Abstract
Children born with an inhibited temperament are at heightened risk for developing anxiety, depression and substance use. Inhibited temperament is believed to have a biological basis; however, little is known about the structural brain basis of this vulnerability trait. Structural MRI scans were obtained from 84 (44 inhibited, 40 uninhibited) young adults. Given previous findings of amygdala hyperactivity in inhibited individuals, groups were compared on three measures of amygdala structure. To identify novel substrates of inhibited temperament, a whole brain analysis was performed. Functional activation and connectivity were examined across both groups. Inhibited adults had larger amygdala and caudate volume and larger volume predicted greater activation to neutral faces. In addition, larger amygdala volume predicted greater connectivity with subcortical and higher order visual structures. Larger caudate volume predicted greater connectivity with the basal ganglia, and less connectivity with primary visual and auditory cortex. We propose that larger volume in these salience detection regions may result in increased activation and enhanced connectivity in response to social stimuli. Given the strong link between inhibited temperament and risk for psychiatric illness, novel therapeutics that target these brain regions and related neural circuits have the potential to reduce rates of illness in vulnerable individuals.
Keywords: behavioral inhibition, shy, salience, anxiety
INTRODUCTION
Individuals with an inhibited temperament exhibit profound behavioral differences that are evident early in childhood (Calkins et al., 1996; Kagan et al., 1998) and persist across development (Fox et al., 2001; Kagan et al., 2007). Inhibited children are quiet, reticent, and avoid novel situations and people. Importantly, these behavioral differences predict long-term psychiatric outcomes. For example, very inhibited children are at increased risk for anxiety (Hirshfeld et al., 1992; Schwartz et al., 1999; Chronis-Tuscano et al., 2009; Essex et al., 2010; Clauss and Blackford, 2012), depression (Caspi et al., 1996; Beesdo et al., 2007) and substance use (Williams et al., 2010; Lahat et al., 2012). Neuroscientifically based preventions promise to reduce the burden of psychiatric illness in these vulnerable children; however, the neurobiological basis of inhibited temperament remains poorly understood.
Inhibited temperament is evolutionarily conserved and is observed across species, including rhesus monkeys (Kalin and Shelton, 1989; Fox et al., 2008; Oler et al., 2010; Shackman et al., 2013) and other mammals (for an extensive review, see Gosling and John, 1999). Although the trait exists across a continuum, ranging from inhibited to uninhibited, a considerable number of individuals are either very inhibited or very uninhibited. These extreme groups may be maintained by natural selection, preserving genes for extreme responses to novelty. Both very inhibited and very uninhibited individuals have an evolutionary advantage, with relative strengths in different contexts (Biro and Post, 2008). For example, in contexts of predator threat, inhibited individuals are more likely to stay close to shelter and survive, whereas uninhibited individuals are more likely to venture out and be attacked by a predator. In contexts of famine, the pattern is opposite: uninhibited individuals are more likely to explore and find food, whereas inhibited individuals are more likely to stay close to shelter and starve. In humans, inhibited and uninhibited temperament can be reliably and validly measured (Cheek and Buss, 1981; Garcia Coll et al., 1984; Jones et al., 1986), and extreme groups are typically defined as the top and bottom 15% of the continuum (Kagan et al., 1998).
Although the behavioral correlates of inhibited temperament have been well characterized, the origin of these differences remains poorly understood. Inhibited temperament likely has a neurobiological origin and is associated with physiological differences—such as a high and stable heart rate, increased salivary cortisol and greater asymmetry in frontal electroencephalography (EEG) (Kagan et al., 1998; Fox et al., 2005a). Furthermore, both inhibited humans and non-human primates consistently show altered amygdala function (Pérez-Edgar et al., 2007; Beaton et al., 2008; Fox et al., 2008; Oler et al., 2010; Blackford et al., 2011; Schwartz et al., 2012; Shackman et al., 2013), which may reflect differences in gene expression within the amygdala (Fox et al., 2012). Given prior work that demonstrates that brain structure is associated with brain function (Kalin et al., 2004; Machado et al., 2008; Bliss-Moreau et al., 2010), we propose that these differences in behavior and brain function may be related to variation in brain structure. One prior study examined cortical thickness and found that inhibited temperament was associated with thicker ventromedial prefrontal cortex and thinner lateral orbitofrontal cortex (Schwartz et al., 2010). To our knowledge, no prior studies have explored the link between inhibited temperament and amygdala volume. We hypothesize that inhibited temperament is associated with larger amygdala volume.
MATERIALS AND METHODS
Participants
Consistent with both the existing animal and human literature, we used an extreme discordant phenotype approach (Nebert, 2000) and compared inhibited (n = 40; 21 female) and uninhibited (n = 44; 26 female) young adults. To focus on a stable trait and ensure that valid groups were identified, the trait was assessed retrospectively (childhood) and currently using the Retrospective Self-Report of Inhibition (RSRI) and the Adult Self-Report of Inhibition (ASRI) (Reznick et al., 1992), respectively. Both questionnaires have excellent reliability (Cronbach’s α = 0.79 for the RSRI and α = 0.78 for the ASRI), demonstrate convergent validity (Reznick et al., 1992; Rohrbacher et al., 2008) and minimize self-report bias by focusing on reports of concrete behaviors in specific situations instead of subjective feelings.
Young adults were selected from a larger, ongoing study (n = 114 at the time of this analysis). Inhibited and uninhibited young adults were recruited using a systematic recruitment strategy designed to target members of extreme groups who identified as being ‘especially shy or outgoing as a child’. Subjects were screened for extreme inhibited or uninhibited temperament on both the RSRI and the ASRI [standard top/bottom 15% guideline used in most human studies, based on normative data (Reznick et al., 1992)]; therefore, all subjects had stable inhibited temperament. For the larger study, 53% of subjects who were screened met criteria for extreme temperament. Subject characteristics are reported in Table 1. For this report, we selected inhibited and uninhibited adults who had completed a structural MRI scan. To minimize environmental effects on brain structure, adults were excluded for significant drug use (defined by substance abuse or dependence within the past 6 months), current psychotropic medication use, major medical illness, or history of brain trauma. To minimize disorder-related effects on brain volume, adults were excluded if they had a psychiatric disorder; however, because anxiety is common in inhibited adults, anxious subjects in the inhibited group were not excluded. Although inhibited temperament is also associated with risk for depression and substance abuse, incidence is lower and likely develops secondary to anxiety (Beesdo et al., 2007). Psychiatric diagnoses were established by trained interviewers using the Structured Clinical Interview for DSM-IV and 10 inhibited adults met criteria for an anxiety disorder (4 social anxiety disorder, 1 generalized anxiety disorder, 2 anxiety not otherwise specified, 1 specific phobia, and 2 comorbid social anxiety disorder and generalized anxiety disorder). Inhibited individuals with an anxiety diagnosis had significantly higher scores on both the RSRI (M = 3.66, s.d. = 0.46 vs M = 3.2, s.d. = 0.48; P = 0.01) and ASRI (M = 3.49, s.d. = 0.57 vs M = 3.1, s.d. = 0.39; P = 0.02) relative to inhibited individuals without an anxiety diagnosis.
