Abstract
The binding and cytochrome P45051 (CYP51) inhibition properties of a novel antifungal compound, VT-1161, against purified recombinant Candida albicans CYP51 (ERG11) and Homo sapiens CYP51 were compared with those of clotrimazole, fluconazole, itraconazole, and voriconazole. VT-1161 produced a type II binding spectrum with Candida albicans CYP51, characteristic of heme iron coordination. The binding affinity of VT-1161 for Candida albicans CYP51 was high (dissociation constant [Kd], ≤39 nM) and similar to that of the pharmaceutical azole antifungals (Kd, ≤50 nM). In stark contrast, VT-1161 at concentrations up to 86 μM did not perturb the spectrum of recombinant human CYP51, whereas all the pharmaceutical azoles bound to human CYP51. In reconstitution assays, VT-1161 inhibited Candida albicans CYP51 activity in a tight-binding fashion with a potency similar to that of the pharmaceutical azoles but failed to inhibit the human enzyme at the highest concentration tested (50 μM). In addition, VT-1161 (MIC = 0.002 μg ml−1) had a more pronounced fungal sterol disruption profile (increased levels of methylated sterols and decreased levels of ergosterol) than the known CYP51 inhibitor voriconazole (MIC = 0.004 μg ml−1). Furthermore, VT-1161 weakly inhibited human CYP2C9, CYP2C19, and CYP3A4, suggesting a low drug-drug interaction potential. In summary, VT-1161 potently inhibited Candida albicans CYP51 and culture growth but did not inhibit human CYP51, demonstrating a >2,000-fold selectivity. This degree of potency and selectivity strongly supports the potential utility of VT-1161 in the treatment of Candida infections.
INTRODUCTION
Candida albicans is responsible for a wide range of human fungal infections ranging in severity from relatively minor but disruptive mucosal infections, such as vaginitis and oral candidiasis, to potentially life-threatening systemic candidemia. The azole class of drugs targeting fungal cytochrome P45051 (CYP51) is widely used as a first-line treatment for fungal infections or as preemptive treatment. Relative to amphotericin B, the approved azoles, such as fluconazole, itraconazole, and voriconazole, have fewer side effects. However, several side effects do indeed exist and are predominantly linked to inhibition of off-target human cytochromes P450 (CYPs). All marketed azoles have multiple drug-drug interactions (DDIs) due to inhibition of such human CYPs as CYP3A4, CYP2C9, and CYP2C19 (1). Because many patients with fungal infections are on therapy for an underlying disease, these DDIs are problematic and contribute to the need for monitoring plasma drug levels. The well-documented visual disturbances caused by voriconazole have been postulated to be due to inhibition of human CYP46 (2). Warnings against the use of voriconazole, itraconazole, and fluconazole during pregnancy result from inhibition of endocrine biosynthetic enzymes, such as CYP19 (3). The mechanism of hepatotoxicity, with itraconazole causing the most pronounced occurrence, resulting in a 10% discontinuation rate (4), is not as well understood but may be in part due to interaction with liver CYPs. Fungal CYP51 inhibitors with greater selectivity for fungal CYP51 than off-target human CYPs could overcome these limitations and would thus be a significant advancement in the field of fungal therapy.
Additionally, the widespread use of azole antifungals, especially for prolonged treatment periods, has led to the emergence of azole-resistant strains of Candida albicans and other Candida species, especially with immunocompromised patients (5–12). Thus, there is a growing need to develop new effective antifungal drugs to combat the increasing occurrence of Candida resistance, especially for the treatment of deep systemic infections. Because many of the marketed azole drugs are limited by a low therapeutic index (13), a drug with a higher therapeutic index might be able to combat resistant pathogens at plasma concentrations still below toxic levels.
In this study, we compared the novel antifungal VT-1161 (14) with four clinical azole antifungal drugs in terms of its potency and selectivity of binding to and inhibition of recombinant C. albicans CYP51 (CaCYP51) compared to human CYP51, wild-type azole-susceptible C. albicans in cellular growth assays, and three critical human xenobiotic-metabolizing CYPs (CYP2C9, CYP2C19, and CYP3A4). Using a combination of ligand binding spectroscopy techniques, CYP enzymatic assays, and analysis of cellular sterol pathway components, VT-1161 was demonstrated to be of therapeutic interest because of its high C. albicans CYP51 binding affinity, potent C. albicans growth inhibition, and minimal interactions with human CYP51 and key drug-metabolizing CYPs.
MATERIALS AND METHODS
Cloning, expression, solubilization, purification, and characterization of CaCYP51 and Δ60HsCYP51 enzymes.
All methods were performed as previously described (15). In Homo sapiens CYP51 with a 60-codon gene truncation (Δ60HsCYP51), the N-terminal transmembrane domain upstream of Pro-61 was replaced with the N-terminal MAKKTSSKGKL sequence from CYP2C3 (16, 17). The first eight amino acids of the full-length CaCYP51 construct were altered to MALLLAVF (ATGGCTCTGTTATTAGCAGTTTTT) to facilitate expression in Escherichia coli (18). Both gene constructs included a 12-base insertion (CATCACCATCAC) encoding a four-histidine tag immediately in front of the stop codon to facilitate protein purification by Ni2+-nitrilotriacetic acid (NTA) agarose affinity chromatography. Previously, we have shown that the properties of binding of the pharmaceutical azole antifungal drugs to the Δ60HsCYP51 protein were comparable to those of binding to full-length HsCYP51 (15). Ni2+-NTA agarose-purified CaCYP51 and Δ60HsCYP51 enzymes were used for all subsequent spectrophotometric binding studies and CYP51 reconstitution assay 50% inhibitory concentration (IC50) determinations.
Antifungal agent binding properties.
Binding assays with 5 μM CaCYP51 and Δ60HsCYP51 proteins were performed as previously described (19) using quartz cuvettes with a 4.5-mm light path and stock 1-, 0.5-, 0.2-, and 0.1-mg ml−1 antifungal agent solutions prepared in dimethyl sulfoxide (DMSO). Antifungal agents were titrated against 5 μM CYP51 proteins in 0.1 M Tris-HCl (pH 8.1) and 25% glycerol at 22°C, with equivalent volumes of DMSO also being added to the CYP51-containing compartment of the reference cuvette. The absorbance difference spectra between 500 and 350 nm were determined after each incremental addition of compound, with binding saturation curves being constructed from the change in the absorbance at 430 nm and that at 412 nm (ΔA430-412) against the antifungal agent concentration. The dissociation constant (Kd) of the enzyme-antifungal agent complex for tight ligand binding was determined by nonlinear regression (Levenberg-Marquardt algorithm) using a rearrangement of the Morrison equation (20, 21). Each binding determination was performed in triplicate. Binding studies using clotrimazole, fluconazole, itraconazole, and voriconazole with 5 μM CaCYP51 and Δ60HsCYP51 were performed previously (15).
