Abstract
The antituberculosis (anti-TB) drug rifampin (RIF) binds to the beta subunit of the RNA polymerase (RpoB) of Mycobacterium tuberculosis, but the bactericidal responses triggered after target interaction are not known. To evaluate whether RIF induced an oxidative burst, lysates of RIF-treated M. tuberculosis were tested for determination of reactive oxygen species (ROS) by the electron paramagnetic resonance (EPR) technique using 1-hydroxy-3-carboxy-pyrrolidine (CPH) and 5,5-dimethyl-1-pyrrolidine-N-oxide (DMPO) as spin traps. M. tuberculosis killing by RIF stimulated an increase in the rate of formation of the CPH radical (CP·). Lysate pretreatment with the O2·− and ·OH scavengers superoxide dismutase (SOD) and thiourea (THIO), respectively, or with the metal chelator diethylene triamine pentaacetic acid (DTPA) inhibited CP· formation, arguing in favor of a metal-catalyzed ROS response. Formation of CP· did not increase following treatment of RIF-resistant strains with RIF, indicating that the ROS were induced after RpoB binding. To identify the ROS formed, lysates of RIF-treated bacilli were incubated with DMPO, a spin trap specific for ·OH and O2·−, with or without pretreatment with SOD, catalase, THIO, or DTPA. Superoxide dismutase, catalase, and THIO decreased formation of the DMPO-OH adduct, and SOD plus DTPA completely suppressed it, suggesting that RIF activated metal-dependent O2·−-mediated mechanisms producing ·OH inside tubercle bacilli. The finding that the metal chelator DTPA reduced the bactericidal activity of RIF supported the possibility that ·OH was generated through these mechanisms and that it participated at least in part in M. tuberculosis killing by the drug.
INTRODUCTION
Tuberculosis (TB), an infectious disease caused by the bacillus Mycobacterium tuberculosis, remains one of the most important public health problems, particularly in low- and middle-income countries. Current treatment for TB includes administration of first-line drugs isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), and ethambutol for 2 months, followed by RIF and INH for 4 months. While molecular targets of these drugs are known (1), less information has been reported on the cellular responses triggered after target interaction. In Gram-negative and -positive bacteria, it is known that bactericidal antibiotics induced a mechanism of cellular death stimulating the production of reactive oxygen species (ROS) (2–5). The most common ROS include superoxide anion (O2·−), hydrogen peroxide (H2O2), and hydroxyl radical (·OH) (6). Using the dye hydroxyphenyl fluorescein (HPF) and flow cytometric enumeration, it was found that aminoglycosides, quinolones, and beta-lactams induced ·OH in Escherichia coli and Staphylococcus aureus through pathways involving alterations in central and iron metabolism driving the Fenton reaction (2). This reaction leads to formation of ·OH through the reduction of H2O2 by ferrous iron and causes damage to DNA, proteins, and lipids that ultimately results in the death of the bacterial cell. Using HPF, INH and PZA were shown to induce ·OH formation also in M. tuberculosis, leading to release of products that stimulated host cell ROS and autophagy (7, 8). By another fluorescent dye, M. tuberculosis was shown to be extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction (9). Noticeably, mycobacterial persisters, a small bacterial population refractory to antibiotic killing, were eradicated after treatment with ·OH-generating antibiotics (10).
However, detection of ROS by cell-penetrating dyes such as HPF, which are oxidized more quickly inside antibiotic-treated bacteria, has been recently questioned (11, 12). For this reason, in this work the ROS formation was determined by electron paramagnetic resonance (EPR) spin trapping, a technique of great specificity and sensitivity for the measurement of free radicals (6, 13, 14). Spin trapping reagents react with short-lived radicals, which are subsequently changed to long-lived radicals called spin adducts. The advantage of EPR over other techniques is that it allows the detection and identification of free radicals by observation of the EPR spectrum of a spin adduct and the determination of the rate of radical formation. The EPR spin trapping technique is a semiquantitative or quantitative method successfully applied to detect various types of radicals, from protein radicals to small molecules.
By using EPR spin trapping, it was shown that the bactericidal drug INH induced reactive intermediates and secondary free radicals that brought about the activity of this agent (15–17). EPR studies were also performed to investigate redox cycling and activity of rifamycin SV in Escherichia coli (18–20), but no major information was given for the rifamycin SV-derived RIF, the companion drug of INH currently used for anti-TB therapy. It is known that RIF inhibits M. tuberculosis transcription by binding to the beta subunit of the RNA polymerase (RpoB) encoded by the rpoB gene (21, 22), but the cellular responses triggered after inhibition of mRNA synthesis are not known. In this study, by using the EPR technique, we found that RIF induced ·OH formation through a metal-dependent O2·−-mediated reaction. This and other observations may provide helpful insight into the role of ROS in the anti-TB activity of RIF.
MATERIALS AND METHODS
Microorganisms and measurement of drug activity.
M. tuberculosis strains were grown in 20- by 125-mm screw-cap tubes containing Dubos Tween-albumin (DTA) broth prepared from Dubos broth base and Dubos medium albumin (Difco, Detroit, MI) and stirred at 250 rpm with 8-mm magnetic bars at 37°C under a humidified 5% CO2 atmosphere (23). The following M. tuberculosis strains were used: RIF-susceptible H37Rv (ATCC 27294), RIF-resistant H37Rv (ATCC 35838) having the rpoB mutation Ser531Leu (24), two RIF-susceptible clinical isolates (strains 5502 and 5050), and three RIF-resistant clinical isolates having the rpoB mutations Ser531Leu (strains 7225 and 4164) and His526Tyr (strain 3063).
