Abstract
Here, we evaluated the in vitro anti-HIV-1 activity of the experimental CCR5 inhibitor VCH-286 as a single agent or in combination with various classes of HIV-1 inhibitors. Although VCH-286 used alone had highly inhibitory activity, paired combinations with different drug classes led to synergistic or additive interactions. However, combinations with other CCR5 inhibitors led to effects ranging from synergy to antagonism. We suggest that caution should be exercised when combining CCR5 inhibitors in vivo.
TEXT
HIV entry inhibitors represent a diverse group of drugs targeting multiple steps of the viral entry process. Among these drugs, the chemokine coreceptor CCR5 antagonist maraviroc is the only approved drug for combination therapy for the treatment of HIV-1 (1–3). The curing of an HIV-infected patient with acute myeloid leukemia treated with the hematopoietic stem/progenitor cells from a CCR5Δ32 homozygous donor highlighted the critical role of the CCR5 coreceptor in HIV infection and disease progression, and it raised hopes for HIV eradication using therapeutic approaches that inactivate CCR5 (4–7). Because of their broad therapeutic potentials, CCR5 inhibitors represent an interesting group of drug candidates. Of note, CCR5 inhibitors are not limited to the treatment of HIV infection, as CCR5 has been implicated in the pathophysiologies of a number of inflammatory diseases, such as transplant rejection, autoimmune diseases (e.g., multiple sclerosis), type 1 diabetes, colitis, and rheumatoid arthritis (8, 9). CCR5 inhibitors have been shown to reduce plaque formation in atherosclerosis and participate in the anti-tumor immune responses mediated by CCR5-expressing leukocytes (9).
CCR5 inhibitors include different members, such as maraviroc (MVC) (UK-42785; Selzentry), vicriviroc (VVC), aplaviroc (AVC), and TAK-779 (10). This group of small-molecule inhibitors binds to the hydrophobic pockets located in the transmembrane domains of the HIV-1 cellular coreceptor CCR5, which induces conformational changes in CCR5. These changes inhibit HIV-1 entry by allosteric mechanisms preventing the binding of the viral protein gp120 to CCR5 (1, 11). Maraviroc (MVC), a phenylpropylamine, was the first CCR5 inhibitor approved by the FDA in 2007 for HIV-1 treatment in combination with other antiretrovirals for treatment-experienced patients, and as a first-line therapy in 2009 (1, 11, 12). The development of vicriviroc (VVC), a piperidinopiperidine and another CCR5 inhibitor tested in clinical trials, was discontinued because of suboptimal efficacy (1, 13, 14). Cenicriviroc, a CCR5/CCR2 antagonist, is currently under development in a phase II study (15). Finally, VCH-286 (a citrate salt, Fig. 1A) from ViroChem, Inc., Canada (now Vertex Pharmaceuticals), is a novel CCR5 antagonist. A phase I clinical study with VCH-286 in healthy volunteers showed favorable pharmacokinetics and safety profiles, and it has recently received phase II regulatory approval (16, 17).
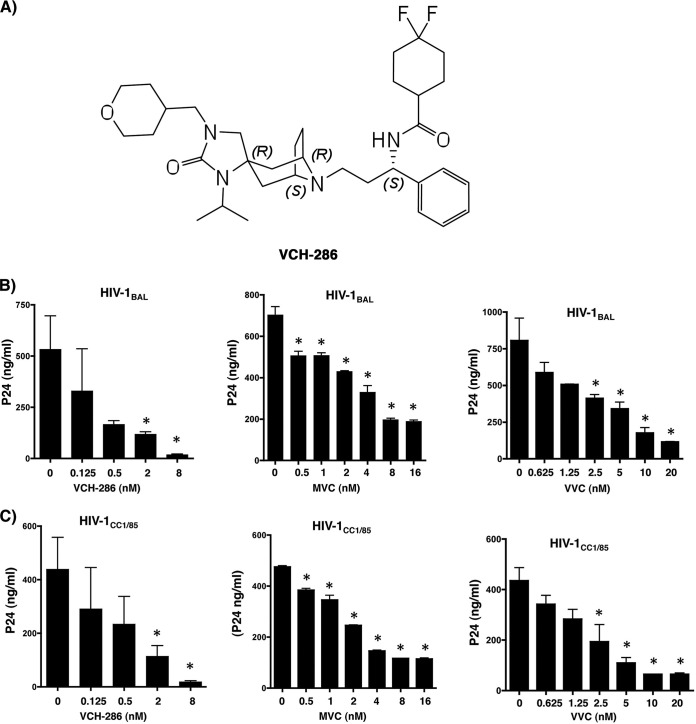
FIG 1.
