Abstract
Comparative genome analysis revealed seven uncharacterized genes, sven0909 to sven0915, adjacent to the previously identified chloramphenicol biosynthetic gene cluster (sven0916–sven0928) of Streptomyces venezuelae strain ATCC 10712 that was absent in a closely related Streptomyces strain that does not produce chloramphenicol. Transcriptional analysis suggested that three of these genes might be involved in chloramphenicol production, a prediction confirmed by the construction of deletion mutants. These three genes encode a cluster-associated transcriptional activator (Sven0913), a phosphopantetheinyl transferase (Sven0914), and a Na+/H+ antiporter (Sven0915). Bioinformatic analysis also revealed the presence of a previously undetected gene, sven0925, embedded within the chloramphenicol biosynthetic gene cluster that appears to encode an acyl carrier protein, bringing the number of new genes likely to be involved in chloramphenicol production to four. Microarray experiments and synteny comparisons also suggest that sven0929 is part of the biosynthetic gene cluster. This has allowed us to propose an updated and revised version of the chloramphenicol biosynthetic pathway.
INTRODUCTION
Chloramphenicol (CHL) is a bacteriostatic antibiotic that inhibits protein synthesis by binding to the 50S subunit of the bacterial ribosome. It is active against both Gram-negative and Gram-positive bacteria, including many multiply drug-resistant strains. CHL is produced by several Gram-positive soil actinomycetes, but its biosynthesis has been analyzed mostly in Streptomyces venezuelae strain ATCC 10712 (1). The initial stages of CHL biosynthesis utilize the shikimate pathway that leads to the production of chorismic acid. Chorismic acid serves as a branch point for aromatic amino acid (phenylalanine, tyrosine, and tryptophan) biosynthesis and for the production of p-aminobenzoic acid (PABA) that is required for folic acid biosynthesis (2). The conversion of chorismic acid to PABA occurs via 4-amino-4-deoxychorismate the precursor for the pathway dedicated to chloramphenicol biosynthesis (3).
Previous analyses of the CHL biosynthetic gene cluster of S. venezuelae resulted in the identification of 12 genes with a proven or likely role in CHL production (3–10). For reasons that we do not understand, we were unable to detect CHL production by S. venezuelae under our laboratory conditions, but we did succeed in expressing the CHL biosynthetic gene cluster from this strain in the heterologous host Streptomyces coelicolor A3 (2, 11).
Bioinformatic analysis has become a powerful tool for the identification of natural product biosynthetic gene clusters (12, 13). By combining comparative genomics with microarray and bioinformatic analyses, we have identified four additional genes likely to be involved in CHL production, and we confirmed the participation of three of these in antibiotic biosynthesis by mutational analyses.
MATERIALS AND METHODS
Strains and general methods.
The strains used and generated in this study are listed in Table 1. Escherichia coli strains were grown and manipulated according to standard methods (14–16). S. coelicolor and S. venezuelae strains were grown as described previously on soya flour mannitol (SFM) or DNA agar medium (17) and in glucose-yeast extract-malt extract (GYM) (18) or in maltose-yeast extract-malt extract (MYM) (19) liquid medium. The plasmids and oligonucleotides used are described in Table 2 and Table S1 in the supplemental material, respectively.
TABLE 1.
Strains used and constructed in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| E. coli BW25113 | K-12 derivative (ΔaraBAD ΔrhaBAD) carrying plasmid pIJ790 | 40 |
| E. coli DH5α | F− ϕ80dlacZΔM15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17 (rK− mK+) phoA supE44 thi-1 gyrA96 relA1 λ−) | 41 |
| E. coli ET12567 | dam-13:: Tn9 dcm-6 hsdM CHLr, carrying helper plasmid pUZ8002 | 42 |
| E. coli TOP10 | DH10B derivative | Invitrogen |
| M. luteus ATCC 4698 | Bioassay indicator microorganism | ATCC |
| S. coelicolor M1152 | M145 derivative Δact Δred Δcpk Δcda rpoB(C1298T) | 11 |
| S. coelicolor M1581 | M1152 containing cosmid pAH91 (S. venezuelae cosmid containing CHL cluster) | This work |
| S. coelicolor M1583 | M1152 pAH91 (Δsven0909–Δsven0912) | This work |
| S. coelicolor M1584 | M1152 pAH91 (Δsven0913–Δsven0915) | This work |
| S. coelicolor M1585 | M1152 pAH91 (Δsven0913) | This work |
| S. coelicolor M1586 | M1152 pAH91 (Δsven0914) | This work |
| S. coelicolor M1587 | M1152 pAH91 (Δsven0915) | This work |
| S. coelicolor M1588 | M1152 pAH91 (Δsven0913) + pIJ12745 | This work |
| S. coelicolor M1589 | M1152 pAH91 (Δsven0914) + pIJ12747 | This work |
| S. coelicolor M1590 | M1152 pAH91 (Δsven0915) + pIJ12748 | This work |
| S. venezuelae ATCC 10712 | Wild-type S. venezuelae strain | ATCC |
| S. venezuelae M1582 | S. venezuelae with pIJ12744 (ermE*p::sven0915) | This work |
| S. venezuelae M1591 | S. venezuelae with pIJ10257 | This work |
TABLE 2.
Vectors and constructs used in this study
| Vector/construct | Descriptiona | Reference or source |
|---|---|---|
| pAH91 | Conjugative and integrative (ϕC31 attB) derivative of cosmid 4P22 containing the CHL gene cluster from S. venezuelae, APRr CARr | 11 |
| pIJ10700 | pBluescript II KS(+) containing hyg-oriT cassette | 16 |
| pUZ8002 | tra, neo, RP4 | 43 |
| pIJ790 | λ-RED (gam, bet, exo), cat, araC, rep101ts | 44 |
| pR9406 | Driver plasmid, CARr derived from pUB307 | 45; David Figurski, personal communication |
| pIJ10257 | oriT, ϕBT1 attB-int, HYGr, ermE*p | 22 |
| pIJ12744 | pIJ10257 with sven0913, HYGr | This work |
| pRT802 | Conjugative and ϕBT1-integrative vector, KANr | 20 |
| pIJ12551 | Conjugative and ϕC31-integrative vector, APRr | 21 |
| pIJ12744 | pIJ10257 with sven0913 under ermE*p control, HYGr | This work |
| pIJ12745 | pRT802 with sven0913 and its promoter region, KANr | This work |
| pIJ12746 | pIJ12551 with sven0914 under ermE*p control, APRr | This work |
| pIJ12747 | pRT802 with sven0914 under ermE*p control, KANr | This work |
| pIJ12748 | pRT802 with sven0915 and its promoter region, KANr | This work |
APRr, apramycin resistance; CARr, carbenicillin resistance; HYGr, hygromycin resistance; KANr, kanamycin resistance.
Construction of deletion mutants.