To determine drug activity and ROS production, tubercle bacilli (optical density at 600 nm of about 0.3) were incubated for 1 and 3 days in the presence of RIF (Sigma Chemicals, St. Louis, MO), at concentrations ranging from 0.125 μg/ml (MIC) (25) to 8 μg/ml (maximum concentration of drug in serum [Cmax]) (23). In some experiments, bacilli were treated for 3 days with 4 μg/ml (Cmax) of moxifloxacin (MX) or 4 μg/ml (Cmax) of the bacteriostatic agent ethambutol (EMB) (23). One milliliter of culture was washed and resuspended in 1 ml of DTA broth, and 0.2 ml of serial 10-fold dilutions was inoculated on Middlebrook 7H10 agar plates for CFU determination; the number of colonies were counted after 3 weeks of incubation at 37°C under a humidified 5% CO2 atmosphere.
Characterization of ROS by EPR.
Tubercle bacilli (6-ml cultures) were collected by centrifugation (1,500 × g for 30 min) and were then washed and resuspended in 1 ml of phosphate-buffered saline (PBS). The samples were transferred into 1.5-ml screw-cap tubes containing approximately 0.5 ml of 0.1-mm zirconia/silica beads and passed at maximum speed through 6 cycles of 50 s each with a 60-s rest on ice between pulses by using a Mini-Beadbeater-8 apparatus (Bio-Spec Products Inc., Bartlesville, OK, USA) to lyse M. tuberculosis cells; this treatment caused a 6.3-log10 CFU/ml decrease.
The lysates were collected and filtered through membranes (pore size of 0.22 μm; Millipore, Malsheim, France). To characterize the ROS formed, 100 μl of lysates was mixed with different EPR spin trapping reagents, including 1-hydroxy-3-carboxy-pyrrolidine (CPH), a reagent detecting different reactive oxidizing species (14, 26), and 5,5-dimethyl-1-pyrrolidine-N-oxide (DMPO), a spin trap more specific than CPH for detecting ·OH and O2·− (13, 20, 27, 28), at the following final concentrations: CPH, 0.5 mM; DMPO, 0.1 M. Samples were then drawn up into a gas-permeable Teflon tube with a 0.81-mm internal diameter and a 0.05-mm wall thickness (Zeus Industrial Products, Raritan, NJ) for EPR measurements.
To be sure that a genuine radical signal was observed, the lysates were treated with CPH or DMPO with or without the addition of the scavengers superoxide dismutase (SOD), thiourea (THIO), catalase (CAT) (for O2·−, ·OH, H2O2, respectively), and the metal chelator diethylene triamine pentaacetic acid (DTPA) (3, 13), at the following final concentrations: SOD, 10 μg/ml; THIO, 100 mM; CAT, 10 μg/ml; DTPA, 1 mM.
EPR spectra were measured at 37°C on a Bruker ECS 106 spectrometer (Bruker, Rheinstetten, Germany) equipped with a variable-temperature unit (ER4111VT). The Teflon tube was folded four times, inserted into a quartz tube, and fixed to the EPR cavity (4108 TMH). The gas flow was air or pure N2 as indicated. For samples containing CPH, the low field shoulder was chosen to quantify the CPH radical (the nitroxyl 3-carboxy-proxyl radical [CP·]) (14), because the middle component centered at g 2.0 overlaps with many other free radical signals found in biological systems. The kinetics of CP· formation was monitored at intervals of 2 min for 20 min, with the first spectrum being acquired exactly 2 min after CPH addition. During the chosen acquisition time, the rate of CP· formation was linear and expressed as μM CP· formed/min (14). To quantify the ROS formation, a calibration curve was obtained by using standard solutions of CP·. For samples containing DMPO (0.1 M), the first spectrum was acquired exactly 2 min after spin trap addition. All spectra were corrected for baseline drift by a linear function of the software supplied by Bruker (ESP 1600 data system). Spectrometer conditions common to all spectra were as follows: modulation frequency, 100 kHz; microwave frequency, 9.4 GHz; microwave power, 20 mW; modulation amplitude, 0.1 mT. The other specific spectrometer conditions were as follows: gain, 1 × 104 (CPH) or 5 × 104 (DMPO); conversion time, 20.5 ms (CPH) or 82 ms (DMPO); time constant, 82 ms (CPH) or 163 ms (DMPO); sweep time, 21 s (CPH) or 84 s (DMPO); number of scans, 2 (CPH) or 20 (DMPO).
Protection assay.
To demonstrate that ·OH formation contributed to M. tuberculosis killing, RIF was added to the cultures either alone or in the presence of 1 mM DTPA. After 1 and 3 days of incubation, 0.2 ml of serial 10-fold-diluted cultures was inoculated in Middlebrook 7H10 agar plates for CFU determination, as described above.
Statistical method.
The significance of the differences between CFU counts and rate of CP· formation was assessed by a two-tailed Student t test. P values of ≤0.05 were considered significant.