Table 1.
Subject characteristics by group
| Inhibited temperament (n = 40) |
Uninhibited temperament (n = 44) |
||||
|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | P-value | |
| Inhibited temperament | |||||
| Child | 3.26 | 0.50 | 1.55 | 0.47 | <0.001 |
| Adult | 3.19 | 0.48 | 1.78 | 0.43 | <0.001 |
| Intracranial volume | 1.71 | 0.16 | 1.71 | 0.18 | ns |
| Age (years) | 23.4 | 4.0 | 22.8 | 3.7 | ns |
| Percent | N | Percent | N | P-value | |
| Gender (% female) | 52.5 | 21 | 59.1 | 26 | ns |
| Handedness (% right) | 80.0 | 32 | 90.9 | 40 | ns |
| Ethnicity | ns | ||||
| % Caucasian | 70.0 | 28 | 70.5 | 31 | |
| % African American | 12.5 | 5 | 20.5 | 9 | |
| % Asian | 17.5 | 7 | 4.5 | 2 | |
| % Other | 0.0 | 0 | 4.5 | 2 | |
ns, not significant.
The study was approved by the Vanderbilt University Institutional Review Board and we obtained written informed consent after providing subjects with a complete study description.
Measures
Gray matter volume
High resolution anatomic images were collected using a 3 Tesla Philips scanner (Philips Healthcare, Inc., Best, The Netherlands). T1-weighted images were obtained in the sagittal direction, with a three-dimensional turbo field echo, 1 mm3 voxel-size, 256 mm field of view, 170 slices, 1 mm slice thickness, 0 mm gap, 8 ms TR and 3.7 ms TE.
Three complimentary methods were used to assess amygdala structure: manual segmentation, surface mapping and voxel-based morphometry (VBM). Manual segmentation was used to identify the size and shape of each subject’s amygdalae. The amygdala is well-suited for manual segmentation and surface mapping because of its small structure, clear boundaries and well-established segmentation methods (Pruessner et al., 2000); the low reliability of automated segmentation methods makes manual segmentation the gold standard (Nugent et al., 2013), but see Hanson et al. (2012). The amygdala was manually traced by two blinded raters (A.L.S. and R.M.V., trained by an expert rater J.U.B.) using 3DSlicer (version 3.4; www.slicer.org), a software package that provides simultaneous visualization in all orientations. Amygdalae were traced according to standard protocols (Honeycutt et al., 1998; Pruessner et al., 2000) with reference to an anatomical atlas (Mai et al., 2008). Consistent with standards for manual segmentation (Pruessner et al., 2000; Bergouignan et al., 2009) and previous studies (Dedovic et al., 2010; van der Plas et al., 2010), images were normalized to a standard T1 Montreal Neurological Institute (MNI) template using standard normalization in SPM5 (affine registration, followed by nonlinear deformations) prior to manual tracing. The amygdala was initially traced from the superior to the inferior border in the axial view, with the coronal and the sagittal views used for confirmation. The posterior boundary of the amygdala was defined using the alveus of the hippocampus or the inferior horn of the lateral ventricle. The lateral border was defined as the vertical border tangential to the most medial adjacent white matter. The medial border was defined in slices superior to the uncus, as the border with the ambient cistern, and in slices inferior to the uncus, as the white matter separating the amygdala from the entorhinal cortex. In superior slices, the anterior border of the amygdala was defined in the axial view as the border with the subarachnoid space. In the inferior slices, the anterior border was defined in the coronal view as the slice just posterior to the slice containing the anterior commissure. The superior border was defined in the coronal view, by drawing a horizontal line between the superolateral part of the optic tract and the inferior portion of the circular sulcus of the insula. Amygdala volume was estimated for each of the subjects in 3-D Slicer. Reliability was estimated using intra-class correlations (Shrout and Fleiss, 1979); the intra-rater correlation and the inter-rater correlation were both 0.81.
Surface models were estimated using weighted spherical harmonic representation methods (Chung et al., 2008). Briefly, amygdala surface was extracted from the manual segmentation in the form of a triangular mesh, and then inflated onto a sphere. A weighted spherical harmonic representation was estimated for each subject’s left and right amygdala. The weighted spherical harmonic representation was projected onto a sphere with 2562 vertices and 5120 faces, and the surface spheres were averaged across all subjects to create an average for the left amygdala and right amygdala. Individual differences in amygdala shape were estimated as the deviation of each subject’s surface from the average surface. Surface models were estimated using Matlab.
Because comparisons of overall amygdala volume can only identify gross structural differences, it is critical to also test for regional differences, especially for brain structures composed of multiple functionally heterogeneous subnuclei, such as the amygdala. Regional differences in gray matter volume were assessed using VBM—an automated technique for comparing gray matter volume in each voxel of the brain (Ashburner and Friston, 2000). With VBM, individual brains are normalized into a common space while retaining the original gray matter volume. For example, for a brain region that is compressed to fit the standard space, gray matter volume will be >1 in the compressed voxels; for brain regions that are expanded, gray matter volume will be <1 in the expanded voxels. VBM analysis was performed using SPM8 (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, London, UK) implemented in Matlab.
Following an established VBM protocol (Bergouignan et al., 2009), preprocessing steps included checking for artifacts and anatomic abnormalities, manually realigning the anterior and posterior commissures to the same horizontal line, and setting the image origin at the anterior commissure. Preprocessed images were segmented into gray matter, white matter and cerebrospinal fluid using the a priori templates included in SPM8 and standard SPM8 segmentation. As traditional normalization is less effective for medial temporal lobe structures (Yassa and Stark, 2009), such as the amygdala, we used an improved method—diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) (Ashburner, 2007). This method is more exact for normalizing medial temporal lobe structures than other automated normalization programs (Bergouignan et al., 2009; Yassa and Stark, 2009). Using DARTEL, segmented images were skull-stripped and averaged to create a template of gray and white matter for all subjects. This average template was used to register and derive Jacobian-modulated warped-tissue class images of gray and white matter for each subject and transform the images into a standard space (MNI T1 template). Finally, the images were smoothed with an isotropic Gaussian kernel of 6 mm at FWHM. The resulting images provided a measure of gray matter volume accounting for shape differences for each 1 mm3 voxel across the entire brain. To adjust for any remaining differences in brain size, total intracranial volume (sum of gray matter, white matter and cerebrospinal fluid volumes) was also computed for use as a covariate. Total intracranial volume did not differ significantly between groups (Table 1).
Functional activation and connectivity
Functional MRIs (fMRIs) were acquired on a subset of 26 subjects (14 inhibited, 12 uninhibited) who had participated in a study of face processing (Blackford et al., 2011). In the fMRI task, novel and familiar neutral faces were presented using a slow event-related design. Twelve novel and 12 familiar faces were presented once per run across four runs, for a total presentation of 48 novel face presentations and 48 familiar face presentations (of the six familiarized faces). No novel faces were repeated across runs. Faces were familiarized by showing them eight times per face across the course of a familiarization block, prior to the present task. Face stimuli were selected from two standard sets of emotional expressions (Lundqvist et al., 1998; Gur et al., 2001). Brain activation was measured during the presentation of neutral faces, relative to baseline. Data from this fMRI study have been previously published and detailed methods are available (Blackford et al., 2011). Importantly, this subsample was representative of the larger sample of subjects; the groups did not differ on any demographic or other study variables (all Ps > 0.13).