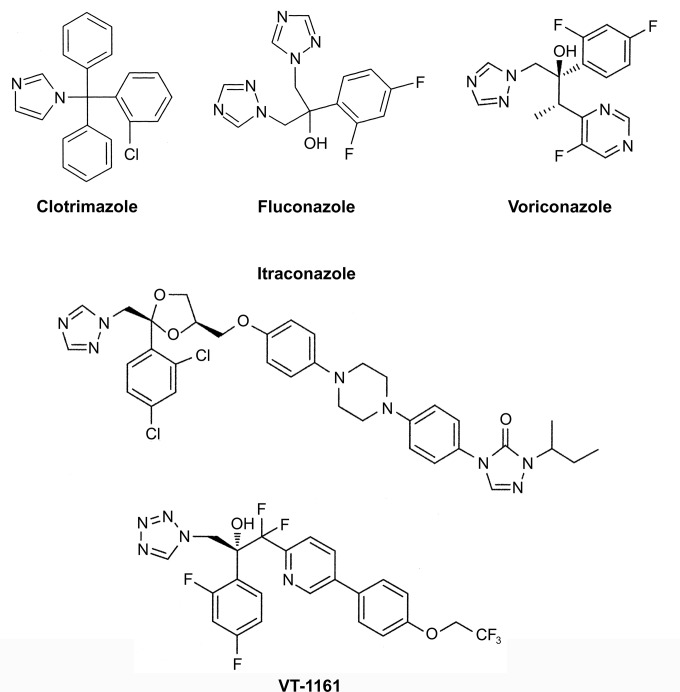
In addition, absolute spectra between 700 and 300 nm for 5 μM CaCYP51 and Δ60HsCYP51 in the absence and presence of 10 or 40 μM antifungal agent were measured using 10-mm-light-path semimicroquartz cuvettes, and the difference were spectra calculated by subtracting the resting-state absolute CYP51 spectra from the azole-treated absolute CYP51 spectra. The chemical structures of the antifungal agents used in this study are shown in Fig. 1.
FIG 1.
Chemical structures of azole antifungal agents. The chemical structures of clotrimazole (molecular weight, 345), fluconazole (molecular weight, 306), itraconazole (molecular weight, 706), voriconazole (molecular weight, 349), and VT-1161 (molecular weight, 527) are shown.
CYP51 reconstitution assays and IC50 determinations.
Lanosterol (15 μg) and dilaurylphosphatidylcholine (DLPC; 100 μg) were dissolved in 0.25 ml of chloroform prior to the addition of 0.2 ml water. The mixture was vortexed to form a white emulsion while the chloroform was simultaneously evaporated off under nitrogen to produce aqueous sterol-DLPC micelles. The CYP51 reconstitution system consisted of 0.2 ml of sterol-DLPC micelles diluted to 0.8 ml with 2.5 nmol CYP51 protein, 10 nmol Saccharomyces cerevisiae cytochrome P450 reductase truncated by 33 amino acids (22), 3 U S. cerevisiae glucose-6-phosphate dehydrogenase, 2 μmol disodium glucose-6-phosphate, and 100 μmol potassium phosphate (pH 6.6). The mixture was preincubated at 37°C for 5 min prior to reaction initiation by the addition of 0.1 ml 20 mM β-NADPH-Na4 in 0.2% NaHCO3, followed by further incubation at 37°C for 60 min with shaking. The final pH of the CYP51 reconstitution assay mixture was 7.0 to 7.2. Addition of 2 ml of 15% KOH in ethanol terminated the reaction, and subsequent incubation at 85°C for 90 min saponified the lipid fraction. Hexane extraction recovered the sterol metabolites, which were derivatized with N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) and trimethylchlorosilane (TMCS) prior to analysis by gas chromatography (GC)-mass spectrometry (MS) (23). The IC50s for antifungal agents were determined by varying the concentration of the antifungal compound in 5 μl of DMSO, which was added to the assay mixture prior to preincubation at 37°C and addition of β-NADPH-Na4.
Antifungal susceptibility testing.
The susceptibilities of the SC5314 and CA14 C. albicans wild-type strains to all antifungals were determined using the standardized CLSI M27-A3 broth dilution method (24).
C. albicans sterol composition determinations.
Single colonies were used to inoculate 15-ml volumes of RPMI 1640, which were incubated for 18 h at 37°C and 180 rpm in the presence of 0.004 μg ml−1 antifungal agent or an equivalent amount of DMSO (untreated control colonies). Cells were harvested by centrifugation and washed with sterile water prior to sterol extraction as previously described (25). Cell pellets were suspended in 2.5 ml methanol, 1.5 ml potassium hydroxide (60%, wt/vol), and 1 ml methanol-dissolved pyrogallol (0.5%, wt/vol), and the suspension was heated at 90°C for 2 h. Nonsaponifiable sterols were extracted into glass vials with three sequential 2-ml volumes of hexane. Extracts were evaporated to dryness using a centrifugal evaporator (Heto Maxi dry plus) and derivatized (BSTFA-TMCS [99:1] plus 50 μl anhydrous pyridine, 70°C) for 2 h. TMCS-derivatized sterols were analyzed and identified using GC-MS with reference to the retention times and fragmentation spectra for known standards (see Fig. S1 in the supplemental material). GC-MS data files were analyzed using Agilent software (Mass Selective Detector Enhanced ChemStation; Agilent Technologies Inc.) to determine the sterol profiles for SC5314 and CA14 and for derivation of integrated peak heights. In addition to the analysis of untreated RPMI 1640-grown SC5314 and CA14, the sterol compositions of both isolates following treatment with a fixed azole concentration of 0.004 μg ml−1 were determined using GC-MS.
Strains and media.
Wild-type (azole-sensitive) Candida albicans strain SC5314 (ATCC MYA-2876) (26) and wild-type clinical isolate CA14 (27) were maintained at 37°C on yeast extract peptone dextrose (YEPD) agar containing 2% glucose, 2% Bacto peptone, 1% yeast extract, and 2% agar (Formedium). RPMI 1640 medium (Sigma) buffered with 0.165 M MOPS (morpholinepropanesulfonic acid; pH 7) was used to culture the isolates for sterol analyses and antifungal susceptibility testing.