Genes carried by pAH91 (Andrew Hesketh, personal communication; Gomez-Escribano and Bibb [11]) were replaced either individually (sven0913, sven0914, and sven0915) or in groups (sven0909 to sven0912 and sven0913–sven0915) with a hygromycin (HYG) resistance cassette amplified from pIJ10700 using the primer pairs listed in Table S1 in the supplemental material, as described by Gust et al. (15). The mutations were confirmed by PCR using flanking primers. Conjugations between E. coli strain ET12567 with plasmid pUZ8002 carrying the oriT-containing pAH91 derivatives and streptomycete strains were carried out as described previously (17).
Complementation of deletion mutants.
To complement the sven0913, sven0914, and sven0915 deletion mutants, PCR products were generated by high-fidelity PCR using the primers listed in Table S1 in the supplemental material. For the complementation of Δsven0913 and Δsven0915, the PCR products extended from the beginning of the upstream intergenic region to the stop codon of each gene. The fragments were cloned into pRT802 (20) to generate pIJ12745 and pIJ12748, respectively, which were then transferred into the corresponding mutant strains using E. coli strain DH10B transformed with either of the plasmids in triparental matings with E. coli TOP10 (Invitrogen) containing the driver plasmid pR9406 and the nonmethylating E. coli strain ET12567, according to standard procedures (17). For the complementation of Δsven0914, the coding sequence between the start and stop codons of the gene was amplified and cloned into pIJ12551 (21) to fuse sven0914 to the constitutive ermE* promoter generating pIJ12746. A BamHI fragment from pIJ12746 containing ermE*p-sven0914 was then cloned into pRT802 to generate pIJ12747, which was manipulated as above.
Overexpression of sven0913.
A PCR product containing sven0913 (extending from the start to stop codons) was generated by high-fidelity PCR using the primers listed in Table S1 in the supplemental material. This fragment was cloned into the integrative vector pIJ10257 (22) to fuse sven0913 to the constitutive ermE* promoter, generating pIJ12744. The vector was transferred into S. venezuelae by conjugation from E. coli ET12567(pUZ8002), as described previously (17).
HPLC analysis.
Chloramphenicol production was quantified by high-performance liquid chromatography (HPLC). The culture supernatants were filtered through VectaSpin Micro polysulfone 0.2-mm columns (Whatman, Maidstone, United Kingdom), injected onto a Spherisorb 5-mm ODS2 4.6 by 250 mm C18 column (Waters, Milford, MA, USA) fitted to an Agilent 1100 HPLC system with a diode array detector and analyzed using a method modified from He et al. (4; A. Hesketh, personal communication): gradient water-methanol; min 0, 0% methanol; min 2, 25% methanol; min 12, 50% methanol, min 14, 100% methanol; min 20, 100% methanol; and min 22, 0% methanol. CHL eluted at about 15.8 min and was detected at 273 nm. CHL (catalog no. C03478; Sigma) was used as a standard.
DNA microarray analysis.
RNA isolation from S. venezuelae and subsequent DNA microarray analysis were carried out as described previously (23).
Real-time PCR analysis.
Streptomyces strains were cultured in liquid GYM medium in triplicate, as described previously (17). RNA was extracted according to published procedures (24) from 2.5 ml of culture sampled after 16 h of growth of the S. venezuelae strains and after 48 h of growth of the S. coelicolor M1152 derivatives. Mycelial pellets were resuspended in 1 ml of RTL buffer with lysing matrix B (MP Biomedicals) and homogenized using a FastPrep instrument (BIO 101). Two pulses of 30 s of intensity 6.0 were applied, with cooling down for 1 min on ice between pulses. The supernatants were centrifuged for 10 min at 13,000 rpm and then treated according to the instructions given in the RNeasy kit (Qiagen, Crawley, United Kingdom). The RNA samples were treated with DNase I (Invitrogen) until they were free of DNA contamination. The RNA was quantified, and equal amounts from each sample were converted to cDNA, according to the manufacturer's instructions (SuperScript; Invitrogen). The oligonucleotide pairs listed in Table S1 in the supplemental material were used to amplify the genes representing each of the putative operons within the CHL biosynthetic cluster, as well as the left and right flanking genes (sven0912 and sven0930, respectively). Amplification was also attempted using the same oligonucleotide pairs on RNA samples that had not been treated with reverse transcriptase to confirm a lack of DNA contamination.
RESULTS
Comparative genome and microarray analyses reveal three putative new members of the chloramphenicol gene cluster.
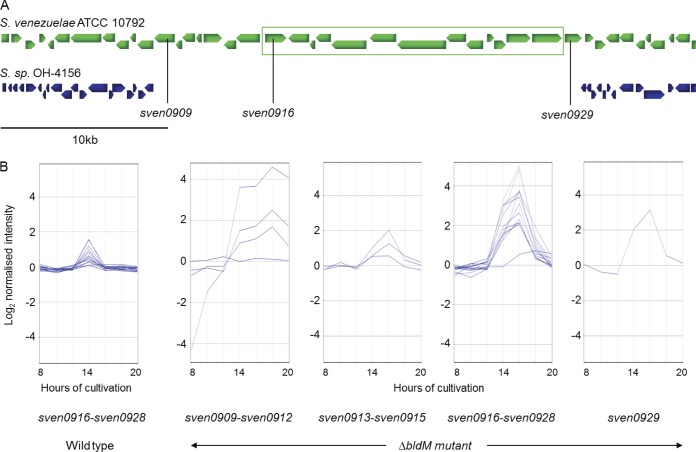
Comparative genome analysis can play an extremely useful role in identifying and determining the extent of natural product biosynthetic gene clusters. The genome mining of Streptomyces sp. strain OH-4156 (25), which does not produce CHL, indicated a high level of nucleotide sequence similarity with the genome of S. venezuelae. The alignment of the Streptomyces sp. strain OH-4156 Solexa contigs on the S. venezuelae genome sequence in the region of the CHL biosynthetic gene cluster revealed the presence of seven genes (sven0909–sven0915) adjacent to the previously identified CHL biosynthetic gene cluster (sven0916–sven0928) that were absent in the nonproducing strain (Fig. 1A). To gain insight into whether any of these newly identified genes might play a role in CHL production, we compared their transcriptional profiles with those of established CHL biosynthetic genes in the microarray data from submerged cultures of S. venezuelae and several of its developmental mutants that are deficient in sporulation. Although we were unable to detect CHL biosynthesis in S. venezuelae, the transcription of sven0916–sven0928 was detected in the wild-type strain at a low level, peaking at 14 h of cultivation (Fig. 1B). Surprisingly, the transcription of these genes was markedly increased in a bldM mutant and showed a pattern of expression similar to that in the wild-type strain, but peaking after 16 h of cultivation (Fig. 1B). Analysis of the expression profiles for sven0909–sven0915 suggested that three of these genes, sven0913–sven0915, might be coordinately regulated with sven0916–sven0928, genes known or believed to be involved in chloramphenicol biosynthesis, and which thus might form part of an extended CHL biosynthetic gene cluster (Fig. 1B). These three genes were predicted to encode a transcriptional activator (sven0913) with 44% amino acid sequence identity to StrR, the pathway-specific activator of the streptomycin biosynthetic gene cluster in Streptomyces griseus, a phosphopantetheinyl transferase (PPTase) (sven0914), and a Na+/H+ antiporter (sven0913). In contrast, the expression patterns of sven0909–sven0912, all encoding hypothetical proteins, were different from those of the known CHL biosynthetic genes, suggesting that they may not be part of the CHL cluster (Fig. 1B).