To determine if gray matter volume (as measured by VBM) was significantly correlated with functional activation in brain regions with significant volume differences, a voxel-to-voxel correlation analysis was performed. Using the Biological Parametric Mapping toolbox (Casanova et al., 2007) implemented in SPM8, voxel-level correlations were estimated between the fMRI images (all faces > baseline) and gray matter volume images (from the VBM analysis). Correlation analyses were performed across all individuals, because the structure–function relationship was expected to be similar for both groups.
To identify the impact of gray matter volume differences on neurocircuit function, a functional connectivity analysis was performed using the Generalized Psychophysiological Interaction Toolbox (McLaren et al., 2012). For this analysis, we used ‘seeds’ based on the regions where group differences in gray matter volume were identified using VBM. We created a map of network connectivity for each seed region (FWE-corrected α = 0.05). To determine the relationship between volume and connectivity, we correlated gray matter volume (using VBM, extracted from the region with significant group differences) and functional connectivity between each seed region and all other voxels in the connectivity map.
Statistical analyses
Structural differences
Group differences in amygdala volume were tested using an analysis of covariance (ANCOVA) with age, gender, race and handedness included as covariates. Statistical analyses (α = 0.05) were performed using SAS (SAS Institute, Cary, NC). Group differences in amygdala shape were tested using a general linear model, implemented in SurfStat (http://www.math.mcgill.ca/keith/surfstat/), with age, gender, race and handedness included as covariates. Group differences were represented by a t-value at each dimension (vertex) of the average amygdala. Uncorrected P-values were estimated using SurfStat and P-values corrected for multiple comparisons were estimated using a false-discovery rate method implemented in Matlab (mafdr function; version 7.5.0; MathWorks, Natick, MA). The specific location of group differences was identified by comparing significant regions with a detailed anatomic atlas of the amygdala (Mai et al., 2008). Group differences in gray matter volume (VBM) within the amygdala were tested using an ANCOVA with age, gender, race and handedness included as covariates. Analyses were restricted to an amygdala region of interest defined by manual tracing on an average study-specific brain. A combination of voxel P-values and cluster-based thresholds was used to control for Type I error due to multiple comparisons (simulations computed using AlphaSim). For the amygdala VBM analysis, family-wise error correction at α = 0.05 was provided by a voxel P = 0.05 and cluster size of 282 voxels (left amygdala) or 275 voxels (right amygdala). For the whole brain analysis, the ANCOVA included age, gender, race, handedness and intracranial volume as covariates. Family-wise error correction at α = 0.05 was provided by a voxel-level P < 0.001 and a cluster size of 295 voxels.
Functional differences
Correlations between gray matter volume and functional activation were computed for the left and right amygdalae, and for other regions identified as having significantly different gray matter volumes across the brain. A one-sample t-test in SPM was used to test for significant correlations. To control for multiple comparisons, AlphaSim was used to determine cluster sizes at voxel-level P < 0.05 and α = 0.05.
To determine the functional impact of greater gray matter volume on functional connectivity, a regression analysis was performed with gray matter volume in the amygdala and any other regions identified as having significantly different gray matter volumes across the brain as the predictor variables. Analyses were performed within the overall functional connectivity map for each region to constrain the analyses to relevant regions (overall map constrained with voxel-level P < 0.05, cluster-corrected at α = 0.05). For correlation analyses, family-wise error was controlled at α = 0.05 with a voxel-level P < 0.05 and a cluster threshold determined by AlphaSim.
Diagnosis effects
To determine the effects of subjects with an anxiety disorder on the results, we compared inhibited subjects with an anxiety diagnosis to inhibited subjects without a diagnosis. Because the inhibited subjects with anxiety disorders also had higher temperament scores, we performed an additional analysis controlling for temperament scores.
RESULTS
Amygdala analyses
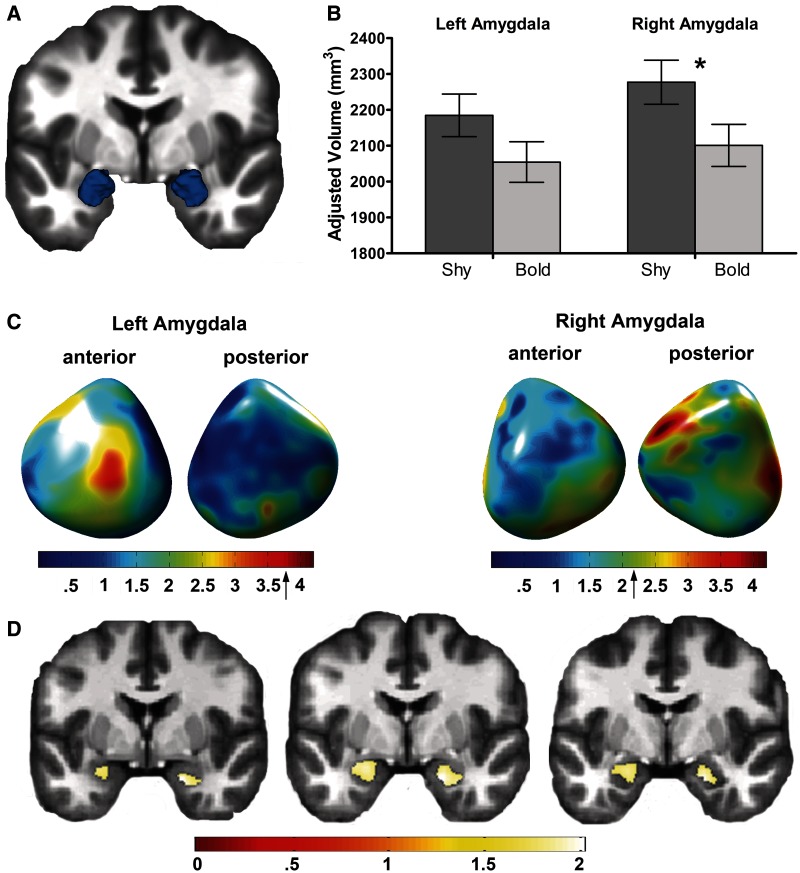
Due to the known role of the amygdala in responding to novel stimuli (Wright et al., 2003; Blackford et al., 2010) and mediating fear and avoidance behaviors (Davis, 1992), we first examined differences in amygdala volume. Using manual segmentation to measure overall amygdala volume, we found that inhibited adults had significantly larger volume in the right amygdala (Figure 1B; 8% larger; P = 0.05), with a similar trend for the left amygdala (6% larger; P = 0.13).
Fig. 1.
