Bennett and Miller, 2010), we have further attempted to replicate our major findings in a second sample.
Fig. 1.
Design. Experimental timeline (A) and structure of trials in the unpredictable (B), predictable (C) and safe (D) trials. Shown is an example of stimulus-condition assignments. Bolt denotes US.
MATERIALS AND METHODS
Participants
All participants provided written informed consent. The study was approved by the local ethics committee [Ärztekammer Hamburg (General Medical Council Hamburg)].
Discovery sample
Twenty-three male, right-handed participants (mean age, 28.8 years) were included in the study, whereof three participants were excluded (due to pathological anatomy, claustrophobia or unavailability on Day8), leaving 20 participants for statistical analyses.
Replication sample
The replication sample represents the placebo group from a double-blind randomized placebo-controlled clinical trial. Nineteen male, right-handed participants (mean age, 29.2 years) were included in the replication sample. All experimental procedures were identical between the discovery and the replication sample with the exception that participants in the replication sample were administered a placebo pill on Day2, right after extinction training and received 160 Euro for their participation (discovery sample: 80 Euro).
Stimulus material
Three background pictures of rooms served as context CSs (CXT) and three geometric symbols (triangle, circle and star) served as cue CSs (cue) (Figure 1). The feasibility of using two-dimensional pictures as contexts has been convincingly demonstrated earlier (Baas et al., 2004; Milad et al., 2005). A black screen with a white fixation cross served as inter-trial intervals (ITIs). An electrotactile stimulus consisting of a train of three square-wave pulses of 2 ms duration each (interval 50 ms) served as the US. The US was delivered through a surface electrode with platinum pin (Specialty Developments, Bexley, UK) on the right dorsal hand using a DS7A electrical stimulator (Digitimer, Welwyn Garden City, UK).
Procedure
Day1 (conditioning)
The procedure included attachment of recording and stimulation electrodes as well as individual US intensity calibration to maximum tolerable pain (range 2.7–20.6 mA, mean 8.6 ± 1.2 mA). Participants were asked to rate the painfulness of the US between 0 (‘I feel nothing’) and 10 (‘maximally unpleasant’) (final rating: range 5–9, mean 7.2 ± 0.3).
Stimuli were presented in trials that corresponded to the 45 s continuous presentation of one of the CXTs. ITI duration was 6–8 s with a mean of 7 s. During a trial, the corresponding cue was presented twice for 5 s during fixed time windows (onsets at 13–15 s and 31–35 s post-trial onset; Figure 1B–D). Trials were always grouped in blocks of 9 (3 of each condition) that were separated by subjective fear ratings.
Three conditions were realized through different predictability of the US. In the unpredictable condition, the cue (UCue) did not signal the US making the context itself (UCXT) the best US predictor (context conditioning). Cue conditioning on the other hand should occur in the predictable condition where the cue (PCue) always co-terminated with the US, making the cue a better US predictor than the context (PCXT). In a safe condition, providing control stimuli SCue and SCXT, no US was given.
During a habituation phase, each of the three trial types was presented in a shortened exemplary version (CXT presentation for 7.5 s with cue onset 2.5 s post-trial onset) without USs. Participants were also familiarized with the fear rating scales and the use of the keypad. Conditioning consisted of 27 trials in 3 blocks (total of 9 trials per condition).
In the unpredictable condition, one, two or three USs per trial were randomly administered in fixed time windows (with onsets between 5–7, 24–26 or 39–41 s after trial onset). To avoid that the UCue acquired safety signal properties, two USs in total were applied during UCue presentations (1 s after cue onset). In the unpredictable condition, on average (including both USs to the UCXT and the UCue), two USs were administered (range 1–3). In the predictable condition, the PCue was always paired with a US occurring 4.8 s after cue onset (100% PCue reinforcement). Thus, in both conditions, the same total number of USs was administered. In the safe condition, no US ever occurred. Participants were not informed about the conditioning contingencies beforehand.
Day2 (extinction)
Approximately 24 h after conditioning, participants returned. Stimulation and recording electrodes were attached at the same positions as the day before, without renewed US intensity calibration. Eighteen trials were presented in two blocks (total of six trials per condition). No US was administered. Participants were not informed beforehand about any change in CS–US contingencies.
In the replication sample, participants received a placebo pill immediately following extinction and remained in the laboratory, while blood pressure and pulse frequency was monitored intermittently.
Day8 (Test1 and Test2)
Participants were placed inside the MR scanner, and stimulation and recording electrodes were attached. There was no additional US calibration. A recall test (Test1) consisted of 18 unreinforced trials in 2 blocks (total of 6 trials per condition) and was followed by the presentation of a gray screen. Five seconds after onset of the gray screen, three unsignaled reinstatement USs were administered (interval 5 s). Two minutes after the last US, a reinstatement test (Test2, corresponding to Test1) was conducted. The interval between reinstatement USs and Test2 served to reduce potential non-associative effects of the USs on subsequent CRs (e.g. sensitization; Rescorla and Heth, 1975).
Reinstatement of fear normally requires presentation of the reinstatement USs in a context identical to the test context (Bouton, 2004). Reinstatement USs were here presented in the same global context (i.e. running fMRI acquisition) as the CSs during Test1 and Test2, but not while any of the experimental context CSs were present. This was done to avoid re-acquisition of any of the context CSs. However, the gray background on which the reinstatement USs were presented (compare, e.g. Hermans et al., 2005; Dirikx et al., 2007; Kull et al., 2012) also introduced a physical distinction from the tests.
Behavioral measures
Fear ratings
At the beginning of each experimental phase as well as after every trial block, participants were asked to rate each CS with respect to the fear/stress/tension that was elicited when they last saw it. Ratings were performed on a computerized visual analog scale [0 (none) − 100 (maximal)], using the keyboard (Days1 and 2) or a button-response box (Day8) with the right hand. Selected rating values had to be confirmed by a key press and were otherwise treated as missing data. Participants were excluded from the analyses (day-wise) if less than one-third of all data points were valid [not missing, discovery sample: N(Day1) = 1, N(Day2) = 1; replication sample: N(Day1) = 2, N(Day2) = 2, N(Day8) = 1]. See Tables 2 and 3 for the exact N included in the different analyses. Ratings prior to the first experimental phase (conditioning, Day1) were not included in the analyses.
Table 2.
