Abstract
Caspofungin exhibits potent antifungal activities against Candida and Aspergillus species. The elimination rate and retinal toxicity of caspofungin were determined in this study to assess its pharmacokinetics and safety in the treatment of fungal endophthalmitis. Intravitreal injections of 50 μg/0.1 ml of caspofungin were administered to rabbits. Levels of caspofungin in the vitreous and aqueous humors were determined using high-performance liquid chromatography (HPLC) at selected time intervals (10 min and 1, 2, 4, 8, 16, 24, and 48 h), and the half-lives were calculated. Eyes were intravitreally injected with caspofungin to obtain concentrations of 10 μg/ml, 50 μg/ml, 100 μg/ml, and 200 μg/ml. Electroretinograms were recorded 4 weeks after injections, and the injected eyes were examined histologically. The concentrations of intravitreal caspofungin at various time points exhibited an exponential decay with a half-life of 6.28 h. The mean vitreous concentration was 6.06 ± 1.76 μg/ml 1 h after intravitreal injection, and this declined to 0.47 ± 0.15 μg/ml at 24 h. The mean aqueous concentration showed undetectable levels at all time points. There were no statistical differences in scotopic a-wave and b-wave responses between control eyes and caspofungin-injected eyes. No focal necrosis or other abnormality in retinal histology was observed. Intravitreal caspofungin injection may be considered to be an alternative treatment for fungal endophthalmitis based on its antifungal activity, lower retinal toxicity, and lower elimination rate in the vitreous. More clinical data are needed to determine its potential role as primary therapy for fungal endophthalmitis.
INTRODUCTION
Fungal endophthalmitis, although uncommon, can cause serious ocular devastation and has an ominous prognosis even with prompt treatment. Fungal endophthalmitis can be either exogenous, such as in cases of ocular surgery, trauma, and keratitis, or endogenous, with infection spreading to the eye, such as in patients receiving immunosuppressive therapy and intravenous infusion from indwelling catheters. According to previous studies, fungal endophthalmitis accounts for 8% to 18% of culture-proven endophthalmitis, and Candida spp. and Aspergillus spp. are the most frequently isolated organisms (1–5).
In the past decade, intravitreal antibiotic injection has become a mainstay of treatment for fungal endophthalmitis. Amphotericin B and voriconazole are the two antifungal agents used for injection into the vitreous. However, amphotericin B may cause retinal necrosis at low concentrations, and a variety of fungal species have shown resistance to it (6–8). Although previous studies have shown voriconazole to have a broad spectrum of activity and to be effective as primary therapy in the treatment of invasive aspergillosis, 50 μg/ml of intravitreal voriconazole has been found to cause small foci of retinal necrosis in animal studies (9–11). Moreover, voriconazole has a relatively rapid elimination rate in the vitreous, and supplementary injection is frequently required in clinical treatment (12, 13). Systemically administered voriconazole which would be given simultaneously with intravitreal injection would penetrate into the vitreous. In addition to voriconazole, fluconazole also penetrates well into the vitreous, and both agents have been effective in treating fungal endophthalmitis through intravenous administration (14, 15). Intravitreal injection of liposomal amphotericin B, which has less toxicity than amphotericin B, was used to treat a patient with bilateral endogenous Candida endophthalmitis (16).
Caspofungin noncompetitively inhibits β(1,3)-d-glucan synthase, an enzyme that is necessary for the synthesis of the cell wall in many fungal species, and exhibits potent in vitro and in vivo antifungal activity against Candida spp. and Aspergillus spp., including pathogens resistant to azole or amphotericin B (17–20). Furthermore, caspofungin's synergistic effects have been observed in combination with a polyene or an azole, and attempts have been made to use these combinations as systemic therapy of endophthalmitis. In vitro studies have shown MICs of caspofungin ranging from 0.03 to 1 μg/ml for Candida species and a MIC of 0.06 μg/ml for the vast majority of Aspergillus species. Caspofungin has low oral bioavailability (less than 0.2%). It is distributed well to tissues through intravenous administration but with minimal penetration into the eye due to its high level of protein binding and high molecular mass (1,213 Da). Intravitreal injection of caspofungin could be considered as an alternative in treating fungal endophthalmitis if lower retinal toxicity and slower elimination in the vitreous could be documented. Because the treatment options for fungal endophthalmitis are limited and caspofungin has good fungicidal and fungistatic activity, the safety and pharmacokinetics of caspofungin as an intravitreal agent need to be evaluated.
In this study, we determined the elimination rate and retinal toxicity of intravitreal caspofungin in rabbits and tried to assess the safety and optimum dosage of intravitreal injection required to maintain therapeutic levels in the vitreous.
MATERIALS AND METHODS
Animals.
Caspofungin (Cancidas; Merck & Co., Albuquerque, NM, USA) was obtained in pure powder form and reconstituted in sterile water to yield a concentration of 50 μg/0.1 ml. Seventeen New Zealand White rabbits weighing 2 to 2.5 kg were acclimated for at least 1 week under standardized temperature (25 to 28°C), humidity (50 to 60%), and light (12 h light-dark) conditions before the experiment. All care and handling of rabbits was performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, with the approval of the Institutional Authority for Laboratory Animal Care at Taichung Veterans General Hospital.