FIG 1.
(A) Alignment of Solexa contigs (shown as filled blue arrows) from the draft genome sequence of Streptomyces sp. strain OH-4156 with the region of the S. venezuelae genome encoding CHL biosynthesis (protein-coding sequences are shown as filled green arrows). A Solexa contig was aligned to the S. venezuelae genome sequence if it exhibited ≥80% identity over a length of ≥100 nucleotides (average level of nucleotide sequence identity between the Solexa contigs and the S. venezuelae genome sequence was 88.5%, with a modal value of 93%). The alignment shows a cluster of 21 genes (sven0909–sven0929) unique to S. venezuelae that includes the 12 previously implicated in CHL biosynthesis (sven0916–sven0928, shown in the green rectangle). (B) Microarray expression profiles of the 21 genes suggested that sven0913–sven0929 may be transcriptionally coregulated. The x axis represents culture age, and the y axis is normalized transcript abundance on a log2 scale.
Deletion of sven0913–sven0915 abolishes CHL production.
To assess whether sven0909–sven0912 and sven0913–sven0915 play a role in CHL biosynthesis, each set of genes was deleted from the cloned CHL biosynthetic gene cluster of pAH91 by PCR targeting. The HYG resistance cassette from pIJ10700 (15) was used to replace sven0909–sven0912 (Δsven0909–Δsven0912) and sven0913–sven0915 (Δsven0913–Δsven0915) in E. coli, and the mutated cosmids were transferred to S. coelicolor M1152 by conjugation, yielding M1583 (Δsven0909–Δsven0912) and M1584 (Δsven0913–Δsven0915), respectively. The integration of each cosmid was confirmed by PCR. The two strains, together with S. coelicolor M1152 carrying pAH91 (M1581), were grown in GYM liquid medium (18), and the supernatants from each culture were spotted onto filter paper discs laid on top of a lawn of Micrococcus luteus (Fig. 2). While the supernatant from M1583 (Δsven0909–Δsven0912, Δ1 in Fig. 2) produced a zone of inhibition identical to that of M1581, that from M1584 (Δsven0913–Δsven0915, Δ2 in Fig. 2) showed no inhibitory activity, and HPLC analysis confirmed the absence of CHL in the supernatant (data not shown), indicating that at least one of the genes sven0913–sven0915 plays an essential role in CHL biosynthesis.
FIG 2.
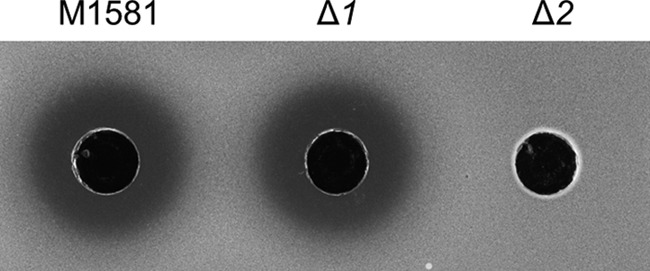
Analysis of the effect on CHL biosynthesis of replacing sven0909–sven0912 or sven0913–sven0915 with the hyg cassette from pIJ10700. S. coelicolor M1581 and its mutant derivatives M1583 (Δ1 for sven0909–sven0912) and M1584 (Δ2 for sven0913–sven0915) were grown for 6 days in GYM liquid medium, and 100 μl of culture supernatant was assayed for antibiotic activity against M. luteus. The absence of an inhibition zone in the Δ2 mutant indicates an essential role for at least one of the sven0913–sven0915 genes in CHL biosynthesis.
Individual deletion of sven0913, sven0914, and sven0915 confirms their role in CHL production.
To investigate the individual roles of sven0913–sven0915 in CHL biosynthesis, PCR targeting was carried out on pAH91 to separately delete each gene. The deletion of sven0913 (yielding M1585), the putative transcriptional activator, abolished antibiotic activity (Fig. 3A) and CHL production (Fig. 3B). In contrast, the deletion of either sven0914 (to give M1586), coding for a putative PPTase, or sven0915 (yielding M1587), encoding a putative Na+/H+ antiporter, decreased the level of CHL production to around 40% of that observed with the wild-type gene cluster. Presumably, the roles that Sven0914 and Sven0915 play in CHL biosynthesis can be at least partially substituted by proteins encoded elsewhere in the S. coelicolor genome.
FIG 3.
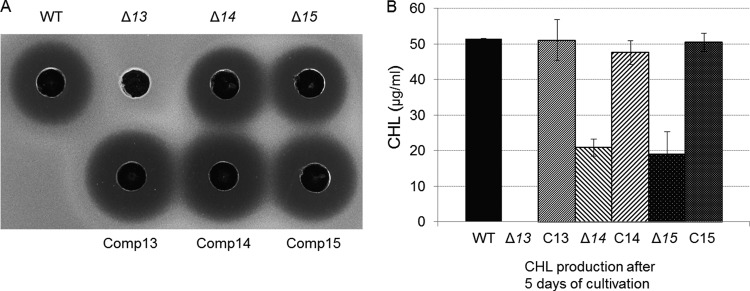
Analysis of the effects of single-gene deletions on CHL production. S. coelicolor M1581 and its mutant derivatives M1585 (Δsven0913 [Δ13]), M1586 (Δsven0914 [Δ14]), and M1587 (Δsven0915 [Δ15]) and their complemented derivatives (Comp13, Comp14, and Comp15, respectively) were grown for 5 days in GYM liquid medium. (A) One hundred microliters of supernatant from each of the cultures was assayed for antibiotic activity against M. luteus. (B) Quantitation of CHL production by each of the strains by HPLC analysis. Bars indicate the standard deviation for three biological samples.
The complementation of each of the three mutant strains was accomplished using integrative vectors containing the gene of interest expressed from either its native promoter (sven0913 and sven0915) or from the constitutive ermE* promoter (sven0914). In each case, CHL production was restored to the level observed with the wild-type gene cluster (Fig. 3).
Constitutive expression of sven0913 activates CHL production in S. venezuelae.