(A) Chemical structure of the new CCR5 inhibitor VCH-286, a citrate salt. (B) Inhibitory effects of VCH-286 (left), MVC (middle), and VVC (right) on HIV-1BAL. (C) Inhibitory effects of VCH-286 (left), MVC (middle), and VVC (right) on HIV-1CC1/85. The core viral protein p24 was measured from the culture supernatant by commercial enzyme-linked immunoassay (PerkinElmer) at day 7 postinfection (mean ± SD from three independent experiments). *, P < 0.05 calculated by paired t test (comparing the p24 production by HIV-infected cells with each drug concentration relative to infected nontreated cells). Cell viability was assessed by the exclusion method using the Trypan blue dye.
As more members of this class of entry inhibitors make their way through the process of development for use in HIV treatment, it is important to evaluate their interactions and rule out any antagonistic effects (4). Therefore, in this work, we aimed to evaluate the in vitro interactions of a new candidate CCR5 inhibitor, VCH-286, with other members of the same class, MVC and VVC, and also with representative candidates from other classes of HIV inhibitors.
We first established the inhibitory effects of the three CCR5 inhibitors MVC, VVC, and VCH-286 using a dose-response inhibitory assay against two HIV-1 R5 isolates, the laboratory strain HIV-1BAL and the clinical isolate HIV-1CC1/85 (18–21). Viral infections were carried out on total peripheral blood mononuclear cells (PBMCs) from three HIV- and hepatitis B virus-seronegative donors (all participants were adults and signed written informed consent approved by the Centre de Recherche du Centre Hospitalier de l'Université de Montréal [CRCHUM] institutional review boards). The cells were isolated by Ficoll-Paque gradient separation and stimulated for 3 days with phytohemagglutinin (PHA) (1 mg/ml) and interleukin-2 (1 μg/ml) in 24-well tissue culture plates, followed by infection with 3,000× the tissue culture infectious doses (TCID) of the HIV-1 R5 viruses. As shown in Fig. 1B and C, viral replication of both HIV strains was readily inhibited by the three CCR5 inhibitors when monitored by the production of the viral core protein p24 (measured by enzyme-linked immunosorbent assay [ELISA]). The 50% inhibitory concentrations (IC50s) (calculated by dose-effect analysis using the CalcuSyn software [Biosoft, Cambridge, United Kingdom]) were used to determine the antiviral activities of the three drugs, as these compounds act at the cell surface and are not dependent on cellular uptake and metabolism. The IC50s against the HIV-1BAL strain for MVC, VVC, and VCH-286 were 1.85 nM, 3.38 nM, and 0.23 nM, respectively (Table 1). The IC50s against HIV-1CC1/85 for MVC, VVC, and VCH-286 were 4.39 nM, 3.78 nM, and 0.34 nM, respectively (Table 1). Of note, no toxicity was observed in the uninfected PBMCs with concentrations up to 1,000 nM with any of these three drugs. These results are thus consistent with earlier reports of strong antiviral activities of MVC and VVC against both HIV-1BAL and HIV-1CC1/85 infections (12, 22). Moreover, VCH-286 showed a significant inhibition of viral replication at drug concentrations that were lower than those of the two other drugs (i.e., IC50s 8- to 14-fold lower than those of MVC and VVC).
TABLE 1.
IC50s obtained for MVC, VVC, and VCH-286 against the R5 viruses HIV-1BAL and HIV-1CC1/85a
| Drug | IC50 (nM) for: |
|
|---|---|---|
| HIV-1BAL | HIV-1CC1/85 | |
| MVC | 1.85 | 4.39 |
| VVC | 3.38 | 3.78 |
| VCH-286 | 0.23 | 0.34 |
IC50, 50% inhibitory concentration; MVC, maraviroc; VVC, vicriviroc.