The rabbits were anesthetized with a mixture of ketamine hydrochloride (35 mg/kg; Parke-Davis, Morris Plains, NJ) and xylazine (5 mg/kg; Astra, Astra Södertälje, Sweden) intramuscularly in the hindquarter. Both eyes of each rabbit were included in the experiment. An anterior chamber paracentesis was performed followed by an injection of 50 μg caspofungin in 0.1 ml sterilized distilled water at a site 2 mm posterior to the limbus. Treatment was administered using a 30-gauge needle attached to a regular insulin syringe with the bevel positioned upward in the midvitreous of the eyes, slowly and under direct visualization. A cotton tipped applicator was applied to the injection site immediately after removal of the needle to prevent fluid reflux from the injection site. Mydriasis was achieved with two to three drops of 1% tropicamide, and the fundus was examined by indirect ophthalmoscopy before and after injections. Aqueous humor samples were obtained using a 30-gauge needle, and the sampling was performed on two rabbits at each time interval (10 min and 1, 2, 4, 8, 16, 24, and 48 h) after injection and before enucleation of eyes. Rabbits were sacrificed with a lethal cardiac injection of pentobarbital sodium and phenytoin sodium (Beuthanasia-D; Schering Animal Health, Kenilworth, NJ). Four eyes per time interval up to 48 h and both eyes of an additional control rabbit were enucleated on the same day and immediately frozen at −80°C. The eyes were dissected while frozen, and the entire vitreous was isolated according to the technique described by Abel and Boyle (21). The vitreous of the control eyes was isolated to obtain standardization curves for HPLC analyses. Assays of caspofungin concentrations in vitreous and aqueous humor samples were performed with high-performance liquid chromatography (HPLC).
HPLC.
Caspofungin concentrations of vitreous and aqueous humors were determined using HPLC. Analysis of the samples was performed in a blinded fashion. The HPLC method of Spriet et al. was adapted (22). The rabbit vitreous samples and caspofungin standard (150 μl) were each pretreated with the addition of 600 μl of 100% methanol followed by vortex mixing at high speed for 1 min at room temperature. The mixtures were centrifuged in a microultracentrifuge (CS 120 GX; Hitachi, Japan) at 45,000 rpm for 30 min at 4°C. A 450-μl portion of supernatant was transferred to a clean tube and dried in a centrifugal vacuum concentrator (Speed Vac Plus SC110; Savant Instruments, Inc., Holbrook, NY). The injection samples were redissolved in 120 μl of 20% methanol containing 0.1% trifluoroacetic acid (TFA) followed by 1 min of vortex mixing. Insoluble particles were removed by ultracentrifugation at 45,000 rpm for 30 min at 4°C. The samples of aqueous humor (90 μl) were extracted with 450 μl of 100% methanol, and 450 μl of supernatant was removed for drying after ultracentrifugation. After drying, the samples were redissolved in 90 μl of 20% methanol containing 0.1% TFA.
The samples were analyzed with an HPLC system including a gradient HPLC pump (Aligent 1100; Aligent Technologies, Germany) and a UV-VIS detector (S-3702; Soma, Japan) interfaced to a Chromato-integrator (D-2500, Hitachi, Japan). The gradient eluting system consisted of 0.1% TFA in deionized water (mobile phase A) versus 0.1% TFA in methanol (mobile phase B) with a flow rate of 1.5 ml/min. A 20-μl volume of each sample was injected onto a reverse-phase (RP) column (LiChrospher 100RP-18e; 250 mm by 4 mm, 5 μm; Aligent Technologies, Germany; column temperature of 35°C), which was pre-equilibrated with 20% mobile phase B. Caspofungin was eluted with a linear gradient of methanol (20% to 60% containing 0.1% TFA).
Caspofungin was monitored by absorbance at 215 nm and identified by coinjection with standard. The area of the caspofungin peak after baseline subtraction was calculated and compared with the area-versus-mass curve for the standard to quantify the amounts of caspofungin in the samples. The standard curve was linear to 20 μg/ml (correlation coefficient = 0.9997 for the range 0.2 to 20.0 μg/ml), and the detection limit was estimated to be approximately 0.1 μg/ml (signal-to-noise ratio > 2). Samples with caspofungin outside the linear range were properly diluted with 20% methanol for further HPLC analysis.
Electroretinogram and histopathologic analysis.
Sixteen New Zealand White albino rabbits were given an intravitreal injection of one of four caspofungin doses: 15, 75, 150, or 300 μg in 0.1 ml. Caspofungin solutions were serially diluted with balanced salt solution (BSS) (Alcon Labs, Inc.) so that the final intravitreal concentrations were 10 μg/ml, 50 μg/ml, 100 μg/ml, and 200 μg/ml, based on the data that adult rabbit vitreous volume is about 1.4 ml. Each animal's non-caspofungin-treated eye served as a control and was injected intravitreally with 0.1 ml of BSS instead of caspofungin. After the injections, all eyes were examined weekly by ophthalmoscopy. Animals were kept under ambient light on a 12-hour light/dark schedule. Four weeks after injection, animals were processed for electroretinogram recordings and subsequent retinal histologic examinations.
Prior to testing, rabbits were allowed to adapt to darkness overnight and were anesthetized with a solution of ketamine and xylazine. Several drops of 0.5% tropicamide (Mydriacyl) and 2.5% phenylephrine for pupil dilatation and a drop of 0.5% proparacaine hydrochloride for corneal anesthesia were applied. A single bright-flash electroretinogram (ERG) (UTAS-E 300; LKC Technology, Gaithersburg, MD, USA) was performed to assess safety and retinal function. A small amount (2.5%) of methylcellulose gel was applied to the eye, and a gold electrode was placed in contact with the center of the cornea. A reference electrode was attached to the shaven skin of the scalp and a ground electrode was clipped to the rabbit's tail. For dark-adapted ERG, the luminance of the stimulus was 3 cd/m2 with a duration of 10 ms. Five responses elicited by identical flashes applied at 10-s intervals were averaged. The amplitude and implicit time of the a and b waves were measured and averaged.