Inhibition of human liver CYP enzymes.
In vitro studies determined the IC50s of the test compounds for CYP2C9, CYP2C19, and CYP3A4 (with either midazolam or testosterone as the substrates) in intact human liver microsomes. A separate series of incubation mixtures was prepared, with the final concentrations of each test compound in the reaction mixture ranging from 0.0128 to 200 μM. Each incubation mixture contained pooled human liver microsomes at an assay concentration of 1 mg ml−1 microsomal protein (Life Technologies, Grand Island, NY) and metabolic substrates of isozymes for CYP2C9, CYP2C19, and CYP3A4 (diclofenac, omeprazole, and midazolam or testosterone, respectively) at their experimentally determined Km concentrations. Active control wells contained microsomes, substrate(s), and the test compound diluent (i.e., DMSO-acetonitrile-phosphate buffer, 5:5:190) substituted for test compound solutions. The reaction was initiated by addition of an enzyme cofactor source (NADPH regenerating solution; BD Biosciences, San Jose, CA), and the mixtures were incubated at 37°C. After 10 min, the incubation mixtures were quenched with acetonitrile, mixed, and centrifuged. The supernatant was analyzed by high-pressure liquid chromatography–MS/MS for the hydroxy metabolite of the substrates. Each product peak area was normalized to be represented as a percentage of the average for the enzyme control. The IC50 for each test compound was determined by fitting a 4-parameter logistical fit to the dose-response data and graphically determining the inhibitor concentration at 50% of the maximal enzymatic response.
Data analysis.
Curve fitting of ligand binding data was performed using the computer program ProFit (version 6.1.12; QuantumSoft, Zurich, Switzerland). Spectral determinations were made using quartz semimicrocuvettes with an Hitachi U-3310 UV-visible spectrophotometer (San Jose, CA).
Chemicals.
All chemicals, including all azole antifungals except voriconazole, were obtained from Sigma Chemical Company (Poole, United Kingdom). Voriconazole was supplied by Discovery Fine Chemicals (Bournemouth, United Kingdom). VT-1161 was supplied by Viamet Pharmaceuticals, Inc. (Durham, NC).
RESULTS
VT-1161 binding properties.
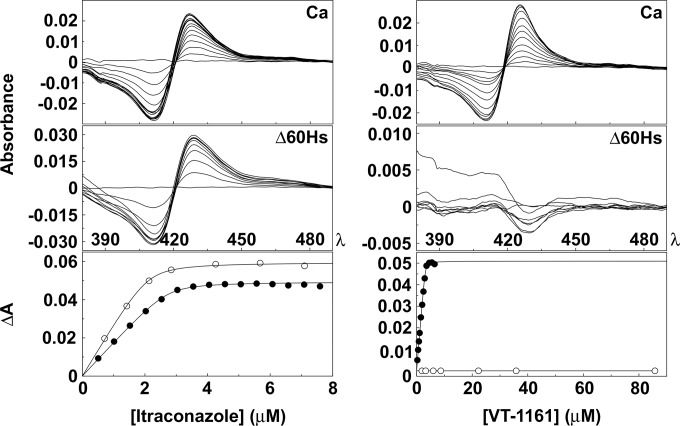
VT-1161 bound tightly to CaCYP51 (Kd, ≤39 nM), producing a strong type II difference spectrum (Fig. 2) with an affinity similar to the affinities of the pharmaceutical antifungal agents clotrimazole, fluconazole, itraconazole, and voriconazole (Kd, near or below 50 nM) (Table 1). Type II binding spectra with a maximum absorbance at 429 to 431 nm and a trough absorbance at 411 to 413 nm arise through the triazole N-4 nitrogen (fluconazole, itraconazole, and voriconazole) or the imidazole ring N-3 nitrogen (clotrimazole), coordinating as the sixth ligand with the heme iron (28, 29) to form a low-spin CYP51-azole complex. The interaction of VT-1161 with the heme ferric ion is through a terminal (N-3 or N-4) tetrazole nitrogen atom (Fig. 1). The N-4 nitrogen was found to be more nucleophilic in heats of formation experiments (data not shown).
FIG 2.
Azole binding properties of CaCYP51 (Ca) and Δ60HsCYP51 (Δ60Hs). VT-1161 and itraconazole were progressively titrated against 5 μM CaCYP51 and 5 μM Δ60HsCYP51, and binding saturation curves were constructed from the change in absorbance (ΔA430-412) against the antifungal concentration for CaCYP51 (filled circles) and Δ60HsCYP51 (hollow circles). All spectral determinations were performed in triplicate, although only one replicate is shown.
TABLE 1.
Binding affinity for CaCYP51 and Δ60HsCYP51 and MICs for wild-type C. albicansf
| Antifungal |
Kd for antifungal (nM)a |
Fold difference in Kd between Δ60HsCYP51 and CaCYP51a | MIC for antifungal (μg ml−1 [nM]) |
||
|---|---|---|---|---|---|
| CaCYP51 | Δ60HsCYP51 | SC5314d | CA14e | ||
| Clotrimazole | 26 (6) | 55 (5) | 2.1 | ||
| Fluconazole | 56 (4) | 30,400 (4,100)b | 543 | 0.125 (408) | 1.0 (3,268) |
| Itraconazole | 19 (5) | 92 (7) | 4.8 | ||
| Voriconazole | 10 (2) | 2,290 (120)b | 229 | 0.004 (11) | 0.0625 (179) |
| VT-1161 | 39 (2) | No bindingc | >2,000c | 0.002 (4) | 0.002 (4) |
The values of the parameters were previously determined (15) for all antifungals except VT-1161.
Where ligand binding was no longer tight, the Michaelis-Menten equation was used to determine Kd values.
No discernible ligand binding spectrum was obtained for VT-1161 with Δ60HsCYP51 at drug concentrations up to 86 μM.
Laboratory wild-type C. albicans strain (26).
Wild-type clinical C. albicans isolate (27).
Binding studies were performed using 5 μM CYP51 proteins. Kd values were calculated using a rearrangement of the Morrison equation for tight-binding ligands (20, 21). Mean Kd values of three replicates are shown, and the associated standard errors are given in parentheses. MICs were determined using the CLSI broth dilution methodology (24) after 48 h growth at 37°C and 180 rpm.