Behavioral data: context conditioning
| Measure | Phase | Sample | N | df | F | P | Eta2 | Contrasts |
|---|---|---|---|---|---|---|---|---|
| Ratings | C | Discovery | 19 | 2,36 | 25.83 | <0.001 | 0.59 | 2 |
| E | 19 | 2,36 | 19.03 | <0.001 | 0.51 | 2 | ||
| T1 | 16 | 2,30 | 6.55 | 0.008 | 0.30 | 2 | ||
| T2 | 18 | 2,34 | 7.14 | 0.004 | 0.30 | 2 | ||
| T2 > T1 | 18 | 1,17 | 3.34 | 0.085 | 0.16 | |||
| C | Replication | 17 | 2,32 | 35.05 | <0.001 | 0.69 | 0 | |
| E | 17 | 2,32 | 16.52 | <0.001 | 0.49 | 2 | ||
| T1 | 17 | 2,32 | 7.95 | 0.009 | 0.33 | 2 | ||
| T2 | 17 | 2,32 | 6.27 | 0.019 | 0.28 | 2 | ||
| T2 > T1 | 17 | 1,16 | <1 | 0.45 | ||||
| SCR | C | Discovery | 18 | 2,34 | 4.5 | 0.02 | 0.21 | 1 |
| E | 19 | 2,36 | <1 | 0.40 | ||||
| T1 | 14 | 2,26 | 3.06 | 0.08 | 0.19 | 1 | ||
| T2 | 14 | 2,26 | <1 | 0.72 | ||||
| T2 > T1 | 14 | 1,13 | 4.90 | 0.045 | 0.27 | – | ||
| C | Replication | 19 | 2,36 | 1.32 | 0.28 | |||
| E | 17 | 2,32 | <1 | 0.51 | ||||
| T1 | 12 | 2,22 | <1 | 0.55 | ||||
| T2 | 12 | 2,22 | <1 | 0.67 | ||||
| T2 > T1 | 12 | 2,11 | 4.50 | 0.057 | 0.25 | – |
Main effects of stimulus (UCXT, PCXT, SCXT) in the discovery and replication samples during conditioning (C, Day1), extinction (E, Day2) and the memory tests on Day8 before (Test1, T1) and after reinstatement (Test2, T2). Main effects of time are given to index changes from T1 to T2 (T2 > T1, indicative of a generalized reinstatement).
Contrasts
0 = all CXTs differ significantly from each other.
1 = UCXT differs significantly from SCXT and at trend level from PCXT; PCXT and SCXT do not differ.
2 = UCXT and PCXT do not differ significantly from each other but both differ significantly from SCXT.
Table 3.
Behavioral data: cue conditioning
| Measure | Day | Sample | N | df | F | P | Eta2 | Contrast |
|---|---|---|---|---|---|---|---|---|
| Ratings | C | Discovery | 19 | 2,36 | 17.98 | <0.001 | 0.5 | 3 |
| E | 19 | 2,36 | 11.81 | <0.001 | 0.4 | 3 | ||
| T1 | 15 | 2,28 | 9.66 | 0.008 | 0.41 | 2 | ||
| T2 | 20 | 2,38 | 8.35 | 0.003 | 0.31 | 2 | ||
| T2 > T1 | 19 | 1,18 | 7.34 | 0.014 | 0.29 | – | ||
| C | Replication | 17 | 2,32 | 35.05 | <0.001 | 0.69 | 2 | |
| E | 17 | 2,32 | 10.25 | 0.002 | 0.39 | 3 | ||
| T1 | 17 | 2,32 | 5.65 | 0.017 | 0.26 | 2 | ||
| T2 | 16 | 2,30 | 6.12 | 0.020 | 0.29 | 2 | ||
| T2 > T1 | 15 | 1,14 | <1.1 | 0.35 | – | |||
| SCR | C | Discovery | 18 | 2,34 | 9.54 | 0.001 | 0.36 | 4 |
| E | 19 | 2,36 | 5.62 | 0.01 | 0.24 | 2 | ||
| T1 | 14 | 2,26 | 5.17 | 0.014 | 0.28 | 1 | ||
| T2 | 14 | 2,26 | 1.30 | 0.29 | ||||
| T2 > T1 | 14 | 1,13 | <1 | 0.60 | – | |||
| C | Replication | 19 | 2,36 | 7.02 | 0.004 | 0.28 | 4 | |
| E | 17 | 2,32 | 2.76 | 0.097 | 0.15 | 1 | ||
| T1 | 12 | 2,22 | <1.4 | 0.29 | ||||
| T2 | 12 | 2,22 | <1.1 | 0.37 | ||||
| T2 > T1 | 12 | 1,11 | 16.33 | 0.002 | 0.60 | – |
Main effects of stimulus (UCue, PCue, SCue) in the discovery and replication samples during conditioning (C, Day1), extinction (E, Day2) and the memory tests on Day8 before (Test1, T1) and after reinstatement (Test2, T2). Main effects of time are given to index changes from T1 to T2 (T2 > T1, indicative of a generalized reinstatement).
Contrasts
1 = only PCue differs from SCue.
2 = PCue and UCue do not differ significantly from each other but both differ significantly from SCue.
3 = PCue and UCue differ tendentially and both differ significantly from SCue.
4 = PCue differs significantly or tendentially from UCue and SCue but UCue and SCue do not differ.
Skin conductance
Skin conductance was measured via self-adhesive Ag/AgCl electrodes placed on the palmar side of the left hand on the distal and proximal hypothenar. Data were down-sampled to 10 Hz and phasic skin conductance responses (SCRs) to the onsets of CXT (Marschner et al., 2008) or cue CS were manually scored off-line using a custom-made computer program. SCR amplitudes (in μS) were scored as the largest response occurring 0.9–4.0 s after CXT or Cue onset (Fowles et al., 1981). As before (Marschner et al., 2008), we did not analyze the rest of the CXT presentation periods as in the predictable condition these are confounded by US reactions. Separately for the three experimental days, logarithms were computed for all values, to normalize the distribution (Venables and Christie, 1980), and these log values were range-corrected (SCR/SCRmax[day]) to account for inter-individual variability (Lykken and Venables, 1971). SCR measurements that showed recording artifacts or excessive baseline activity were discarded and treated as missing data. Due to technical difficulties, SCR data from a limited number of participants had insufficient data quality and were thus excluded (day-wise) from the analyses [discovery sample: N(Day1) = 2, N(Day2) = 1, N(Day8) = 6; replication sample: N(Day2) = 2, N(Day8) = 7]. See Tables 2 and 3 for the exact N included in the different analyses. The high numbers of excluded subjects on Day8 are due to the technical challenges posed by the combined acquisition of psychophysiological and fMRI data.
SCRs were averaged over blocks of three (context conditioning) or six (cue conditioning) trials, resulting in three blocks on Day1, two blocks on Day2 and two blocks per Test on Day8 (as in Figure 2).
