Abstract
Giardiasis is highly prevalent in the developing world, and treatment failures with the standard drugs are common. This work deals with the proposal of omeprazole as a novel antigiardial drug, focusing on a giardial glycolytic enzyme used to follow the cytotoxic effect at the molecular level. We used recombinant technology and enzyme inactivation to demonstrate the capacity of omeprazole to inactivate giardial triosephosphate isomerase, with no adverse effects on its human counterpart. To establish the specific target in the enzyme, we used single mutants of every cysteine residue in triosephosphate isomerase. The effect on cellular triosephosphate isomerase was evaluated by following the remnant enzyme activity on trophozoites treated with omeprazole. The interaction of omeprazole with giardial proteins was analyzed by fluorescence spectroscopy. The susceptibility to omeprazole of drug-susceptible and drug-resistant strains of Giardia lamblia was evaluated to demonstrate its potential as a novel antigiardial drug. Our results demonstrate that omeprazole inhibits giardial triosephosphate isomerase in a species-specific manner through interaction with cysteine at position 222. Omeprazole enters the cytoplasmic compartment of the trophozoites and inhibits cellular triosephosphate isomerase activity in a dose-dependent manner. Such inhibition takes place concomitantly with the cytotoxic effect caused by omeprazole on trophozoites. G. lamblia triosephosphate isomerase (GlTIM) is a cytoplasmic protein which can help analyses of how omeprazole works against the proteins of this parasite and in the effort to understand its mechanism of cytotoxicity. Our results demonstrate the mechanism of giardial triosephosphate isomerase inhibition by omeprazole and show that this drug is effective in vitro against drug-resistant and drug-susceptible strains of G. lamblia.
INTRODUCTION
Infectious diseases are public health problems all around the world; moreover, infections caused by resistant microorganisms often fail to respond to conventional treatment, resulting in prolonged illness and a greater risk of death. This condition reduces the efficacy of treatment because patients remain infectious longer and spread resistant microorganisms to others.
The protozoan Giardia lamblia (also known as Giardia duodenalis or Giardia intestinalis) is a parasite that displays drug resistance and undergoes antigenic variation (1). G. lamblia is the causative agent of giardiasis and inhabits the upper small intestine of humans and other vertebrates. Giardiasis is the most common diarrheal disease caused by the protozoan, with cosmopolitan distribution, 280 million symptomatic cases, and some 500,000 new cases every year (2). Infection rates of close to 100% have been reported for some developing countries, whereas infections are less common in developed countries. Nonetheless, incidence rates of up to 70% have been observed in some areas (3). G. lamblia is also the most common cause of waterborne outbreaks of diarrheal disease in humans in developed countries (4), and it severely affects domestic animals (5).
The clinical impact of giardiasis seems to be stronger in the first 3 years of life and in undernourished or immunodeficient individuals (2, 6–8). A rising incidence of giardiasis has been noted in children in day care centers, which has led to the designation of giardiasis as a reemerging infectious disease in the developed world (9). In addition, as it is a waterborne human parasite, for several years, the Centers for Disease Control and Prevention in the United States has classified G. lamblia as a bioterrorism category B organism.
A variety of drugs, such as 5-nitroimidazole compounds, nitazoxanide, benzimidazole, quinacrine, furazolidone, and paromomycin, have been used in therapy for giardiasis (10, 11). Metronidazole and nitazoxanide are used as first-line therapies because of their effectiveness (12–14).
Unfortunately, these drugs have relevant side effects (15, 16); single-drug and multidrug resistance have been demonstrated or induced in vitro, and distinct isolates from human patients with reduced drug susceptibility have been described (10, 17, 18). An approximately 20% prevalence rate of clinical resistant cases of giardiasis has been reported for metronidazole, with recurrence rates of up to 90% (19). This has led to the search for novel experimental strategies and the evaluation of other treatment regimens. Strategies ranging from the development of vaccines to the search for new molecular targets are currently being addressed (1, 20). The enzymes of the glycolytic pathway in G. lamblia have been proposed as potential targets for drug design (21, 22) because the organism lacks oxidative phosphorylation (23). Our group has demonstrated the mechanism of inactivation of the giardial glycolytic enzyme triosephosphate isomerase (GlTIM) by chemical modification with thiol-reactive compounds (22, 24). In line with this, omeprazole (5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole) drew our attention because it is a drug that is safely used in humans and shows a molecular mechanism of action based on the chemical modification of cysteines (Cys). Omeprazole is one of the most important proton pump inhibitors (PPIs), which irreversibly inhibits H+/K+-ATPase by modification of the Cys813 residue (25). Earlier reports from our group (G. López-Velázquez and H. Reyes-Vivas, 6 October 2008, Mexican Patent Office) and others (26) have proposed omeprazole as a compound with antigiardial effects.
This work demonstrates the species-specific mechanism of omeprazole as a GlTIM inhibitor and analyzes the cytotoxic effect in vitro of omeprazole on G. lamblia trophozoites, on strains that are resistant to either metronidazole or nitazoxanide, and on drug-susceptible strains. Our results support the possibility of omeprazole as an alternative antigiardial drug for use in the near future.
MATERIALS AND METHODS
Cysteine mutants of GlTIM.
In a previous work, we generated by site-directed mutagenesis the single mutants for all five positions occupied by Cys in GlTIM (24). The kinetic constants were calculated and reported (24) and were in the range of those of the wild-type GlTIM (wt-GlTIM); thus, mutagenesis did not affect the activity or stability of the enzymes (24). Those mutants were used in this work.
Recombinant enzymes.
We used transformed Escherichia coli strain BL21(DE3)(pLysS) cells containing the wild-type hstim, wild-type gltim, and mutant gltim genes to produce the recombinant enzymes. Purification was performed as described previously (27), with the additional use of anion-exchange (Q-Sepharose fast flow [FF]; 1.5 cm by 12 cm) and gel filtration (Superdex 75 prep grade; 1.6 cm by 60 cm) chromatography to obtain an enzyme purity of >95%. We calculated the protein concentrations of the mutants and wt-GlTIM by using the ε280 of 26,600 M−1 cm−1 and ε280 of 32,595 M−1 cm−1 for human triosephosphate isomerase (HsTIM).
Inactivation of recombinant enzymes.
The activities of recombinant HsTIM and GlTIMs were determined in the direction of glyceraldehyde 3-phosphate (GAP) to dihydroxyacetone phosphate (DHAP) using a coupled system by following the decay of NADH at 340 nm and 25°C, as described elsewhere (27). The inactivation assays were performed at a protein concentration of 0.2 mg/ml (7.2 nM) in Tris-EDTA (TE) buffer (100 mM triethanolamine, 10 mM EDTA [pH 7.4]) at 37°C. Omeprazole (Sigma, Aldrich) was freshly prepared before any assay by dissolving it in dimethyl sulfoxide (DMSO) to obtain a stock solution of 20 mM omeprazole and 10% DMSO. To acid activate the omeprazole, a stock solution of 200 mM omeprazole in 90% DMSO and 10% 0.1 M HCl was made and incubated for 1 and 3 h at room temperature, in the dark, before being used in the dilution needed for each assay. The omeprazole used in the assays contained <5% DMSO. All the experiments were performed in triplicate.
The pseudo-first-order inactivation rate constant was obtained at omeprazole concentrations ranging from 300 to 700 μM (neutral pH); the concentration of GlTIM was 0.2 mg ml−1 (7.2 nM) in TE buffer at 37°C. Every 10 min (from 0 to 120 min), aliquots were removed from the samples and assayed for residual activity in the standard reaction mixture. The data were adjusted to a monoexponential decay model, AR = A0(e−kt), where AR is the residual activity at time t, A0 is the activity at time zero, and k is the pseudo-first-order inactivation rate constant (24).
Enzyme activity of cellular NADH oxidase and NADPH oxidoreductase.
Approximately 1.2 × 107 trophozoites were centrifuged at 2,755 × g for 10 min at 4°C; the supernatant was discarded and pellet resuspended in phosphate-buffered saline (PBS) buffer, and this procedure was repeated three times. Next, the trophozoites were sonicated by 4 cycles of 20 s at 4°C in a buffer containing 100 mM HEPES (pH 7.2). The sample was centrifuged at 12,754 × g for 30 min, and the supernatant was collected. Enzyme activity was spectrophotometrically measured at 25°C following the decay of absorbance at 340 nm, during the first 5 min of recording; the data are reported as nmol of NADH or NADPH oxidized min−1 mg−1 protein−1. The reaction mixture to measure the activity of NADH oxidase contained 0.2 mM NADH and 100 mM HEPES (pH 7.2). The reaction mixture to measure the activity of NADPH oxidoreductase contained 0.2 mM NADPH, 0.1 mM flavin adenine dinucleotide (FAD), and 100 mM HEPES (pH 7.2).