In contrast, VT-1161 was unique as an azole-based antifungal agent, giving no discernible binding spectrum with Δ60HsCYP51 at concentrations up to 86 μM, indicating no disturbance of the heme environment. These data suggest that VT-1161 either binds to Δ60HsCYP51 in an orientation that does not perturb the heme environment or does not interact with Δ60HsCYP51 at this concentration. Clotrimazole and itraconazole, however, bound tightly to Δ60HsCYP51 (Table 1), producing characteristic type II binding spectra (Fig. 2 and 3) with Kd values of 55 and 92 nM, respectively, while voriconazole and fluconazole bound weakly to Δ60HsCYP51 (Kd values, 2.3 and 30 μM, respectively). These results were in agreement with previous findings for truncated human CYP51 (30), where clotrimazole bound tightly (Kd, <0.1 μM) and fluconazole bound weakly (Kd, 70 μM). In addition, we have previously shown that full-length human CYP51 bound pharmaceutical azole antifungal agents with affinities similar to those of the truncated Δ60HsCYP51 enzyme (15).
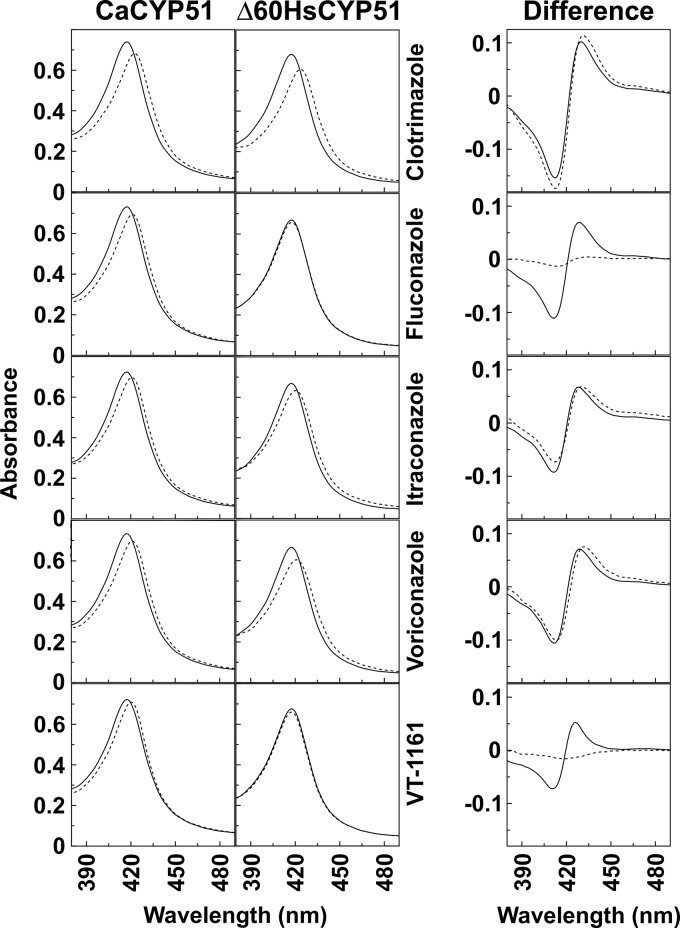
FIG 3.
Red shift of the heme Soret peak induced by azole ligand binding. Absolute spectra of 5 μM CaCYP51 and 5 μM Δ60HsCYP51 were measured in the absence (solid lines) and presence (dashed lines) of 10 μM antifungal compound. The 380- to 490-nm region of the spectra is shown to highlight any red shift of the Soret peak in response to ligand binding. The difference spectra for CaCYP51 (solid lines) and Δ60HsCYP51 (dashed lines), obtained by subtracting the resting-state absolute CYP51 spectra from the +10 μM azole-treated absolute CYP51 spectra, are also shown.
The differences in antifungal agent binding potency observed between CaCYP51 and Δ60HsCYP51 using ligand binding difference spectroscopy were confirmed by measuring the absolute spectra of the CYP51 proteins in the absence and presence of 10 μM compound (Fig. 3). When bound to CaCYP51, VT-1161 and the four pharmaceutical azoles induced a red shift of the 417-nm Soret peak to 420 nm with VT-1161, 423 nm with clotrimazole, and 421 nm with fluconazole, itraconazole, and voriconazole. These shifts are consistent with basicity governing the magnitude, with the most basic imidazole giving the greatest shift, followed by triazole and then tetrazole (31). Subtraction of the resting-state absolute spectrum for ferric CaCYP51 from the antifungal-bound absolute spectra gave the characteristic type II difference spectra (Fig. 3). In contrast, the addition of 10 μM or 40 μM VT-1161 to Δ60HsCYP51 did not cause any shift of the heme Soret peak, which explained the lack of any type II difference spectrum during the titration of VT-1161 with Δ60HsCYP51 (Fig. 2). Clotrimazole, itraconazole, and voriconazole, however, induced a red shift of the Soret peak of Δ60HsCYP51 from 417 nm to 423, 420, and 421 nm, respectively (Fig. 3). In contrast, fluconazole caused only a 1-nm red shift of the Soret peak of Δ60HsCYP51 to 418 nm, confirming the weak type II difference spectrum previously observed for fluconazole with Δ60HsCYP51 (15).
IC50 determinations with CaCYP51 and Δ60HsCYP51.
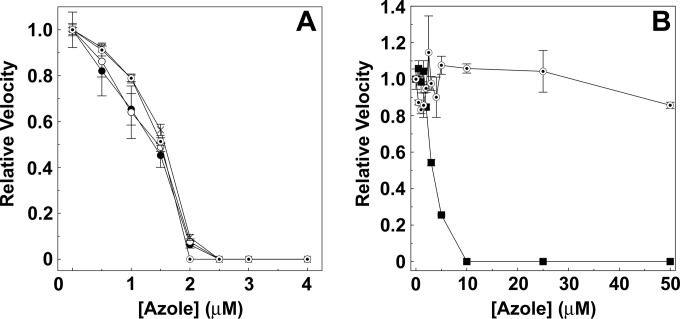
VT-1161 potently inhibited the sterol 14α-demethylase activity of isolated CaCYP51 (Fig. 4A), as did fluconazole, itraconazole, and voriconazole, with IC50s that were approximately half the CaCYP51 concentration present (1.4 to 1.6 μM). This behavior is typical of tight-binding enzyme inhibitors. VT-1161, however, caused no significant inhibition of Δ60HsCYP51 activity even at a concentration of 50 μM (Fig. 4B), again consistent with VT-1161 not binding to Δ60HsCYP51. In contrast, clotrimazole strongly inhibited Δ60HsCYP51 sterol 14α-demethylase activity (IC50, 3.2 μM) in vitro. Previously, we have shown that fluconazole only weakly inhibited Δ60HsCYP51 activity (15) with an IC50 of ∼1,300 μM and that itraconazole also inhibited Δ60HsCYP51 relatively poorly (IC50 ∼70 μM) in comparison to clotrimazole.