Inhibited adults have larger amygdalae. (A) Average manually traced amygdala displayed in 3-D. (B) Overall amygdala volume was greater in the inhibited compared with the uninhibited group in the right amygdala (inhibited: 2277 ± 62 mm3; uninhibited: 2101 ± 59 mm3; P = 0.045). (C) In the inhibited group, shape analysis reveals areas of greater convexity in the left and right amygdala. The peak areas of convexity are shown in red. Group differences in surface amygdala shape are displayed on an average amygdala surface. (D) Inhibited adults had regions of significantly larger gray matter volume in both the left and right amygdalae (P < 0.05 FWE corrected at the cluster level). For the left amygdala, the significant cluster was 442 voxels (35% of 1252 voxels; peak voxel: x = −21, y = −4, z = −16). For the right amygdala, the significant cluster was 402 voxels (33% of 1217 voxels; peak voxel: x = 18, y = −3, z = −22). Areas of significant between-group differences are illustrated on multiple coronal slices of an average brain.
To examine differences in amygdala shape, we used surface modeling methods (Chung et al., 2008). Relative to uninhibited adults, inhibited adults had regions of increased convexity (Figure 1C; right amygdala peak difference: corrected P = 0.02, uncorrected P < 0.0001; left amygdala peak difference: corrected P = 0.055, uncorrected P = 0.0003) located primarily in the basolateral and lateral subnuclei (Mai et al., 2008), regions known to play a crucial role in both fear conditioning and anxiety (Davis, 1992; Phelps and LeDoux, 2005; Etkin and Wager, 2007).
Next, we tested for regional differences in amygdala gray matter volume using VBM. Inhibited adults, relative to uninhibited adults, had regions of greater gray matter volume in both the left and right amygdalae (Figure 1C; P < 0.05 FWE corrected at the cluster level). The regions of greater volume were mainly in the basal and lateral subnuclei, consistent with the amygdala shape analysis. Together, these findings provide converging evidence that inhibited adults have overall larger amygdalae with specific volume increases in the basal and lateral amygdala subnuclei.
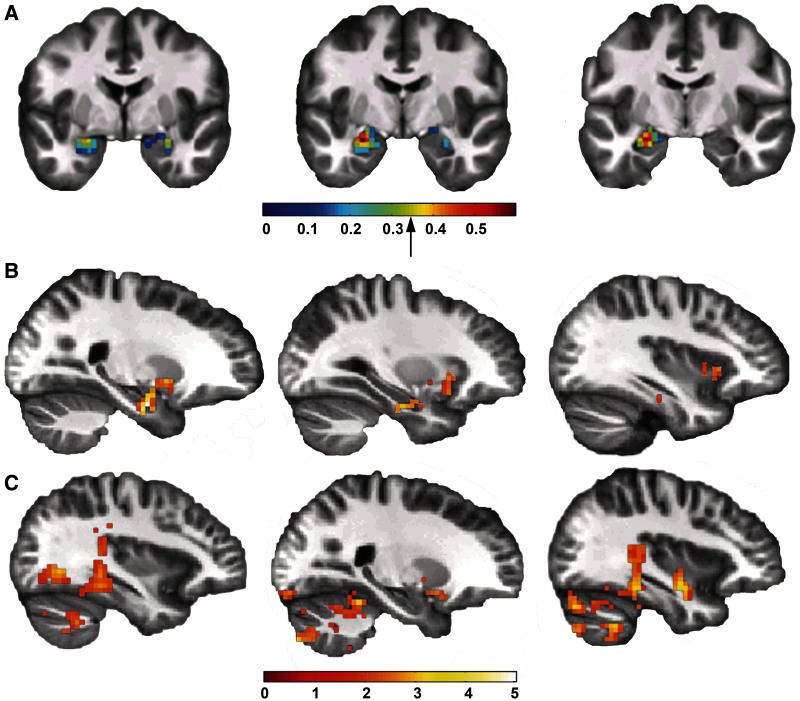
To determine the functional impact of greater amygdala volume in these regions, we tested for structure–function relationships in a subsample with fMRI data. Left amygdala gray matter volume was positively correlated with left amygdala activation to faces (P < 0.05 FWE, k > 10; Figure 2A), suggesting that the functional impact of greater amygdala volume is increased activation to biologically salient stimuli.
Fig. 2.
Larger amygdala volume predicted greater activation and connectivity. (A) Gray matter volume was correlated with activation to faces in the left amygdala. Correlations are shown on three coronal slices of an average structural brain. The correlations are thresholded at r = 0.01 for illustration; the arrow on the color bar indicates P = 0.05, corrected (r = 0.33). (B) Greater gray matter volume in the left amygdala was associated with greater connectivity between the left amygdala and multiple other brain regions including the right temporal lobe, right putamen and right insula (P < 0.05 FWE corrected at the cluster level). Functional connectivity is illustrated across multiple sagittal slices of an average brain. (C) Greater gray matter volume in the right amygdala was associated with greater connectivity between the right amygdala and multiple other brain regions including the right temporal lobe, bilateral medial visual cortex, right insula and bilateral cerebellum (P < 0.05 FWE corrected at the cluster level). Functional connectivity is illustrated across multiple sagittal slices of an average brain.
To determine the impact of amygdala activation on neurocircuit function, we tested whether amygdala volume predicted differences in amygdala functional connectivity across the brain. Larger volume in the left amygdala was correlated with greater connectivity between the left amygdala and the right temporal lobe (including the parahippocampal gyrus, hippocampus and amygdala), right putamen and right insula (all P < 0.05 FWE, k > 99; Figure 2B and Table 2). Greater volume in the right amygdala was correlated with greater connectivity between the right amygdala and the right temporal lobe (including the parahippocampal gyrus, amygdala and hippocampus), bilateral medial visual cortex (including the lingual gyrus, parahippocampal gyrus and fusiform gyrus), right insula and bilateral cerebellum (all P < 0.05 FWE, k > 94; Figure 2C and Table 2). There were no regions where larger amygdala volume was associated with less functional connectivity. The regions of enhanced connectivity are consistent with neural networks activated during face perception (Haxby et al., 2000) and social interactions (Blakemore, 2008).
Table 2.
Brain regions that show significant positive correlations between amygdala gray matter volume and functional connectivity
| Cluster (volume mm3) | Gyrus/region |
|---|---|
| Connectivity with the left amygdala | |
| Temporo-basal gangliar (3699) | |
| Temporal | R hippocampus, R parahippocampal gyrus, R amygdala |
| Basal ganglia | R putamen |
| Connectivity with the right amygdala | |
| Temporo-occipito-cerebellar (65 610) | |
| Temporal | B hippocampus, B parahippocampal gyrus, B superior temporal gyrus, B inferior temporal gyrus, B transverse temporal gyrus |
| Occipital | B lingual gyrus, B fusiform gyrus, B middle occipital gyrus, B inferior occipital gyrus |
| Cerebellum | B dentate, B vermis |
| Temporo-basal gangliar-insular (2592) | |
| Temporal | R parahippocampal gyrus, R amygdala, R hippocampus |
| Basal ganglia | R putamen |
| Insula | R insula |
B, bilateral; L, left hemisphere; R, right hemisphere.