Cys quantification.
The Cys residues were determined using Ellman's reagent under denaturing conditions (28). For the recombinant enzymes, samples with or without 1 mM omeprazole (at 37°C for 2 h) were filtered to remove the reagent that did not react (29) and added to TE buffer, 1 mM 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), and 5% (wt/vol) SDS. To measure the thiol groups (free Cys) from the cellular proteins, we incubated the cultures with or without 1 mM omeprazole for 12 h. After harvesting, the pellets were washed 5 times in phosphate-buffered saline (PBS); the proteins were precipitated using acetone (at −70°C) overnight. The pellets were dried and resuspended in TE buffer and 5% SDS. We used the bicinchoninic acid method with bovine serum albumin as a standard (30) to quantify the total proteins in the extracts from trophozoites. The number of Cys residues in recombinant enzymes (per monomer) or in the total proteins was quantified by following the increase of absorbance at 412 nm (ε412, 13.6 mM−1 cm−1).
Giardia strains.
G. lamblia WB strain was acquired from the American Type Culture Collection (ATCC), cultured, harvested, and maintained as previously described (31). Metronidazole resistance was induced in WB by conventional methods. Briefly, the trophozoites were treated with 1.5 μM metronidazole (Sigma-Aldrich) for 48 h and allowed to recover before the next metronidazole exposure. The concentration of metronidazole was sequentially increased to allow the survival of 10 to 20% of the trophozoites. After 4 months, the time of exposition was reduced to 24 h and was continued for 4 months. The final concentration of metronidazole used in the induction was 40 μM, and after that, the cultures were maintained in the presence of 0.4 g/ml metronidazole. Resistance to metronidazole was observed as reported in Table 1 at the time of the assays.
TABLE 1.
In vitro susceptibilities to omeprazole, metronidazole, nitazoxanide, and albendazole in drug-susceptible and drug-resistant strains of G. lamblia
| Compound | LD50 (μM) for method: |
|||||
|---|---|---|---|---|---|---|
| 1 |
2 |
|||||
| Drug susceptible | Metronidazole resistant | Nitazoxanide resistant | Drug susceptible | Metronidazole resistant | Nitazoxanide resistant | |
| Omeprazole, nonactivated | 319.68 | 385.38 | 297.66 | 0.116 | 0.26 | 0.544 |
| Omeprazole, acid activated | 0.072 | 0.214 | 0.127 | |||
| Metronidazole | 1.24 | 2.9 | 0.84 | |||
| Nitazoxanide | 0.016 | 0.172 | 1.42 | |||
| Albendazole | 0.05 | 0.118 | 0.277 | |||
G. lamblia N1-INP strain was isolated and axenized from a Mexican child previously treated with nitazoxanide and was clinically diagnosed as resistant to this drug. Briefly, trophozoites from duodenal fluid aspirate were cultured in TYI-S-33 medium (pH 7.2) with an antibiotic mixture containing 110.4 μM ceftazidime pentahydrate, 1.7 mM ampicillin, 195 μM amikacin, and 2.68 μM amphotericin B. Every 24 h, for 7 days, the culture medium was carefully decanted, the trophozoites that adhered to the glass were retained, and the tube was replenished with fresh culture medium with the additives described above. The axenic strains were maintained in the presence of 0.5 μg/ml nitazoxanide. It is important to note that after treatment for 3 days with nitazoxanide, the patient was still having symptoms of giardiasis until metronidazole was used per a doctor's prescription. Indeed, the patient showed malabsorption syndrome until metronidazole was taken.
Cellular GlTIM activity.
Cultures of both drug-susceptible and drug-resistant strains were cultured in the presence of increasing concentrations of omeprazole for 24 h at 37°C. At the end of the treatment period, the tubes were decanted in order to conserve the adhered trophozoites only (trying to preserve living cells only, without mixing with dead cells). Nonetheless, the living and dead trophozoites were counted from these supernatants in order to register the total viability after treatment. The tubes were washed three times with PBS (pH 7.4) and chilled on ice for 20 min, and then the trophozoites were counted in order to standardize the assays by the number of cells. The cells were disrupted by sonication at 4°C, and then five cycles of 20 s with 2-min resting intervals in 50 mM Tris-HCl (pH 7.4), 1 mM phenylmethylsulfonyl fluoride, and 1% (vol/vol) DMSO were performed. The sonicates were centrifuged at 15,700 × g for 20 min at 4°C, and the supernatants were withdrawn for analysis of residual GlTIM activity using the coupled method mentioned above at 37°C. We considered 100% to be the enzyme activity registered for the cultures without omeprazole. The enzyme activity was assayed using 30,000 trophozoites per condition, and the assays were repeated ≥3 times. NADH consumption by enzymes other than GlTIM was calculated by measuring the change of NADH to NAD+ at 340 nm in the presence of cellular extracts and coupled system but without GAP (the GlTIM substrate). After a few minutes, GAP was added to this mixture, and the resulting activity was taken as 100%. The difference in ΔOD (optical density at time 2 − optical density at time 1) before and after the addition of GAP was considered to be the consumption of NADH by enzymes other than GlTIM.
Fluorescence spectroscopy in trophozoites.
Cultures of G. lamblia with approximately 12 × 106 trophozoites per tube were incubated in the presence of 1 mM omeprazole for 6 or 9 h at 37°C. To prevent remaining omeprazole in the samples, 1 ml of culture medium from the bottom of the tubes was discarded using a Pasteur pipette; the tubes were chilled on ice for 20 min and centrifuged at 453 × g at 4°C for 10 min. The supernatant was discarded and pellet resuspended in 500 μl of PBS (4°C) and transferred to a microtube (1.5 ml). The samples were centrifuged at 13,400 × g in a microcentrifuge, and the supernatant was discarded and resuspended in 500 μl of PBS (4°C); this last step was repeated 5 times. The cells were resuspended in 200 μl and counted with a hemocytometer. The samples without omeprazole (negative control) and without cells (“control of wash”) underwent the same extensive washing process. We measured the fluorescence emission spectra at 25°C with an LS55 spectrofluorometer (PerkinElmer) and recorded them from 300 to 600 nm, with an integration time of 1 s, a size step of 0.5 nm, and an excitation wavelength (λExc) of 335 nm. The assays were carried out using 3 million cells from each sample or the volume corresponding to this number of cells after sonication. The negative controls and controls of wash used the same volume (∼40 μl), and all the samples were exposed to UV light for 15 min before analysis in the spectrofluorometer. Each spectrum was the average of 2 scans. The background fluorescence of the buffer was not subtracted from the sample reading in order to make a comparison with the negative controls and controls of wash. The wavelength emission of maximum fluorescence in nm (λmax) was calculated by selecting the point that exhibited the highest intensity.
Confocal microscopy.
The cells were grown on glass coverslips into six-well cell culture clusters (Costar) with TYI-S-33 medium for 48 h, followed by incubation with 1 mM omeprazole diluted in TYI-S-33 medium, or without omeprazole (control) for 6 and 9 h. Afterward, the coverslips were washed three times with PBS for 5 min each and mounted with mounting medium for fluorescence (Vectashield; Vector). The mounted samples were irradiated for 15 min in a UV chamber (homemade with a fluorescent black-light tube; WKO model F8T5BL) and immediately analyzed and photographed with a laser scanning confocal microscope (Olympus FV1000), using a violet diode laser of 405 nm. Taking into account that green is an easier color to distinguish by the naked eye, a green pseudocolor was assigned to the signal instead of blue, due to its emission being near 412 nm. The emission wavelength of omeprazole impedes the use of 4′,6′-diamidino-2-phenylindole (DAPI) counterstains.
Susceptibility assays.