Following the electroretinogram tests, the animals were sacrificed. Both eyes from all animals were enucleated and fixed immediately in 4% formaldehyde in 0.1 M phosphate buffer (pH, 7.4). The eye was cut along the cornea-optic nerve axis into halves after the lens was removed. Specimens were further fixed in 4% paraformaldehyde and 0.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). Histological sections were obtained for light-microscopic examination after the tissues were embedded in paraffin, sectioned at a thickness of 6 μm, and stained with hematoxylin-eosin.
RESULTS
Indirect ophthalmoscopy of the rabbit eyes revealed no retinal damage, hemorrhage, or detachment after intravitreal injection of 50 μg/0.1 ml caspofungin. The mean caspofungin levels measured for vitreous and aqueous humors at all sampling times are listed in Table 1. The vitreous concentration declined rapidly with time. The mean vitreous concentration was 6.06 ± 1.76 μg/ml 1 h after injection and declined to 3.396 ± 0.42 μg/ml at 4 h and 0.47 ± 0.15 μg/ml at 24 h, respectively. An exponential decay model was used to fit the data, and a least-squares regression analysis was performed. The elimination half-life was calculated from the slope of the line of log concentration versus time. The vitreous caspofungin concentration showed an exponential decay with a half-life of 6.28 h. The mean aqueous concentration was much lower and showed undetectable levels in all samples after injection (Table 1).
TABLE 1.
Levels of caspofungin at different times after intravitreal injection of 50 μg/0.1 ml in rabbits
| Time | Caspofungin concn (μg/ml) in the vitreous (n = 4)a |
|---|---|
| 10 min | 13.98 ± 0.87 |
| 1 h | 6.06 ± 1.76 |
| 2 h | 4.71 ± 2.03 |
| 4 h | 3.39 ± 0.42 |
| 8 h | 2.98 ± 0.57 |
| 16 h | 2.04 ± 0.94 |
| 24 h | 0.47 ± 0.15 |
| 48 h | Undetectable |
Values are means ± standard deviations. Caspofungin in all aqueous samples was below the detection limit (0.1 μg/ml) after injection.
To directly evaluate rod photoreceptor function, we measured the waves of ERG, which arises almost exclusively from the rod photoreceptors. The responses to an intense flash, which saturated the rod photoreceptors, were recorded. The saturated a-wave and b-wave amplitudes and implicit time for the eyes with intravitreal caspofungin injection of 10, 50, 100, and 200 μg/ml and measurements from control eyes are summarized in Table 2. There were no statistical differences in a-wave and b-wave responses between control eyes and any caspofungin-injected eyes using an unpaired t test. The statistical comparison of the amplitudes and the implicit times of a waves and b waves from electroretinograms between control eyes and the eyes with injection of various concentrations of caspofungin are shown in Table 2 and Fig. 1.
TABLE 2.
Amplitudes and implicit times of a waves and b waves from electroretinograms and b/a ratios at various intravitreal concentrations of caspofungina
| Caspofungin concn (μg/ml) |
a waves (n = 4) |
b waves (n = 4) |
b/a ratio |
|||||
|---|---|---|---|---|---|---|---|---|
| Amplitude (μV) (mean ± SD) | P | Implicit time (ms) | Amplitude (μV) (mean ± SD) | P | Implicit time (ms) | Mean ratio ± SD | P | |
| 10 | 55.33 ± 12.34 | 0.750 | 51 ± 8 | 172.67 ± 16.17 | 0.552 | 65 ± 4 | 3.24 ± 0.90 | 0.857 |
| Control | 58.00 ± 5.57 | 48 ± 5 | 180.33 ± 12.50 | 56 ± 9 | 3.13 ± 0.41 | |||
| 50 | 57.67 ± 8.39 | 0.630 | 45 ± 2 | 162.00 ± 19.00 | 0.739 | 73 ± 14 | 2.85 ± 0.56 | 0.806 |
| Control | 53.33 ± 11.72 | 48 ± 6 | 155.33 ± 26.10 | 69 ± 3 | 2.95 ± 0.35 | |||
| 100 | 55.00 ± 13.00 | 0.593 | 52 ± 6 | 158.33 ± 24.38 | 0.720 | 68 ± 7 | 2.97 ± 0.67 | 0.749 |
| Control | 59.67 ± 5.03 | 56 ± 6 | 165.33 ± 20.03 | 60 ± 4 | 2.80 ± 0.54 | |||
| 200 | 52.33 ± 10.97 | 0.786 | 52 ± 9 | 160.33 ± 10.60 | 0.853 | 75 ± 5 | 3.14 ± 0.54 | 0.788 |
| Control | 55.00 ± 11.53 | 50 ± 3 | 158.00 ± 17.52 | 71 ± 10 | 2.98 ± 0.80 | |||
Four animals were included for each concentration.
FIG 1.