We further evaluated the impact of drug interactions through paired combinations between MCV, VVC, and VCH-286 on HIV replication, using the same experimental settings. Drug combinations using CCR5 inhibitors may represent an interesting approach, as the association and dissociation rates to the CCR5 receptor may differ between the drug candidates, thus providing a pharmacodynamic advantage in maintaining adequate receptor occupancy (23). Therefore, we opted to define whether different drug combinations would result in synergistic effects. Synergism takes place when the combination is more effective than single-agent use; one of the agents increases the actions of the second drug. Antagonism is when the combination is less effective than with the use of single agents; one of the agents counteracts the actions of the other. We also employed a multiple-drug effect analysis. This multiple-drug effect analysis is based on the median-effect principle and the isobologram technique (24). While the IC50, IC75, and IC90 are the concentrations required to inhibit 50%, 75%, and 90%, respectively, the combination indexes (CI) CI50, CI75, and CI90 of any given combination of two drugs provide information on the nature and extent of drug interaction at the IC50, IC75, and IC90 of each drug, respectively. A combination was defined as synergistic when the CI value was <1, additive when the CI was 1, and antagonistic when the CI was >1, as described earlier (25). Combinations of CCR5 inhibitors showed interactions ranging from synergy to antagonism, as illustrated by the combination indices (CI) shown in Table 2. The interaction of MVC and VCH-286 was highly synergistic under all tested concentrations against both viral isolates, with CI90 values of 0.41 (mean, 0.47 and 0.35 from two independent experiments) and 0.43 (mean, 0.44 and 0.42) for HIV-1BAL and HIV-1CC1/85, respectively. In contrast, combinations of MVC with VVC showed highly antagonistic interactions against both HIV isolates under the different inhibitory concentrations tested, with CI90 values of 5.61 (mean, 5.99 and 5.24 from two independent experiments) and 1.86 (mean, 1.37 and 2.34) for HIV-1BAL and HIV-1CC1/85, respectively. Meanwhile, the interaction between VVC and VCH-286 was additive, with a CI90 value of 1.08 (mean, 1.1 and 1.05 from two independent experiments) against HIV-1BAL. However, this same combination performed in an antagonistic fashion against HIV-1CC1/85, with a CI90 value of 2.22 (mean, 2.52 and 1.92 from two independent experiments).
TABLE 2.
Combination indices for MVC, VVC, and VCH-286 against the R5 viruses HIV-1BAL and HIV-1CC1/85a
| Virus | Drug combination | CI50 | SD | CI75 | SD | CI90 | SD | Interpretationb |
|---|---|---|---|---|---|---|---|---|
| HIV-1BAL | MVC + VCH-286 | 0.76 | 0.05 | 0.56 | 0.03 | 0.41 | 0.08 | Synergy |
| MVC + VVC | 9.53 | 0.629 | 7.49 | 0.377 | 5.61 | 0.53 | Antagonism | |
| VVC + VCH-286 | 0.96 | 0.06 | 1.055 | 0.007 | 1.08 | 0.03 | Additive | |
| HIV-1CC1/85 | MVC + VCH-286 | 0.61 | 0.01 | 0.50 | 0.002 | 0.43 | 0.014 | Synergy |
| MVC + VVC | 10.17 | 0.7 | 4.22 | 0.54 | 1.86 | 0.68 | Antagonism | |
| VVC + VCH-286 | 0.68 | 0.23 | 1.91 | 0.098 | 2.22 | 0.42 | Antagonism |
Data are presented as the means from two independent experiments (three replicates per condition for each experiment) ± standard deviations (SD). The ranges of doses used for MVC, VVC, and VCH-286 were as follows: 0.0128, 0.064, 0.32, 1.6, 8, 40, 200, and 1,000 nM.
Combination index (CI) interpretation: <1, synergy; 1, additive; and >1, antagonism.