RESULTS
M. tuberculosis killing by RIF induced formation of radicals.
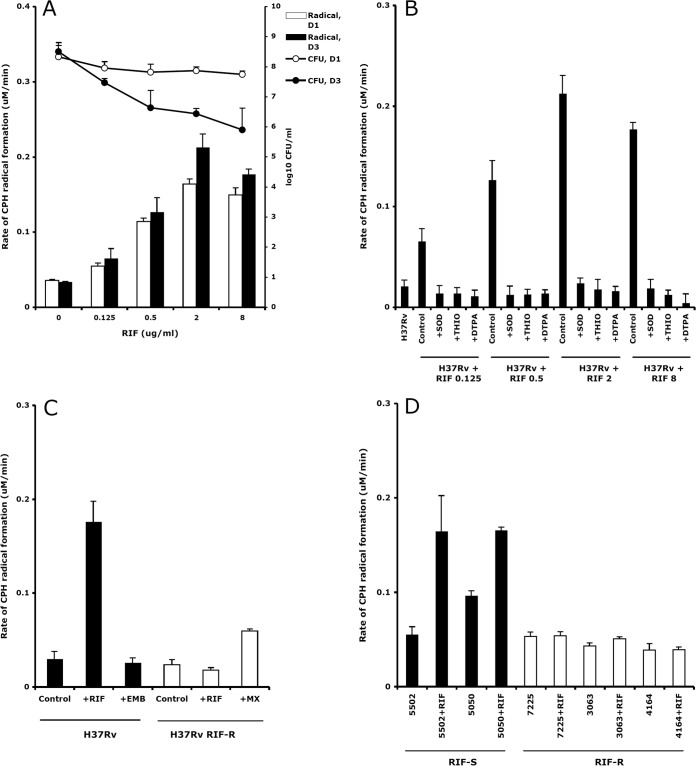
The numbers of CFU and the rate of formation of CP· after treatment of the RIF-susceptible M. tuberculosis strain H37Rv with RIF concentrations increasing from 0.125 μg/ml to 8 μg/ml for 1 and 3 days are shown in Fig. 1A. Rifampin killed tubercle bacilli in a dose-response fashion, with the number of CFU decreasing from 57% to 75% on day 1 and from 85% to 99% on day 3. The rate of CP· formation on days 1 and 3 increased 4.7 and 6.2 times at 2 μg/ml RIF, respectively, and then slightly decreased at 8 μg/ml.
FIG 1.
(A) Killing of M. tuberculosis H37Rv by RIF generated an increase in the rate of CPH radical formation. Shown are M. tuberculosis survival (log10 CFU/ml) and rate of CPH radical formation (μM/min) after 1 day (D1) and 3 days (D3) of treatment with RIF. Means and standard deviations (SD) from three experiments are shown. (B) Rate of CPH radical formation (μM/min ± SD) after 3 days of incubation of M. tuberculosis H37Rv alone (H37Rv) and in the presence of 0.125, 0.5, 2, 8 μg/ml RIF, with or without pretreatment of the lysates with 10 μg/ml SOD, 100 mM THIO, 1 mM DTPA. (C) Rate of CPH radical formation (μM/min ± SD) after 3 days of incubation of M. tuberculosis H37Rv with 8 μg/ml RIF or 4 μg/ml EMB and of RIF-resistant H37Rv (H37Rv RIF-R) with 8 μg/ml RIF or 4 μg/ml MX. (D) Rate of CPH radical formation (μM/min ± SD) after 1 day of incubation of RIF-susceptible (strains 5502 and 5050) and RIF-resistant (strains 7225, 3063, and 4164) clinical isolates, alone and in the presence of 8 μg/ml RIF. RIF-S, RIF susceptible; RIF-R, RIF resistant.
To confirm the occurrence of a genuine radical signal, the rate of CP· formation in the lysates of RIF-untreated and RIF-treated (0.125, 0.5, 2, and 8 μg/ml) H37Rv strains was determined by performing competition experiments with the O2·− and ·OH scavengers SOD and THIO, respectively (Fig. 1B). The presence of transition metals in the M. tuberculosis lysates was evaluated by measuring the rate of CP· formation after adding the chelating agent DTPA. Superoxide dismutase and THIO completely inhibited the CP· increase induced by RIF concentrations ranging from 0.125 to 8 μg/ml, suggesting that a broad RIF-dependent induction of O2·− and ·OH, respectively, occurred. Under these conditions, DTPA fully inhibited CP· formation, strongly indicating that RIF induced a ROS response through a metal-dependent O2·−-mediated reaction.
Rifampin induced formation of radicals after rpoB binding.
The knowledge that in other microorganisms bactericidal drugs induced harmful ·OH after drug-target interaction prompted us to measure the rate of CP· formation after treatment of RIF-susceptible and -resistant (rpoB-mutated) strains with RIF (Fig. 1C and D). The drug concentration used to perform these and the next experiments was 8 μg/ml. Following RIF exposure, the CP· signal increased in the H37Rv strain (P < 0.05 compared to untreated bacilli) but not in the RIF-resistant H37Rv strain (H37Rv RIF-R), in comparison with RIF-untreated controls (Fig. 1C). No increase in the rate of CP· formation was observed after treatment of H37Rv with EMB. Instead, the rate of CP· formation increased after treatment of H37Rv RIF-R with MX (P < 0.05 compared to untreated bacilli).