Whole brain analyses
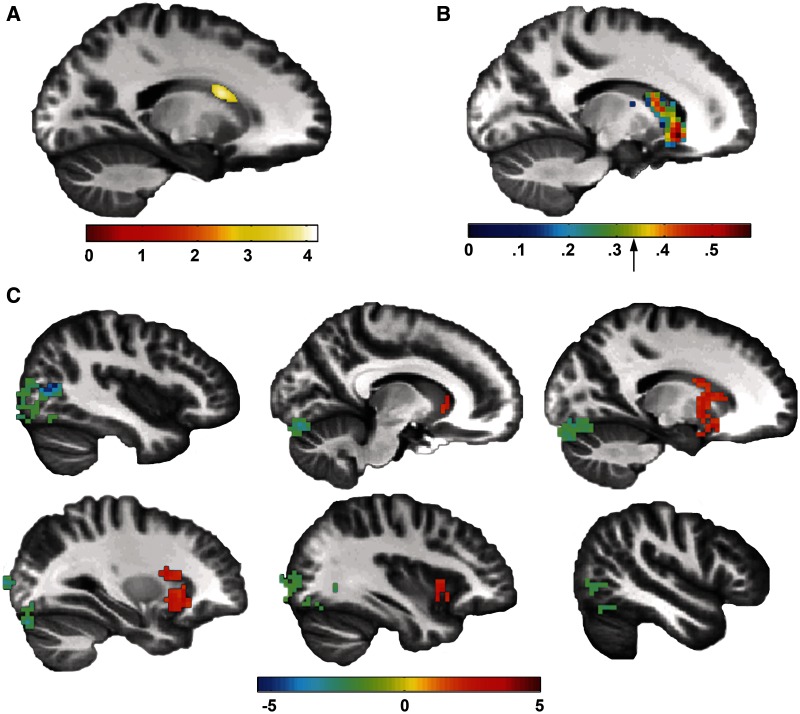
Next, we hypothesized that other brain regions, in addition to the amygdala, might show structural differences in inhibited adults. To identify novel structural substrates of inhibited temperament, we tested for group differences in gray matter volume across the whole brain using VBM. Inhibited adults had significant larger volume in a region of the left caudate than the uninhibited adults (P < 0.05 FWE corrected at the cluster level; Figure 3A).
Fig. 3.
Greater caudate volume and function in inhibited adults. (A) Inhibited adults had greater gray matter volume in the left caudate (496 voxels; P < 0.05 FWE corrected at the cluster level; peak voxel: x = −19, y = 6, z = 17). The region of group difference is illustrated on a sagittal slice of an average brain. (B) Gray matter volume was positively correlated with functional activation to faces in the left caudate. The correlations are thresholded at 0.01 for illustration; the arrow on the color bar marking P < 0.05 FWE corrected at the cluster level (r = 0.33). (C) Larger gray matter volume in the left caudate was associated with greater functional connectivity between the left caudate and right caudate, putamen, and insula (P < 0.05 FWE corrected at the cluster level) and with reduced functional connectivity between the left caudate and lateral occipital complex and cerebellum. Functional connectivity is illustrated across multiple sagittal slices of an average brain.
To assess the functional impact of caudate volume differences, we tested for a correlation between caudate gray matter volume and activation to faces. Gray matter volume was positively correlated with activation to faces (P < 0.05 FWE, k > 23; Figure 3B). Caudate volume differences also predicted differences in functional connectivity. Individuals with larger caudate volumes had greater connectivity between the left caudate and regions which respond to social stimuli, including the right caudate, putamen and insula. Larger caudate volume was also associated with reduced connectivity between the left caudate and the cerebellum and lateral occipital complex (P < 0.05 FWE, k > 94; Figure 3C and Table 3).
Table 3.
Brain regions that show significant correlations between left caudate gray matter volume and functional connectivity
| Cluster (volume mm3) | Gyrus/region |
|---|---|
| Positive correlation | |
| Basal gangliar-insular (5238) | |
| Basal ganglia | R putamen, R caudate, R globus pallidus |
| Insula | R insula |
| Negative correlation | |
| Temporo-occipital (6075) | |
| Temporal | L middle temporal gyrus, L inferior temporal gyrus |
| Occipital | L middle occipital gyrus, L inferior occipital gyrus |
| Insular (3294) | L insula |
| Temporo-occipito-cerebellar (7182) | |
| Temporal | R inferior temporal gyrus, R middle temporal gyrus |
| Occipital | R middle occipital gyrus, R inferior occipital gyrus |
| Cerebellum | R declive, R posterior lobe |
B, bilateral; L, left hemisphere; R, right hemisphere.
Diagnosis effects
As a post hoc analysis, we examined the impact of anxiety diagnosis on our results. Specifically, we compared inhibited individuals with an anxiety diagnosis to those without on each of the structural findings. Inhibited subjects with a diagnosis had a region of significantly larger left amygdala volume (VBM; P = 0.02) relative to inhibited individuals without a diagnosis, and a trend toward larger regional volume in the right amygdala (VBM; P = 0.06). The groups were similar on right amygdala volume (manual; P = 0.38) and left caudate volume (VBM; P = 0.29). Because inhibited individuals with an anxiety diagnosis also had more extreme inhibited temperament scores which could have contributed to volume differences, we performed an additional analysis covarying for temperament score. The inhibited group with a diagnosis had a trend toward a larger left amygdala volume (VBM; P = 0.07). The groups were not significantly different for right amygdala volume (VBM; P = 0.27, manual P = 0.30) or left caudate volume (VBM; P = 0.50).
DISCUSSION
Inhibited adults had structural brain differences in two key regions that distinguished them from uninhibited adults, providing compelling evidence for a structural basis to this foundational temperament trait. Using three complimentary methods, we demonstrated that inhibited adults have significantly larger amygdalae and critically, these anatomical differences had functional consequences for both activation and connectivity. We also identified a novel brain region that distinguished inhibited adults—the caudate. Together these findings provide converging evidence for a structural and functional correlates of inhibited temperament.
Inhibited adults had overall amygdalae volumes that were 8% larger in the left hemisphere and 6% larger in the right hemisphere. These differences are consistent with volume differences reported for individuals with severe behavioral alterations; for example, the well-documented hippocampal atrophy in schizophrenia represents a 4% reduction in volume (Nelson et al., 1998). The structural differences identified using VBM and surface mapping converged on the basal and lateral subnuclei of the amygdala. These regions have widespread efferent projections from sensory cortices and afferent projections to prefrontal cortical regions that modulate the amygdala (Stefanacci and Amaral, 2000). Interestingly, increases in basolateral amygdala volume produce increases in anxious behavior in rodents (Vyas et al., 2002; Mitra and Sapolsky, 2008). In humans, alterations in amygdala structure and function are associated with anxiety, depression and substance abuse (De Bellis et al., 2000; Etkin and Wager, 2007; Shin and Liberzon, 2010; Price and Drevets, 2012). In support of this view, the inhibited subjects with an anxiety diagnosis in this sample had larger amygdala volumes and more extreme inhibited temperament. Based on these findings, we propose that increased amygdala volume in inhibited individuals may produce the characteristic shy and cautious behavior, increased amygdala activation to faces, and increased risk for psychiatric illness.