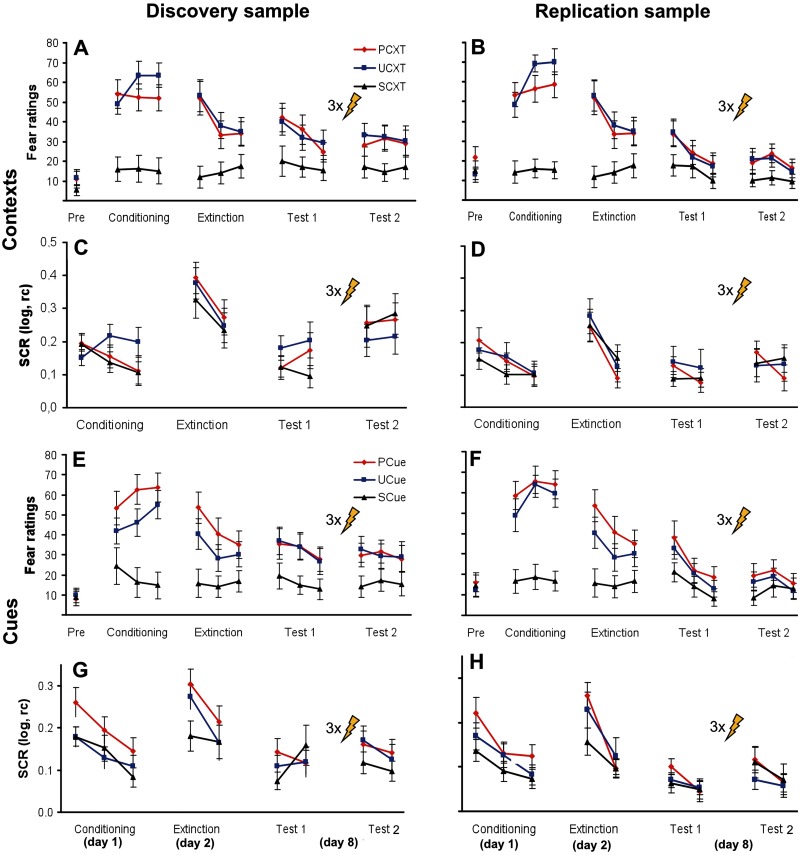
Fig. 2.
Behavioral data. Fear ratings and SCRs for context CSs in the discovery (A, C) and replication samples (B, D) and for cue CSs in the discovery (E, G) and replication samples (F, H). PCXT, UCXT, SCXT: context CSs in the predictable, unpredictable and safe conditions, respectively. PCue, UCue, SCue: cue CSs in the predictable, unpredictable and safe conditions, respectively. Data show mean ± s.e.m. Log, logarithmized; rc, range-corrected. Bolt denotes reinstatement USs.
Data analysis
Behavioral data were analyzed separately for the three experimental days as well as the two phases on Day8 (Test1, Test2), using SPSS 18 for Windows. Please note, that different SCR equipment was used inside (Day8) and outside the scanner (Day1, Day2) for methodological reasons. For fear ratings, repeated-measures ANOVAs with stimulus (3) as the within-subject variable were applied, while for SCRs stimulus (3) × time (block) repeated-measures ANOVAs for Days1 and 2 were calculated. In contrast to fear ratings where only few data points throughout the experimental sessions exist, the factor block was included for SCR’s analyses to provide a more fine-grained picture of the learning curves. For Day8, the ANOVAs testing memory expression before (Test1) as well as after (Test2) reinstatement were restricted to stimulus effects (3) in the first blocks of each test, to account for on-line extinction. A potential enhanced fear memory expression after vs before reinstatement (Test2 > Test1) was assessed using stimulus (3) × time (2) repeated-measures ANOVAs on the last block before and the first block after reinstatement. Thereby, a main effect of time would be indicative of a generalized reinstatement effect [as frequently observed in human studies (e.g. Dirikx et al., 2007, 2009)], while a differential reinstatement effect as observed by others (e.g. Hermans et al., 2005) would be obvious from a stimulus × time interaction. An α-level of P < 0.05 was considered significant, and Greenhouse–Geisser correction was applied if necessary.
ANOVAs were followed by contrasts of interest defined a priori. We were particularly interested in the contrasts showing whether participants successfully discriminated between truly US-predictive stimuli (UCXT, PCue) and the corresponding non-predictive stimuli (here in particular SCXT and SCue), that is, UCXT > SCXT (for context conditioning) and PCue > SCue (for cue conditioning). This was complemented by the more ‘demanding’ comparisons UCXT > PCXT (for context conditioning) and PCue > UCue (for cue conditioning) (see, e.g. Marschner et al., 2008), based on the idea that responding to PCXT and UCue is indicative of false, generalizing threat attributions governed by mere US presence in the temporal surrounding of the stimulus. Note also that comparing only stimuli of the same kind (cues with cues, contexts with contexts) avoids problems related to different scaling of event- and block-type regressors (cue and context responses) in the imaging data analysis (which actually prohibits cue-to-context comparisons).
Imaging (Day8)
Data acquisition and pre-processing
fMRI data were obtained with a 3 T MR scanner (MAGNETOM trio, Siemens Germany) using a 32-channel head coil. Pre-processing [SPM8 (www.fil.ion.ucl.ac.uk/spm) running on MatlabR2009b (The MathWorks, Natick, MA, USA)] involved realignment, unwarping co-registration and normalization to a sample-specific template, using DARTEL (Ashburner, 2007). See Supplementary data for more information.
Correction for multiple comparison at an α-level of P < 0.05 was restricted to pre-defined regions of interest (ROIs; Table 1) and used small volume correction (SVC) based on Gaussian random field theory (family-wise error rate method; Friston et al., 2006).
Table 1.
ROI center coordinates and literature sources
| ROI | x | y | z | References |
|---|---|---|---|---|
| vmPFC | 0 | 40 | −12 | Phelps et al. (2004) |
| Kalisch et al. (2006) | ||||
| Milad et al. (2007) | ||||
| Milad et al. (2009) | ||||
| Spoormaker et al. (2010) | ||||
| dmPFC | 0 | 43 | 29 | Kalisch et al. (2009) |
| Milad et al. (2009) | ||||
| Anterior hippocampus | ±29 | −16 | −25 | Kalisch et al. (2006) |
| Milad et al. (2007) | ||||
| Milad et al. (2009) | ||||
| Posterior hippocampus | ±36 | −32 | −14 | Kalisch et al. (2006) |
| Kalisch et al. (2009) | ||||
| Amygdala | Harvard-Oxford cortical and subcortical structural atlases (Desikan et al., 2006) | |||
ROI center coordinates in the vmPFC, the anterior and posterior hippocampus as well as two key regions of the fear network (amygdala, dmPFC; Sehlmeyer et al., 2009; Mechias et al., 2010; Etkin et al., 2011) were determined by averaging peak-effect coordinates reported in prior studies of fear and extinction expression, provided the reported effects had survived appropriate correction for multiple comparisons. Because no such data were available for the amygdala, a probabilistic anatomical mask was used (http://www.cma.mgh.harvard.edu; threshold 0.7; Desikan et al., 2006). As in earlier work (Kalisch et al., 2009), subcortical ROIs were spheres of 6 mm radius around the corresponding unilateral center coordinate. For cortical ROIs (vmPFC, dmPFC), the x-coordinate was set to 0 [averaging from coordinates reported in the listed studies ignored laterality (sign of the x-coordinate) to create homologous unilateral ROIs] and the resulting midline-centered coordinates were used as the center of a box of dimensions 20 × 16 × 16 mm that equally covered both hemispheres (see Raczka et al., 2010; Paret et al., 2011). Coordinates are in Montreal Neurological Institute (MNI) space.