In vitro susceptibility assays were performed using two methods for all strains tested; both methods were essentially derived from previously described works (32). The first method was implemented to mimic the concentrations obtained from the inactivation assays performed with recombinant GlTIM. Briefly, subconfluent cultures of G. lamblia with approximately 12 × 106 trophozoites were incubated for 24 h at 37°C with different concentrations of omeprazole (range, 2 mM to 25 μM) added as a solution in freshly made TYI-S-33. At the end of the treatment period (24 h), the trophozoites were counted with a hemocytometer, and the 50% lethal dose (LD50) was calculated by means of Probit analysis (SPSS package). The experiments were carried out using triplicate tubes and were repeated at least three times for each strain. The other method used was subculture, applied as a conventional method to calculate the LD50 (33). Briefly, cultures with 50,000 trophozoites/ml of TYI-S-33 medium were incubated for 48 h at 37°C, with omeprazole concentrations between 0.001 μg/ml and 1 μg/ml. After this time, the samples were chilled on ice for 20 min, washed, and resuspended. Fifteen-microliter aliquots were taken from each sample, diluted in 1 ml of fresh TYI-S-33 medium without omeprazole, and incubated at 37°C for 48 h; the trophozoites were counted with a hemocytometer, and the LD50 was calculated by means of Probit analysis (SPSS package). We calculated the LD50 for omeprazole at a neutral pH (nonactivated), omeprazole previously incubated at an acidic pH (acid activated), metronidazole, nitazoxanide, and albendazole. Table 1 displays the complete data set.
Processing of RNA samples and real-time qPCR.
To quantify the gene expression of the variant surface proteins TSA417, GlTIM, and NADH oxidase by real-time quantitative PCR (qPCR), trophozoites of drug-susceptible and drug-resistant strains were grown in the absence of drugs until confluence was reached. The cells were harvested and RNA was extracted using TRIzol reagent (Invitrogen), according to the manufacturer's instructions. DNase I (Thermo Scientific) digestion was used to remove residual genomic DNA. First-strand cDNA was synthesized using 100 ng of RNA and RevertAid reverse transcriptase (Thermo Scientific), previously quantified in a μDrop plate (Thermo Scientific), mixed with 0.5 μg of oligo(dT) (Fermentas) in 15 μl of H2O-diethyl pyrocarbonate (DEPC), and incubated at 70°C for 5 min. All qPCR amplification reactions were performed using the StepOne real-time PCR system (Applied Biosystems) in a 20-μl total reaction volume containing 10 μl of Fast SYBR green master mix (Applied Biosystems), 10 ng of cDNA, and 10 μM forward and reverse primers. The amplification reaction mixture included a forward and reverse primer of each gene assayed: TSA417qF, 5′-GCG AAA GTG ATA GCA ATG GG-3′, and TSA417qR, 5′-TGA GGT AAC AGA GGA CGG AGC-3′, to quantify TSA417; TIMqF, 5′-ACA CGG GCT CGT AAG CAA T-3′, and TIMqR, 5′-AGG AGC TCG GAG AGT CCA A-3′, to quantify GlTIM; NADHqF, 5′-GCA CCA TAT GGC TTC AAC GG-3′, and NADHqR, 5′-CAG GCC TGT CCG TGT CAT TA-3′, to quantify NADH oxidase; and ACTqF, 5′-TTG CCG TAC CTG CCT TCT AT-3′, and ACTqR, 5′-GCC CGG AAC TGT AGA GAG C-3′, for actin as a reference. The reference gene (Act) and test (voltage sensor probe [VSP], GlTIM, and NADH oxidase) primers were designed with a desired length of 19 to 21 nucleotides and an annealing temperature of 60°C. The amplification of the reporter and reference gene fragments used the gene-specific primers and yielded 93-, 60-, 98-, and 60-bp PCR products for the VSP, GlTIM, NADH oxidase, and actin genes, respectively. PCR was started at 95°C for 20 s; subsequent amplification was done in 40 cycles of denaturation (at 95°C for 3 s) and annealing/extension (at 60°C for 30 s). Templates and amplification controls were developed for each primer set, and all cDNA samples were prepared in triplicate. The mean threshold cycle (CT) value was calculated for each sample from triplicates and normalized against the Act reference gene. The variability in expression in the VSP, GlTIM, and NADH oxidase genes was evaluated by use of the ΔΔCT relative quantification method, according to the following equations (CT [threshold cycle]): ΔCT = CTtarget − CTnormalized; ΔΔCT = ΔCTexperimental − ΔCTcontrol; and comparative expression level = 2−ΔΔCT (34).
RESULTS
Omeprazole inactivates recombinant GlTIM with no adverse effects on HsTIM.
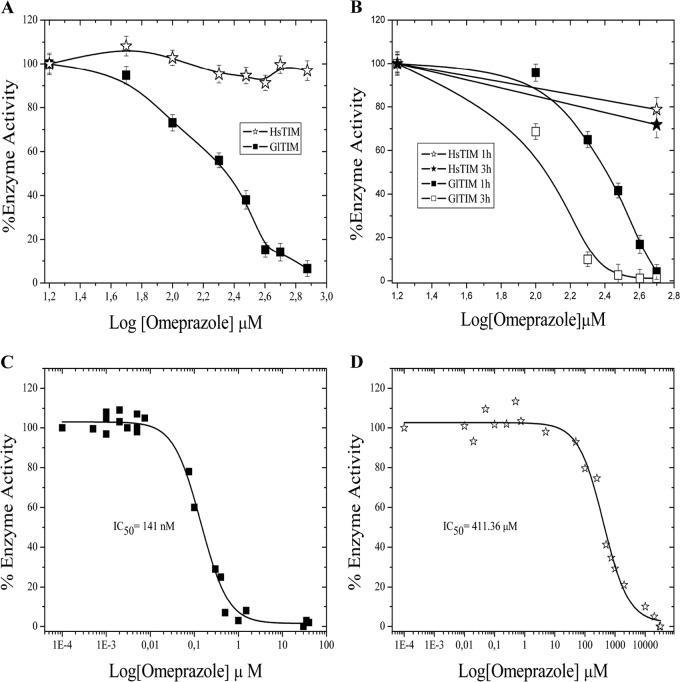
We already demonstrated the role of thiol-reactive compounds on the inactivation of GlTIM (22, 24). Taking into account the mechanism of action of omeprazole on proton pump enzymes, which is based on its capacity to react with Cys, we performed enzyme inactivation assays using omeprazole against recombinant GlTIM and recombinant human TIM (HsTIM). Figure 1A depicts the residual activities of GlTIM and HsTIM with increasing concentrations of omeprazole at neutral pH (7.0); this is important in light of the reported inhibitory actions of omeprazole and the fact that the pH at mid-jejunum is between 7.0 and 9.0. While the enzyme activity of GlTIM is completely abolished at 750 μM, the activity of HsTIM retains almost the level of its original activity. Moreover, when GlTIM reached 50% inactivation, HsTIM retained >90% activity (Fig. 1A). We generated recombinant GlTIM from the N1-INP strain, and its behavior was essentially the same as that of GlTIM from WB (data not shown). The efficacy of omeprazole is reported to be dependent on acid pH in parietal cells and in other cases with Leishmania donovani (35). Our results demonstrate the efficacy and species-specific action of omeprazole on GlTIM under neutral pH conditions (Fig. 1A). Interestingly, inactivation assays using previously acid-activated omeprazole (see Materials and Methods) show an improvement on the effect of omeprazole against GlTIM (Fig. 1B); such improvement is dependent on the acidification time of omeprazole. Under the least favorable conditions for GlTIM in this assay (3 h of preincubation of omeprazole at acid pH), HsTIM retained >90% activity when GlTIM reached 50% inactivation (Fig. 1 B).
FIG 1.
GlTIM inactivation by omeprazole. One hundred percent of the enzyme activities were fitted with the activity registered for the enzymes incubated without omeprazole (3,870 μmol min−1 mg−1 and 4,230 μmol min−1mg−1 for GlTIM and HsTIM, respectively). (A) Efficacy and species-specific inactivation of omeprazole at pH 7.0. (B) Efficiency and species-specific inactivation of acid-activated omeprazole for 1 and 3 h. Values are means ± standard deviations (SD) from at least three independent experiments. Omeprazole IC50 under neutral pH conditions are shown for GlTIM (C) and HsTIM (D).
In order to obtain the 50% inhibitory concentration (IC50) for recombinant GlTIM using low concentrations of omeprazole under neutral pH conditions, we performed overnight enzyme inactivation assays using increasing concentrations of omeprazole and adjusting to sigmoidal curves. The inflection point of the resulting sigmoidal curve represents the IC50, and the obtained value was 141 nM (Fig. 1C). The same experiment was done with HsTIM, obtaining an IC50 of 411 μM (Fig. 1D), which represents almost 3,000 times more resistance to omeprazole than that of GlTIM.
Finally, the pseudo-first-order inactivation rate constant for omeprazole at neutral pH was 0.6 M−1 s−1. It is lower than that for DTNB (43.4 M−1 s−1) and 2-carboxyethyl methanethiosulfonate (MTSCE) (7.1 M−1 s−1), also previously reported under neutral pH conditions (24).