As mentioned previously, we were unable to detect chloramphenicol production in S. venezuelae under a variety of growth conditions (Fig. 4). Since our results suggest that sven0913 likely acts as a transcriptional activator of the CHL biosynthetic gene cluster, we attempted to overexpress this gene in S. venezuelae. To achieve this, the sven0913 coding sequence was cloned in the integrative vector pIJ10257 (22) under the control of the constitutive ermE* promoter, and the resulting plasmid was transferred to S. venezuelae by conjugation and integration confirmed by PCR, yielding M1582. The supernatants obtained from this strain and from wild-type S. venezuelae were analyzed by HPLC for CHL production (Fig. 4). The constitutive expression of sven0913 led to CHL production levels of approximately 3.5 μg/ml after 72 h of growth of M1582, while CHL biosynthesis remained undetectable in the vector-only control strain (M1591), consistent with the proposed role of sven0913 as a cluster-situated transcriptional activator of the CHL biosynthetic gene cluster.
FIG 4.
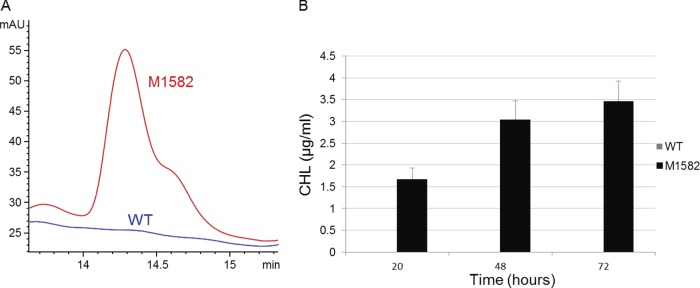
CHL production in wild-type (WT) S. venezuelae and in M1582 (with sven0913 expressed from the ermE* promoter). (A) HPLC chromatogram of CHL present in supernatants of 72 h cultures measured at a wavelength of 273 nm (see Materials and Methods). (B) CHL production, estimated as in panel A by HPLC analysis, after 20, 48, and 72 h of culture. Bars indicate the standard deviation for three biological samples.
RT-PCR analyses confirm that Sven0913 is a transcriptional activator of the CHL biosynthetic cluster.
To confirm that sven0913 plays a role in activating transcription of the CHL biosynthetic gene cluster, real-time PCR (RT-PCR) analysis was performed on the following strains: wild-type S. venezuelae, M1582 (S. venezuelae with constitutive expression of sven0913), M1581 (S. coelicolor M1152 carrying pAH91), and M1585 (M1581 with sven0913 deleted). RNA was extracted from each of the cultures and RT-PCR conducted using primers amplifying the transcripts from several regions of the CHL gene cluster as well as from flanking genes (see Table S1 in the supplemental material). While the constitutive expression of sven0913 in S. venezuelae resulted in elevated levels of expression of the genes predicted to lie within the CHL gene cluster, it had no effect on the flanking genes sven0912 and sven0930 (Fig. 5). Conversely, the deletion of sven0913 in S. coelicolor (M1585) reduced the levels of expression of the predicted CHL biosynthetic genes but had no effect on those of the flanking genes. These results confirm that Sven0913 is a previously unidentified transcriptional activator that plays a crucial role in the regulation of CHL biosynthesis.
FIG 5.
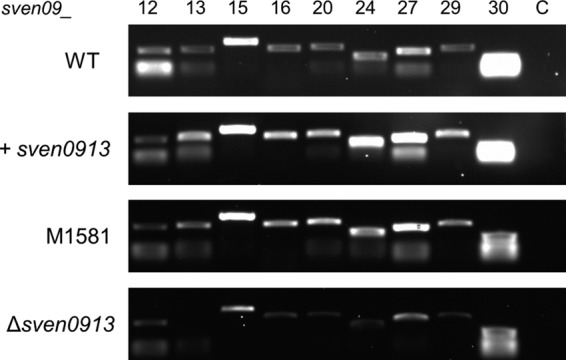
RT-PCR analysis of selected genes from the CHL biosynthetic gene cluster. RNA was isolated from 16 h cultures of the S. venezuelae strains and from 5-day cultures of the S. coelicolor M1152 derivatives and subjected to RT-PCR analysis. Top, wild-type S. venezuelae and M1582 (with sven0913 expressed from the ermE* promoter [+sven0913]). Bottom, S. coelicolor M1581 and M1585 (with sven0913 deleted [Δsven0913]). The primer pairs listed in Table S1 in the supplemental material were used. The numbers at the top refer to the last two digits of the individual sven genes. A control (C) without reverse transcriptase and using primers corresponding to sven0930 was used to confirm the absence of DNA contamination.
sven0925, a previously unidentified gene encoding a putative acyl carrier protein.
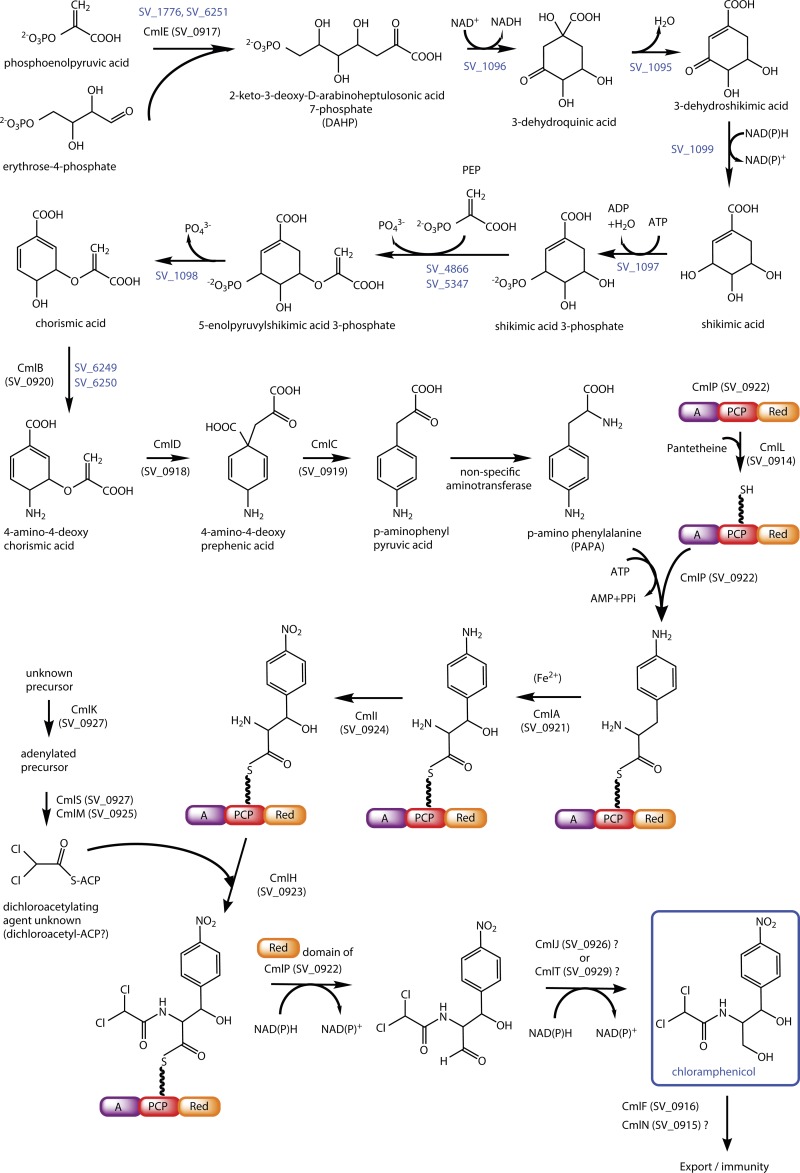
Our earlier annotation of the genome sequence of S. venezuelae (GenBank accession no. FR845719) had identified an additional previously undetected gene in the CHL biosynthetic gene cluster, sven0925, whose 108-amino-acid product failed to show clear similarity to the proteins of known function in the NCBI database. A more recent BLASTp search revealed a large number of homologues present in the genome sequences, some of which were annotated as acyl carrier proteins (ACPs). Based on this observation, Sven0925 was subjected to analysis using the Phyre2 protein structure prediction server (26) (http://www.sbg.bio.ic.ac.uk/phyre2). The program predicts with 97% to 98% confidence that Sven0925 is a homologue of structurally characterized ACPs from a variety of bacterial species; for example, 77 residues of Sven0925 (71% of its sequence) were modeled with 98.0% confidence to the structure of the holo-acyl carrier protein ne2163 from Nitrosomonas europaea (see Fig. S1 in the supplemental material). Moreover, Sven0925 possesses a conserved serine at position 50 that is predicted to be the catalytic residue needed for the addition of the 4′-phosphopantetheine moiety required for the activity of the holo-ACP (see Fig. S2 in the supplemental material). Together with the expression profile of sven0925, which conforms to that of the other CHL biosynthetic genes, we conclude that Sven0925 is indeed an ACP involved in CHL biosynthesis (see Fig. 6).