RESULTS
Behavioral data
Context conditioning
Fear ratings showed robust conditioning effects in both the replication (Figure 2A) and the discovery sample (Figure 2B), as indicated by significant main effects of stimulus that were maintained throughout extinction and the memory tests before (Test1) and after (Test2) reinstatement (all P < 0.019; see Table 2 for statistical details). Generally, participants reported more fear of both the UCXT and the PCXT than the SCXT, but did not discriminate between the UCXT and the PCXT (see ‘Contrasts’ in Table 2). This suggests generalization of fear from the truly US-predictive stimuli (UCXT and PCue) to the context in the predictable condition (PCXT). The reinstatement manipulation did not further enhance ratings.
In SCRs, in contrast, main effects of stimulus were observed only during conditioning and only in the discovery sample (P < 0.02; Table 2; Figure 2C). Nevertheless, there was a generalized reinstatement in both samples (main effect of time in absence of an interaction with stimulus; discovery sample: P = 0.045, replication sample: P = 0.057, trend level). In view of the weak stimulus effects in SCRs during Days1 and 2, the observed reinstatement effects on Day8 can be considered substantial (compare also the relative increase of responding in Figure 2C and D).
Cue conditioning
Like for contextual fear, ratings of cued fear also showed robust conditioning effects, both in the discovery sample (Figure 2E) and in the replication sample (Figure 2F). This was indicated by significant main effects of stimulus that were maintained throughout extinction and the memory tests before (Test1) and after (Test2) reinstatement (all P < 0.020; Table 3). The ‘demanding’ discrimination between the predictable and the unpredictable conditions was generally better than in context conditioning, that is, PCue mostly evoked more fear than UCue (see ‘Contrasts’ in Table 3). A generalized reinstatement effect (Test2 > Test1) was observed in the discovery sample only (P = 0.014).
In SCRs, main effects of stimulus were present during conditioning, extinction and before reinstatement in the discovery sample (all P < 0.014; Table 3; Figure 2G) and during conditioning (P = 0.002) and extinction (P = 0.097, trend level) in the replication sample (Figure 2H). The replication sample, but not the discovery sample, also showed a generalized reinstatement effect (Test2 > Test1, P = 0.002). In view of the globally good sensitivity of cue conditioning measures for stimulus effects on all 3 days, the inconsistency of reinstatement effects on Day8 suggests a lesser success of the reinstatement procedure in reactivating cued as compared with contextual fear. This might be related to the presentation of the reinstatement USs on a background [gray screen as in previous studies on human reinstatement (Hermans et al., 2005; Dirikx et al., 2007; Kull et al., 2012)] that was different from the background on which cue CSs were presented during Test2 (context CSs; see 2.5 Procedure; Bouton, 2004). The unsignaled reinstatement USs might imbue the general test situation with a sense of danger and enhanced US expectancy and therefore establish a superordinate, US-associated context that facilitates the return of contextual fear by gating the retrieval of the initial context CS–US association (Bouton, 2004; Vervliet et al., 2013a) rather than the initial cued CS–US association. An alternative explanation for the disparate return of cued and contextual fear might be that the expression of fear to contextual CSs may be the appropriate defensive response (acquired through unpredictable US administrations during Day1) after experiencing unpredictable USs during reinstatement.
Imaging data (Day8)
Our main hypotheses were vmPFC and anterior hippocampus activation before the reinstatement procedure, where the retrieval and/or expression of extinction should prevail (Test1), as well as posterior hippocampus, dmPFC and amygdala activation after the reinstatement procedure, where the retrieval and/or expression of fear should prevail (Test2). Above behavioral analysis of memory expression suggested that this relative dichotomy should be clearer in the case of context conditioning (where behavioral reinstatement effects were comparatively strong). We focused on contrasts UCXT > SCXT (for context conditioning) and PCue > SCue (for cue conditioning) as there was not much evidence for UCXT > PCXT and PCue > UCue discrimination in behavior.
Context conditioning
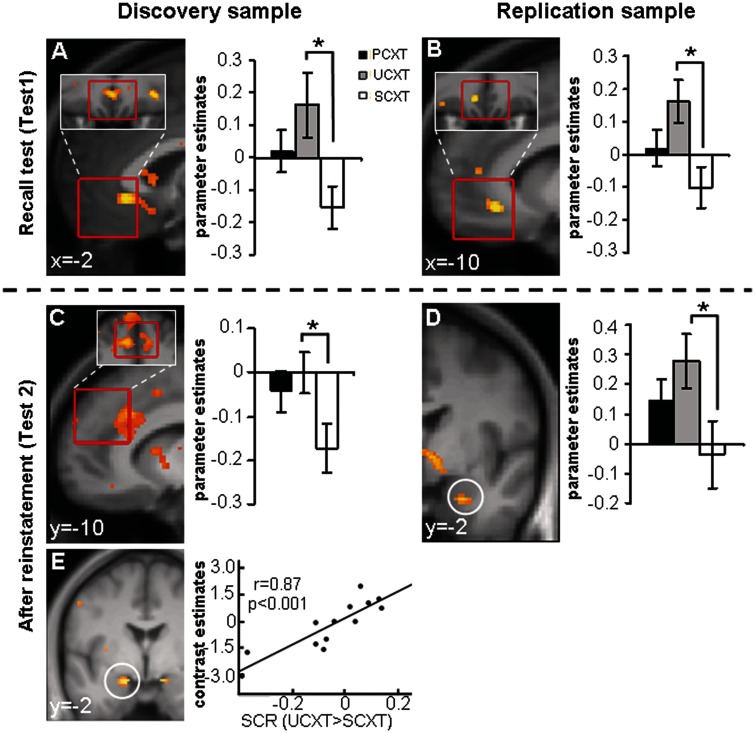
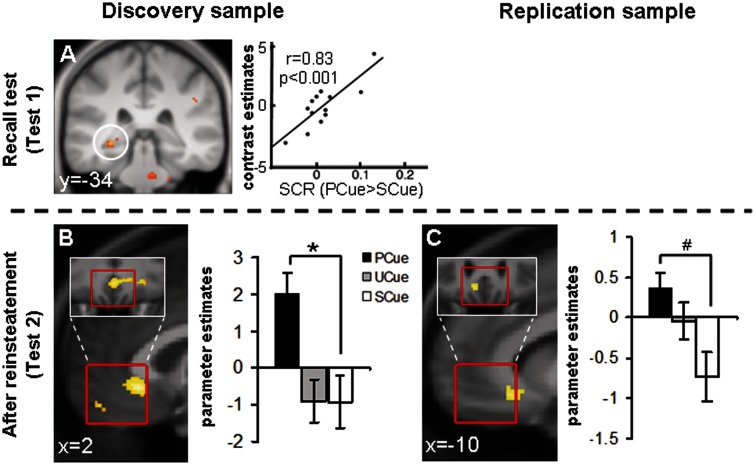
Before reinstatement (Test1), a subgenual area of the vmPFC ROI exhibited larger categorical responses to the UCXT than the SCXT in both the discovery and replication sample (Table 4; Figure 3A and B). Unexpectedly, at Test1, there was no significant anterior hippocampal activation. After reinstatement (Test2), in the discovery sample, the UCXT > SCXT comparison yielded categorical activation in the dmPFC (Table 4, Figure 3C) and a weak linearly decreasing activation in the right amygdala [x, y, z = 34, 4, −20, k = 10, Z = 2.93, P(uc) = 0.002]. Of note, amygdala activation in this sample was significantly correlated with the individual SCR index of contextual fear (UCXT > SCXT; Figure 3E) [left amygdala: x, y, z = −16, −2, −18, k = 38, Z = 3.33, P(SVC) = 0.033; right amygdala: x, y, z = 14, −4, −18, k = 12, Z = 3.10, P(SVC) = 0.071]. In the replication sample, the amygdala effect manifested as a significant categorical UCXT > SCXT difference that was independent of SCRs (Table 4; Figure 3D). There was, however, no significant dmPFC activation.