Omeprazole targets Cys 222 in GlTIM.
As we previously described, the wild-type (wt) GlTIM is a homodimer with 5 Cys residues per monomer, with the Cys residue at position 222 being responsible for the inactivation of GlTIM with thiol-reactive compounds (24). In order to identify the target of omeprazole on GlTIM, we performed enzyme inactivation assays using omeprazole against single mutants of its five different Cys residues at positions 14, 127, 202, 222, and 228; these mutants were previously made by us, and their kinetics were demonstrated to be similar to those of wt-GlTIM (24). For example, the kcat (turnover number) values were 4.6 ± 0.16, 5 ± 0.16, 3.5 ± 0.1, 2.9 ± 0.14, 5 ± 0.4, 1.87 ± 0.19 per 105 min for the wt, C14A, C127A, C202A, C222A, and C228A mutants, respectively (24). Similarly to other thiol-reactive compounds, such as DTNB [5,5-dithio-bis(2-nitrobenzoic acid)] and MTSCE (2-carboxyethyl methanethiosulfonate) (24), omeprazole completely inactivates all the single mutants of Cys, except for that without Cys 222 (Fig. 2A). Although omeprazole modifies more than one Cys on each single mutant and wt-GlTIM (Table 2), only the presence of Cys 222 resulted in the total inactivation of the enzyme (Fig. 2A). Our data demonstrate that the interaction between omeprazole and Cys 222 is responsible for the major inhibition of GlTIM. Moreover, the susceptibility to omeprazole of the mutant C222A is practically the same when omeprazole is used at neutral pH as when it is used with previous incubation for 1 or 3 h at acid pH (Fig. 2B).
FIG 2.
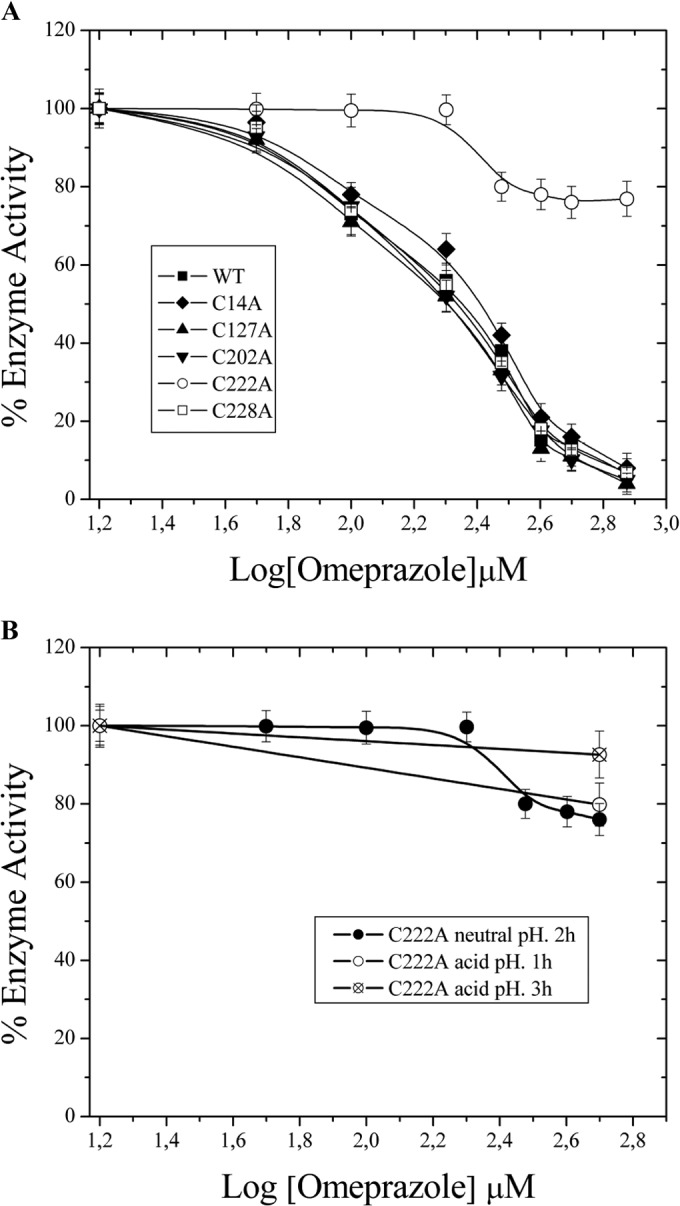
Omeprazole inactivates GlTIM by means of interaction with Cys at position 222. (A) Each single mutant of Cys is inactivated in the same way as wt GlTIM, except for that without Cys 222 (for 2 h at 37°C). (B) C222A resists inactivation with omeprazole either under acid-activated and nonactivated conditions. The specific activities at baseline were 3,870, 4,006, 3,994, 3,204, 3,853, and 4,201 μmol min−1 mg−1 for wt-GlTIM, C14A, C127A, C202A, C222A, and C228A, respectively. The values are means ± SD from at least three independent experiments.
TABLE 2.
Modified cysteines on each single mutant and wt-GlTIM by omeprazole
| Enzyme | Modified Cys/monomer |
|---|---|
| wt-GlTIM | 3 ± 0.14 |
| C14A | 3 ± 0.18 |
| C127A | 3 ± 0.12 |
| C202A | 2 ± 0.13 |
| C222A | 2 ± 0.07 |
| C228A | 2 ± 0.09 |
Fluorescence of GlTIM-omeprazole adducts.
Based on the capacity of omeprazole to fluoresce after exposure to UV light (36, 37), we demonstrated that recombinant GlTIM incubated with omeprazole at concentrations of ≥200 μM (pH 7.0) (at 37°C for 2 h) shows a fluorescent band of 28 kDa in denaturing gels (Fig. 3A). Figure 3B shows the fluorescent binding on C222A and HsTIM with 0.7 mM omeprazole (the concentration that showed good fluorescence, as shown in Fig. 3A) in the time course from 0 to 2 h. With omeprazole concentrations of <0.5 mM, the band was hardly distinguishable in the gels. After incubation with omeprazole, GlTIM and HsTIM were loaded onto a Sephadex G-25 column and filtered by centrifugation in order to remove excess omeprazole. We also analyzed the fluorescence spectrum of recombinant GlTIM incubated with omeprazole (λmax, 490 nm; λExc, 335 nm) and found that the fluorescence intensity (FI) of GlTIM with omeprazole was 5.5 times higher than that of GlTIM alone (Fig. 3C).
FIG 3.