To confirm the observations obtained with the reference strains, the rate of CP· formation was measured in M. tuberculosis clinical isolates (Fig. 1D). Again, while after treatment with RIF the CP· signal increased in RIF-susceptible strains (strains 5502 and 5050) (P < 0.05 compared to untreated bacilli), no difference between RIF-treated and RIF-untreated RIF-resistant strains (strains 7225, 3063, 4164) was observed. Overall, these observations indicated that the ROS were generated after binding of RIF to the RpoB target.
Rifampin induced metal-dependent O2·−-mediated formation of ·OH.
To identify the ROS formed, the lysates of RIF-exposed M. tuberculosis H37Rv were treated with DMPO (Fig. 2). Spectrum A showed that, in the absence of RIF, the addition of DMPO resulted in the formation of the 1:2:2:1 quartet with hyperfine splitting constants (aH = aN = 14.9 G) characteristic of the DMPO-hydroxyl (DMPO-OH) adduct (20, 27, 28). In RIF-exposed H37Rv lysates, the intensity of the DMPO-OH adduct consistently increased with respect to that shown in the absence of the drug (spectrum B). Moreover, a weak six-line spectrum with hyperfine splitting constants of aN = 15.4 G and aβH = 23 G was concomitantly detected. The latter adduct could be attributed to the trapping of a carbon-centered radical likely generated via the oxidation of an impurity of commercial DMPO, as previously reported (29). To investigate the source of DMPO-OH adduct formation, RIF-exposed M. tuberculosis lysates were incubated for 10 min before DMPO addition with the ROS scavengers SOD and CAT (for O2·− and H2O2, respectively) and THIO (for ·OH). Superoxide dismutase and CAT (spectra C and D, respectively) efficiently inhibited the RIF-dependent increase of DMPO-OH adducts, whose levels were comparable to those measured in the absence of the drug (compare spectra A, C, and D). In the presence of THIO, no EPR-detectable signals were observed (spectrum E), suggesting that both RIF-dependent and -independent (RIF-untreated) mechanisms generated ·OH. The contribution of metals to the DMPO-OH formation was suggested by the lack of adduct formation when the metal chelator DTPA was added simultaneously to SOD (spectrum F). The detection of DMPO-OH signal does not always prove that ·OH is produced in the experimental system. DMPO-OH can indeed arise from either direct trapping of authentic ·OH or by decomposition of adduct with O2·− (DMPO-O2H), the half-life of which is less than 1 min. To unequivocally establish the ·OH formation, it is essential to perform kinetic-based competition experiments in the presence of ·OH scavengers. These compounds efficiently compete with DMPO for ·OH, forming carbon-centered radicals that can subsequently be trapped by DMPO (28). When the ·OH scavenger ethanol was added to RIF-exposed M. tuberculosis lysates, the formation of DMPO-OH was markedly inhibited (spectrum G). Concomitantly, the characteristic six-line DMPO adduct with hydroxyethyl radical DMPO-CHCH3OH was formed (aN = 15.4 G and aβH = 22.7 G) (28), superimposed to the weak preexistent six-line spectrum (compare spectra B and G). Finally, adduct formation was completely dependent on dissolved oxygen, as shown by the lack of any detectable signal when lysates were bubbled with 100% N2 for 15 min before the addition of DMPO (spectrum H). No adduct was formed by DMPO in PBS (spectrum I). Collectively, these results indicated that RIF promoted ·OH formation in M. tuberculosis through metal-dependent, O2·−-mediated mechanisms.
FIG 2.
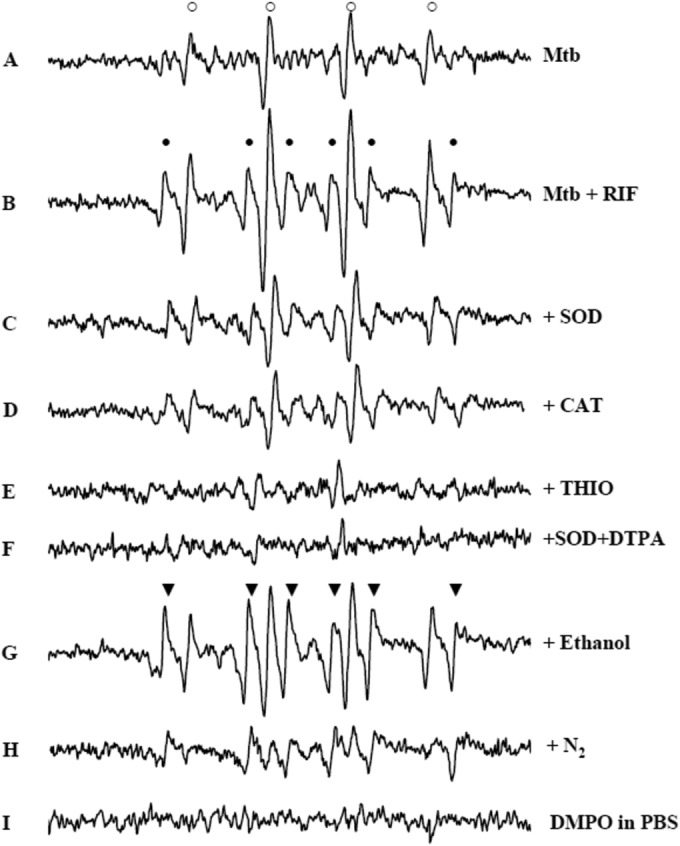
EPR spectra of DMPO adducts obtained in RIF-treated and -untreated M. tuberculosis suggest ·OH involvement. DMPO (0.1 M) was added to lysates of M. tuberculosis H37Rv grown for 3 days in the absence of RIF (spectrum A) or in the presence of 8 μg/ml RIF (spectrum B). Lysates from samples grown in the presence of RIF were treated with 10 μg/ml SOD (spectrum C), 10 μg/ml CAT (spectrum D), 100 mM THIO (spectrum E), 10 μg/ml SOD and 1 mM DTPA (spectrum F), or 1% ethanol (spectrum G) or treated in hypoxic conditions by 15 min N2 bubbling (spectrum H). No signals were detected in PBS in the absence of M. tuberculosis lysates (spectrum I). Adduct attribution: DMPO-OH (aH = aN = 14.9 G; marked with ○), DMPO oxidation product (aN = 15.4 G and aβH = 23 G; marked with ●), and DMPO-CHCH3OH (aN = 15.4 G and aβH = 22.7 G; marked with ▼). M. tuberculosis lysates were incubated with scavengers at 37°C, 10 min before DMPO addition. Spectra were acquired at 37°C, 2 min after DMPO addition. For instrument settings, see Materials and Methods. The EPR spectra obtained after 1 day of incubation with RIF were superimposable to those obtained after 3 days shown here. Mtb, M. tuberculosis.