VCH-286 was further evaluated in dual combinations with representative drugs from each of the currently approved antiretroviral classes: the nucleoside reverse transcriptase inhibitors zidovudine (AZT) and lamivudine (3TC), the nonnucleoside reverse transcriptase inhibitors nevirapine (NVP) and efavirenz (EFV), the protease inhibitors lopinavir (LPV) and saquinavir (SQV), the integrase inhibitor raltegravir (RTG), and the fusion inhibitor enfuvirtide (Fuzeon, T-20) (Table 3). The laboratory-adapted strain HIV-1BAL and the clinical isolate HIV-1CC1/85 were both susceptible to almost all the antiretroviral drug combinations with VCH-286 used in this study, and synergistic or additive interactions were observed, as shown in Table 3. The synergistic and additive effects of the combination of VCH-286 with other drug candidates are consistent with our earlier observations (26) and those of others (12) on the combination of the CCR5 inhibitors in vitro. Only two exceptions with moderate and significant antagonistic effects were observed for HIV-1BAL and HIV-1CC1/85 when combining VCH-286 with lopinavir and 3TC, respectively (Table 3). The CI90 for the combination of VCH-286 with lopinavir against HIV-1BAL was 1.39 (mean, 1.69 and 1.09 from two independent experiments), whereas the CI90 for the combination of VCH-286 with 3TC against HIV-1CC1/85 was 2.03 (mean, 2.2 and 1.85). Although we did not study the mechanism(s) underlying the clear antagonism between the CCR5 inhibitor VCH-286 and the nucleoside reverse transcriptase inhibitor (NRTI) 3TC, this might be related to a potential interference with the cell activation process. The NRTI 3TC is known to be dependent on the cellular machinery in order to be transformed from the initial monophosphate to the triphosphate active form (27), a step that might be affected by the interference with CCR5 signaling by VCH-286. On the other hand, the moderate antagonism with lopinavir might be related to an unappreciated low level of cytotoxicity mediated by the drug combination. Of note, the cytotoxicities for all single drugs and drug combinations were assessed by treating noninfected cells with the highest concentrations used in the current study. Cell viability was tested by the Trypan blue exclusion method and showed negligible effects.
TABLE 3.
Combination indices for VCH-286 and reverse transcriptase, protease, integrase, and fusion inhibitors at various inhibitory concentrations against the R5 viruses HIV-1BAL and HIV-1CC1/85a
| Virus | Drug | CI50 | SD | CI75 | SD | CI90 | SD | Interpretationb |
|---|---|---|---|---|---|---|---|---|
| HIV-1BAL | AZT | 0.780 | 0.145 | 0.762 | 0.030 | 0.768 | 0.064 | Synergy |
| 3TC | 1.620 | 0.083 | 1.168 | 0.032 | 0.899 | 0.028 | Synergy | |
| NVP | 0.683 | 0.022 | 0.621 | 0.141 | 0.633 | 0.235 | Synergy | |
| EFV | 1.070 | 0.101 | 1.913 | 0.075 | 0.824 | 0.209 | Additive | |
| LPV | 1.143 | 0.914 | 1.235 | 0.701 | 1.397 | 0.421 | Moderate antagonism (33) | |
| SQV | 0.297 | 0.271 | 0.170 | 0.049 | 0.390 | 0.015 | Synergy | |
| RTG | 0.776 | 0.158 | 0.551 | 0.058 | 0.427 | 0.156 | Synergy | |
| T-20 | 0.704 | 0.285 | 0.674 | 0.015 | 0.722 | 0.218 | Synergy | |
| HIV-1CC1/85 | AZT | 2.859 | 3.085 | 1.167 | 0.602 | 0.747 | 0.094 | Synergy |
| 3TC | 1.900 | 0.136 | 2.037 | 0.033 | 2.031 | 0.252 | Antagonism | |
| NVP | 0.686 | 0.081 | 0.911 | 0.086 | 0.741 | 0.003 | Synergy | |
| EFV | 1.039 | 0.145 | 0.883 | 0.033 | 0.790 | 0.161 | Synergy | |
| LPV | 1.243 | 0.144 | 0.849 | 0.013 | 0.568 | 0.036 | Synergy | |
| SQV | 0.342 | 0.207 | 0.237 | 0.144 | 0.479 | 0.148 | Synergy | |
| RTG | 0.969 | 0.034 | 0.933 | 0.035 | 0.992 | 0.003 | Synergy | |
| T-20 | 0.741 | 0.056 | 0.734 | 0.003 | 0.802 | 0.134 | Synergy |
Data are presented as the means from two independent experiments (three replicates per condition for each experiment) ± standard deviations (SD). The ranges of doses used for MVC, VVC, and VCH-286 were as follows: 0.0128, 0.064, 0.32, 1.6, 8, 40, 200, and 1,000 nM.