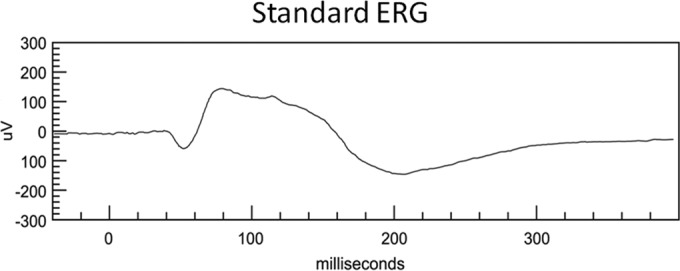
ERG responses recorded from a rabbit eye after an intravitreal injection of 200 μg/ml of caspofungin.
Gross examination of eye specimens showed no retinal hemorrhages or signs of infection in any caspofungin-injected or control eyes. Histologic examination with light microscopy did not reveal any retinal abnormality in the eyes injected with BSS as controls. In eyes injected with caspofungin at intravitreal concentrations of 10 μg/ml to 200 μg/ml, no focal necrosis or other abnormality could be observed in any layer of retina (Fig. 2).
FIG 2.

Histologic examination of the retina from a rabbit eye injected with 200 μg/ml of caspofungin. The sensory retina is preserved almost completely. H&E staining was used. Magnification, ×40.
DISCUSSION
Although fungus is a common causative organism of systemic infection, it is an unusual cause of endophthalmitis and often carries an ominous prognosis. The difficulty in treatment is due to a combination of the growth characteristics of fungi, a scarcity of effective antifungal agents, and poor tissue penetration. Severe fungal infections may result in extensive retinal damage, acute inflammation, and rapid vision loss. Despite current advances in antifungal therapy, the management of acute fungal endophthalmitis remains a formidable challenge.
The antifungal agent used most often for treatment of fungal endophthalmitis is amphotericin B. It was the first polyene compound shown to be effective in treating systemic mycosis and was the treatment of choice against yeasts and natamycin-resistant filamentous fungi, notably Aspergillus (23, 24). In vitro activities of amphotericin B vary, with the MICs ranging from less than 0.5 to 6.73 μg/ml and, for Fusarium solani infections, from 1.56 to 100 μg/ml (25, 26). Intravitreous injection of 5 to 10 μg of amphotericin B is a widely used treatment for fungal endophthalmitis. While amphotericin B is an irritant, intravitreal liposomal amphotericin B is well tolerated in monkey and rabbit eyes and has been used in the treatment of Candida endophthalmitis (16, 27, 28). Another broad-spectrum antifungal agent administered intravitreally for treatment of fungal endophthalmitis is voriconazole, which inhibits the fungal enzyme cytochrome P450 demethylase. In vitro studies have shown that voriconazole MICs range from 0.06 to 0.25 μg/ml for Candida species, 0.5 μg/ml for Aspergillus species, and 2 to 8 μg/ml for Fusarium oxysporum and Fusarium solani (29–32). Clinically, intravitreal voriconazole injections have been effective in treating fungal endophthalmitis caused by Aspergillus flavus, Scedosporium apiospermum, and Fusarium species and Candida albicans (33). However, amphotericin B can induce intraocular inflammation, and voriconazole has a rapid clearance in the vitreous. In addition, both agents can cause retinal necrosis at lower concentrations. Fluconazole is highly effective against Candida spp., but it shows high MICs (>100 μg/ml) against Aspergillus (34). Fluconazole has excellent penetration into the vitreous (35). A case of endogenous Candida albicans endophthalmitis in a newborn refractory to intravenous amphotericin B that responded to fluconazole has been reported (14). Itraconazole is active against a variety of fungi and is well absorbed orally in doses of 50 to 400 mg daily. However, systemically administered itraconazole penetrates poorly into noninflamed aqueous or vitreous. Caspofungin is fungicidal in vitro and in vivo against a broad range of Candida spp., including species that are intrinsically resistant to azoles (Candida krusei and Candida glabrata) or amphotericin B (Candida lusitaniae) and emerging species (e.g., Candida famata and Candida rugosa) (36–38). Caspofungin MIC90s range between 0.06 and 1 μg/ml (39–42).
Caspofungin is distributed well in tissue but penetrates minimally into the eye intravenously due to its high level of protein binding and high molecular weight. The high level of protein binding could limit the amount of caspofungin available for activity in plasma (38, 43, 44). Consequently, failure of intravenously administered caspofungin in treatment of fungal endophthalmitis has been reported (45). Caspofungin exerts its fungicidal effect against Candida spp. in a concentration-dependent manner over a broad concentration range in vivo. Furthermore, caspofungin also possesses prolonged postantifungal effects, remaining at a high concentration in tissue and maintaining antifungal efficacy even after the serum concentration falls below the MIC (46). Due to the characteristics of high-level protein binding and high molecular weight, the efficacy, toxicity, and residence times of caspofungin within the vitreous may change. Assuming that the protein binding in the vitreous is similar to that in plasma, only 3% of caspofungin will be present as a free drug. In such a case, an intravitreal concentration of 100 μg/ml of caspofungin will result in a free-drug level of 3 μg/ml, which is about 3 times the MIC of caspofungin against Aspergillus and Candida isolates. However, the protein level in the vitreous is low, and the caspofungin levels detected in the vitreous in this study were much higher than we expected. Therefore, a therapeutic level of caspofungin in the vitreous was achieved. Although the relationship between caspofungin concentration and antifungal activity in the vitreous has not been determined, caspofungin exhibits linear pharmacokinetics with a lower elimination rate than voriconazole in the vitreous and has a prolonged postantifungal effect, in which case a potent antifungal efficacy of caspofungin in vitreal cavities would be expected. In a study of experimentally induced Candida endophthalmitis, a single intravitreal injection of 100 μg of caspofungin produced a greater improvement in symptoms and a greater decrease in colony counts than 50 μg of voriconazole or 10 μg of amphotericin B (47).