The metal chelator DTPA protected M. tuberculosis against killing by RIF.
To further ascertain whether metal-dependent mechanisms were involved in the killing of M. tuberculosis H37Rv by RIF, we determined the numbers of CFU/ml after 1 and 3 days of treatment with RIF, at concentrations increasing from 0.125 to 8 μg/ml, both alone and in the presence of the metal chelator DTPA (Fig. 3). While no CFU differences were observed between RIF and RIF-DTPA after 1 day of incubation, treatment with RIF-DTPA for 3 days reduced killing activity of RIF by 0.3 to 0.7 log10. DTPA alone did not inhibit bacterial growth, because no major CFU differences between RIF and RIF-DTPA were observed in RIF-untreated cells (0 μg/ml RIF).
FIG 3.
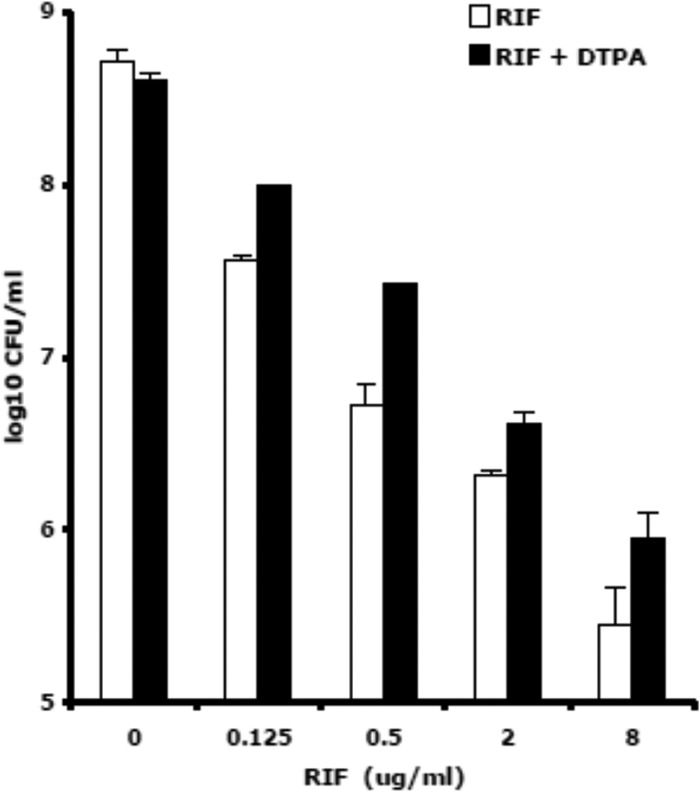
Killing of M. tuberculosis strain H37Rv by RIF was reduced by the metal chelator DTPA. Shown is survival (log10 CFU/ml ± SD) after 3 days of treatment with 8 μg/ml RIF and 8 μg/ml RIF plus 1 mM DTPA. A representative experiment out of three is shown.
DISCUSSION
Rifamycins are a group of antibiotics first isolated in 1957 from Nocardia mediterranei as a mixture of components designated A, B, C, D, and E (30). Chemical modifications of rifamycin B led to the discovery of rifamycin SV and of its derivative RIF, introduced for oral treatment of pulmonary TB in 1971. Inhibition of DNA-dependent RNA polymerase seems to be the common mechanism for all antibacterial rifamycins, and the binding site of the drug is located at the beta subunit of the RNA polymerase encoded by the rpoB gene (21, 31). However, few data have been reported on the cellular responses that occur as a consequence of drug-target interaction. In studies on the activity of rifamycin SV against E. coli, the ·OH was not detected by flow cytometry with HPF (2) but was found by EPR techniques and was suggested to participate in the inhibition of bacterial growth (18). To our knowledge, no study on the ROS generated following treatment of M. tuberculosis with the pivotal anti-TB drug RIF has been reported. Here, we present data indicating that ·OH was induced by RIF, which contributes to M. tuberculosis killing.