Combination index (CI) interpretation: <1, synergy; 1, additive; and >1, antagonism.
Altogether, our results clearly show that the new CCR5 inhibitor VCH-286 performed well when used as a single agent, with IC50s 8- to 14-fold lower than those of the two other CCR5 inhibitor drugs. It also showed favorable combination indexes with drug candidates from different classes of viral inhibitors. However, its interactions with other CCR5 inhibitors ranged from synergistic to antagonistic, depending on the agent with which it was combined and the viral isolate. This variation in interactions suggests that overlapping binding sites of drugs on the CCR5 protein are likely involved in the antagonistic effects. Small-molecule inhibitors of CCR5 bind to the pocket formed by the transmembrane (TM) domain of CCR5 in helices 1, 2, 3, 5, and 7 (28). Although all inhibitors bind to the same hydrophobic pocket, they occupy different subcavities (29). The nature of the specific interactions within this pocket is unique to each molecule, as they have different electrostatic shapes and polarities. Interestingly, the key residues involved in the interactions of CCR5 with MVC and VVC are similar (Glu283 on TM7, Tyr108 on TM3, Ile198 on TM5, and Tyr251 on TM6 [30]), and this overlapping binding is likely to explain the antagonistic effect that we observed upon combining these drugs in vitro. Our results are therefore consistent with earlier reports on the combination of CCR5 inhibitors. Nakata et al. (31) reported that a combination of the CCR5 inhibitor aplaviroc (AVC) and other members from the same class, such as TAK-779 and SCH-C, leads to mild synergism and additivity, respectively. Similarly, Murga et al. (32) observed a significant synergy for the humanized CCR5 monoclonal antibody (MAb) PRO 140 in combination with three small-molecule CCR5 inhibitors (maraviroc, vicriviroc, and TAK-779), with CI values from 0.36 to 0.61, but additive effects were observed with the combination of MVC and VVC.
In vitro studies of drug interactions have proven to be beneficial in predicting which drug combination regimens should be evaluated in a clinical setting (12–14). In the present study, we also evaluated the interactions between VCH-286 and representatives from each class of currently available antiretroviral agents in vitro. We have found that in the nanomolar range, VCH-286 exerted synergistic activity against two HIV-1 R5 viruses when it was combined with AZT, NVP, SQV, RTG, and T-20.
In conclusion, our current study highlights the efficacy of VCH-286 as a new antiviral agent inhibiting HIV-1 binding to CCR5. It has favorable drug interactions with antiretrovirals (ARVs) used in the clinic to treat HIV/AIDS, such as reverse transcriptase, protease, integrase, and fusion inhibitors, thus suggesting that VCH-286 may be a useful anti-HIV drug in combination therapy. However, we raise the possibility that antagonistic effects with the combination of CCR5 inhibitors, including this new drug candidate, may take place in vivo; hence, caution should be exercised when considering this type of combination in a potential treatment regimen.
ACKNOWLEDGMENTS
This work was supported by an unrestricted educational grant from CANFAR and Réseau FRQS-SIDAmi. C.L.T. is a scholar from the “Fonds de Recherche du Québec en Santé” and is Pfizer/University of Montreal chair on HIV translational research.
We thank ViroChem, Inc., Canada (now Vertex Pharmaceuticals), for providing the VCH-286 compound and its chemical structure for the current study. We also thank Jean Bédard (ViroChem) for his guidance and discussions.