FIG 4.
IC50 determinations for antifungal agents with CaCYP51 and Δ60HsCYP51. CYP51 reconstitution assays containing either 2.5 μM CaCYP51 (A) or 2.5 μM Δ60HsCYP51 (B) were performed. For CaCYP51, the concentrations of fluconazole (filled circles), itraconazole (hollow circles), voriconazole (crosses), and VT-1161 (bullets) were varied from 0 to 4 μM. For Δ60HsCYP51, the concentrations of clotrimazole (filled squares) and VT-1161 (bullets) were varied from 0 to 50 μM. The DMSO concentration was kept constant at 0.5% in all assays. Mean relative enzyme velocities from three replicate data points along with the associated standard error bars are shown. Relative velocities of 1.0 correspond to actual velocities of 0.32 ± 0.04 nmol min−1 for CaCYP51 and 0.69 ± 0.18 nmol min−1 for Δ60HsCYP51.
Antifungal agent susceptibility of wild-type C. albicans strains.
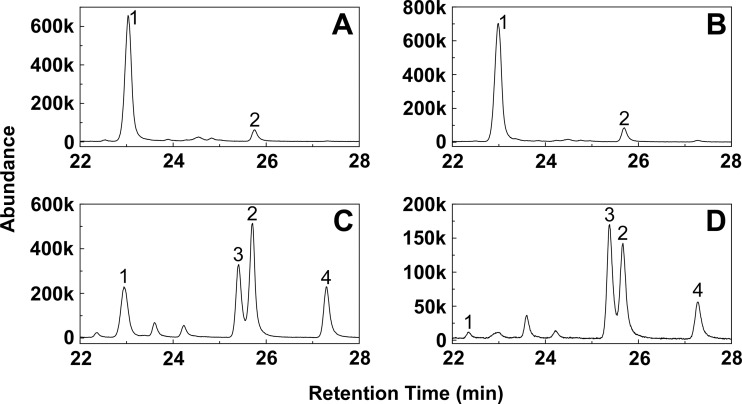
Fluconazole and voriconazole inhibited both wild-type C. albicans strains. However, the CA14 wild-type clinical strain was 8-fold less susceptible toward fluconazole and 16-fold less susceptible toward voriconazole (Table 1) than the wild-type SC5314 laboratory strain. In contrast, VT-1161 was equally effective against the wild-type strains, with a MIC value of 0.002 μg ml−1 (or 3.8 nM) (Table 1). The mode of action of VT-1161 as a sterol 14α-demethylase inhibitor was confirmed by the sterol composition of VT-1161-treated SC5314 C. albicans cells, which was compared to that of untreated or fluconazole- and voriconazole-treated cells (Fig. 5 and Table 2). The untreated cell sterol fraction contained ∼92% ergosterol, indicating that sterol biosynthesis was fully functional. The fluconazole-treated cell sterol fraction contained only marginally less ergosterol (∼88%), as the fluconazole concentration used (0.004 μg ml−1) was ∼30-fold lower than the MIC value (Table 1). The voriconazole-treated cell sterol fraction contained far less ergosterol (∼16%), increased levels of lanosterol (∼36%) and eburicol (∼16%), and an accumulation of the fungistatic metabolite 14α-methylergosta-8,24(28)-dien-3β,6α-diol (∼23%). This metabolite is the end product of the sequential action of ERG25, ERG26, ERG27, and ERG3 enzymes on eburicol, indicative of the disruption of the sterol biosynthesis pathway by inhibition of CYP51 activity (27, 32, 33). VT-1161 caused the potent disruption of sterol biosynthesis in C. albicans, resulting in a dramatic reduction (∼95%) in the ergosterol content from ∼92% to just ∼3% of the total sterol fraction and the accumulation of lanosterol (∼33%), eburicol (∼13%), and 14α-methylergosta-8,24(28)-dien-3β,6α-diol (∼40%) (Table 2).
FIG 5.
Gas chromatograms of the sterol fraction isolated from untreated and antifungal-treated C. albicans. Wild-type C. albicans strain SC5134 (26) was treated with 0.004 μg ml−1 antifungal agents and an untreated control received DMSO alone, followed by 18 h growth at 37°C and 180 rpm. The sterol content was extracted and analyzed as previously described (27). Gas chromatograms of the extracted sterols are shown for the untreated C. albicans cells (A) and the cells treated with the antifungals fluconazole (B), voriconazole (C), and VT-1161 (D). The four most abundant sterols present were ergosterol (peak 1), lanosterol (peak 2), 14α-methylergosta-8,24(28)-dien-3β,6α-diol (peak 3), and eburicol (peak 4). The data on the abundance axis are expressed in units of 1,000 (e.g., 600k = 600,000).
TABLE 2.
Sterol composition of untreated and antifungal-treated wild-type C. albicans strainsa
| Sterol | Sterol composition (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Untreated |
With fluconazole |
With voriconazole |
With VT-1161 |
|||||
| SC5134 | CA14 | SC5134 | CA14 | SC5134 | CA14 | SC5134 | CA14 | |
| Ergosterol | 91.6 | 79.6 | 88.3 | 80.5 | 16.2 | 22.0 | 2.6 | 5.0 |
| 14α-Methylfecosterol | 4.8 | 4.6 | 8.6 | 7.5 | ||||
| 4,14α-Dimethylcholesta-8,24-dienol | 3.9 | 2.5 | 3.0 | 2.5 | ||||
| 14α-Methylergosta-8,24(28)-dien-3β,6α-diol | 23.1 | 42.9 | 39.8 | 60.2 | ||||
| Lanosterol | 8.4 | 13.2 | 10.2 | 14.1 | 35.9 | 21.3 | 33.2 | 19.9 |
| Eburicol | 7.2 | 1.5 | 5.4 | 16.2 | 6.7 | 12.8 | 5.0 | |
The C. albicans strains used included the laboratory wild-type SC5134 strain (26) and the clinical wild-type isolate CA14 (27). Both isolates were treated with antifungal agent at a concentration of 0.004 μg ml−1, followed by 18 h growth at 37°C and 180 rpm, and the sterol content was extracted and analyzed as previously described (27).