To set up the EPR protocol, we performed preliminary experiments with CPH. First, no differences in the baseline rate of CP· formation were observed between RIF-containing and no-RIF culture medium. Furthermore, we found that CPH was unable to penetrate inside RIF-treated and -untreated M. tuberculosis. Indeed, the rate of CP· formation increased after the addition of CPH to lysates of cultures treated for 1 or 3 days with RIF but not to lysates in which CPH was added to the cultures together with RIF on day 0. Finally, to exclude that the level of CP· signal was due to limited CPH availability, time course experiments were performed with 0.5, 1, and 2 mM CPH. No differences between these concentrations were observed; therefore, 0.5 mM CPH was used throughout the study.
The rate of CP· formation was measured after exposure of M. tuberculosis to increasing concentrations of RIF. The highest CP· levels were seen after 1 day of treatment, due to consistent (57 to 75%) killing, and then the rate of CP· formation increased slowly on day 3, in keeping with the lower number of bacilli remaining. A decrease in the rate of CP· formation was observed when the RIF concentration increased from 2 to 8 μg/ml; a similar observation was seen in norfloxacin-treated E. coli (12).
Competition experiments with scavengers and pretreatment with a metal chelating agent confirmed that a genuine radical response was induced by various RIF concentrations. Because ·OH can be produced by the interaction of O2·−, H2O2, and metal ions through the Fenton and/or the Haber-Weiss reactions (6, 9), inhibition of the CP· signal by SOD indicated that O2·− was generated and likely participated in ·OH formation. The occurrence of ·OH production through these reactions was also suggested by inhibition of the CP· signal with the iron chelator DTPA and with THIO, a ·OH scavenger decreasing the damaging effect of this radical (2–5). Overall, these data indicated that the interaction between RIF and M. tuberculosis triggered an ROS response, including metal-dependent O2·−-mediated production of ·OH.
To explore the relationship between ROS production and interaction of RIF with its target, the rate of CP· formation in RIF-susceptible and -resistant (rpoB-mutated) strains was measured. In the drug-susceptible strain H37Rv, the CP· signal was triggered by RIF but not by the bacteriostatic agent EMB, in keeping with previous observations suggesting that ROS production is specific for bactericidal antibiotics (2). The ROS were generated only after interaction between RIF and its target RpoB, because in drug-resistant strains (both H37Rv RIF-R and clinical isolates), RIF, but not MX, a bactericidal agent targeting DNA gyrase (1, 23), failed to trigger CP· formation. These observations also exclude that ROS were produced intracellularly by auto-oxidation of RIF, as mentioned in previous studies (18).
A further demonstration that RIF stimulated ·OH formation was obtained by performing EPR experiments with DMPO. Drug-untreated M. tuberculosis generated a spectrum characteristic of DMPO-OH spin adduct formed after ·OH trapping by DMPO (20, 27, 28). After RIF exposure, the intensity of DMPO-OH signal substantially increased in comparison with that of untreated M. tuberculosis. The RIF-dependent signal was reduced to the level of the drug-untreated control by SOD and CAT, indicating that O2·− and/or H2O2 were essential for the generation of the DMPO-OH adduct and that its formation likely occurred after their production. Both RIF-dependent and -independent (RIF-untreated) ·OH levels were strongly suppressed by THIO, in keeping with inhibition of radical formation by THIO observed by using CPH. Finally, when SOD and DTPA were added to lysates, a complete inhibition of the DMPO-OH signal was seen, strongly arguing in favor of a metal catalysis. A further demonstration that the DMPO-OH detected in the above-described systems was due to ·OH trapping by DMPO was that lysate treatment with the ·OH scavenger ethanol generated a spectrum typical of the hydroxyethyl radical (28). Overall, EPR data indicated that RIF induced ROS formation in M. tuberculosis and that the DMPO adduct formed was due to the trapping of ·OH. On the basis of these data, we hypothesized that RIF stimulated ·OH formation by pathways involving the Fenton and/or the Haber-Weiss reactions that are known to occur in M. tuberculosis (6, 9).
The finding that the metal chelator DTPA reduced at least in part the bactericidal activity of RIF supported this hypothesis. While no effect was seen after 1 day of incubation with the drug, likely due to low penetration of DTPA inside M. tuberculosis cells, this compound reduced the killing activity of RIF on day 3, confirming intracellular activation of metal-dependent mechanisms. On the other hand, 3-day treatment of M. tuberculosis with 150 mM THIO (10) did not inhibit bacterial growth, and 3-day cotreatment with 8 μg/ml RIF and 150 mM THIO did not protect M. tuberculosis cells from drug effect (P ≥ 0.20, seven experiments). These results were likely due to lack of penetration of THIO inside M. tuberculosis, because no difference in the rate of CP· formation was seen between RIF and RIF-THIO-treated bacilli (unpublished data).
Overall, our observations on the tuberculocidal activity of RIF are in keeping with studies using flow cytometry showing that in Gram-negative and -positive microorganisms, the bactericidal antibiotics induced formation of toxic ·OH after drug-target interaction (2, 4, 5, 7, 10). This view was recently challenged by studies in E. coli showing that a shift in HPF fluorescence and protection by THIO occurred also under anaerobic conditions (11, 12) and that O2·− had both protective and detrimental roles in response to antibiotics (32). Here, we determined the ROS levels by EPR, a technique more specific and sensitive than fluorescence for measurement of radicals, and used two different spin trapping reagents to detect and identify the ROS formed. Collectively, we showed that RIF induced ·OH in M. tuberculosis and that a metal chelator reduced at least in part the bactericidal activity of RIF. These results are in keeping with the knowledge that mycobacterial persisters were eradicated by antibiotic-generated ·OH (10) and that M. tuberculosis was sensitive to killing by a vitamin C-induced Fenton reaction (9). In our study, we detected ROS after bacterial lysis; thus, we cannot exclude the possibility that ROS were produced in the lysates from substrates produced intracellularly as a result of the rpoB-rifampin interaction.