The concentration of a drug in the vitreal cavity depends on its dosage, the volume of distribution, and the elimination rate. The elimination of a drug in the vitreal cavity may be affected by a variety of factors, including its molecular weight, protein binding, and tissue absorption. The clearance of a drug in the vitreal cavity can be via the anterior route passage into the aqueous humor and the posterior route by active transport across the retina. Generally, drugs eliminated from vitreous cavities using a retinal pump mechanism have shorter half-lives than drugs eliminated via the anterior chamber (28, 29). This study showed that caspofungin in the vitreous had an exponential decay and a half-life of 6.28 h. Therefore, its antifungal efficacy against Candida and Aspergillus species would persist up to 24 h. Given the elimination rate and low aqueous concentrations achieved, our data suggest that caspofungin is eliminated primarily via the posterior route. With the normal volume of the vitreal cavity in rabbits assumed to be 1.4 ml, the injected dose of 50 μg/0.1 ml in rabbit eyes resulted in an initial vitreous concentration of 33.33 μg/ml. The peak vitreous levels achieved were thus nearly 30 times greater than the MICs of caspofungin against most Candida and Aspergillus spp.
Some antifungal agents may be toxic to retinal structures. However, caspofungin has an excellent safety profile with reduced toxicities, compared to other antifungal agents applied intravitreally in the rabbit. Because electroretinograms measure a panretinal response, they are employed in monitoring retinal toxicity. Different ERG components are related to different retinal structures. Negative a waves and b waves reflect the function of photoreceptors and bipolar and Müller cells, respectively (48). Moreover, the ratio of b-wave to a-wave amplitude represents the response of a given stimulus in the inner and outer retina. It is difficult to standardize ERG responses, because they are influenced by a number of factors, such as pupil size, electrodes, stimulus intensity, dark adaptation time, age, and body temperature (49, 50). There were no significant differences in the values of amplitude and the implicit time, which suggests that the inner and outer retina were not functionally impaired by the dose of intravitreal caspofungin that was used in this study. In addition, there were no differences in the ratios of b-wave to a-wave amplitude between the eyes that were injected with caspofungin and control eyes, which suggests that intravitreal concentrations of up to 200 μg/ml of caspofungin do not cause the deterioration of retinal function.
Although intravitreal injection of caspofungin does not lead to electrophysiological changes in the rabbit retina based on ERG, focal damage to the retina may occur, because full-field ERG is a mass effect of light stimulation on the retina (51, 52). In our results, there was no evidence of histologic damage to the retina with intravitreal concentrations of up to 200 μg/ml. This nontoxic dose of caspofungin in the retina is more than 200 times the MIC90 for Candida spp. and Aspergillus spp. The tolerance doses of caspofungin in the retina should be safe and sufficient for the treatment of fungal endophthalmitis, even with cumulative doses in retinal tissue after repeated injections. Based on the results of our ERG tests and retinal histopathologic studies, intravitreal concentrations of up to 200 μg/ml of caspofungin do not cause photoreceptor functional impairment or structural changes in the rabbit retina. However, intravitreal concentrations of caspofungin caused retinal toxicity among some animal species. Mojumder et al. showed that vitreal concentrations from 0.41 to 4.1 μM in mice did not significantly alter their ERG waveforms. However, at a concentration of 41 μM (50 μg/ml), the a-wave and b-wave amplitudes were reduced, and a decrease in the number of cells in the ganglion cell layer was observed (53). Kernt and Kampik reported that caspofungin did not cause significant toxic effects in human retinal pigment epithelium (RPE) cells after 24 h treatment at vitreal concentrations between 5 and 75 μg/ml in a cell culture study, but doses of caspofungin above 150 μg/ml led to a rapid and significant reduction of viability in RPE cells (54). In a study of experimentally induced Candida endophthalmitis in rabbit eyes, 100 μg/0.1 ml of intravitreally injected caspofungin had no toxic effect on the retinal layers when evaluated histopathologically under light microscopy (47). In addition to the different levels of tolerance among species, the characteristics of tissue absorption and protein binding may result in higher concentrations in the mouse retina than in the white rabbit retina even at the same vitreous concentrations, which means that caspofungin is more toxic in the mouse retina.
The results of this study indicate that intravitreal injection of caspofungin may be an alternative in the treatment of fungal endophthalmitis. In comparison with the current antifungal agents, caspofungin has several advantages. (i) Caspofungin is less toxic to the retina than amphotericin B and voriconazole in the rabbit, though it is known to cause local irritation at the site of injection. Intravitreal concentrations of amphotericin B between 4.1 and 8.3 μg/ml have been found to produce retinal toxicity, and voriconazole at a concentration of 50 μg/ml also results in retinal focal necrosis. In addition, amphotericin B is proinflammatory. (ii) Caspofungin has a lower elimination rate and exhibits prolonged postantifungal effects, perhaps because of its high molecular weight and high rate of tissue absorption. (iii) Synergistic action with the azole group of antifungals and amphotericin B and the biofilm-eradicating effect are other advantages of caspofungin, although there is, as yet, no evidence to suggest that these will improve efficacy in endophthalmitis. As a result of caspofungin's unique mechanism of action and the high morbidity of fungal endophthalmitis, there is considerable interest in using this antifungal agent as an alternative or as part of a combination antifungal therapy. Caspofungin is also a promising agent as first-line therapy for endophthalmitis and as salvage therapy for damage caused by Candida spp. and Aspergillus spp. However, caspofungin has limitations in the antifungal spectrum, including its limited efficacy against two significant ophthalmic pathogens, Fusarium and Scedosporium spp. More clinical data are needed to define caspofungin's role as primary therapy for fungal endophthalmitis and its role in antifungal combination therapy.