Table 4.
Imaging data: findings from Test1 (before reinstatement) and Test2 (after reinstatement)
| Sample | Contrast | Region | k(ROI) | P(SVC) | P(uc) | Z | MNI coordinates |
||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Test1 | |||||||||
| Discovery | UCXT > SCXT | vmPFC | 42 | 0.031 | <0.001 | 3.57 | −2 | 32 | −6 |
| Replication | UCXT > SCXT | vmPFC | 14 | 0.019 | <0.001 | 3.67 | −10 | 34 | −10 |
| Test2 | |||||||||
| Discovery | UCXT > SCXT | dmPFC | 73 | 0.038 | <0.001 | 3.46 | −10 | 36 | 28 |
| Replication | UCXT > SCXT | R amygdala | 15 | 0.046 | 0.001 | 3.09 | 24 | −2 | −26 |
| Discovery | PCue > SCue | vmPFC | 80 | 0.044 | <0.001 | 3.49 | 2 | 32 | −6 |
| Replication | PCue > SCue | vmPFC | – | n.s. | <0.001 | 3.17 | −10 | 26 | −12 |
Significant activation clusters within the ROIs from context and cue conditioning contrasts during the memory tests before (Test1) and after reinstatement (Test2). k(ROI), number of voxels in cluster inside ROI; uc, uncorrected; R, right.
Fig. 3.
Imaging data, context conditioning: major findings from Test1 (before reinstatement) and Test2 (after reinstatement). Contrast UCXT > SCXT at Test1 in the vmPFC ROI in the discovery (A) and replication samples (B) as well as at Test2 in the dmPFC ROI in the discovery sample (C) and in the right amygdala ROI in the replication sample (D). Correlation of the SCR index for contextual fear (UCXT > SCXT) at Test2 with left amygdala activation at Test2 in the discovery sample (E). Statistical parametric maps are superimposed on an average structural image and thresholded at P = 0.01 on sagittal (A–C) or coronal (D, E) views, respectively. Red borders indicate the exact locations of the ROIs. Bar graphs show parameter estimates. *P(SVC) < 0.05.
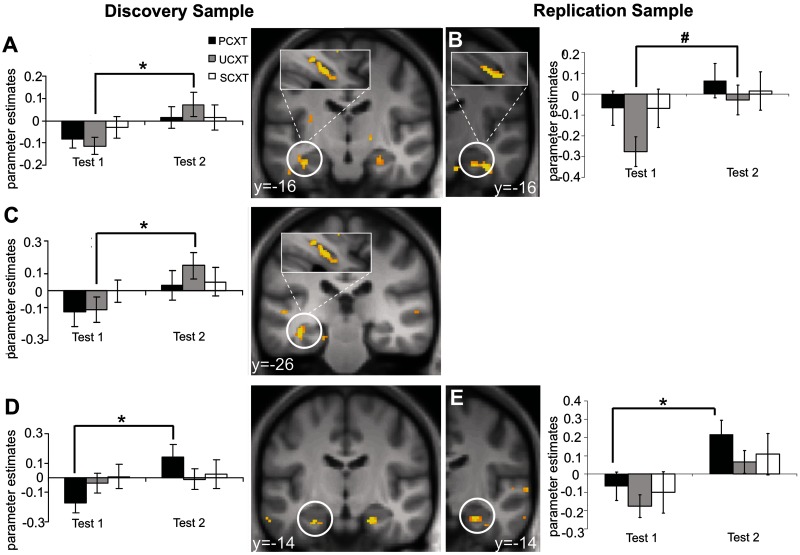
More critically, comparing UCXT-evoked activity after minus before reinstatement (Test2 > Test1) in the discovery sample yielded wide-spread categorical activation in the left posterior (Table 5; Figure 4C) and the left (and, at trend level, right) anterior hippocampal (Table 5; Figure 4A) ROIs. As Figure 4A and C illustrate, left hippocampal activation was predominantly anterior. In the replication sample, right anterior hippocampal activation (Table 5; Figure 4B) just missed significance and, generally, hippocampal activation was even more restricted to anterior parts and extended into peri-hippocampal areas. The anterior hippocampal focus of reinstatement effects was contrary to our predictions. The generalized reinstatement effects in the behavioral data are mirrored in similar anterior hippocampal effects observed for the PCXT (Table 5; Figure 4D and E), whereas no reinstatement effects were found for the SCXT. That is, SCRs show enhanced reactions in general (e.g. also to the SCXT), while neural activation pattern reveal no reinstatement effect to the SCXT. A dissociation with respect to differential vs generalized reinstatement effects in different dependent measures (e.g. SCR, Fear potentiated startle and fear ratings) has already been observed in previous studies (Vervliet et al., 2013b).
Table 5.
Imaging data, context conditioning: reinstatement effects (Test2 > Test1)
| Context | Sample | Region | k | P(SVC) | P(uc) | Z | MNI coordinates |
||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| UCXT | Discovery | L ant. hippocampus | 8 | 0.027 | 0.001 | 3.07 | −34 | −18 | −20 |
| Replication | – | – | – | – | – | – | – | – | |
| Discovery | R ant. hippocampus | 8 | 0.064# | 0.003 | 2.72 | 26 | −18 | −20 | |
| Replication | R ant. hippocampus | 26 | 0.056# | 0.003 | 2.76 | 34 | −16 | −24 | |
| Discovery | L post. hippocampus | 4 | 0.025 | 0.001 | 3.10 | −36 | −26 | −14 | |
| Replication | – | – | – | – | – | – | – | – | |
| PCXT | Discovery | L ant. hippocampus | 6 | 0.043 | 0.002 | 2.88 | −26 | −14 | −26 |
| Replication | L ant. hippocampus | 1 | 0.098# | 0.006 | 2.51 | −26 | −20 | −20 | |
| Discovery | R ant. hippocampus | 3 | 0.069# | 0.004 | 2.68 | 24 | −16 | −24 | |
| Replication | R ant. hippocampus | 14 | 0.034 | 0.001 | 2.97 | 24 | −14 | −22 | |
Significant or trend-level activation clusters inside the ROIs from the comparison after reinstatement (Test2) > before reinstatement (Test1). #P < 0.1 trend-level significance.
Fig. 4.