Given that most theories of inhibited temperament have focused on brain regions mediating fear and anxiety, larger caudate volume in inhibited adults is a novel and intriguing finding. The caudate is a diverse brain region involved in many functions including motor learning and habit formation (Jueptner et al., 1997; Kesner and Gilbert, 2006), cognition (Grahn et al., 2008) and reward (Knutson et al., 2000; Choi et al., 2014). One possible explanation is that increased caudate volume and activity are associated with the caudate’s role in reward processing. Consistent with this view, increased caudate activation has been reported in inhibited and anxious adolescents during anticipation of reward (Guyer et al., 2006, 2012) and loss of reward (Helfinstein et al., 2011). However, we propose an alternative explanation. An emerging perspective is that the caudate is sensitive to saliency and is only engaged under conditions where response choice matters (Tricomi et al., 2004; Zink et al., 2004), consistent with previous findings in other regions of the basal ganglia (Cooper and Knutson, 2008; Bar-Haim et al., 2009; Zaehle et al., 2013). In this study, larger caudate volume predicted greater caudate activation during face processing, consistent with the idea that faces are more salient for inhibited individuals. Therefore, although the amygdala has been studied most extensively in inhibited temperament, due to its role in fear and threat evaluation, the current findings highlight the need to broaden our ideas about the inhibited brain. Instead of focusing on neural systems governed by heightened threat detection, fear and avoidance, we propose that the inhibited brain is governed by increased sensitivity to salient stimuli, including aversive, novel, rewarding and social stimuli. This sensitivity is likely manifest in multiple ways including heightened reactivity to salient stimuli (Schwartz et al., 2003) and broader windows of what stimuli elicit brain responses (Blackford et al., 2011). Interestingly, a recent study found that adults with high dispositional mindfulness—a trait associated with low stress reactivity—have smaller amygdala and caudate volumes (Taren et al., 2013), providing a possible link between enhanced volume, salience detection and stress reactivity.
These findings raise interesting questions. For example, ‘What are the origins of these brain differences?’ Inhibited temperament has genetic origins—it is conserved across evolution, is heritable (Plomin and Daniels, 1986; Emde et al., 1992; Robinson et al., 1992; Eley et al., 2003), and linked to specific genes (Arbelle et al., 2003; Fox et al., 2005b; Smoller et al., 2005). Although nature is clearly important, we propose that nurture is equally important. Key to this proposition is the fact that not all inhibited children become inhibited adults (Essex et al., 2010; Clauss and Blackford, 2012). Developmental research has highlighted the contributions of parental environment (Williams et al., 2009; Kiel and Buss, 2011; Marakovitz et al., 2011; Tang et al., 2012) and family stress (Volbrecht and Goldsmith, 2010) to the developmental trajectories of inhibited children. As with many other biological processes, we expect that nature and nurture are intertwined and have reciprocal influences on inhibited temperament across development. Many factors interact between nature and nurture, including intra-uterine environment (Buss et al., 2012), epigenetic influences (McEwen, 2012; Monk et al., 2012) and shaping of the environment by parents (Tang et al., 2012). In this study, the observed structural differences may reflect both innate differences and the effect of being inhibited over many years. Much remains to be discovered about how genetic and environmental influences shape brain development in inhibited children.
Another question is ‘What is the mechanism by which structure may impact function?’ Studies in rodents and humans provide compelling evidence that brain structure and function can be altered by both learning (Markham and Greenough, 2004; Zatorre et al., 2012) and drugs (Mitra and Sapolsky, 2008). Larger volume and greater functional activation may reflect underlying differences in gray matter—including axon sprouting, dendritic branching and synaptogenesis, neurogenesis, glial changes, angiogenesis—or white matter, for example, changes in myelin formation, myelin remodeling or fiber organization (for a review, see Zatorre et al., 2012). In an attempt to link rodent studies with human MRI findings, a recent study found that structural MRI changes in rodents due to learning reflect changes in presynaptic terminal morphology. Although we cannot draw conclusions about the specific mechanism, future advances in technology may improve the ability to examine cellular changes in vivo and to link these changes to altered brain function and behavior in humans.
A limitation of the study is that the fMRI data were only available on a subsample of subjects. Although the sample size was similar to other functional studies and the subjects were representative of the larger sample, the functional findings should be considered preliminary. Also, the correlational nature of the study prevents us from drawing strong conclusions about the direction of the effects. We propose that these differences may be the underlying neurobiological basis of inhibited temperament; however, the differences may instead reflect the consequence of years of being inhibited. Animal models will be critical for teasing apart the directionality of this relationship. For example, a non-human primate model of inhibited temperament (anxious temperament) has been well-established (Fox et al., 2008; Oler et al., 2010; Shackman et al., 2013) and has the potential to inform the question of whether larger amygdala and caudate volume is a cause or a consequence of inhibited temperament.
In conclusion, this study provides evidence that inhibited temperament is associated with structural brain differences and that these differences have functional consequences for face processing. Given that inhibited individuals are at high risk for adverse long-term outcomes, these structural differences may also inform our understanding of why inhibited individuals have increased rates of developing psychiatric disorders. Inhibited individuals are more sensitive to the environment and more likely to avoid novel stimuli, which may result in heightened anxiety, and subsequently, depression and substance use. We propose that larger amygdala and caudate volume may mediate this enhanced environmental sensitivity. Studies aimed at identifying the cellular and molecular basis of larger amygdala and caudate volume may provide novel and specific targets for treatment. For example, studies in rodents have identified that increases in amygdala dendritic arborization are associated with anxious behavior (Vyas et al., 2002). Novel therapeutics that target dendritic branching or increase inhibitory projections to the amygdala may reduce inhibited or anxious behavior in humans. In addition, prevention strategies that focus on enriching the environments of high-risk children hold promise (Fox et al., 2013; Rapee, 2013).
Acknowledgments
Research reported in this publication was supported by funding from the National Institute of Mental Health (K01-MH083052, J.U.B.; F30-MH097344, J.A.C.; T32-MH018921, J.A.C.), the National Institute of Drug Abuse (R21-DA020149, R.L.C.; K12-DA000357 NIDA/AACAP, M.M.B.), the National Institute for General Medical Studies (T32-GM07347 to Vanderbilt Medical Scientist Training Program, J.A.C.), the Vanderbilt Medical Scholars Program (J.A.C.), the Vanderbilt Institute for Clinical and Translational Research (TL1-RR024978, UL1 TR000445-06, National Center for Advancing Translational Sciences) and the Vanderbilt University Institute of Imaging Science. The authors thank Ned Kalin, Karoly Mirnics, Andy Tomarken and David Zald for helpful critiques and feedback on the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Portions of this work were presented on 13 April 2011 at the Wisconsin Symposium on Emotion, Madison, WI, 2011, on 12 May 2011 at the Society for Biological Psychiatry, San Francisco, CA, 2011 and on 6 April 2013 at the Anxiety Disorders Association of America.