Footnotes
Published ahead of print 29 September 2014
REFERENCES
- 1.Berro R, Klasse PJ, Jakobsen MR, Gorry PR, Moore JP, Sanders RW. 2012. V3 determinants of HIV-1 escape from the CCR5 inhibitors maraviroc and vicriviroc. Virology 427:158–165. 10.1016/j.virol.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuritzkes DR. 2009. HIV-1 entry inhibitors: an overview. Curr. Opin. HIV AIDS 4:82–87. 10.1097/COH.0b013e328322402e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veiga AS, Santos NC, Castanho MA. 2006. An insight on the leading HIV entry inhibitors. Recent Pat. Antiinfect. Drug Discov. 1:67–73. 10.2174/157489106775244046. [DOI] [PubMed] [Google Scholar]
- 4.Haqqani AA, Tilton JC. 2013. Entry inhibitors and their use in the treatment of HIV-1 infection. Antiviral Res. 98:158–170. 10.1016/j.antiviral.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Allers K, Hutter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, Schneider T. 2011. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood 117:2791–2799. 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 6.Hütter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, Schneider T, Hofmann J, Kucherer C, Blau O, Blau IW, Hofmann WK, Thiel E. 2009. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 360:692–698. 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 7.Hütter G, Ganepola S. 2011. Eradication of HIV by transplantation of CCR5-deficient hematopoietic stem cells. ScientificWorldJournal 11:1068–1076. 10.1100/tsw.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribeiro S, Horuk R. 2005. The clinical potential of chemokine receptor antagonists. Pharmacol. Ther. 107:44–58. 10.1016/j.pharmthera.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Corbeau P, Reynes J. 2009. CCR5 antagonism in HIV infection: ways, effects, and side effects. AIDS 23:1931–1943. 10.1097/QAD.0b013e32832e71cd. [DOI] [PubMed] [Google Scholar]
- 10.Briz V, Poveda E, Soriano V. 2006. HIV entry inhibitors: mechanisms of action and resistance pathways. J. Antimicrob. Chemother. 57:619–627. 10.1093/jac/dkl027. [DOI] [PubMed] [Google Scholar]
- 11.Maeda K, Das D, Nakata H, Mitsuya H. 2012. CCR5 inhibitors: emergence, success, and challenges. Expert Opin. Emerg. Drugs 17:135–145. 10.1517/14728214.2012.673584. [DOI] [PubMed] [Google Scholar]
- 12.Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, Mori J, Rickett G, Smith-Burchnell C, Napier C, Webster R, Armour D, Price D, Stammen B, Wood A, Perros M. 2005. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 49:4721–4732. 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berro R, Klasse PJ, Jakobsen MR, Gorry PR, Moore JP, Sanders RW. 2012. V3 determinants of HIV-1 escape from the CCR5 inhibitors maraviroc and vicriviroc. Virology 427:158–165. 10.1016/j.virol.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strizki JM, Tremblay C, Xu S, Wojcik L, Wagner N, Gonsiorek W, Hipkin RW, Chou CC, Pugliese-Sivo C, Xiao Y, Tagat JR, Cox K, Priestley T, Sorota S, Huang W, Hirsch M, Reyes GR, Baroudy BM. 2005. Discovery and characterization of vicriviroc (SCH 417690), a CCR5 antagonist with potent activity against human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 49:4911–4919. 10.1128/AAC.49.12.4911-4919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagan RM, Johnson EP, Siaw MF, Van Baelen B, Ogden R, Platt JL, Pesano RL, Lefebvre E. 2014. Comparison of genotypic and phenotypic HIV type 1 tropism assay: results from the screening samples of Cenicriviroc Study 202, a randomized phase II trial in treatment-naive subjects. AIDS Res. Hum. Retroviruses 30:151–159. 10.1089/aid.2013.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhopale GM. 2012. Emerging drugs for the treatment of human immunodeficiency virus. Recent Pat. Antiinfect. Drug Discov. 7:45–52. 10.2174/157489112799829729. [DOI] [PubMed] [Google Scholar]
- 17.Proulx LC, Clermont N, Laterreur PJ, Thibert R. 2008. Results of a phase I study to evaluate the safety, tolerability, pharmacokinetics (with and without ritonavir) and food-effect of VCH-286. J. Int. AIDS Soc. 11(Suppl 1):298. 10.1186/1758-2652-11-S1-P298. [DOI] [Google Scholar]