Inhibition of human liver drug-metabolizing CYPs.
The levels of inhibition of three critical xenobiotic-metabolizing CYPs by the four approved azole drugs and VT-1161 are shown in Table 3. Where available, the IC50s for the marketed agents from the literature (34–36) agree well with those measured in this study. The imidazole-containing clotrimazole was the most potent CYP inhibitor, inhibiting all activities with sub- or low-micromolar potency. The three triazole-containing agents had variable inhibitory potencies, with itraconazole potently inhibiting CYP3A4 with either substrate (0.08 and 0.13 μM), voriconazole inhibiting all activities with a relatively tight range of potencies (4 to 13 μM), and fluconazole showing a slightly broader range (6 to 34 μM). In contrast, VT-1161 weakly inhibited each of these activities (65 to 140 μM).
TABLE 3.
Inhibition of human liver CYPs by fungal CYP51 inhibitors
| Inhibitor | IC50 (μM)a |
|||
|---|---|---|---|---|
| CYP2C9 | CYP2C19 | CYP3A4b | CYP3A4c | |
| Clotrimazole | 1.4 (0.1) | 0.6 (0.2) | 0.03 (0.01) | 0.045 (0.001) |
| Fluconazole | 34 (10) | 13 (9) | 32 (5) | 6 (2) |
| Itraconazole | 80 (28) | 78 (31) | 0.08 (0.02) | 0.13 (0.06) |
| Voriconazole | 10 (5) | 10 (4) | 13 (4) | 3.8 (0.2) |
| VT-1161 | 99 (33) | 72 (26) | 65 (30) | 140 (10) |
Values are averages of 2 to 4 separate determinations, and standard deviations are given in parentheses.
With testosterone as the substrate.
With midazolam as the substrate.
DISCUSSION
A potential new antifungal drug candidate for the treatment of systemic Candida infection should ideally have high potency against the intended CaCYP51 target enzyme and minimal interaction with human CYP51 (and other off-target human CYP enzymes). VT-1161 meets both these criteria by binding tightly to CaCYP51 with a high affinity similar to that of other pharmaceutical azole antifungal agents and binding either extremely weakly or not at all to the host human CYP51 in vitro. Binding studies (Fig. 2 and 3) can provide useful preliminary information on the likely effectiveness of cyclized nitrogen-containing antifungal drug candidates inhibiting CYP51 activity. However, only IC50 determinations using a CYP51 reconstitution assay system can determine the functional activity of each compound as a CYP51 inhibitor.
The CYP51-antifungal agent IC50 determinations confirmed that VT-1161 was a strong inhibitor of CaCYP51 activity but did not significantly inhibit Δ60HsCYP51 activity at drug concentrations up to 50 μM, meeting the selection criteria of high potency against the fungal drug target (CaCYP51) and minimal if any activity against the human CYP51 enzyme. Therefore, VT-1161 is a good candidate for the treatment of systemic Candida infections and was subjected to testing for efficacy against wild-type clinical C. albicans strains. According to the MICs, the activity of VT-1161 was more potent than the Kd value for binding to CaCYP51, suggesting that the Morrison equation-calculated Kd value was an overestimate of the in vivo value in part due to the relatively high CYP51 protein concentrations required for in vitro binding studies and also that VT-1161 was readily taken up by the C. albicans cells to exert a potent effect. The VT-1161-induced reduction in ergosterol content was greater than that caused by an equal concentration of voriconazole, a desirable attribute for any new antifungal drug candidate in conjunction with the extremely high selectivity observed. Using a simplified equation relating percent inhibition and IC50 (37) and on the basis of the extent of inhibition (∼95%) of the sterol pathway at a VT-1161 concentration of ∼8 nM, the biochemical IC50 for inhibiting fungal CYP51 was estimated to be ≤1 nM.
When predicting a clinical drug-drug interaction (DDI) potential on the basis of in vitro CYP data, it has been postulated (38) that free drug concentrations should be used in the calculations. For example, fluconazole binds plasma protein poorly and largely exists as free drug in the blood (39). Thus, using in vitro potencies consistent with those listed in Table 3, fluconazole was predicted to have significant DDIs with substrates of CYP2C9, CYP2C19, and CYP3A4 (38). Similarly, when the high plasma protein binder itraconazole was modeled for DDIs with CYP3A4 substrates, it too was predicted to cause DDIs due to the high potency of CYP3A4 inhibition. Importantly, there were strong correlations between these modeling predictions and documented clinical DDIs (38). Given that VT-1161 binds human plasma protein strongly (>99%) (E. P. Garvey, W. J. Hoekstra, R. J. Schotzinger, J. A. Sobel, E. A. Lilly, and P. L. Fidel, unpublished data) and given the weak in vitro potencies for VT-1161, no DDIs were predicted for VT-1161 for these three CYPs shown in Table 3 (or for CYP1A2, CYP2B6, CYP2C8, and CYP2D6 on the basis of in vitro IC50 data not shown). It remains to be demonstrated in clinical DDI studies if these predictions will be borne out.
In summary, VT-1161 potently bound C. albicans CYP51 (Kd, ≤39 nM) with a type II difference spectrum indicative of a heme interaction and inhibited C. albicans CYP51 sterol 14α-demethylase activity in a manner consistent with tight-binding inhibition. In C. albicans cellular studies, VT-1161 nearly completely inhibited the sterol biosynthesis pathway at a concentration of 0.004 μg ml−1 (7.6 nM). In stark contrast, VT-1161 did not bind to human CYP51 at a concentration of 86 μM, nor did it inhibit human CYP51 activity at a concentration of 50 μM. Furthermore, VT-1161 inhibition of three critical human drug-metabolizing CYPs was weak (IC50s, ≥65 μM). Using an estimated biochemical IC50 of the fungal target of ≤1 nM and the very weak inhibition of human cytochrome P450 enzymes studied in this report, the selectivity of VT-1161 was ∼10,000 for its target compared to that for human liver CYPs. This degree of potency and selectivity of VT-1161 is markedly superior to that of approved CYP51 inhibitors, and the potential for a high degree of safety has been corroborated in preclinical and clinical phase I studies. The activity spectrum of VT-1161 against several pathogenic fungi (Candida albicans and non-albicans Candida species, Cryptococcus neoformans, endemic fungus, and mold species), including strains that possess intrinsic and acquired resistance to current azole antifungal therapeutics, has been investigated and will be reported elsewhere. VT-1161 is currently in phase IIa investigations of fungal infections and has the strong potential to significantly advance the field of fungal CYP51 therapeutics.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the Engineering and Physical Sciences Research Council National Mass Spectrometry Service Centre at Swansea University for assistance in GC-MS analyses and to OpAns, LLC (Durham, NC), for performing the CYP2C9, CYP2C19, and CYP3A4 assays.