ACKNOWLEDGMENTS
We thank the Biostatistics Task Force of Taichung Veterans General Hospital for statistical assistance.
This study was supported in part by research grants from Taichung Veterans General Hospital, Taiwan, Republic of China.
We have no financial conflicts of interest to declare regarding this paper.
Footnotes
Published ahead of print 22 September 2014
REFERENCES
- 1.Benz MS, Scott IU, Flynn HW, Jr, Unonius N, Miller D. 2004. Endophthalmitis isolates and antibiotic sensitivities: a 6-year review of culture-proven cases. Am. J. Ophthalmol. 137:38–42. 10.1016/S0002-9394(03)00896-1. [DOI] [PubMed] [Google Scholar]
- 2.Essman TF, Flynn HW, Jr, Smiddy WE, Brod RD, Murray TG, Davis JL, Rubsamen PE. 1997. Treatment outcomes in a 10-year study of endogenous fungal endophthalmitis. Ophthalmic Surg. Lasers 28:185–194. [PubMed] [Google Scholar]
- 3.Kunimoto DY, Das T, Sharma S, Jalali S, Majji AB, Gopinathan U, Athmanathan S, Rao TN. 1999. Microbiologic spectrum and susceptibility of isolates: part I. Postoperative endophthalmitis. Endophthalmitis Research Group. Am. J. Ophthalmol. 128:240–242. [DOI] [PubMed] [Google Scholar]
- 4.Narang S, Gupta A, Gupta V, Dogra MR, Ram J, Pandav SS, Chakrabarti A. 2001. Fungal endophthalmitis following cataract surgery: clinical presentation, microbiological spectrum, and outcome. Am. J. Ophthalmol. 132:609–617. 10.1016/S0002-9394(01)01180-1. [DOI] [PubMed] [Google Scholar]
- 5.Nayak N. 2008. Fungal infections of the eye: laboratory diagnosis and treatment. Nepal Med. Coll. J. 10:48–63. [PubMed] [Google Scholar]
- 6.Axelrod AJ, Peyman GA, Apple DJ. 1973. Toxicity of intravitreal injection of amphotericin B. Am. J. Ophthalmol. 76:578–583. 10.1016/0002-9394(73)90753-8. [DOI] [PubMed] [Google Scholar]
- 7.Baldinger J, Doft BH, Burns SA, Johnson B. 1986. Retinal toxicity of amphotericin B in vitrectomised versus non-vitrectomised eyes. Br. J. Ophthalmol. 70:657–661. 10.1136/bjo.70.9.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallis HA, Drew RH, Pickard WW. 1990. Amphotericin B: 30 years of clinical experience. Rev. Infect. Dis. 12:308–329. 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 9.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B, Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408–415. 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 10.Gao H, Pennesi ME, Shah K, Qiao X, Hariprasad SM, Mieler WF, Wu SM, Holz ER. 2004. Intravitreal voriconazole: an electroretinographic and histopathologic study. Arch. Ophthalmol. 122:1687–1692. 10.1001/archopht.122.11.1687. [DOI] [PubMed] [Google Scholar]
- 11.Gao H, Pennesi M, Shah K, Qiao X, Hariprasad SM, Mieler WF, Wu SM, Holz ER. 2003. Safety of intravitreal voriconazole: electroretinographic and histopathologic studies. Trans. Am. Ophthalmol. Soc. 101:183–189. [PMC free article] [PubMed] [Google Scholar]
- 12.Shen YC, Wang MY, Wang CY, Tsai TC, Tsai HY, Lee YF, Wei LC. 2007. Clearance of intravitreal voriconazole. Invest. Ophthalmol. Vis. Sci. 48:2238–2241. 10.1167/iovs.06-1362. [DOI] [PubMed] [Google Scholar]
- 13.Shen YC, Wang MY, Wang CY, Tsai TC, Tsai HY, Lee HN, Wei LC. 2009. Pharmacokinetics of intracameral voriconazole injection. Antimicrob. Agents Chemother. 53:2156–2157. 10.1128/AAC.01125-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dotto PF, Rodrigues LD, Brandao-Fernandes ML. 2005. Intravenous fluconazole use in the treatment of fungal endogenous endophthalmitis: case report. Arq. Bras. Oftalmol. 68:543–546. 10.1590/S0004-27492005000400022. [DOI] [PubMed] [Google Scholar]
- 15.Durand ML, Kim IK, D'Amico DJ, Loewenstein JI, Tobin EH, Kieval SJ, Martin SS, Azar DT, Miller FS, III, Lujan BJ, Miller JW. 2005. Successful treatment of Fusarium endophthalmitis with voriconazole and Aspergillus endophthalmitis with voriconazole plus caspofungin. Am. J. Ophthalmol. 140:552–554. 10.1016/j.ajo.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Koç A, Onal S, Yenice O, Kazokoglu H. 2010. Pars plana vitrectomy and intravitreal liposomal amphotericin B in the treatment of candida endophthalmitis. Ophthalmic Surg. Lasers Imaging 9:1–3. 10.3928/15428877-20100215-35. [DOI] [PubMed] [Google Scholar]
- 17.Denning DW. 2002. Echinocandins: a new class of antifungal. J. Antimicrob. Chemother. 49:889–891. 10.1093/jac/dkf045. [DOI] [PubMed] [Google Scholar]