Imaging data, context conditioning: reinstatement effects (Test2 > Test1). UCXT responses at Test2 > Test1 in the anterior hippocampus ROI in the discovery sample (A) and the right anterior hippocampus ROI in the replication sample (B) as well as in the left posterior hippocampus ROI in the discovery sample (C). Contrast PCXT at Test2 > Test1 in the left anterior hippocampus ROI in the discovery sample (D) and in the right anterior hippocampus ROI in the replication sample (E). Activations are superimposed on an average structural image and thresholded at P = 0.01 on coronal views. Bar graphs show parameter estimates. *P(SVC) < 0.05, #P(SVC) < 0.1.
Cue conditioning
In comparison to context conditioning, the behavioral analysis of cued fear expression in our paradigm had found globally stronger and more discriminative fear memory acquisition and expression, including in Test1, but little evidence for return of fear by reinstatement. Unlike in context conditioning, we did not observe any vmPFC activation before reinstatement (Test1), in line with weak or absent retrieval/expression of extinction. However, categorical hemodynamic responses to cued fear (PCue > SCue) in the discovery sample were positively correlated with the SCR index of cued fear (PCue > SCue) in the left posterior hippocampus [x, y, z = −30, −34, −6, Z = 3.32, P(uc) < 0.001] (Figure 5A) and negatively correlated with SCRs in the right anterior hippocampus [x, y, z = 26, −22, −12, Z = 3.76, P(uc) < 0.001]. These activations fell just outside of our conservatively defined ROIs. These effects might reflect posterior hippocampal influences promoting the processing of threat associations and anterior hippocampal influences promoting the processing of safety associations.
Fig. 5.
Imaging data, cue conditioning: major findings from Test1 (before reinstatement) and Test2 (after reinstatement). Correlation of the SCR index for cued fear (PCue > SCue) at Test1 with left posterior hippocampus activation at Test1 in the discovery sample (A). Contrast PCue > SCue at Test2 in the vmPFC ROI in the discovery (B) and replication samples (C). Activations are superimposed on an average structural image and thresholded at P = 0.01 on coronal (A) or sagittal (B, C) views, respectively. Red borders indicate the exact locations of the ROIs. Bar graphs show parameter estimates. *P(SVC) < 0.05, #P(SVC) < 0.1.
After reinstatement (Test2), we observed categorical PCue > SCue activation in a subgenual part of the vmPFC ROI in the discovery sample (Table 4; Figure 5B); in the replication sample, the activation focus was located just outside our ROI and significant at an uncorrected threshold of P < 0.001 (Table 4; Figure 5C). There was no activation enhancement by reinstatement (Test2 > Test1).
DISCUSSION
Our study investigated long-term expression of cued and contextual extinction memory as well as return of fear induced by reinstatement. Based on the results from two independent samples, we provide three new pieces of information on the neural bases of fear and extinction memory expression. First, a context previously (Day1, conditioning) associated with unpredictable USs (UCXT) that is subsequently extinguished (Day2, extinction) elicits hemodynamic responses in the subgenual vmPFC during long-term recall (Day8, Test1). This stands in analogy to results derived from extinction of cued fear in animals (Morgan and LeDoux, 1995; Milad and Quirk, 2002, 2012) and humans (Kalisch et al., 2006; Milad et al., 2007) and suggests vmPFC activation might be a general feature of extinction (memory) expression, independent of the mode of conditioning. Second, for the first time, our study provides evidence for an involvement of the human amygdala in the return of contextual fear, as produced here using a reinstatement manipulation (Day8, Test2). The maintained neural differentiation between predictive (UCXT) and safe (SCXT) context CSs after reinstatement indicates that this amygdala effect represents CS–US association retrieval/expression (Fanselow and Gale, 2003). Activation of the amygdala is in line with recent reports in rodents (Laurent and Westbrook, 2010; Lin et al., 2011) and extends reports of human amygdala activation during return of cued fear (Kalisch et al., 2006, 2009; Agren et al., 2012; Lonsdorf et al., 2013). In addition, our data from the discovery sample provide preliminary evidence for a role of the dorsal ACC/dmPFC in the return of fear. However, as this finding was not replicated in the replication sample, we refrain from a detailed discussion here.
Third, we show that activation of the anterior hippocampus is enhanced after the reinstatement of contextual fear relative to before (Day8, Test2 > Test1). The significance of this finding is discussed in the following. A fourth finding that was replicated in both samples was vmPFC activation to PCue > SCue after reinstatement (Test2). This was unexpected but might be related to the lack of strong evidence in the behavioral data for reinstatement of cued fear. No activation of areas ascribed to the fear network was observed for cued CSs following reinstatement. PCues might also have been processed as safety signals relative to the surrounding context (PCXT) which showed return-of-fear effects in both behavior and fMRI. Thus, vmPFC activation after reinstatement might reflect resistance to additional return of cued fear in this particular instantiation of the reinstatement manipulation in our paradigm. Lack of reinstatement for cued CSs in fear potentiated startle responses as well as fear ratings was recently also observed in a large sample using the same paradigm in our laboratory (Haaker et al., 2013). Our initial hypothesis was that prevailing extinction expression before reinstatement (Test1) would be accompanied by anterior hippocampus activation, and prevailing fear expression after reinstatement (Test2) would be accompanied by posterior hippocampus activation. In cue conditioning, where there was evidence for fear expression already before reinstatement but not for additional fear expression after reinstatement, we observed a positive correlation of posterior hippocampal activity, and a negative correlation of anterior hippocampal activity, with SCRs to predictive CSs (PCue) relative to control CSs (SCue) in the test before reinstatement. This could be taken to support the hypothesized anterior–posterior dissociation with respect to fear vs extinction expression and to reflect a competitive co-existence of fear (posterior hippocampus) and extinction memories (anterior hippocampus) linked to the same CS. Such putatively balanced states may be particularly sensitive for interference (e.g. pharmacological, behavioral) or for modulation by individual predispositions that render one memory trace dominant.
With respect to contextual CSs, we observed hippocampal activation that was most pronounced in anterior parts after reinstatement as compared to before (Test2 > Test1) and that accompanied relatively enhanced behavioral fear expression as well as post-reinstatement activation of the amygdala. We did not observe anterior hippocampus activation before reinstatement nor any particularly pronounced and consistent posterior hippocampus activation after reinstatement, in contrast to our initial hypothesis. This might suggest that hippocampus activation, at least in the case of contextual memories, may not be a function of memory valence (Maren, 2011).
Our initial proposal was based on results from a cue conditioning paradigm where we observed that the anterior hippocampus activated specifically when an extinguished CS was presented in the extinction context, but not the conditioning context. In addition, this activation co-varied with activation of the vmPFC (Kalisch et al., 2006). In a similar vein, Milad et al. (2007, 2009) have demonstrated that an extinguished, compared to a non-extinguished cue CS, also activates the anterior hippocampus, again in concert with the vmPFC. Conversely, the posterior hippocampus in our first study activated mainly to CS presentations in the conditioning context. This was later extended by a finding that pharmacological enhancement of generalized fear expression across both conditioning and extinction contexts also boosted posterior hippocampus activation (Kalisch et al., 2009).