This work was supported in part by the European Regional Development Fund/Welsh Government-funded BEACON research program and by Viamet Pharmaceuticals, Inc. (Durham, NC).
Footnotes
Published ahead of print 15 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03707-14.
REFERENCES
- 1.Nivoix Y, Leveque D, Herbrecht R, Koffel J-C, Beretz L, Ubeaud-Sequier G. 2008. The enzymatic basis of drug-drug interactions with systemic triazole antifungals. Clin. Pharmacokinet. 47:779–792. 10.2165/0003088-200847120-00003. [DOI] [PubMed] [Google Scholar]
- 2.Shafaati M, Mast N, Beck O, Nayef R, Heo GY, Bjorkhem-Bergman L, Lutjohann D, Bjorkhem I, Pkuleva IA. 2010. The antifungal drug voriconazole is an efficient inhibitor of brain cholesterol 24S-hydroxylase in vitro and in vivo. J. Lipid Res. 51:318–323. 10.1194/jlr.M900174-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kragie L, Turner SD, Patten CJ, Crespi CL, Stresser DM. 2002. Assessing pregnancy risks of azole antifungals using a high throughput aromatase inhibition assay. Endocr. Res. 28:129–140. 10.1081/ERC-120015045. [DOI] [PubMed] [Google Scholar]
- 4.Wang J-L, Chang C-H, Young-Xu Y, Chan KA. 2010. Systematic review and meta-analysis of the tolerability and heptotoxicity of antifungals in empirical and definitive therapy for invasive fungal infection. Antimicrob. Agents Chemother. 54:2409–2419. 10.1128/AAC.01657-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkateswarlu K, Denning DW, Manning NJ, Kelly SL. 1996. Reduced accumulation of drug in Candida krusei accounts for itraconazole resistance. Antimicrob. Agents Chemother. 40:2443–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eddouzi J, Parker JE, Vale-Silva LA, Coste A, Ischer F, Kelly S, Manai M, Sanglard D. 2013. Molecular mechanisms of drug resistance in clinical Candida species isolated from Tunisian hospitals. Antimicrob. Agents Chemother. 57:3182–3193. 10.1128/AAC.00555-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hull CM, Parker JE, Bader O, Weig M, Gross U, Warrilow AG, Kelly DE, Kelly SL. 2012. Facultative sterol uptake in an ergosterol-deficient clinical isolate of Candida glabrata harboring a missense mutation in ERG11 and exhibiting cross-resistance to azoles and amphotericin B. Antimicrob. Agents Chemother. 56:4223–4232. 10.1128/AAC.06253-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133–163. 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson MD. 2005. Changing patterns and trends in systemic fungal infections. J. Antimicrob. Chemother. 56(Suppl S1):i5–i11. 10.1093/jac/dki218. [DOI] [PubMed] [Google Scholar]
- 10.Sims CR, Ostrosky-Zeichner L, Rex JH. 2005. Invasive candidiasis in immunocompromised hospitalized patients. Arch. Med. Res. 36:660–671. 10.1016/j.arcmed.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Coste A, Selmecki A, Forche A, Diogo D, Bougnoux ME, d'Enfert C, Berman J, Sanglard D. 2007. Genotypic evolution of azole resistance mechanisms in sequential Candida albicans isolates. Eukaryot. Cell 6:1889–1904. 10.1128/EC.00151-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morio F, Loge C, Besse B, Hennequin C, Le Pape P. 2010. Screening for amino acid substitutions in the Candida albicans Erg11 protein of azole-susceptible and azole-resistant clinical isolates: new substitutions and a review of the literature. Diagn. Microbiol. Infect. Dis. 66:373–384. 10.1016/j.diagmicrobio.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki Y, Tokimatsu I, Sato Y, Kawasaki K, Sato Y, Goto T, Hashinaga K, Itoh H, Hiramatsu K, Kadota J. 2013. Association of sustained high plasma trough concentration of voriconazole with the incidence of hepatotoxicity. Clin. Chim. Acta 424:119–122. 10.1016/j.cca.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 14.Hoekstra WJ, Garvey EP, Moore WR, Rafferty SW, Yates CM, Schotzinger RJ. 2014. Design and optimization of highly-selective fungal CYP51 inhibitors. Bioorg. Med. Chem. Lett. 24:3455–3458. 10.1016/j.bmcl.2014.05.068. [DOI] [PubMed] [Google Scholar]
- 15.Warrilow AGS, Parker JE, Kelly DE, Kelly SL. 2013. Azole affinity of sterol 14α-demethylase (CYP51) enzymes from Candida albicans and Homo sapiens. Antimicrob. Agents Chemother. 57:1352–1360. 10.1128/AAC.02067-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lepesheva GI, Park HW, Hargrove TY, Vanhollebeke B, Wawrzak Z, Harp JM, Sundaramoorthy M, Nes WD, Pays E, Chaudhuri M, Villalta F, Waterman MR. 2010. Crystal structures of Trypanosoma brucei sterol 14α-demethylase and implications for selective treatment of human infections. J. Biol. Chem. 285:1773–1780. 10.1074/jbc.M109.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Wachenfeldt C, Richardson TH, Cosme J, Johnson EF. 1997. Microsomal P450 2C3 is expressed as a soluble dimer in Escherichia coli following modifications of its N-terminus. Arch. Biochem. Biophys. 339:107–114. 10.1006/abbi.1996.9859. [DOI] [PubMed] [Google Scholar]
- 18.Barnes HJ, Arlotto MP, Waterman MR. 1991. Expression and enzymatic activity of recombinant cytochrome P450 17α-hydroxylase in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 88:5597–5601. 10.1073/pnas.88.13.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamb DC, Kelly DE, Waterman MR, Stromstedt M, Rozman D, Kelly SL. 1999. Characteristics of the heterologously expressed human lanosterol 14α-demethylase (other names: P45014DM, CYP51, P45051) and inhibition of the purified human and Candida albicans CYP51 with azole antifungal agents. Yeast 15:755–763. . [DOI] [PubMed] [Google Scholar]