However, the contribution of ROS to antimicrobial activity may be more complex than initially envisioned (2, 22), requiring the examination of different microorganisms, drugs, and experimental conditions (11, 12, 32, 33). The recent observation that drugs accelerate oxidative stress in M. tuberculosis within infected macrophages is in keeping with this view (34). Apart from this, we think that the EPR technique can be a powerful tool for studying free radicals and should be considered when investigating the activity of anti-TB drugs and their combinations.
ACKNOWLEDGMENTS
This work was supported in part by the European project StopLATENT-TB, grant agreement 200999.
We thank Armand van Deun, Institute of Tropical Medicine, Antwerp, Belgium, Coordinator of the WHO Supranational Reference Laboratory (SRL) network, for sending the five M. tuberculosis clinical isolates with known rpoB mutations to L. Fattorini (SRL of Rome) for annual proficiency testing of anti-TB drugs.
Footnotes
Published ahead of print 6 October 2014
REFERENCES
- 1.Zumla A, Nahid P, Cole ST. 2013. Advances in the development of new tuberculosis drugs and treatment regimens. Nat. Rev. Drug Discov. 12:388–404. 10.1038/nrd4001. [DOI] [PubMed] [Google Scholar]
- 2.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810. 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Zhao X. 2009. Contribution of oxidative damage to antimicrobial lethality. Antimicrob. Agents Chemother. 53:1395–1402. 10.1128/AAC.01087-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Liu X, Qu Y, Wang X, Li L, Zhao X. 2012. Inhibitors of reactive oxygen species accumulation delay and/or reduce the lethality of several antistaphylococcal agents. Antimicrob. Agents Chemother. 56:6048–6050. 10.1128/AAC.00754-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampson TR, Liu X, Schroeder MR, Kraft CS, Burd EM, Weiss DS. 2012. Rapid killing of Acinetobacter baumannii by polymyxins is mediated by a hydroxyl radical death pathway. Antimicrob. Agents Chemother. 56:5642–5649. 10.1128/AAC.00756-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohno M. 2010. Applications of electron spin resonance spectrometry for reactive oxygen species and reactive nitrogen species research. J. Clin. Biochem. Nutr. 47:1–11. 10.3164/jcbn.10-13R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JJ, Lee HM, Shin DM, Kim W, Yuk JM, Jin HS, Lee SH, Cha GH, Kim JM, Lee ZW, Shin SJ, Yoo H, Park YK, Park JB, Chung J, Yoshimori T, Jo EK. 2012. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe 11:457–468. 10.1016/j.chom.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Goletti D, Petruccioli E, Romagnoli A, Piacentini M, Fimia GM. 2013. Autophagy in Mycobacterium tuberculosis infection: a passepartout to flush the intruder out? Cytokine Growth Factor Rev. 24:335–343. 10.1016/j.cytogfr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Vilchèze C, Hartman T, Weinrick B, Jacobs WR. 2013. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat. Commun. 10.1038/ncomms2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant SS, Kaufmann BB, Chand NS, Haseley N, Hung DT. 2012. Eradication of bacterial persisters with antibiotic-generated hydroxyl radicals. Proc. Natl. Acad. Sci. U. S. A. 109:12147–12152. 10.1073/pnas.1203735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Imlay JA. 2013. Cell death from antibiotics without the involvement of reactive oxygen species. Science 339:1210–1213. 10.1126/science.1232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keren I, Wu Y, Inocencio J, Mulcahy LR, Lewis K. 2013. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science 339:1213–1216. 10.1126/science.1232688. [DOI] [PubMed] [Google Scholar]
- 13.Buettner GR, Mason R. 2003. Spin-trapping methods for detecting superoxide and hydroxyl free radicals in vitro and in vivo, p 27–38 In Cutler RG, Rodriguez H. (ed), Critical reviews of oxidative stress and aging: advances in basic science, diagnostics and intervention, vol 1 World Scientific, Hong Kong, China. [Google Scholar]
- 14.Straface E, Marchesi A, Gambardella L, Metere A, Tarissi de Jacobis I, Viora M, Giordani L, Villani A, Del Principe D, Malorni W, Pietraforte D. 2012. Does oxidative stress play a critical role in cardiovascular complications of Kawasaki disease? Antioxid. Redox Signal. 17:1441–1446. 10.1089/ars.2012.4660. [DOI] [PubMed] [Google Scholar]
- 15.Rickman KA, Swancutt KL, Mezyk SP, Kiddle JJ. 2013. Isoniazid: radical-induced oxidation and reduction chemistry. Bioorg. Med. Chem. Lett. 23:3096–3100. 10.1016/j.bmcl.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Timmins GS, Deretic V. 2006. Mechanisms of action of isoniazid. Mol. Microbiol. 62:1220–1227. 10.1111/j.1365-2958.2006.05467.x. [DOI] [PubMed] [Google Scholar]