FIG 6.
Revised pathway for CHL biosynthesis in S. venezuelae. SV_, protein designations from StrepDB (http://strepdb.streptomyces.org.uk/); those involved in the shikimate pathway and not part of the CHL gene cluster are shown in blue.
sven0929, part of the CHL biosynthetic gene cluster, but not required for CHL production.
Synteny comparisons of sven0900–sven0940 with the draft genome sequences of both Streptomyces sp. strain OH-4156 (Fig. 1A) and Micromonospora carbonacea strain ATCC 39149 (see below; see also Fig. S3 in the supplemental material) suggested that sven0929 might be part of the CHL biosynthetic gene cluster. This inference was further supported by the microarray analysis (Fig. 1B) and RT-PCR experiments (Fig. 5), which clearly demonstrate that sven0929 is transcriptionally coregulated with genes involved in CHL biosynthesis. However, the previous deletion of sven0929 (ORF13 of Piraee, White, and Vining [9]), which appears to encode an aldo-keto reductase, had no apparent effect on CHL biosynthesis. We believe that sven0929 is indeed part of the CHL biosynthetic gene cluster and that its role in CHL biosynthesis, like that of sven0914 and sven0915 in S. coelicolor, can be fulfilled by a functional homologue located elsewhere in the S. venezuelae genome.
DISCUSSION
New developments in next-generation sequencing combined with bioinformatic analysis greatly facilitate the comparison of genome sequences of related bacteria. We have taken advantage of these advances and combined them with microarray analysis to identify four new members of the CHL biosynthetic gene cluster: sven0913, encoding a transcriptional activator; sven0914, encoding a putative PPTase; sven0915, encoding a putative ion antiporter; and sven0925, encoding a putative ACP.
For reasons we do not understand, while the transcription of the CHL biosynthetic gene cluster can be detected by microarray analysis in our wild-type isolate of S. venezuelae (Fig. 1A), albeit at a low level, we were unable to detect CHL production, despite using a range of culture media. However, the constitutive expression of sven0913 resulted in readily detectable levels of antibiotic production, further highlighting the value of ectopically expressing cluster-situated regulatory genes to activate natural product biosynthesis (for an example, see reference 27).
The deletion of either sven0914 or sven0915, which appear to be cotranscribed, decreased CHL production by approximately 60%. Production was restored to wild-type levels when each mutant strain was complemented with the respective gene, demonstrating that the phenotype of the Δsven0914 mutant is not simply the result of a polar effect of the deletion of sven0915 and that both genes play a role in CHL production. Sven0914 is predicted to encode a PPTase. PPTases are responsible for the conversion of the inactive apo form of an ACP or peptidyl carrier protein (PCP) to the active holo form by covalent attachment of a coenzyme-A-derived phosphopantetheine group to a specific serine residue (28). The posttranslational phosphopantetheinylation of the apo-ACP/PCP domains is essential for the activities of many multienzyme synthases and synthetases responsible for the generation of a variety of natural products, most notably polyketides and nonribosomally synthesized peptides (29). Sven0914 contains all of the conserved motifs found in the F/KES subfamily of Sfp-type PPTases (30, 31). While many members of this subfamily utilize PCPs as substrates, there are exceptions, most notably the Sco6673-like PPTase of Streptomyces ambofaciens, which is able to accept both ACP and PCP domains as substrates (32) and which shares 53% amino acid sequence identity with Sven0914. Consequently, we cannot reliably predict whether Sven0914 is involved in the modification of the PCP domain of Sven0922 (see below), the ACP Sven0929, or both.
Interestingly, not all natural products that require the activity of a PPTase for their synthesis contain the corresponding gene within their biosynthetic gene clusters, and instead, they utilize a PPTase encoded elsewhere in the genome. Consequently, it is not too surprising that the deletion of sven0914 reduced but did not abolish CHL production. Indeed, the CHL biosynthetic gene cluster of M. carbonacea ATCC 39149 (33) (GenBank accession no. GG657738) lacks a sven0914 homologue, although all of the other genes required for CHL production are present and arranged in precisely the same manner as in S. venezuelae (see Fig. S3 in the supplemental material). A BLASTp search to find the possible homologues of Sven0914 in the heterologous host S. coelicolor identified Sco6673, with 54% amino acid sequence identity. A Pfam database search (34) identified a PPTase domain within the C-terminal region of Sco6673 and may explain why the deletion of sven0914 still results in some CHL production in the heterologous host (the Sco6673 and the Sco6673-like proteins of S. ambofaciens share 83% identity).
sven0915 is predicted to encode an Na+/H+ ion antiporter. Divergently transcribed from sven0915 is sven0916, which is predicted to encode an efflux permease from the major facilitator superfamily, and it is presumably involved in CHL export. These efflux pumps are often involved in multidrug resistance, exporting toxic compounds to the outside of the cell, and they are usually driven by the energy stored in ion gradients to catalyze the transport of drugs across the membrane. Given their proximity in the CHL gene cluster, it is conceivable that sven0915 provides the energy required for sven0916 to export CHL outside the cell. The deletion of sven0915 reduced CHL production by >60% in the heterologous host S. coelicolor. Several homologues of sven0915 occur in the S. coelicolor M1152 genome, any one or more of which may partially compensate for the loss of Sven0915, thus allowing continued CHL export, although at lower levels, in the deletion mutant.