One common theme in the described experiments engaging the anterior hippocampus might be the existence of situational uncertainty, or ambiguity, about the validity of previously learned predictions of the presented CSs. In the described cue conditioning study (Kalisch et al., 2006), subjects may have been unsure whether not expecting reinforcement in the extinction context was really appropriate, given the novel test situation of intermixed, alternating presentations of conditioning and extinction contexts and the administration of reinstatement USs at the onset of conditioning contexts. In the Milad et al. studies (Milad et al., 2007, 2009), the extinguished CS was tested in the same experimental phase like a comparison CS that had not been present before during extinction, again leaving room for uncertainty about a potential change in reinforcement rules. In the present context conditioning experiment, situational uncertainty might have been introduced by the sudden appearance of reinstatement USs that followed upon two phases of unreinforced presentations of the contextual CSs (extinction on Day2 and Test1 on Day8).
Situational uncertainty or ambiguity, also termed ‘unexpected uncertainty’ (Yu and Dayan, 2005), is generally caused by unsignaled switches in the global situation that go along with strongly unexpected events or contingency changes. Unexpected uncertainty is different from the ‘expected uncertainty’ that is inherent to most associative learning tasks, including partial reinforcement schedules or also extinction. Its role may be to raise attention and motivate new learning and exploration. Evidence suggests that such alerting uncertainty signals are carried by the neurotransmitter norepinephrine (Yu and Dayan, 2005) and encoded in the anterior hippocampus (Chumbley et al., 2012). An interpretation of anterior hippocampal activity in the described conditioning experiments as reflecting situational ambiguity would resonate with the idea that the hippocampus helps disambiguate between different meanings associated with an identical stimulus (Rudy, 2009). This function may be particularly required in extinction experiments where the same stimulus (the CS) can signal two conflicting outcomes (US, no US) and contextual factors have a strong impact on how the stimulus is interpreted (Bouton, 2004). Reinstatement manipulations may call on the anterior hippocampus in particular when reinstatement USs are presented directly after unreinforced CSs (i.e. on the same day) and thus constitute a novel, surprising change of the situation (see also LaBar and Phelps, 2005, for concordant lesion results). Hence, the anterior hippocampus may register situational ambiguity and/or resolve ambiguity by comparing present to past contexts and biasing memory expression in the situationally more appropriate direction (Hirsh, 1974; Corcoran and Maren, 2004; Rudy, 2009; Fanselow, 2010; Maren, 2011). This function may be valence-independent and applicable to both cue and context conditioning. Our hypothesis of an involvement of the anterior hippocampus in ambiguity detection may be tested directly, in an ABC renewal design that should reveal enhanced anterior hippocampus activation as compared to an ABB design.
This modified account of anterior hippocampus in human conditioning/extinction would only leave valence-dependent (pro-fear) processing to posterior hippocampus. Given that fear CRs require preparation or execution of motor responses toward or away from the CS, the posterior hippocampus may also simply be involved in fear expression due to its connectivity with areas involved in locomotion and orientation (Fanselow and Dong, 2010). In contrast, the anterior hippocampus might fulfill its disambiguating and memory-regulating function via its projections to memory storage areas such as the vmPFC and the amygdala (Fanselow and Dong, 2010). While further research will be required to evaluate this modified account of hippocampus function in human fear/extinction expression, it raises the interesting perspective that abnormalities in anterior hippocampus anatomy or function contribute to vulnerability to fear/anxiety disorders (Gilbertson et al., 2002, 2007) by compromising patients’ ability to produce appropriate responses whenever there is situational ambiguity about reinforcement rules. Furthermore, given the role of sex hormones in fear conditioning and extinction (e.g. Milad et al., 2006; Merz et al., 2012), it has to be noted the study was conducted in male participants only, and future studies are needed to examine if the findings can be generalized to females and whether sex hormones have an impact on the processes studied.
CONCLUSIONS
In summary, we implicate the human vmPFC in contextual extinction expression and show an involvement of the human amygdala in reinstatement of contextual fear. We also show generalized up-regulation of anterior hippocampal activation by reinstatement that we interpret as reflecting disambiguation and the balancing-out of competing extinction and fear memory traces.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
We thank Dr Alexandra Thanellou for help with paradigm development and Dr Matthias Gamer for providing software for the SCR scoring. Jonna Löffler for help with SCR scoring as well as Timo Krämer, Kathrin Müller and Katrin Wendt for help with scanning. This work was supported by the State of Hamburg excellence initiative (neurodapt! consortium) and the Deutsche Forschungsgemeinschaft (DFG grants KA 1623/3-1 and KA 1623/4-1) as well as the SFB TRR58 (subproject B06).
REFERENCES
- Agren T, Engman J, Frick A, et al. Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science. 2012;337:1550–2. doi: 10.1126/science.1223006. [DOI] [PubMed] [Google Scholar]
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. Journal of Neuroscience. 2008;28:6211–9. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Baas JM, Nugent M, Lissek S, Pine DS, Grillon C. Fear conditioning in virtual reality contexts: a new tool for the study of anxiety. Biological Psychiatry. 2004;55:1056–60. doi: 10.1016/j.biopsych.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Bennett CM, Miller MB. How reliable are the results from functional magnetic resonance imaging? Annals of the New York Academy of Sciences. 2010;1191:133–55. doi: 10.1111/j.1749-6632.2010.05446.x. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning and Memory. 2004;11:485–94. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learning and Motivation. 1979a;10:445–66. [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. Journal of Experimental Psychology: Animal Behavior Processes. 1979b;5:368–78. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Chumbley JR, Flandin G, Bach DR, et al. Learning and generalization under ambiguity: an fMRI study. PLoS Computational Biology. 2012;8:e1002346. doi: 10.1371/journal.pcbi.1002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learning and Memory. 2004;11:598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dirikx T, Hermans D, Vansteenwegen D, Baeyens F, Eelen P. Reinstatement of conditioned responses in human differential fear conditioning. Journal of Behavior Therapy and Experimental Psychiatry. 2007;38:237–51. doi: 10.1016/j.jbtep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Dirikx T, Vansteenwegen D, Eelen P, Hermans D. Non-differential return of fear in humans after a reinstatement procedure. Acta Psychologica. 2009;130:175–82. doi: 10.1016/j.actpsy.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Signaled shock-free periods and preference for signaled shock. Journal of Experimental Psychology: Animal Behavior Processes. 1980;6:65–80. [Google Scholar]