REFERENCES
- Arbelle S, Benjamin J, Golin M, Kremer I, Belmaker RH, Ebstein RP. Relation of shyness in grade school children to the genotype for the long form of the serotonin transporter promoter region polymorphism. American Journal of Psychiatry. 2003;160:671–6. doi: 10.1176/appi.ajp.160.4.671. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, et al. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychological Science. 2009;20:1009–18. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton EA, Schmidt LA, Schulkin J, Antony MM, Swinson RP, Hall GB. Different neural responses to stranger and personally familiar faces in shy and bold adults. Behavioral Neuroscience. 2008;122:704–9. doi: 10.1037/0735-7044.122.3.704. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Bittner A, Pine DS, et al. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Archives of General Psychiatry. 2007;64:903–12. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- Bergouignan L, Chupin M, Czechowska Y, et al. Can voxel based morphometry, manual segmentation and automated segmentation equally detect hippocampal volume differences in acute depression? Neuroimage. 2009;45:29–37. doi: 10.1016/j.neuroimage.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Biro PA, Post JR. Rapid depletion of genotypes with fast growth and bold personality traits from harvested fish populations. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2919–22. doi: 10.1073/pnas.0708159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Cowan RL, Shelton RC, Zald DH. Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Social Cognitive and Affective Neuroscience. 2011;6:621–9. doi: 10.1093/scan/nsq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Buckholtz JW, Avery SN, Zald DH. A unique role for the amygdala in novelty detection. Neuroimage. 2010;50:1188–93. doi: 10.1016/j.neuroimage.2009.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S-J. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9:267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, Toscano JE, Bauman MD, Mason WA, Amaral DG. Neonatal amygdala or hippocampus lesions influence responsiveness to objects. Developmental Psychobiology. 2010;52:487–503. doi: 10.1002/dev.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E1312–9. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development. 1996;67:523–40. [PubMed] [Google Scholar]
- Casanova R, Srikanth R, Baer A, et al. Biological parametric mapping: a statistical toolbox for multimodality brain image analysis. Neuroimage. 2007;34:137–43. doi: 10.1016/j.neuroimage.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Archives of General Psychiatry. 1996;53:1033–9. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Cheek JM, Buss AH. Shyness and sociability. Journal of Personality and Social Psychology. 1981;41:330–9. [Google Scholar]
- Choi JM, Padmala S, Spechler P, Pessoa L. Pervasive competition between threat and reward in the brain. Social Cognitive and Affective Neuroscience. 2014;9(6):737–50. doi: 10.1093/scan/nst053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, et al. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:928–35. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Dalton KM, Davidson RJ. Tensor-based cortical surface morphometry via weighted spherical harmonic representation. IEEE Transactions on Medical Imaging. 2008;27:1143–51. doi: 10.1109/TMI.2008.918338. [DOI] [PubMed] [Google Scholar]
- Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:1066–75. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JC, Knutson B. Valence and salience contribute to nucleus accumbens activation. Neuroimage. 2008;39:538–47. doi: 10.1016/j.neuroimage.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Casey BJ, Dahl RE, et al. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biological Psychiatry. 2000;48:51–7. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Engert V, Duchesne A, et al. Cortisol awakening response and hippocampal volume: vulnerability for major depressive disorder? Biological Psychiatry. 2010;68:847–53. doi: 10.1016/j.biopsych.2010.07.025. [DOI] [PubMed] [Google Scholar]
- Eley TC, Bolton D, O’Connor TG, Perrin S, Smith P, Plomin R. A twin study of anxiety-related behaviours in pre-school children. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2003;44:945–60. doi: 10.1111/1469-7610.00179. [DOI] [PubMed] [Google Scholar]
- Emde RN, Plomin R, Robinson JA, et al. Temperament, emotion, and cognition at fourteen months: the MacArthur Longitudinal Twin Study. Child Development. 1992;63:1437–55. [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Slattery MJ, Goldsmith HHH, Kalin NH. Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. American Journal of Psychiatry. 2010;167:40–6. doi: 10.1176/appi.ajp.2009.07010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Shelton SE, et al. Central amygdala nucleus (Ce) gene expression linked to increased trait-like Ce metabolism and anxious temperament in young primates. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18108–13. doi: 10.1073/pnas.1206723109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS One. 2008;3:e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Barker TV, White LK, Suway JG, Pine DS. Commentary: To intervene or not? Appreciating or treating individual differences in childhood temperament—remarks on Rapee (2013) Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2013;7:789–90. doi: 10.1111/jcpp.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005a;56:235–62. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Nichols KE, Henderson HA, et al. Evidence for a gene-environment interaction in predicting behavioral inhibition in middle childhood. Psychological Science. 2005b;16:921–6. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- Garcia Coll C, Kagan J, Reznick JS. Behavioral inhibition in young children. Child Development. 1984;55:1005–19. [Google Scholar]
- Gosling SD, John OP. Personality dimensions in nonhuman animals: a cross-species review. Current Directions in Psychological Science. 1999;8:69–75. [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Progress in Neurobiology. 2008;86:141–55. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, et al. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25:766–76. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Detloff A, et al. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. American Journal of Psychiatry. 2012;169:205–12. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26:6399–405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Suh JW, Nacewicz BM, et al. Robust automated amygdala segmentation via multi-atlas diffeomorphic registration. Frontiers in Neuroscience. 2012;6:166. doi: 10.3389/fnins.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J, Hoffman E, Gobbini M. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4:223–33. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Helfinstein SM, Benson B, Perez-Edgar K, et al. Striatal responses to negative monetary outcomes differ between temperamentally inhibited and non-inhibited adolescents. Neuropsychologia. 2011;49:479–85. doi: 10.1016/j.neuropsychologia.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfeld DR, Rosenbaum JF, Biederman J, et al. Stable behavioral-inhibition and its association with anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:103–11. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Honeycutt NA, Smith PD, Aylward E, et al. Mesial temporal lobe measurements on magnetic resonance imaging scans. Psychiatry Research. Neuroimaging. 1998;83:85–94. doi: 10.1016/s0925-4927(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Jones WH, Briggs SR, Smith TG. Shyness: conceptualization and measurement. Journal of Personality and Social Psychology. 1986;51:629–39. doi: 10.1037//0022-3514.51.3.629. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. II. Subcortical structures and learning by trial and error. Journal of Neurophysiology. 1997;77:1325–37. doi: 10.1152/jn.1997.77.3.1325. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1998;240:167–71. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N, Kahn V, Towsley S. The preservation of two infant temperaments into adolescence. Monographs of the Society for Research in Child Development. 2007;72:1–75. doi: 10.1111/j.1540-5834.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–21. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience. 2004;24:5506–15. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE. The role of the medial caudate nucleus, but not the hippocampus, in a matching-to sample task for a motor response. European Journal of Neuroscience. 2006;23:1888–94. doi: 10.1111/j.1460-9568.2006.04709.x. [DOI] [PubMed] [Google Scholar]