Our studies have identified four new genes that play, or that are likely to play, a role in CHL biosynthesis in S. venezuelae, prompting us to present an updated view of the CHL biosynthetic pathway (Fig. 6 and Table 3). Although previous studies have sometimes used different nomenclatures to represent the same gene, we have adopted that of Piraee, White, and Vining (9) to denote previously identified CHL biosynthetic genes, and we propose five new cml designations: cmlR, cmlL, cmlN, and cmlM for the newly identified genes described in this paper and cmlT for sven0929.
TABLE 3.
Genes involved in CHL biosynthesis
| sven no. (StrepDB) | Proposed nomenclatureb | Function of gene product | Reference (of function) |
|---|---|---|---|
| sven0913a | cmlR | Transcriptional activator | This work |
| sven0914 | cmlL | Phosphopantetheinyl transferase | Cosmid annotation/BLASTp |
| sven0915 | cmlN | Integral membrane ion antiporter | Cosmid annotation/BLASTp |
| sven0916 | cmlF | Chloramphenicol efflux pump | 4, 9 |
| sven0917 | cmlE | DAHP synthase | 4 |
| sven0918 | cmlD | 4-Amino-4-deoxychorismate mutase | 46 |
| sven0919a | cmlC | 4-Amino-4-deoxyprephenate dehydrogenase | 46 |
| sven0920a | cmlB | 4-Amino-4-deoxychorismate synthase | 46 |
| sven0921 | cmlA | Nonheme iron monooxygenase catalyzing β-hydroxylation of l-PAPAc | 47 |
| sven0922a | cmlP | Adenylation, PCP and reductase domains | 4, 9 |
| sven0923a | cmlH | Amidase | BLASTp; 4, 9 |
| sven0924 | cmlI | N-Oxygenase, nonheme diiron oxygenase. | 10 |
| sven0925 | cmlM | Putative acyl carrier protein | Phyre2; 26 |
| sven0926a | cmlJ | Short-chain dehydrogenase | 4 |
| sven0927 | cmlK | Acyl-CoA-ACP synthetase, AMP-ligase | 9 |
| sven0928 | cmlS | Flavin-dependent halogenase (chlorination of chloramphenicol) | 9, 48 |
| sven0929 | cmlT | Aldo-keto reductase; not essential for CHL biosynthesis | 9 |
Deletion of that particular gene abolishes CHL production.
Previous or alternative names given in the literature: cmlD, papB (46); cmlC, papC (46); cmlB, pabAB (4, 6), papA (46); cmlP, cmlH (GenBank accession no. AAG21975.2); cmlH, cmlG (8) (based on GenBank accession no. AAG21974.1).
l-PAPA, l-p-aminophenylalanine; CoA, coenzyme A.
As previously noted, CHL biosynthesis utilizes the shikimate pathway, which leads to chorismic acid and hence aromatic amino acid production. Some of the chorismic acid is converted to 4-amino-4-deoxychorismate, which is used for synthesis of the essential metabolite PABA. 4-Amino-4-deoxychorismate is also utilized as the precursor for the pathway dedicated to CHL production. BLASTp searches of the S. venezuelae proteome confirmed that genes encoding homologues of two of the primary metabolic enzymes involved in 4-amino-4-deoxychorismate biosynthesis, 2-keto-3-deoxy-d-arabino-heptulosonate-7-phosphate (DAHP) synthase and 4-amino-4-deoxychorismate synthase, were present in the cml gene cluster (sven0917 [cmlE] and sven0920 [previously pabAB {9}, cmlB here], respectively. Homologues of sven0917 and sven0920 are also located elsewhere in the S. venezuelae genome and are presumably involved in primary metabolism. DAHP synthesis is the first committed step of the shikimate pathway and is often subject to feedback inhibition or repression in other microorganisms (35). Our transcriptional profiling suggests that CHL biosynthesis occurs at the end of rapid vegetative growth in S. venezuelae, when proteolytic degradation of existing proteins may provide an intracellular amino acid pool for continued morphological development. Such a source of aromatic amino acids might act to inhibit the expression and/or activity of the primary metabolic DAHP synthase and thus restrict the precursor pool for CHL biosynthesis. We speculate that the existence of a gene, cmlE, within the CHL biosynthetic gene cluster that encodes a DHAP synthase homologue may serve to ensure sufficient flux through the shikimate pathway for sustained CHL biosynthesis. Similarly, the presence of a 4-amino-4-deoxychorismate synthase homologue, cmlB, in the CHL biosynthetic gene cluster may act to direct flux toward CHL biosynthesis rather than continued aromatic amino acid production.
The work reported in this paper extends previous studies that led to an increasingly informed understanding of CHL production in S. venezuelae (3–6, 9, 36), and it allows us to present a consolidated and updated view of the CHL biosynthetic pathway (Fig. 6 and Table 3). Although the functions of many genes can be assigned with certainty or a high level of confidence, the roles of those involved in the dichloroacetylation of the CHL precursor remain obscure. Partly by a process of elimination, these genes are likely to include sven0925 (cmlM), sven0926 (cmlJ), sven0927 (cmlK), and sven0929 (cmlT), as well as sven0928 (cmlS), encoding a flavin-dependent halogenase. All five of these genes are clustered together and may well be contained in a single operon, consistent with a common function. Based on the similarity of CmlK to the adenylating enzymes PchD and DhbE, which utilize salicylic acid and 2,3-dihydroxybenzoic acid as substrates, respectively, Piraee, White, and Vining (9) speculated that dichloroacetylation occurs through CmlK-mediated adenylation of an aromatic carboxylic acid. The recognition of the putative ACP CmlM in this study suggests that CmlM may also be a substrate for CmlK, tethering the carboxylic acid prior to halogenation (Fig. 6).
Surprisingly, microarray analyses revealed that the expression of the CHL biosynthetic gene cluster was markedly enhanced in bldM, whiI, bldN, and whiG (data not shown) mutants of S. venezuelae. BldM and WhiI are both orphan atypical response regulators that play a crucial role in morphological differentiation. Recent work has demonstrated that BldM and WhiI can form a heterodimer that activates transcription from so-called group II promoters, presumably integrating signals from two distinct developmental pathways (37). Chromatin immunoprecipitation sequencing (ChIP-Seq) analysis by the same authors failed to reveal binding of either protein to any of the potential promoter regions in the CHL biosynthetic gene cluster, nor did we find a sequence motif corresponding to the consensus recognition sequence for group II promoters anywhere in the gene cluster. It thus seems unlikely that a BldM-WhiI heterodimer can directly repress CHL gene expression in the wild-type strain. The elevated levels of CHL gene transcription observed in both bldM and whiI mutants may thus reflect the existence of an as-yet-unidentified gene that negatively regulates CHL production and whose expression is dependent on the BldM-WhiI heterodimer. bldM is transcribed from two promoters, one of which (p1) is directly activated by BldN at the onset of development (23, 38), and thus the effect of deleting bldN on CHL gene transcription is presumably mediated through decreased levels of bldM transcription. Similarly, the transcription of whiI is activated from a single WhiG-dependent promoter (39), presumably explaining the effect of mutation of whiG on cml gene transcription.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported financially by the Biotechnological and Biological Sciences Research Council (BBSRC) Institute Strategic Programme grant “Understanding and exploiting plant and microbial secondary metabolism” (BB/J004561/1) and by an EU COST Action CM 0804 STSM grant to C. Borsetto.