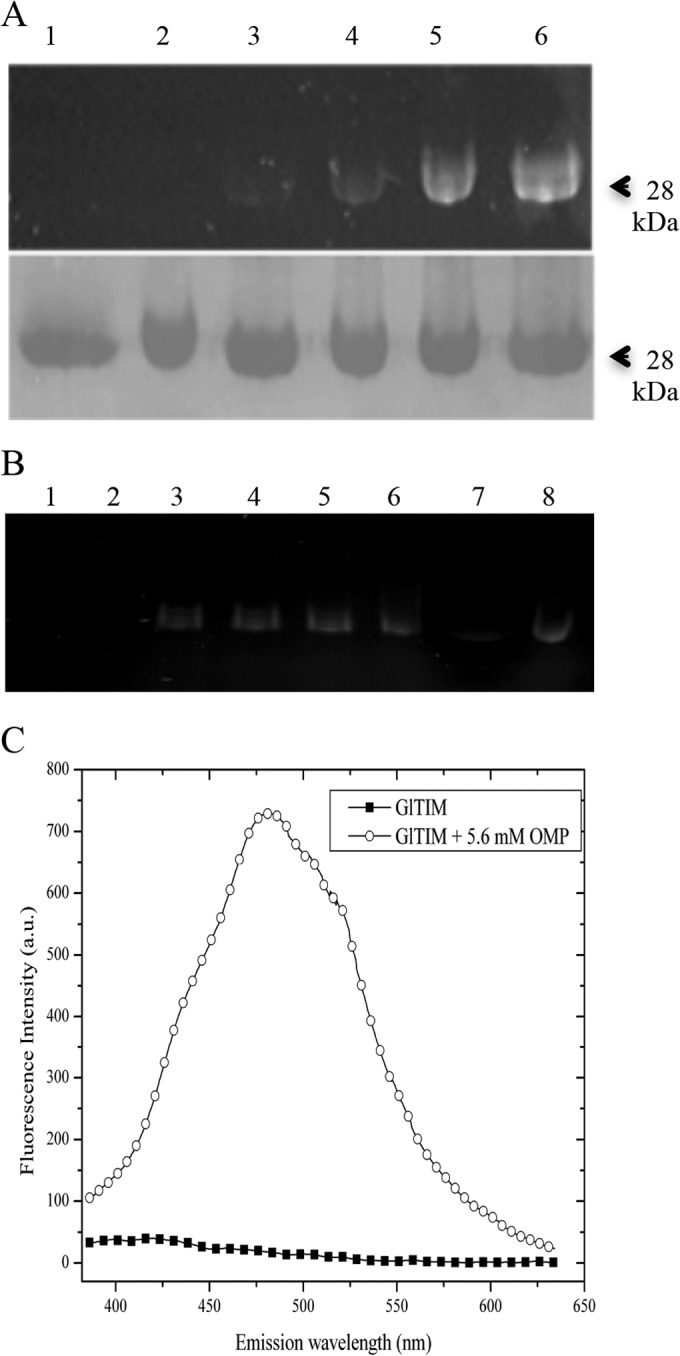
Fluorescence of GlTIM-omeprazole adducts. (A) Top, gel without staining and photographed in a UV transilluminator. Bottom, same gel stained with Coomassie brilliant blue. Lane 1, GlTIM without omeprazole; lanes 2 to 6, GlTIM plus 0.1, 0.2, 0.5, 0.75, and 2.5 mM omeprazole, respectively. (B) Gel without staining and photographed in a UV transilluminator. Lane 1, C222A without omeprazole; lanes 2 to 6, C222A plus 0.7 mM omeprazole at 15, 30, 60, 90, and 120 min, respectively; and lane 7, HsTIM without omeprazole; lane 8, HsTIM plus 0.7 mM omeprazole at 120 min. Each lane was loaded with 10 μg of enzyme. (C) Emission spectra of recombinant GlTIM (0.6 mg/ml protein), plotted for data before (filled squares) and after (circles) incubation with 5.6 mM omeprazole (OMP). a.u., absorbance units.
Omeprazole has a cytotoxic effect on Giardia, decreasing cellular GlTIM activity.
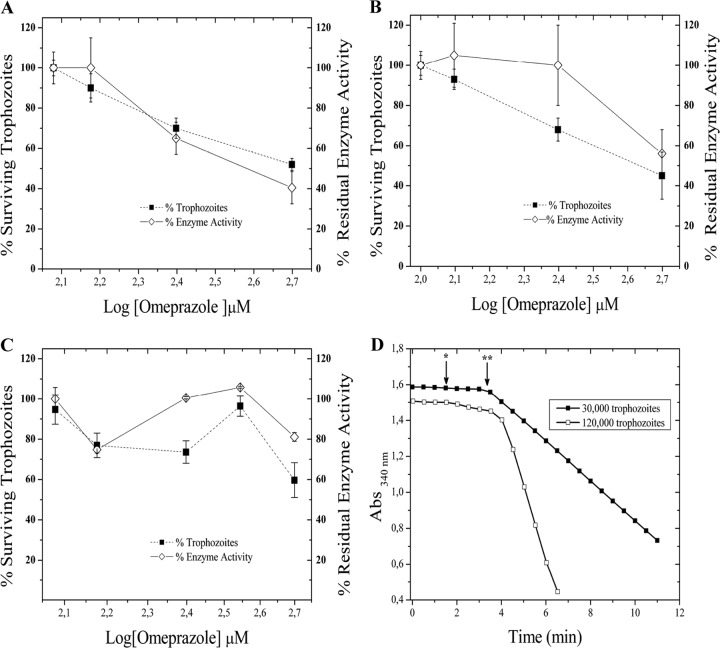
After the incubation of drug-susceptible G. lamblia trophozoites with omeprazole, we demonstrated a decrease in the viability and residual enzyme activity of cellular GlTIM in a dose-dependent manner (Fig. 4A). Similar to the drug-susceptible G. lamblia trophozoites, metronidazole-resistant G. lamblia incubated with different concentrations of omeprazole demonstrated marginal inhibition of cellular GlTIM activity (Fig. 4B). In the case of the nitazoxanide-resistant strain (N1-INP), omeprazole had a cytotoxic effect in trophozoites, but the GlTIM activity showed slight inhibition under these experimental conditions (Fig. 4C). The acid-activated omeprazole did not enhance the cytotoxic effect or the inhibition of cellular GlTIM under these experimental conditions with drug-resistant strains (data not shown).
FIG 4.
Cytotoxic effect of omeprazole and concomitant inhibition of cellular GlTIM. Shown are data for drug-susceptible (A), metronidazole-resistant (B), and nitazoxanide-resistant (C) strains. Values are means ± SD from at least three independent experiments. (D) Non-GlTIM consumption of NADH and cellular GlTIM activity. The arrows show the time of addition for the cellular lysates (*) and 1 mM GAP (**) to the reaction mixture. Abs, absorbance.
To demonstrate the specificity of the assay for GlTIM in the lysates of trophozoites, we measured the conversion of NADH to NAD+ without glyceraldehyde 3-phosphate (GAP) (the substrate of triosephosphate isomerase). The non-GlTIM consumption of NADH represented only 4.38% ± 0.78% of the total (Fig. 4D).
Omeprazole interacts with giardial proteins.
As mentioned above, omeprazole fluoresces after exposure to UV light. Based on this, we tracked omeprazole in the trophozoites of Giardia to address the possibility of its interaction with cytoplasmic proteins, including GlTIM. We analyzed the fluorescence spectrum of trophozoites at a λExc of 335 nm after 6 and 9 h of incubation with 1 mM omeprazole. We named the samples containing all the reactants except for the trophozoites the controls of wash. The controls of wash were used to eliminate any possibility of artifacts due to residual omeprazole. Before UV irradiation, the blank (buffer), controls of wash, nontreated trophozoites, and treated trophozoites showed the same fluorescence intensity (FI). After 15 min of UV irradiation, only the treated trophozoites increased in their FI proportionally to the time of incubation with omeprazole (Fig. 5A). On the other hand, trophozoites that were incubated for 9 h with omeprazole had a 900% increase in FI (λmax, 402 nm) compared with that of the negative control (trophozoites without omeprazole) (Fig. 5B). When the trophozoites were broken by sonication, the cytoplasmic and membrane fractions showed 83% and 35% FI, respectively (Fig. 5B), indicating that a high percentage of omeprazole was in the cytoplasmic compartment.
FIG 5.
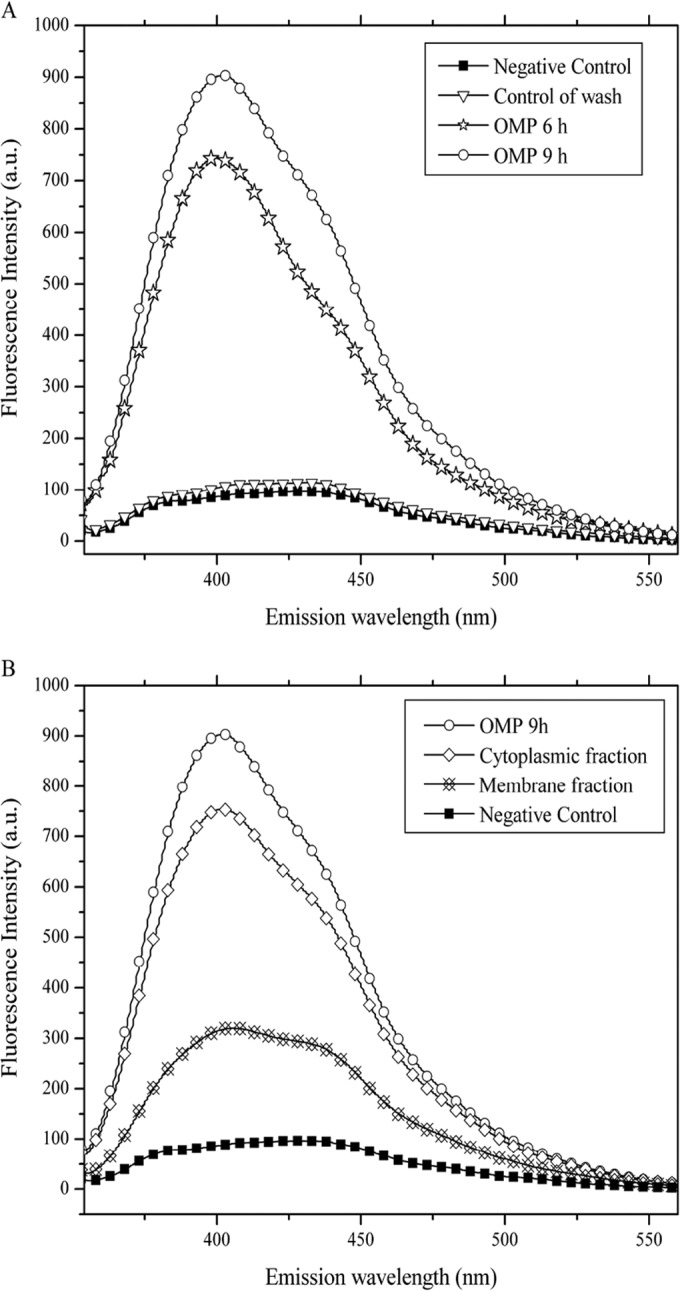
Fluorescence spectra of trophozoites incubated with 1 mM omeprazole (OMP). (A) FI increases according to the time of incubation with omeprazole. The negative control and control of wash showed almost the same FI. (B) When fluorescence of membrane and cytoplasmic fractions is analyzed, the cytoplasmic fraction shows the highest FI, just below the FI of intact trophozoites.