- Fanselow MS. From contextual fear to a dynamic view of memory systems. Trends in Cognitive Sciences. 2010;14:7–15. doi: 10.1016/j.tics.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Annals of the New York Academy of Sciences. 2003;985:125–34. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Fowles DC, Christie MJ, Edelberg R, Grings WW, Lykken DT, Venables PH. Publication recommendations for electrodermal measurements. Psychophysiology. 1981;18:232–9. doi: 10.1111/j.1469-8986.1981.tb03024.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, Penny WD. Statistical Parametric Mapping: The Analysis of Functional Brain Images. Academic Press; 2006. [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience. 2002;5:1242–7. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Williston SK, Paulus LA, et al. Configural cue performance in identical twins discordant for posttraumatic stress disorder: theoretical implications for the role of hippocampal function. Biological Psychiatry. 2007;62:513–20. doi: 10.1016/j.biopsych.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golkar A, Öhman A. Fear extinction in humans: effects of acquisition-extinction delay and masked stimulus presentations. Biological Psychology. 2012;91:292–301. doi: 10.1016/j.biopsycho.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Cornwell B, Johnson L. Context conditioning and behavioral avoidance in a virtual reality environment: effect of predictability. Biological Psychiatry. 2006;60:752–9. doi: 10.1016/j.biopsych.2006.03.072. [DOI] [PubMed] [Google Scholar]
- Haaker J, Lonsdorf TB, Thanellou A, Kalisch R. Multimodal assessment of long-term memory recall and reinstatement in a combined cue and context fear conditioning and extinction paradigm in humans. PLoS ONE. 2013;8:e76179. doi: 10.1371/journal.pone.0076179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Fromm S, Alvarez RP, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. Journal of Neuroscience. 2007;27:6313–9. doi: 10.1523/JNEUROSCI.5369-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D, Dirikx T, Vansteenwegen D, et al. Reinstatement of fear responses in human aversive conditioning. Behavior Research and Therapy. 2005;43:533–51. doi: 10.1016/j.brat.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Hirsh R. The hippocampus and contextual retrieval of information from memory: a theory. Behavioral Biology. 1974;12:421–44. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- Huff NC, Hernandez JA, Blanding NQ, LaBar KS. Delayed extinction attenuates conditioned fear renewal and spontaneous recovery in humans. Behavioral Neuroscience. 2009;123:834–43. doi: 10.1037/a0016511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indovina I, Robbins TW, Núñez-Elizalde AO, Dunn BD, Bishop SJ. Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron. 2011;69:563–71. doi: 10.1016/j.neuron.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–58. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Holt B, Petrovic P, et al. The NMDA agonist D-cycloserine facilitates fear memory consolidation in humans. Cerebral Cortex. 2009;19:187–96. doi: 10.1093/cercor/bhn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. Journal of Neuroscience. 2006;26:9503–11. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull S, Müller BH, Blechert J, Wilhelm FH, Michael T. Reinstatement of fear in humans: autonomic and experiential responses in a differential conditioning paradigm. Acta Psychologica. 2012;140:43–9. doi: 10.1016/j.actpsy.2012.02.007. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behavioral Neuroscience. 2005;119:677–86. doi: 10.1037/0735-7044.119.3.677. [DOI] [PubMed] [Google Scholar]
- Lang S, Kroll A, Lipinski SJ, et al. Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. European Journal of Neuroscience. 2009;29:823–32. doi: 10.1111/j.1460-9568.2009.06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Role of the basolateral amygdala in the reinstatement and extinction of fear responses to a previously extinguished conditioned stimulus. Learning and Memory. 2010;17:86–96. doi: 10.1101/lm.1655010. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H-C, Tseng Y-C, Mao S-C, Chen P-S, Gean P-W. GABAA receptor endocytosis in the basolateral amygdala is critical to the reinstatement of fear memory measured by fear-potentiated startle. Journal of Neuroscience. 2011;31:8851–61. doi: 10.1523/JNEUROSCI.0979-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommen MJJ, Engelhard IM, Sijbrandij M, van den Hout MA, Hermans D. Pre-trauma individual differences in extinction learning predict posttraumatic stress. Behaviour Research and Therapy. 2013;51:63–7. doi: 10.1016/j.brat.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Haaker J, Kalisch R. No evidence for enhanced extinction memory consolidation through noradrenergic reuptake inhibition—delayed memory test and reinstatement in human fMRI. Psychopharmacology. 2013 doi: 10.1007/s00213-013-3338-8. doi 10.1007/s00213-013-3338-8. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Venables PH. Direct measurement of skin conductance: a proposal for standardization. Psychophysiology. 1971;8:656–72. doi: 10.1111/j.1469-8986.1971.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Maren S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70:830–45. doi: 10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Büchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. The Journal of Neuroscience. 2008;28:9030–6. doi: 10.1523/JNEUROSCI.1651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechias M-L, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage. 2010;49:1760–8. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, et al. Neuronal correlates of extinction learning are modulated by sex hormones. Social Cognitive and Affective Neuroscience. 2012;7:819–30. doi: 10.1093/scan/nsr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Goldstein JM, Orr SP, et al. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behavioral Neuroscience. 2006;120:1196–203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42:456–64. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66:1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annual Review of Psychology. 2012;63:129–51. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62:446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behavioral Neuroscience. 1995;109:681–8. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Molecular Psychiatry. 2007;12:120–50. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Paret C, Brenninkmeyer J, Meyer B, et al. A test for the implementation-maintenance model of reappraisal. Frontiers in Psychology. 2011;2:216. doi: 10.3389/fpsyg.2011.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. Journal of Neuroscience. 2003;23:8800–7. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachman S. The return of fear: review and prospect. Clinical Psychology Review. 1989;9:147–68. [Google Scholar]
- Raczka KA, Gartmann N, Mechias ML, et al. A neuropeptide S receptor variant associated with overinterpretation of fear reactions: a potential neurogenetic basis for catastrophizing. Molecular Psychiatry. 2010;15:1045, 1067–74. doi: 10.1038/mp.2010.79. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Spontaneous recovery. Learning and Memory. 2004;11:501–9. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. Journal of Experimental Psychology: Animal Behavior Processes. 1975;1:88–96. [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. Journal of Neuroscience. 2003;23:11054–64. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learning & Memory. 2009;16:573–85. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Schöning S, Zwitserlood P, et al. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS ONE. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoormaker VI, Sturm A, Andrade KC, et al. The neural correlates and temporal sequence of the relationship between shock exposure, disturbed sleep and impaired consolidation of fear extinction. Journal of Psychiatric Research. 2010;44:1121–8. doi: 10.1016/j.jpsychires.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Schrick C, Guzman YF, et al. Segregated populations of hippocampal principal CA1 neurons mediating conditioning and extinction of contextual fear. Journal of Neuroscience. 2009;29:3387–94. doi: 10.1523/JNEUROSCI.5619-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables PH, Christie MJ. Electrodermal activity. In: Martin I, Venables PH, editors. Techniques in Psychophysiology. Chichester, UK: Wiley; 1980. [Google Scholar]
- Vervliet B, Baeyens F, Van den Bergh O, Hermans D. Extinction, generalization, and return of fear: a critical review of renewal research in humans. Biological Psychology. 2013a;92:51–8. doi: 10.1016/j.biopsycho.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Craske MG, Hermans D. Fear extinction and relapse: state of the art. Annual Review of Clinical Psychology. 2013b;9:215–48. doi: 10.1146/annurev-clinpsy-050212-185542. [DOI] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–92. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.