We thank Barrie Wilkinson for comments on the manuscript.
Footnotes
Published ahead of print 29 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04272-14.
REFERENCES
- 1.Vining LC, Stuttard C. 1995. Chloramphenicol. Biotechnology 28:505–530. [DOI] [PubMed] [Google Scholar]
- 2.Vining LC, Westlake D. 1984. Chloramphenicol: properties, biosynthesis and fermentation, p 387–409 In Vandamme EJ. (ed), Biotechnology of industrial antibiotics. Marcel Dekker, New York, NY. [Google Scholar]
- 3.Chang Z, Sun Y, He J, Vining LC. 2001. p-Aminobenzoic acid and chloramphenicol biosynthesis in Streptomyces venezuelae: gene sets for a key enzyme, 4-amino-4-deoxychorismate synthase. Microbiology 147(Pt 8):2113–2126. [DOI] [PubMed] [Google Scholar]
- 4.He J, Magarvey N, Piraee M, Vining LC. 2001. The gene cluster for chloramphenicol biosynthesis in Streptomyces venezuelae ISP5230 includes novel shikimate pathway homologues and a monomodular non-ribosomal peptide synthetase gene. Microbiology 147(Pt 10):2817–2829. [DOI] [PubMed] [Google Scholar]
- 5.Gross F, Lewis EA, Piraee M, van Pée KH, Vining LC, White RL. 2002. Isolation of 3′-O-acetylchloramphenicol: a possible intermediate in chloramphenicol biosynthesis. Bioorg. Med. Chem. Lett. 12:283–286. 10.1016/S0960-894X(01)00739-9. [DOI] [PubMed] [Google Scholar]
- 6.Brown MP, Aidoo KA, Vining LC. 1996. A role for pabAB, a p-aminobenzoate synthase gene of Streptomyces venezuelae ISP5230, in chloramphenicol biosynthesis. Microbiology 142:1345–1355. 10.1099/13500872-142-6-1345. [DOI] [PubMed] [Google Scholar]
- 7.Doull J, Ahmed Z, Stuttard C, Vining LC. 1985. Isolation and characterization of Streptomyces venezuelae mutants blocked in chloramphenicol biosynthesis. J. Gen. Microbiol. 131:97–104. [DOI] [PubMed] [Google Scholar]
- 8.Pacholec M, Sello JK, Walsh CT, Thomas MG. 2007. Formation of an aminoacyl-S-enzyme intermediate is a key step in the biosynthesis of chloramphenicol. Org. Biomol. Chem. 5:1692–1694. 10.1039/b703356g. [DOI] [PubMed] [Google Scholar]
- 9.Piraee M, White RL, Vining LC. 2004. Biosynthesis of the dichloroacetyl component of chloramphenicol in Streptomyces venezuelae ISP5230: genes required for halogenation. Microbiology 150:85–94. 10.1099/mic.0.26319-0. [DOI] [PubMed] [Google Scholar]
- 10.Lu H, Chanco E, Zhao H. 2012. CmlI is an N-oxygenase in the biosynthesis of chloramphenicol. Tetrahedron 68:7651–7654. 10.1016/j.tet.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez-Escribano JP, Bibb MJ. 2011. Engineering Streptomyces coelicolor for heterologous expression of secondary metabolite gene clusters. Microb. Biotechnol. 4:207–215. 10.1111/j.1751-7915.2010.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zerikly M, Challis GL. 2009. Strategies for the discovery of new natural products by genome mining. Chembiochem 10:625–633. 10.1002/cbic.200800389. [DOI] [PubMed] [Google Scholar]
- 13.Owen JG, Reddy BV, Ternei MA, Charlop-Powers Z, Calle PY, Kim JH, Brady SF. 2013. Mapping gene clusters within arrayed metagenomic libraries to expand the structural diversity of biomedically relevant natural products. Proc. Natl. Acad. Sci. U. S. A. 110:11797–11802. 10.1073/pnas.1222159110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 15.Gust B, O'Rourke S, Bird N, Kieser T, Chater KF. 2003. Recombineering in Streptomyces coelicolor. John Innes Foundation, Norwich, United Kingdom: http://streptomyces.org.uk/redirect/RecombineeringFEMSMP-2006-5.pdf. [Google Scholar]
- 16.Gust B, Chandra G, Jakimowicz D, Yuqing T, Bruton CJ, Chater KF. 2004. Lambda red-mediated genetic manipulation of antibiotic-producing Streptomyces. Adv. Appl. Microbiol. 54:107–128. 10.1016/S0065-2164(04)54004-2. [DOI] [PubMed] [Google Scholar]
- 17.Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. 2000. Practical Streptomyces genetics. John Innes Foundation, Norwich, United Kingdom: https://www.jic.ac.uk/science/molmicro/Strepmanual/Manual.htm. [Google Scholar]
- 18.Shima J, Hesketh A, Okamoto S, Kawamoto S, Ochi K. 1996. Induction of actinorhodin production by rpsL (encoding ribosomal protein S12) mutations that confer streptomycin resistance in Streptomyces lividans and Streptomyces coelicolor A3(2). J. Bacteriol. 178:7276–7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuttard C. 1982. Temperate phages of Streptomyces venezuelae: lysogeny and host specificity shown by phages SV1 and SV2. Microbiology 128:115–121. 10.1099/00221287-128-1-115. [DOI] [Google Scholar]
- 20.Gregory MA, Till R, Smith MC. 2003. Integration site for Streptomyces phage phiBT1 and development of site-specific integrating vectors. J. Bacteriol. 185:5320–5323. 10.1128/JB.185.17.5320-5323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherwood EJ, Hesketh AR, Bibb MJ. 2013. Cloning and analysis of the planosporicin lantibiotic biosynthetic gene cluster of Planomonospora alba. J. Bacteriol. 195:2309–2321. 10.1128/JB.02291-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong HJ, Hutchings MI, Hill LM, Buttner MJ. 2005. The role of the novel Fem protein VanK in vancomycin resistance in Streptomyces coelicolor. J. Biol. Chem. 280:13055–13061. 10.1074/jbc.M413801200. [DOI] [PubMed] [Google Scholar]
- 23.Bibb MJ, Domonkos A, Chandra G, Buttner MJ. 2012. Expression of the chaplin and rodlin hydrophobic sheath proteins in Streptomyces venezuelae is controlled by σ(BldN) and a cognate anti-sigma factor, RsbN. Mol. Microbiol. 84:1033–1049. 10.1111/j.1365-2958.2012.08070.x. [DOI] [PubMed] [Google Scholar]
- 24.Tunca S, Barreiro C, Sola-Landa A, Coque JJR, Martín JF. 2007. Transcriptional regulation of the desferrioxamine gene cluster of Streptomyces coelicolor is mediated by binding of DmdR1 to an iron box in the promoter of the desA gene. FEBS J. 274:1110–1122. 10.1111/j.1742-4658.2007.05662.x. [DOI] [PubMed] [Google Scholar]