Although conventional confocal microscopes are not equipped with a laser with a λExc near 335 nm, it was possible to follow an enrichment of the signal inside the trophozoites of drug-susceptible strains as a result of the increase in incubation time with omeprazole and after a short time of exposure to UV light (Fig. 6). The results with drug-resistant strains were similar (see Fig. S1 in the supplemental material).
FIG 6.
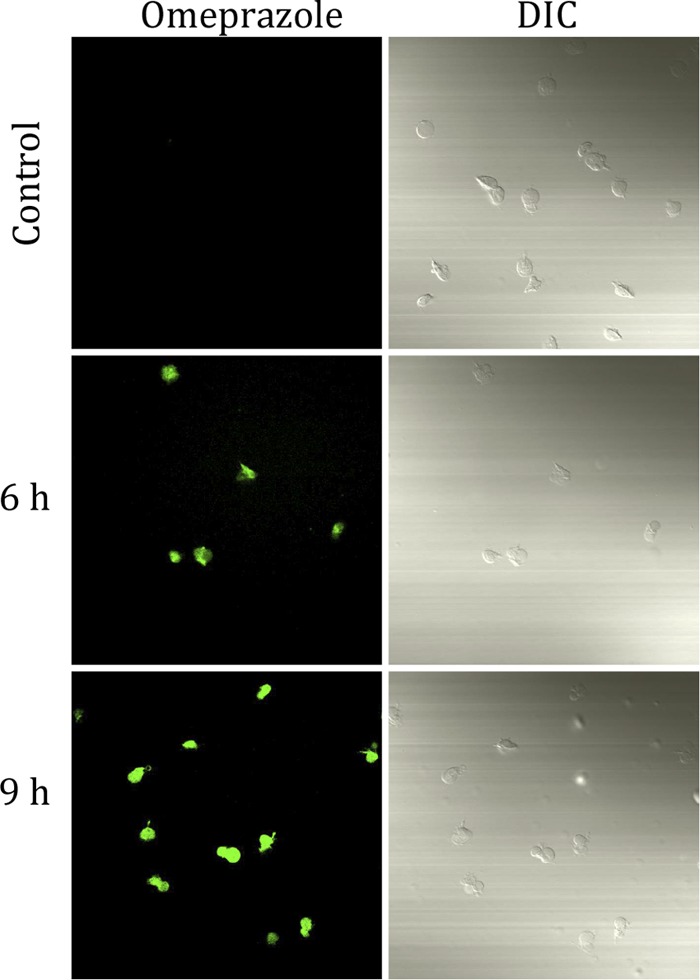
Confocal microscopy of drug-susceptible Giardia trophozoites incubated without and with 1 mM omeprazole. Magnification, ×60. DIC, differential inference contrast.
Omeprazole acts as a thiol-reactive compound with the giardial proteins.
Following Ellman's method, we quantified the number of modified Cys in the proteins from the cytoplasmic fractions of trophozoites incubated with omeprazole. The cultures exposed to omeprazole had 45% more modified Cys compared to that of cultures without omeprazole (95.8 versus 147 nmol of free Cys/mg of proteins). Overall, our results support the hypothesis that Cys is modified by omeprazole through the formation of disulfide bridges.
Omeprazole has a cytotoxic effect on drug-resistant and drug-susceptible Giardia strains.
Cytotoxicity was evaluated in trophozoite cultures incubated with different concentrations of omeprazole equivalent to those used for the inactivation assays with recombinant enzymes. After 24 h of incubation, the surviving and dead cells were counted, and the LD50 was calculated using this method (Table 1, method 1). This effect was corroborated using cultures with omeprazole diluted in TYI-S-33 medium or PBS only; under both conditions, there was no pH variation during the assays. Since the treatment failure of giardiasis with conventional drugs occurs at a rate of approximately 20% (38–42), we tested omeprazole in the strains of G. lamblia resistant to metronidazole and nitazoxanide; the LD50 were calculated for each (Table 1, method 1). The conventional method for calculating LD50 (method 2) was performed in resistant and nonresistant Giardia strains (Table 1, method 2) as well. Additionally, we calculated the LD50 for omeprazole at neutral pH and with acid-activated omeprazole (Table 1, method 2, nonactivated and acid activated).
Omeprazole was effective in vitro as an antigiardial compound in all the tested strains, even under neutral pH and acid-activated conditions (Table 1). These results are also the strongest evidence of drug resistance that we obtained for the assayed strains. Additionally, a human cell line of epithelial cells was incubated with a concentration of omeprazole at approximately the LD50 determined for Giardia strains using the concentration calculated by method 1, and under the same conditions as those for the trophozoites. The results showed no differences in viability among the mammalian cells incubated with or without 300 μM omeprazole (see Fig. S2 in the supplemental material).
VSP TSA417 gene expression is highly different between drug-susceptible and drug-resistant strains, while that of NADH oxidase and GlTIM does not change.
Several molecules have been studied for their expression and function to find potential markers of drug resistance. VSP TSA417 (the major surface antigen of G. lamblia WB) gene expression was found to be increased in G. lamblia WB susceptible to drugs in comparison to that of strains resistant to metronidazole and nitazoxanide induced in vitro (33). We found important differences in the gene expression of VSP TSA417 between drug-susceptible and drug-resistant strains but, while Müller et al. (33) found virtually nondetectable expression levels in drug-resistant clones, we found a significant increase in both metronidazole- and nitazoxanide-resistant strains compared with that in the drug-susceptible strain (see Fig. S3A in the supplemental material). It is important to note that others have previously found an overexpression of VSPs in drug-resistant clones of Giardia (43), which means that this feature is more frequent than it seems. Indeed, there is evidence that correlates the overexpression of VSPs with high virulence in Giardia isolates (44). This overexpression demonstrates another relevant characteristic of these omeprazole-susceptible strains.
Conversely, the gene expression of NADH oxidase and GlTIM did not show differences among the assayed strains (see Fig. S3A in the supplemental material). Previous works showed a differential gene expression of these molecules in Giardia strains resistant to albendazole (45). Based on our results, we can speculate that the mechanism of resistance to metronidazole and nitazoxanide shown by these Giardia strains is different from the others previously described.
Enzyme activity of NADH oxidase and NADPH oxidoreductase shows differences among omeprazole-susceptible strains.
The enzyme activities of NADH oxidase and NADPH oxidoreductase have been used to characterize drug-resistant versus drug-susceptible strains at the metabolic level (46). The activity of NADH oxidase was significantly different (P < 0.05) among the strains (see Fig. S3B in the supplemental material), even though the gene expression was similar (see Fig. S3A in the supplemental material). The activity of NADPH oxidoreductase was similar between both drug-resistant strains but different from that of the drug-susceptible strain (P < 0.05) (see Fig. S3C in the supplemental material). All of this reinforces the idea of a particular mechanism of drug resistance not characterized before.