- 17.Ranguelova K, Suarez J, Magliozzo RS, Mason RP. 2008. Spin trapping investigation of peroxide- and isoniazid-induced radicals in Mycobacterium tuberculosis catalase-peroxidase. Biochemistry 47:11377–11385. 10.1021/bi800952b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kono Y. 1982. Oxygen enhancement of bactericidal activity of rifamycin SV on Escherichia coli and aerobic oxidation of rifamycin SV to rifamycin S catalyzed by manganous ions: the role of superoxide. J. Biochem. 91:381–395. [DOI] [PubMed] [Google Scholar]
- 19.Kono Y, Sugiura Y. 1982. Electron spin resonance studies on the oxidation of rifamycin SV catalyzed by metal ions. J. Biochem. 91:397–401. [DOI] [PubMed] [Google Scholar]
- 20.Rao DNR, Cederbaum AI. 1997. A comparative study of the redox-cycling of a quinone (rifamycin S) and a quinonimine (rifabutin) antibiotic by rat liver microsomes. Free Radic. Biol. Med. 22:439–446. 10.1016/S0891-5849(96)00335-8. [DOI] [PubMed] [Google Scholar]
- 21.Koch A, Mizrahi V, Warner DF. 2014. The impact of drug resistance on Mycobacterium tuberculosis physiology: what can we learn from rifampicin? Emerg. Microbes Infect. 3:e17. 10.1038/emi.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohanski MA, Dwyer DJ, Collins JJ. 2010. How antibiotics kill bacteria: from targets to networks. Nat. Rev. Microbiol. 8:423–435. 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccaro G, Giannoni F, Filippini P, Mustazzolu A, Fattorini L. 2013. Activity of drug combinations against Mycobacterium tuberculosis grown in aerobic and hypoxic acidic conditions. Antimicrob. Agents Chemother. 57:1428–1433. 10.1128/AAC.02154-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia L, Alonso-Sanz M, Rebollo MJ, Tercero JC, Chaves F. 2001. Mutations in the rpoB gene of rifampin-resistant Mycobacterium tuberculosis isolates in Spain and their rapid detection by PCR-enzyme-linked immunosorbent assay. J. Clin. Microbiol. 39:1813–1818. 10.1128/JCM.39.5.1813-1818.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luna-Herrera J, Reddy VM, Daneluzzi D, Gangadharam PR. 1995. Antituberculosis activity of clarithromycin. Antimicrob. Agents Chemother. 39:2692–2695. 10.1128/AAC.39.12.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dikalov S, Skatchkov M, Bassenge E. 1997. Spin trapping of superoxide radicals and peroxynitrite by 1-hydroxy-3-carboxy-pyrrolidine and 1-hydroxy-2,2,6, 6-tetramethyl-4-oxo-piperidine and the stability of corresponding nitroxyl radicals towards biological reductants. Biochem. Biophys. Res. Commun. 231:701–704. 10.1006/bbrc.1997.6174. [DOI] [PubMed] [Google Scholar]
- 27.Pou S, Cohen MS, Britigan BE, Rosen GM. 1989. Spin-trapping and human neutrophils. Limits of detection of hydroxyl radical. J. Biol. Chem. 264:12299–12302. [PubMed] [Google Scholar]
- 28.Zhu BZ, Zhao HT, Kalyanaraman B, Frei B. 2002. Metal-independent production of hydroxyl radicals by halogenated quinones and hydrogen peroxide: an ESR spin trapping study. Free Radic. Biol. Med. 32:465–473. 10.1016/S0891-5849(01)00824-3. [DOI] [PubMed] [Google Scholar]
- 29.Makino K, Imaishi H, Morinishi S, Hagiwara T, Takeuchi T, Murakami A, Nishi M. 1989. An artifact in the ESR spectrum obtained by spin trapping with DMPO. Free Radic. Res. Commun. 6:19–28. 10.3109/10715768909073424. [DOI] [PubMed] [Google Scholar]
- 30.Sensi P. 1983. History of the development of rifampin. Rev. Infect. Dis. 5(Suppl 3):S402–S406. [DOI] [PubMed] [Google Scholar]
- 31.Floss HG, Yu TW. 2005. Rifamycin-mode of action, resistance, and biosynthesis. Chem. Rev. 105:621–632. 10.1021/cr030112j. [DOI] [PubMed] [Google Scholar]
- 32.Mosel M, Li L, Drlica K, Zhao X. 2013. Superoxide-mediated protection of Escherichia coli from antimicrobials. Antimicrob. Agents Chemother. 57:5755–5759. 10.1128/AAC.00754-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dwyer DJ, Belenky PA, Yang JH, MacDonald IC, Martell JD, Takahashi N, Chan CT, Lobritz MA, Braff D, Schwarz EG, Ye JD, Pati M, Vercruysse M, Ralifo PS, Allison KR, Khalil AS, Ting AY, Walker GC, Collins JJ. 2014. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc. Natl. Acad. Sci. U. S. A. 111:E2100–E2109. 10.1073/pnas.1401876111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhaskar A, Chawla M, Mehta M, Parikh P, Chandra P, Bhave D, Kumar D, Carroll KS, Singh A. 2014. Reengineering redox sensitive GFP to measure mycothiol redox potential of Mycobacterium tuberculosis during infection. PLoS Pathog. 10:e1003902. 10.1371/journal.ppat.1003902. [DOI] [PMC free article] [PubMed] [Google Scholar]