- 18.Connor RI, Mohri H, Cao Y, Ho DD. 1993. Increased viral burden and cytopathicity correlate temporally with CD4+ T-lymphocyte decline and clinical progression in human immunodeficiency virus type 1-infected individuals. J. Virol. 67:1772–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhmann SE, Pugach P, Kunstman KJ, Taylor J, Stanfield RL, Snyder A, Strizki JM, Riley J, Baroudy BM, Wilson IA, Korber BT, Wolinsky SM, Moore JP. 2004. Genetic and phenotypic analyses of human immunodeficiency virus type 1 escape from a small-molecule CCR5 inhibitor. J. Virol. 78:2790–2807. 10.1128/JVI.78.6.2790-2807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR. 1997. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621–628. 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. 1986. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 233:215–219. 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 22.Westby M, Smith-Burchnell C, Mori J, Lewis M, Mosley M, Stockdale M, Dorr P, Ciaramella G, Perros M. 2007. Reduced maximal inhibition in phenotypic susceptibility assays indicates that viral strains resistant to the CCR5 antagonist maraviroc utilize inhibitor-bound receptor for entry. J. Virol. 81:2359–2371. 10.1128/JVI.02006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swinney DC, Beavis P, Chuang KT, Zheng Y, Lee I, Gee P, Deval J, Rotstein DM, Dioszegi M, Ravendran P, Zhang J, Sankuratri S, Kondru R, Vauquelin G. 2014. A study of the molecular mechanism of binding kinetics and long residence times of human CCR5 receptor small molecule allosteric ligands. Br. J. Pharmacol. 171:3364–3375. 10.1111/bph.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou TC, Talalay P. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22:27–55. 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 25.Chou TC. 1991. The median-effect principle and the combination index for quantitation of synergism and antagonism, p 61–102 In Chou TC, Rideout DC. (ed), Synergism and antagonism in chemotherapy. Academy Press, New York, NY. [Google Scholar]
- 26.Tremblay CL, Giguel F, Kollmann C, Guan Y, Chou TC, Baroudy BM, Hirsch MS. 2002. Anti-human immunodeficiency virus interactions of SCH-C (SCH 351125), a CCR5 antagonist, with other antiretroviral agents in vitro. Antimicrob. Agents Chemother 46:1336–1339. 10.1128/AAC.46.5.1336-1339.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furman PA, Fyfe JA, St Clair MH, Weinhold K, Rideout JL, Freeman GA, Lehrman SN, Bolognesi DP, Broder S, Mitsuya H. 1986. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc. Natl. Acad. Sci. U. S. A. 83:8333–8337. 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dragic T, Trkola A, Thompson DA, Cormier EG, Kajumo FA, Maxwell E, Lin SW, Ying W, Smith SO, Sakmar TP, Moore JP. 2000. A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc. Natl. Acad. Sci. U. S. A. 97:5639–5644. 10.1073/pnas.090576697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan Q, Zhu Y, Li J, Chen Z, Han GW, Kufareva I, Li T, Ma L, Fenalti G, Li J, Zhang W, Xie X, Yang H, Jiang H, Cherezov V, Liu H, Stevens RC, Zhao Q, Wu B. 2013. Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex. Science 341:1387–1390. 10.1126/science.1241475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kondru R, Zhang J, Ji C, Mirzadegan T, Rotstein D, Sankuratri S, Dioszegi M. 2008. Molecular interactions of CCR5 with major classes of small-molecule anti-HIV CCR5 antagonists. Mol. Pharmacol. 73:789–800. 10.1124/mol.107.042101. [DOI] [PubMed] [Google Scholar]
- 31.Nakata H, Steinberg SM, Koh Y, Maeda K, Takaoka Y, Tamamura H, Fujii N, Mitsuya H. 2008. Potent synergistic anti-human immunodeficiency virus (HIV) effects using combinations of the CCR5 inhibitor aplaviroc with other anti-HIV drugs. Antimicrob. Agents Chemother. 52:2111–2119. 10.1128/AAC.01299-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murga JD, Franti M, Pevear DC, Maddon PJ, Olson WC. 2006. Potent antiviral synergy between monoclonal antibody and small-molecule CCR5 inhibitors of human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 50:3289–3296. 10.1128/AAC.00699-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chou TC. 2006. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 58:621–681. 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]