DISCUSSION
Giardiasis is a neglected illness that is highly prevalent in the developing world, and treatment failures with standard drugs are common. Added to this, the VSPs of Giardia confer antigenic variation (1), and their overexpression is related to high virulence (44). Such conditions have driven our group to search for new antigiardial drugs, with a special interest in triosephosphate isomerase as a molecular target (22, 24, 31). Based on our previous data, we proposed omeprazole and its isomers (G. López-Velázquez and H. Reyes-Vivas, 6 October 2008, Mexican Patent Office) as drugs with a potential to inhibit GlTIM and kill Giardia trophozoites. In the present work, we demonstrated that omeprazole inhibits GlTIM in a species-specific manner, with a deleterious effect only when targeting Cys 222 in GlTIM, even under neutral pH and acid-activated conditions. This indicates that not all Cys present in a protein are reached by omeprazole and, even if a Cys is modified by omeprazole (see above), it does not always have a deleterious effect on the protein. As an example of this, HsTIM (a homodimer with 5 Cys per monomer) shows a Cys residue at position 217, which is equivalent to the Cys 222 in GlTIM. Although omeprazole modifies 1 of its 5 Cys in the same way as when we used DTNB (22), it does not inactivate HsTIM; indeed, it is almost 3,000 times more resistant than GlTIM. This is due to the properties of the region surrounding Cys 222 in GlTIM and its influence on the affinity for the substrate by the enzyme when such a residue is modified (24, 27, 31). The fluorescence of GlTIM-omeprazole adducts observed in recombinant enzymes (see Fig. 3) represents a potential tool for following omeprazole into the cells and identifying its targets (including the non-GlTIM targets). Nonetheless, experimental conditions must be adjusted in the future because there are several factors, (i.e., UV light exposure and molecular environment changes) that influence the signal emitted by the adducts. The changes in GlTIM activity of the surviving treated trophozoites strongly suggest that both molecules interact in the cell. However, this must be directly demonstrated with experiments in which their copurification from the trophozoites can be successful (we are currently investigating this topic). It is known that any molecule proposed for use as an antiparasitic should react with the parasite and, if the proposed target is intracellular, the drug has to enter the cell. Taking advantage of the fluorescent properties of omeprazole and by means of quantifying the modified Cys in the total proteins of the trophozoites, we demonstrated an interaction of omeprazole with giardial proteins by means of Cys modification. Moreover, our data on the fluorescence of omeprazole from cytoplasmic fractions support the idea that omeprazole enters the cytoplasm of trophozoites. Confocal microscopy suggests the internalization of omeprazole (after UV irradiation), but the conditions must be adjusted to understand its cellular localization. Moreover, it is necessary to determine if it accumulates in an organelle (i.e., lysosome-like peripheral vacuoles) in order to be acid activated or if its action is at neutral pH, as occurs in the assays done directly with the recombinant enzymes. Our results show a direct relationship between the cytotoxic effect of omeprazole on G. lamblia and its effect on cellular GlTIM in drug-susceptible strains.
On the other hand, although omeprazole exerted its antigiardial effect in the drug-susceptible and drug-resistant strains used in this work, it seems that the condition of resistance to a drug somehow confers a reduction in membrane permeability to omeprazole and delays its effect (see Fig. 4 and Table 1). In fact, the acid-activated omeprazole does not enhance the deleterious effect on cellular GlTIM (data not shown), and the cytotoxic effect is enhanced until the time of exposure is prolonged to >48 h (see Table 1). It is possible that VSPs capture omeprazole in the external surface of trophozoites through an interaction with cysteines, as can be inferred from those strains with high expression of VSPs (see Fig. S3A in the supplemental material). Without dismissing our proposal regarding the involvement of GlTIM, there might be several levels of effects that can converge to reach the cytotoxic effect of omeprazole in Giardia. The final effect must be differentially influenced by the origin of the Giardia strains studied and the molecules targeted by omeprazole. Conversely to what occurs with the commonly described mechanism of drug resistance in Giardia, the results on the gene expression in conjunction with the enzyme activity of NADH oxidase and NADPH oxidoreductase indicate that these strains might be using an “outer shield” of molecules as VSPs to deal with hostile conditions (i.e., drugs) instead of metabolic modifications to counteract oxidative stress. At this point, the most important contribution is that omeprazole was effective against all these strains even though they show resistance to metronidazole and nitazoxanide (see Table 1). At the same time, the expression of certain genes and their properties as markers of resistance must be revisited to understand their relationship to each other.
While our study focused primarily on the inactivation process of GlTIM and its role in the cytotoxic effect of omeprazole, the whole results provide a framework for further analysis of the effects of the omeprazole in Giardia. In line with this, the possibility that omeprazole targets molecules as ecto-ATPases by means of its acid-activated conformation, as demonstrated in L. donovani (35), must be mentioned. In spite of being acid activated, the cytotoxic effect on Giardia strains is not enhanced with omeprazole with high expression of VSP TSA417 at times of <48 h. Therefore, it appears that instead of its external localization, an ecto-ATPase is not the target of omeprazole, at least not a target that plays a role in the cytotoxic effect. Further studies must be done to confirm this.
It is relevant to point out that others have demonstrated that triosephosphate isomerase has a cytotoxic effect on other parasites (47–49). Having a well-characterized molecular target (i.e., GlTIM) will help to develop more efficient and specific antigiardial drugs that can be used as a coadjuvant treatment or in cases of extremely virulent and resistant strains. As we previously demonstrated, the modification of the Cys 222 region perturbs the structure and function of GlTIM (50); therefore, omeprazole can be the moiety to generate a more efficient antigiardial drug specifically directed to GlTIM. Our results using omeprazole at neutral pH and in an acid-activated form support our proposals. It does not matter if omeprazole passes through the acidic environment of the stomach and reaches the small intestine or if it reaches the small intestine by the blood; both pH conditions will be effective to inactivate GlTIM and cause the antigiardial effect. This is a benefit of using omeprazole against Giardia, taking into account that most PPIs are covered with an enteric coating, and others are administered intravenously (i.v.). One of the most important conclusions is that omeprazole is effective against Giardia-susceptible and -resistant drugs (or is highly virulent) at concentrations in the range of those of the first-line drugs used for giardiasis. Indeed, omeprazole was effective against a nitazoxanide-resistant clone, which is almost 89 times more resistant than the susceptible ones. Those concentrations (omeprazole LD50) can be reached with a conventional dose of 20 mg/day under neutral or acidic pH conditions.
Omeprazole has been used safely in humans, and its efficacy as a PPI is linked to previous acid activation (sulfenamide formation). Nonetheless, it is reported to have enzyme-inhibitory effects under neutral pH conditions (51). Others speculate on the possibility that hypochlorhydria induced by omeprazole can cause the development of giardiasis (52); in light of the increasing use of omeprazole and its isomers, further studies, such as clinical trials, must be done in order to demonstrate the effectiveness of omeprazole against human giardiasis.
We cannot yet guarantee that omeprazole will be more efficient than other anti-Giardia drugs in humans or other hosts. However, if the results of further studies in animals and humans (i.e., clinical trials) are in accordance with our results, its safety, low side effects, possibility of being used in conjunction with another drug, and potential to be used against drug-resistant strains (or highly virulent ones) make omeprazole a promising anti-Giardia drug.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by CONACyT J43022-M, 62321, and 154570. R.F.-S. was the recipient of a fellowship from CONACyT J43022-M.
We thank Silvestre Frenk Freud and Martha Livier Bustos for their critical review of the manuscript.
Footnotes
Published ahead of print 15 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02900-14.