- 25.Claesen J, Bibb M. 2010. Genome mining and genetic analysis of cypemycin biosynthesis reveal an unusual class of posttranslationally modified peptides. Proc. Natl. Acad. Sci. U. S. A. 107:16297–16302. 10.1073/pnas.1008608107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelley LA, Sternberg MJE. 2009. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4:363–371. 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 27.Laureti L, Song L, Huang S, Corre C, Leblond P, Challis GL, Aigle B. 2011. Identification of a bioactive 51-membered macrolide complex by activation of a silent polyketide synthase in Streptomyces ambofaciens. Proc. Natl. Acad. Sci. U. S. A. 108:6258–6263. 10.1073/pnas.1019077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh CT, Gehring AM, Weinreb PH, Quadri LE, Flugel RS. 1997. Post-translational modification of polyketide and nonribosomal peptide synthases. Curr. Opin. Chem. Biol. 1:309–315. 10.1016/S1367-5931(97)80067-1. [DOI] [PubMed] [Google Scholar]
- 29.Lambalot RH, Gehring AM, Flugel RS, Zuber P, LaCelle M, Marahiel MA, Reid R, Khosla C, Walsh CT. 1996. A new enzyme superfamily–the phosphopantetheinyl transferases. Chem. Biol. 3:923–936. 10.1016/S1074-5521(96)90181-7. [DOI] [PubMed] [Google Scholar]
- 30.Copp JN, Neilan BA. 2006. The phosphopantetheinyl transferase superfamily: phylogenetic analysis and functional implications in cyanobacteria. Appl. Environ. Microbiol. 72:2298–2305. 10.1128/AEM.72.4.2298-2305.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asghar AH, Shastri S, Dave E, Wowk I, Agnoli K, Cook AM, Thomas MS. 2011. The pobA gene of Burkholderia cenocepacia encodes a group I Sfp-type phosphopantetheinyltransferase required for biosynthesis of the siderophores ornibactin and pyochelin. Microbiology 157:349–361. 10.1099/mic.0.045559-0. [DOI] [PubMed] [Google Scholar]
- 32.Bunet R, Riclea R, Laureti L, Hôtel L, Paris C, Girardet JM, Spiteller D, Dickschat JS, Leblond P, Aigle B. 2014. A single Sfp-type phosphopantetheinyl transferase plays a major role in the biosynthesis of PKS and NRPS derived metabolites in Streptomyces ambofaciens ATCC 23877. PLoS One 9:e87607. 10.1371/journal.pone.0087607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puar MS, Chan TM, Hegde V, Patel M, Bartner P, Ng KJ, Pramanik BN, MacFarlane RD. 1998. Sch 40832: a novel thiostrepton from Micromonospora carbonacea. J. Antibiot. (Tokyo) 51:221–224. 10.7164/antibiotics.51.221. [DOI] [PubMed] [Google Scholar]
- 34.Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, Forslund K, Ceric G, Clements J, Heger A, Holm L, Sonnhammer ELL, Eddy SR, Bateman A, Finn RD. 2012. The Pfam protein families database. Nucleic Acids Res. 40:D290–D301. 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrmann KM. 1995. The shikimate pathway as an entry to aromatic secondary metabolism. Plant Physiol. 107:7–12. 10.1104/pp.107.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosher RH, Ranade NP, Schrempf H, Vining LC. 1990. Chloramphenicol resistance in Streptomyces: cloning and characterization of a chloramphenicol hydrolase gene from Streptomyces venezuelae. J. Gen. Microbiol. 136:293–301. 10.1099/00221287-136-2-293. [DOI] [PubMed] [Google Scholar]
- 37.Al-Bassam MM, Bibb MJ, Bush MJ, Chandra G, Buttner MJ. 2014. Response regulator heterodimer formation controls a key stage in Streptomyces development. PLoS Genet. 10:e1004554. 10.1371/journal.pgen.1004554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bibb MJ, Molle V, Buttner MJ. 2000. SigmaBldN, an extracytoplasmic function RNA polymerase sigma factor required for aerial mycelium formation in Streptomyces coelicolor A3(2). J. Bacteriol. 182:4606–4616. 10.1128/JB.182.16.4606-4616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aínsa JA, Parry HD, Chater KF. 1999. A response regulator-like protein that functions at an intermediate stage of sporulation in Streptomyces coelicolor A3(2). Mol. Microbiol. 34:607–619. 10.1046/j.1365-2958.1999.01630.x. [DOI] [PubMed] [Google Scholar]
- 40.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645. 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557–580. 10.1016/S0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 42.MacNeil DJ, Occi JL, Gewain KM, MacNeil T, Gibbons PH, Ruby CL, Danis SJ. 1992. Complex organization of the Streptomyces avermitilis genes encoding the avermectin polyketide synthase. Gene 115:119–125. 10.1016/0378-1119(92)90549-5. [DOI] [PubMed] [Google Scholar]
- 43.Paget MS, Chamberlin L, Atrih A, Foster SJ, Buttner MJ. 1999. Evidence that the extracytoplasmic function sigma factor sigmaE is required for normal cell wall structure in Streptomyces coelicolor A3(2). J. Bacteriol. 181:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gust B, Challis GL, Fowler K, Kieser T, Chater KF. 2003. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. U. S. A. 100:1541–1546. 10.1073/pnas.0337542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piffaretti JC, Arini A, Frey J. 1988. pUB307 mobilizes resistance plasmids from Escherichia coli into Neisseria gonorrhoeae. Mol. Gen. Genet. 212:215–218. 10.1007/BF00334687. [DOI] [PubMed] [Google Scholar]
- 46.Yanai K, Sumida N, Okakura K, Moriya T, Watanabe M, Murakami T. 2004. Para-position derivatives of fungal anthelmintic cyclodepsipeptides engineered with Streptomyces venezuelae antibiotic biosynthetic genes. Nat. Biotechnol. 22:848–855. 10.1038/nbt978. [DOI] [PubMed] [Google Scholar]
- 47.Makris TM, Chakrabarti M, Münck E, Lipscomb JD. 2010. A family of diiron monooxygenases catalyzing amino acid beta-hydroxylation in antibiotic biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 107:15391–15396. 10.1073/pnas.1007953107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Latimer R, Podzelinska K, Soares A, Bhattacharya A, Vining LC, Jia Z, Zechel DL. 2009. Expression, purification and preliminary diffraction studies of CmlS. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 65:260–263. 10.1107/S1744309108043091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.