- 18.Kim R, Khachikian D, Reboli AC. 2007. A comparative evaluation of properties and clinical efficacy of the echinocandins. Expert Opin. Pharmacother. 8:1479–1492. 10.1517/14656566.8.10.1479. [DOI] [PubMed] [Google Scholar]
- 19.Wagner C, Graninger W, Presterl E, Joukhadar C. 2006. The echinocandins: comparison of their pharmacokinetics, pharmacodynamics and clinical applications. Pharmacology 78:161–177. 10.1159/000096348. [DOI] [PubMed] [Google Scholar]
- 20.Bartizal K, Gill CJ, Abruzzo GK, Flattery AM, Kong L, Scott PM, Smith JG, Leighton CE, Bouffard A, Dropinski JF, Balkovec J. 1997. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743872). Antimicrob. Agents Chemother. 41:2326–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abel R, Jr, Boyle GL. 1976. Dissecting ocular tissue for intraocular drug studies. Invest. Ophthalmol. 15:216–219. [PubMed] [Google Scholar]
- 22.Spriet I, Annaert P, Meersseman P, Hermans G, Meersseman W, Verbesselt R, Willems L. 2009. Pharmacokinetics of caspofungin and voriconazole in critically ill patients during extracorporeal membrane oxygenation. J. Antimicrob. Chemother. 63:767–770. 10.1093/jac/dkp026. [DOI] [PubMed] [Google Scholar]
- 23.Abad JC, Foster CS. 1996. Fungal keratitis. Int. Ophthalmol. Clin. 36(3):1–15. [DOI] [PubMed] [Google Scholar]
- 24.Yee RW, Boone DE, Rinaldi MG. 1996. Antifungal agents, p 249–267 In Tabbara KF, Hyndiuk RA. (ed), Infections of the eye. Little Brown, Boston, MA. [Google Scholar]
- 25.Arikan S, Lozano-Chiu M, Paetznick V, Nangia S, Rex JH. 1999. Microdilution susceptibility testing of amphotericin B, itraconazole, and voriconazole against clinical isolates of Aspergillus and Fusarium species. J. Clin. Microbiol. 37:3946–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sekhon AS, Padhye AA, Garg AK, Ahmad H, Moledina N. 1994. In vitro sensitivity of medically significant Fusarium species to various antimycotics. Chemotherapy 40:239–244. 10.1159/000239199. [DOI] [PubMed] [Google Scholar]
- 27.Barza M, Baum J, Tremblay C, Szoka F, D'Amico DJ. 1985. Ocular toxicity of intravitreally injected liposomal amphotericin B in rhesus monkeys. Am. J. Ophthalmol. 100:259–263. 10.1016/0002-9394(85)90791-3. [DOI] [PubMed] [Google Scholar]
- 28.Cannon JP, Fiscella R, Pattharachayakul S, Garey KW, De Alba F, Piscitelli S, Edward DP, Danziger LH. 2003. Comparative toxicity and concentrations of intravitreal amphotericin B formulations in a rabbit model. Invest. Ophthalmol. Vis. Sci. 44:2112–2117. 10.1167/iovs.02-1020. [DOI] [PubMed] [Google Scholar]
- 29.Marco F, Pfaller MA, Messer SA, Jones RN. 1998. Antifungal activity of a new triazole, voriconazole (UK-109, 496), compared with three other antifungal agents tested against clinical isolates of filamentous fungi. Med. Mycol. 36:433–436. 10.1080/02681219880000691. [DOI] [PubMed] [Google Scholar]
- 30.Espinel-Ingroff A, Boyle K, Sheehan DJ. 2001. In vitro antifungal activities of voriconazole and reference agents as determined by NCCLS methods: review of the literature. Mycopathologia 150:101–115. 10.1023/A:1010954803886. [DOI] [PubMed] [Google Scholar]
- 31.Ghannoum MA, Kuhn DM. 2002. Voriconazole—better chances for patients with invasive mycoses. Eur. J. Med. Res. 7:242–256. [PubMed] [Google Scholar]
- 32.Marangon FB, Miller D, Giaconi JA, Alfonso EC. 2004. In vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens. Am. J. Ophthalmol. 137:820–825. 10.1016/j.ajo.2003.11.078. [DOI] [PubMed] [Google Scholar]
- 33.Sen P, Gopal L, Sen PR. 2006. Intravitreal voriconazole for drug-resistant fungal endophthalmitis. Retina 26:935–939. 10.1097/01.iae.0000250011.68532.a2. [DOI] [PubMed] [Google Scholar]
- 34.Dermoumi H. 1994. In vitro susceptibility of fungal isolates of clinically important specimens to itraconazole, fluconazole and amphotericin B. Chemotherapy 40:92–98. 10.1159/000239178. [DOI] [PubMed] [Google Scholar]
- 35.Riddell J, IV, Comer GM, Kauffman CA. 2011. Treatment of endogenous fungal endophthalmitis: focus on new antifungal agents. Clin. Infect. Dis. 52:648–653. 10.1093/cid/ciq204. [DOI] [PubMed] [Google Scholar]