- Kiel EJ, Buss KA. Prospective relations among fearful temperament, protective parenting, and social withdrawal: the role of maternal accuracy in a moderated mediation framework. Journal of Abnormal Child Psychology. 2011;39:953–66. doi: 10.1007/s10802-011-9516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–7. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Lahat A, Pérez-Edgar K, Degnan KA, et al. Early childhood temperament predicts substance use in young adults. Translational Psychiatry. 2012;2:e157. doi: 10.1038/tp.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. The Karolinska Directed Emotional Faces - KDEF, CD ROM from Department of Clinical Neuroscience, Psychology section, Karolinska Institutet, ISBN 91-630-7164-9. 1998. [Google Scholar]
- Machado CJ, Emery NJ, Capitanio JP, Mason WA, Mendoza SP, Amaral DG. Bilateral neurotoxic amygdala lesions in rhesus monkeys (Macaca mulatta): consistent pattern of behavior across different social contexts. Behavioral Neuroscience. 2008;122:251–66. doi: 10.1037/0735-7044.122.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T. Atlas of the Human Brain. 3rd edn. New York: Elsevier; 2008. [Google Scholar]
- Marakovitz SE, Wagmiller RL, Mian ND, Briggs-Gowan MJ, Carter AS. Lost toy? Monsters under the bed? Contributions of temperament and family factors to early internalizing problems in boys and girls. Journal of Clinical Child and Adolescent Psychology. 2011;40:233–44. doi: 10.1080/15374416.2011.546036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biology. 2004;1:351–63. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Brain on stress: how the social environment gets under the skin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl.):17180–5. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–86. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5573–8. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Spicer J, Champagne FA. Linking prenatal maternal adversity to developmental outcomes in infants: the role of epigenetic pathways. Development and Psychopathology. 2012;24:1361–76. doi: 10.1017/S0954579412000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW. Extreme discordant phenotype methodology: an intuitive approach to clinical pharmacogenetics. European Journal of Pharmacology. 2000;410:107–20. doi: 10.1016/s0014-2999(00)00809-8. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging. Archives of General Psychiatry. 1998;55:433–40. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Luckenbaugh DA, Wood SE, Bogers W, Zarate CA, Drevets WC. Automated subcortical segmentation using FIRST: test-retest reliability, interscanner reliability, and comparison to manual segmentation. Human Brain Mapping. 2013;34:2313–29. doi: 10.1002/hbm.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, et al. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466:864–8. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Roberson-Nay R, Hardin MG, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35:1538–46. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Plomin R, Daniels D. Genetics and shyness. In: Jones WH, Cheek JM, Briggs SR, editors. Shyness: Perspectives on Research and Treatment. New York: Plenum; 1986. pp. 63–80. [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends in Cognitive Sciences. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cerebral Cortex. 2000;10:433–42. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Rapee RM. The preventative effects of a brief, early intervention for preschool-aged children at risk for internalising: follow-up into middle adolescence. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2013;54:780–8. doi: 10.1111/jcpp.12048. [DOI] [PubMed] [Google Scholar]
- Reznick JS, Hegeman IM, Kaufman ER, Woods SW, Jacobs M. Retrospective and concurrent self-report of behavioral-inhibition and their relation to adult mental-health. Development and Psychopathology. 1992;4:301–21. [Google Scholar]
- Robinson JL, Reznick JS, Kagan J, Corley R. The heritability of inhibited and uninhibited behavior: a twin study. Developmental Psychology. 1992;28:1030–7. [Google Scholar]
- Rohrbacher H, Hoyer J, Beesdo K, et al. Psychometric properties of the Retrospective Self Report of Inhibition (RSRI) in a representative German sample. International Journal of Methods in Psychiatric Research. 2008;17:80–8. doi: 10.1002/mpr.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Kunwar PS, Greve DN, Kagan J, Snidman NC, Bloch RB. A phenotype of early infancy predicts reactivity of the amygdala in male adults. Molecular Psychiatry. 2012;17:1042–50. doi: 10.1038/mp.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Kunwar PS, Greve DN, et al. Structural differences in adult orbital and ventromedial prefrontal cortex predicted by infant temperament at 4 months of age. Archives of General Psychiatry. 2010;67:78–84. doi: 10.1001/archgenpsychiatry.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1008–15. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300:1952–53. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE. Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6145–50. doi: 10.1073/pnas.1214364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–91. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Yamaki LH, Fagerness JA, et al. The corticotropin-releasing hormone gene and behavioral inhibition in children at risk for panic disorder. Biological Psychiatry. 2005;57:1485–92. doi: 10.1016/j.biopsych.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Topographic organization of cortical inputs to the lateral nucleus of the macaque monkey amygdala: a retrograde tracing study. The Journal of Comparative Neurology. 2000;421:52–79. doi: 10.1002/(sici)1096-9861(20000522)421:1<52::aid-cne4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Tang AC, Reeb-Sutherland BC, Romeo RD, McEwen BS. Reducing behavioral inhibition to novelty via systematic neonatal novelty exposure: the influence of maternal hypothalamic-pituitary-adrenal regulation. Biological Psychiatry. 2012;72:150–6. doi: 10.1016/j.biopsych.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Taren AA, Creswell JD, Gianaros PJ. Dispositional mindfulness co-varies with smaller amygdala and caudate volumes in community adults. PLoS One. 2013;8:1–7. doi: 10.1371/journal.pone.0064574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–92. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- van der Plas EAA, Boes AD, Wemmie JA, Tranel D, Nopoulos P. Amygdala volume correlates positively with fearfulness in normal healthy girls. Social Cognitive and Affective Neuroscience. 2010;5:424–31. doi: 10.1093/scan/nsq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volbrecht MM, Goldsmith H. Early temperamental and family predictors of shyness and anxiety. Developmental Psychology. 2010;46:1192–205. doi: 10.1037/a0020616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LR, Degnan KA, Perez-Edgar KE, et al. Impact of behavioral inhibition and parenting style on internalizing and externalizing problems from early childhood through adolescence. Journal of Abnormal Child Psychology. 2009;37:1063–75. doi: 10.1007/s10802-009-9331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LR, Fox NA, Lejuez CW, et al. Early temperament, propensity for risk-taking and adolescent substance-related problems: a prospective multi-method investigation. Addictive Behaviors. 2010;35:1148–51. doi: 10.1016/j.addbeh.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, Schwartz CE, et al. Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex. Neuroimage. 2003;18:660–9. doi: 10.1016/s1053-8119(02)00037-x. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. Neuroimage. 2009;44:319–27. doi: 10.1016/j.neuroimage.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Zaehle T, Bauch EM, Hinrichs H, et al. Nucleus accumbens activity dissociates different forms of salience: evidence from human intracranial recordings. Journal of Neuroscience. 2013;33:8764–71. doi: 10.1523/JNEUROSCI.5276-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nature Neuroscience. 2012;15:528–36. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–17. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]