- 20.Lutz JD, Dixit V, Yeung CK, Dickmann LJ, Zelter A, Thatcher JA, Nelson WL, Isoherranen N. 2009. Expression and functional characterization of cytochrome P450 26A1, a retinoic acid hydroxylase. Biochem. Pharmacol. 77:258–268. 10.1016/j.bcp.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison JF. 1969. Kinetics of the reversible inhibition of enzyme-catalysed reactions by tight-binding inhibitors. Biochim. Biophys. Acta 185:269–286. 10.1016/0005-2744(69)90420-3. [DOI] [PubMed] [Google Scholar]
- 22.Venkateswarlu K, Lamb DC, Kelly DE, Manning NJ, Kelly SL. 1998. The N-terminal membrane domain of yeast NADPH-cytochrome P450 (CYP) oxidoreductase is not required for catalytic activity in sterol biosynthesis or in reconstitution of CYP activity. J. Biol. Chem. 273:4492–4496. 10.1074/jbc.273.8.4492. [DOI] [PubMed] [Google Scholar]
- 23.Venkateswarlu K, Denning DW, Manning NJ, Kelly SL. 1995. Resistance to fluconazole in Candida albicans from AIDS patients correlated with reduced intracellular accumulation of drug. FEMS Microbiol. Lett. 131:337–341. 10.1111/j.1574-6968.1995.tb07797.x. [DOI] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A3, 3rd ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 25.Parker JE, Merkamm M, Manning NJ, Pompon D, Kelly SL, Kelly DE. 2008. Differential azole antifungal efficacies contrasted using a Saccharomyces cerevisiae strain humanized for sterol 14 alpha-demethylase at the homologous locus. Antimicrob. Agents Chemother. 52:3597–3603. 10.1128/AAC.00517-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng S, Clancy CJ, Nguyen KT, Clapp W, Nguyen H. 2007. A Candida albicans petite mutant strain with uncoupled oxidative phosphorylation overexpresses MDR1 and has diminished susceptibility to fluconazole and voriconazole. Antimicrob. Agents Chemother. 51:1855–1858. 10.1128/AAC.00182-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martel CM, Parker JE, Bader O, Weig M, Gross U, Warrilow AGS, Kelly DE, Kelly SL. 2010. A clinical isolate of Candida albicans with mutations in ERG11 (encoding sterol 14α-demethylase) and ERG5 (encoding C22-desaturase) is cross-resistant to azoles and amphotericin B. Antimicrob. Agents Chemother. 54:3578–3583. 10.1128/AAC.00303-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jefcoate CR, Gaylor JL, Calabrese RL. 1969. Ligand interactions with cytochrome P450. I. Binding of primary amines. Biochemistry 8:3455–3463. [DOI] [PubMed] [Google Scholar]
- 29.Jefcoate CR. 1978. Measurement of substrate and inhibitor binding to microsomal cytochrome P-450 by optical-difference spectroscopy. Methods Enzymol. 52:258–279. 10.1016/S0076-6879(78)52029-6. [DOI] [PubMed] [Google Scholar]
- 30.Strushkevich N, Usanov SA, Park H-W. 2010. Structural basis of human CYP51 inhibition by antifungal azoles. J. Mol. Biol. 397:1067–1078. 10.1016/j.jmb.2010.01.075. [DOI] [PubMed] [Google Scholar]
- 31.Locuson CW, Hutzler JM, Tracy TS. 2007. Visible spectra of type II cytochrome P450-drug complexes: evidence that “incomplete” heme coordination is common. Drug Metab. Dispos. 35:614–622. 10.1124/dmd.106.012609. [DOI] [PubMed] [Google Scholar]
- 32.Kelly SL, Lamb DC, Kelly DE, Loeffler J, Einsele H. 1996. Resistance to fluconazole and amphotericin B in Candida albicans from AIDS patients. Lancet 348:1523–1524. [DOI] [PubMed] [Google Scholar]
- 33.Watson PF, Rose ME, Ellis SW, England H, Kelly SL. 1989. Defective sterol C5-6 desaturation and azole resistance: a new hypothesis for the mode of action of azole antifungals. Biochem. Biophys. Res. Commun. 164:1170–1175. 10.1016/0006-291X(89)91792-0. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Ramamoorthy Y, Kilicarslan T, Nolte H, Tyndale RF, Sellers EM. 2002. Inhibition of cytochromes P450 by antifungal imidazole derivatives. Drug Metab. Dispos. 30:314–318. 10.1124/dmd.30.3.314. [DOI] [PubMed] [Google Scholar]
- 35.Niwa T, Shiraga T, Takagi A. 2005. Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol. Pharm. Bull. 28:1805–1808. 10.1248/bpb.28.1805. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Pillai VC, Mada SR, Strom S, Venkataramanan R. 2012. Effect of voriconazole and other azole antifungal agents on CYP3A activity and metabolism of tacrolimus in human liver microsomes. Xenobiotica 42:409–416. 10.3109/00498254.2011.631224. [DOI] [PubMed] [Google Scholar]
- 37.Gao F, Johnson DL, Ekins S, Janiszewski J, Kelly KG, Meyer RD, West M. 2002. Optimizing higher throughput methods to assess drug-drug interactions for CYP1A2, CYP2C9, CYP2C19, CYP2D6, rCYP2D6, and CYP3A4 in vitro using a single point IC(5). J. Biomol. Screen. 7:373–382. 10.1089/108705702320351231. [DOI] [PubMed] [Google Scholar]
- 38.Obach RS, Walsky RL, Venkatakrishnan K, Gaman EA, Houston JB, Tremaine LM. 2006. The utility of in vitro cytochrome P450 inhibition data in the prediction of drug-drug interactions. J. Pharmacol. Exp. Ther. 316:336–348. 10.1124/jpet.105.093229. [DOI] [PubMed] [Google Scholar]
- 39.Humphrey MJ, Jevons S, Tarbit MH. 1985. Pharmacokinetic evaluation of UK-49,858, a metabolically stable triazole antifungal drug, in animals and humans. Antimicrob. Agents Chemother. 28:648–653. 10.1128/AAC.28.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.