- 36.Hajdu R, Thompson R, Sundelof JG, Pelak BA, Bouffard FA, Dropinski JF, Kropp H. 1997. Preliminary animal pharmacokinetics of the parenteral antifungal agent MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2339–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eschenauer G, DePestel DD, Carver PL. 2007. Comparison of echinocandin antifungals. Ther. Clin. Risk Management. 3:71–97. 10.2147/tcrm.2007.3.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vazquez JA, Sobel JA. 2006. Anidulafungin: a novel echinocandin. Clin. Infect. Dis. 43:215–222. 10.1086/505204. [DOI] [PubMed] [Google Scholar]
- 39.Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, Diekema DJ. 2008. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micoafungin: six years of global surveillance. J. Clin. Microbiol. 46:150–156. 10.1128/JCM.01901-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfaller MA, Boyken L, Hollis RJ, Messer SA, Tendolkar S, Diekema DJ. 2006. In vitro susceptibilities of Candida spp. to caspofungin: four years of global surveillance. J. Clin. Microbiol. 44:760–763. 10.1128/JCM.44.3.760-763.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antachopoulos C, Meletiadis J, Sein T, Roilides E, Walsh TJ. 2008. Comparative in vitro pharmacodynamics of caspofungin, micafungin, and anidulafungin against germinated and nongerminated Aspergillus conidia. Antimicrob. Agents Chemother. 52:321–328. 10.1128/AAC.00699-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, Diekema DJ. 2009. In vitro susceptibility of clinical isolates of Aspergillus spp. to anidulafungin, caspofungin, and micafungin: a head-to-head comparison using the CLSI M38-A2 broth microdilution method. J. Clin. Microbiol. 47:3323–3325. 10.1128/JCM.01155-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denning DW. 2003. Echinocandin antifungal drugs. Lancet 362:1142–1151. 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 44.Chandrasekar PH, Sobel JD. 2006. Micafungin: a new echinocandin. Clin. Infect. Dis. 42:1171–1178. 10.1086/501020. [DOI] [PubMed] [Google Scholar]
- 45.Gauthier GM, Nork TM, Price R, Andes D. 2005. Therapeutic ocular penetration caspofungin and associated treatment failure in penetration caspofungin and associated treatment failure in Candida albicans endophthalmitis. Clin. Infect. Dis. 41:e27–28. 10.1086/431761. [DOI] [PubMed] [Google Scholar]
- 46.Louie A, Deziel M, Liu W, Drusano MF, Gumbo T, Drusano GL. 2005. Pharmacodynamics of caspofungin in a murine model of systemic candidiasis: importance of persistence of caspofungin in tissues to understanding drug activity. Antimicrob. Agents Chemother. 49:5058–5068. 10.1128/AAC.49.12.5058-5068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kusbeci T, Avci B, Cetinkaya Z, Ozturk F, Yavas G, Ermis SS, Inan UU. 2007. The effects of caspofungin and voriconazole in experimental Candida endophthalmitis. Curr. Eye Res. 32:57–64. 10.1080/02713680601107157. [DOI] [PubMed] [Google Scholar]
- 48.Asi H, Perlman I. 1992. Relationships between the electroretinogram a-wave, b-wave and oscillatory potentials and their application to clinical diagnosis. Doc. Ophthalmol. 79:125–139. 10.1007/BF00156572. [DOI] [PubMed] [Google Scholar]
- 49.Hebert M, Lachapelle P, Dumont M. 1995. Reproducibility of electroretinograms recorded with DTL electrodes. Doc. Ophthalmol. 91:333–342. 10.1007/BF01214651. [DOI] [PubMed] [Google Scholar]
- 50.Marmor MF, Holder GE, Seeliger MW, Yamamoto S. 2004. Standard for clinical electroretinography (2004 update). Doc. Ophthalmol. 108:107–114. 10.1023/B:DOOP.0000036793.44912.45. [DOI] [PubMed] [Google Scholar]
- 51.Xu W, Wang H, Wang F, Jiang Y, Zhang X, Wang W, Qian J, Xu X, Sun X. 2010. Testing toxicity of multiple intravitreal injections of bevacizumab in rabbit eyes. Can. J. Ophthalmol. 45:386–392. 10.3129/i10-024. [DOI] [PubMed] [Google Scholar]
- 52.Inan UU, Avci B, Kusbeci T, Kaderli B, Avci R, Temel SG. 2007. Preclinical safety evaluation of intravitreal injection of full-length humanized vascular endothelial growth factor antibody in rabbit eyes. Invest. Ophthalmol. Vis. Sci. 48:1773–1781. 10.1167/iovs.06-0828. [DOI] [PubMed] [Google Scholar]
- 53.Mojumder DK, Concepcion FA, Patel SK, Barkmeier AJ, Carvounis PE, Wilson JH, Holz ER, Wensel TG. 2010. Evaluating retinal toxicity of intravitreal caspofungin in the mouse eye. Invest. Ophthalmol. Vis. Sci. 51:5796–5803. 10.1167/iovs.10-5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kernt M, Kampik A. 2011. Intraocular caspofungin: in vitro safety profile for human ocular cells. Mycoses 54:e110–121. 10.1111/j.1439-0507.2009.01853.x. [DOI] [PubMed] [Google Scholar]