REFERENCES
- 1.Prucca CG, Rivero FD, Luján HD. 2011. Regulation of antigenic variation in Giardia lamblia. Annu. Rev. Microbiol. 65:611–630. 10.1146/annurev-micro-090110-102940. [DOI] [PubMed] [Google Scholar]
- 2.Lane S, Lloyd D. 2002. Current trends in research into the waterborne parasite Giardia. Crit. Rev. Microbiol. 28:123–147. 10.1080/1040-840291046713. [DOI] [PubMed] [Google Scholar]
- 3.Flanagan PA. 1992. Giardia–diagnosis, clinical course and epidemiology. A review. Epidemiol. Infect. 109:1–22. [PMC free article] [PubMed] [Google Scholar]
- 4.Slifko TR, Smith HV, Rose JB. 2000. Emerging parasite zoonoses associated with water and food. Int. J. Parasitol. 30:1379–1393. 10.1016/S0020-7519(00)00128-4. [DOI] [PubMed] [Google Scholar]
- 5.Thompson RC, Smith A. 2011. Zoonotic enteric protozoa. Vet. Parasitol. 24:70–78. 10.1016/j.vetpar.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Farthing MJG. 1989. Host parasite interactions in human giardiasis. Q. J. Med. 70:191–204. [PubMed] [Google Scholar]
- 7.de Weerth A, Gocht A, Seewald S, Brand B, van Lunzen J, Seitz U, Thonke F, Fritscher-Ravens A, Soehendra N. 2000. Duodenal nodular lymphoid hyperplasia caused by giardiasis infection in a patient who is immunodeficient. Gastrointest. Endosc. 4:605–607. 10.1067/mge.2002.120786. [DOI] [PubMed] [Google Scholar]
- 8.Feitosa G, Bandeira AC, Sampaio DP, Badaró R, Brites C. 2001. High prevalence of giardiasis and stronglyloidiasis among HIV-infected patients in Bahia, Brazil. Braz. J. Infect. Dis. 6:339–344. 10.1590/S1413-86702001000600008. [DOI] [PubMed] [Google Scholar]
- 9.Thompson RC. 2000. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int. J. Parasitol. 30:1259–1267. 10.1016/S0020-7519(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 10.Upcroft JA, Upcroft P. 1993. Drug resistance and Giardia. Parasitol. Today 9:187–190. 10.1016/0169-4758(93)90144-5. [DOI] [PubMed] [Google Scholar]
- 11.Upcroft JA, Dunn LA, Wright JM, Benakli K, Upcroft P, Vanelle P. 2006. 5-Nitroimidazole drugs effective against metronidazole-resistant Trichomonas vaginalis and Giardia duodenalis. Antimicrob. Agents Chemother. 50:344–347. 10.1128/AAC.50.1.344-347.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lloyd D, Pedersen JZ. 1985. Metronidazole radical-anion generation in vivo in Trichomonas vaginalis oxygen quenching is enhanced in the drug-resistant strain. J. Gen. Microbiol. 131:87–92. [DOI] [PubMed] [Google Scholar]
- 13.Müller M. 1983. Mode of action of metronidazole on anaerobic bacteria and protozoa. Surgery 93:165–171. [PubMed] [Google Scholar]
- 14.Edwards DI. 1986. Reduction of nitroimidazoles in vitro and DNA damage. Biochem. Pharmacol. 35:53–58. 10.1016/0006-2952(86)90554-X. [DOI] [PubMed] [Google Scholar]
- 15.Petri WA., Jr 1999. Therapy of intestinal protozoa. Trends Parasitol. 19:523–526. [DOI] [PubMed] [Google Scholar]
- 16.Karabay O, Tamer A, Gunduz H, Kayas D, Arinc H, Celebi H. 2004. Albendazole versus metronidazole treatment of adult giardiasis: an open randomized clinical study. World J. Gastroenterol. 10:1215–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner TB, Hill DR. 2001. Treatment of giardiasis. Clin. Microbiol. Rev. 14:114–128. 10.1128/CMR.14.1.114-128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller J, Sterk M, Hemphill A, Müller N. 2007. Characterization of Giardia lamblia WB C6 clones resistant to nitazoxanide and to metronidazole. J. Antimicrob. Chemother. 60:280–287. 10.1093/jac/dkm205. [DOI] [PubMed] [Google Scholar]
- 19.Lalle M. 2010. Giardiasis in the post genomic era: treatment, drug resistance and novel therapeutic perspectives. Infect. Disord. Drug Targets 10:283–294. 10.2174/187152610791591610. [DOI] [PubMed] [Google Scholar]
- 20.Rivero FD, Saura A, Prucca CG, Carranza PG, Torri A, Lujan HD. 2010. Disruption of antigenic variation is crucial for effective parasite vaccine. Nat. Med. 16:551–557. 10.1038/nm.2141. [DOI] [PubMed] [Google Scholar]
- 21.Galkin A, Kulakova L, Melamud E, Li L, Wu C, Mariano P, Dunaway-Mariano D, Nash TE, Herzberg O. 2007. Characterization, kinetics, and crystal structures of fructose-1,6-bisphosphate aldolase from the human parasite, Giardia lamblia. J. Biol. Chem. 282:4859–4867. 10.1074/jbc.M609534200. [DOI] [PubMed] [Google Scholar]
- 22.Enríquez-Flores S, Rodríguez-Romero A, Hernández-Alcántara G, De la Mora-De la Mora I, Gutierrez-Castrellon P, Carvajal K, Lopez-Velazquez G, Reyes-Vivas H. 2008. Species-specific inhibition of Giardia lamblia triosephosphate isomerase by localized perturbation of the homodimer. Mol. Biochem. Parasitol. 157:179–186. 10.1016/j.molbiopara.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Tovar J, León-Avila G, Sánchez LB, Sutak R, Tachezy J, van der Giezen M, Hernández M, Müller M, Lucocq JM. 2003. Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature 426:172–176. 10.1038/nature01945. [DOI] [PubMed] [Google Scholar]
- 24.Enríquez-Flores S, Rodríguez-Romero A, Hernández-Alcántara G, Oria-Hernández J, Gutiérrez-Castrellón P, Pérez-Hernández G, de la Mora-de la Mora I, Castillo-Villanueva A, García-Torres I, Méndez ST, Gómez-Manzo S, Torres-Arroyo A, López-Velázquez G, Reyes-Vivas H. 2011. Determining the molecular mechanism of inactivation by chemical modification of triosephosphate isomerase from the human parasite Giardia lamblia: a study for antiparasitic drug design. Proteins 79:2711–2724. 10.1002/prot.23100. [DOI] [PubMed] [Google Scholar]
- 25.Besancon M, Simon A, Sachs G, Shin JM. 1997. Sites of reaction of the gastric H,K-ATPase with extracytoplasmic thiol reagents. J. Biol. Chem. 272:22438–22446. 10.1074/jbc.272.36.22438. [DOI] [PubMed] [Google Scholar]
- 26.Pérez-Villanueva J, Romo-Mancillas A, Hernández-Campos A, Yépez-Mulia L, Hernández-Luis F, Castillo R. 2011. Antiprotozoal activity of proton-pump inhibitors. Bioorg. Med. Chem. Lett. 21:7351–7354. 10.1016/j.bmcl.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 27.López-Velázquez G, Molina-Ortiz D, Cabrera N, Hernández-Alcántara G, Peon-Peralta J, Yépez-Mulia L, Peréz-Montfort R, Reyes-Vivas H. 2004. An unusual triosephosphate isomerase from the early divergent eukaryote Giardia lamblia. Proteins 55:824–834. 10.1002/prot.20097. [DOI] [PubMed] [Google Scholar]
- 28.Ellman GL. 1958. A colorimetric method for determining low concentrations of mercaptans. Arch. Biochem. Biophys. 74:443–450. 10.1016/0003-9861(58)90014-6. [DOI] [PubMed] [Google Scholar]
- 29.Penefsky HS. 1977. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J. Biol. Chem. 252:2891–2899. [PubMed] [Google Scholar]
- 30.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76–85. 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 31.Reyes-Vivas H, Diaz A, Peon J, Mendoza-Hernández G, Hernández-Alcántara G, De la Mora-De la Mora I, Enríquez-Flores S, Dominguez-Ramírez L, López-Velázquez G. 2007. Disulfide bridges in the mesophilic triosephosphate isomerase from Giardia lamblia are related to oligomerization and activity. J. Mol. Biol. 365:752–763. 10.1016/j.jmb.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 32.Cedillo-Rivera R, Muñoz O. 1992. In-vitro susceptibility of Giardia lamblia to albendazole, mebendazole, and other chemotherapeutic agents. J. Med. Microbiol. 37:221–224. 10.1099/00222615-37-3-221. [DOI] [PubMed] [Google Scholar]
- 33.Müller J, Sterk M, Hemphill A, Müller N. 2007. Characterization of Giardia lamblia WB C6 clones resistant to nitazoxanide and to metronidazole. J. Antimicrob. Chemother. 60:280–287. 10.1093/jac/dkm205. [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Jiang S, Meadows J, Anderson SA, Mukkada AJ. 2002. Antileishmanial activity of the antiulcer agent omeprazole. Antimicrob. Agents Chemother. 46:2569–2574. 10.1128/AAC.46.8.2569-2574.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peralta CM, Fernández LP, Masi AN. 2012. Precision improvement for omeprazole determination through stability evaluation. Drug Test. Anal. 4:48–52. 10.1002/dta.243. [DOI] [PubMed] [Google Scholar]
- 37.Takeguchi N, Yamanouchi T, Sakai H, Morii M. 1992. New fluorescent probes E3810 and methoxy E3810 for determining distributions of the apical membrane and the acidic compartment of gastric acid secreting cells. Jpn. J. Physiol. 42:75–88. 10.2170/jjphysiol.42.75. [DOI] [PubMed] [Google Scholar]
- 38.Canete R, Escobedo AA, González ME, Almirall P, Cantelar N. 2006. A randomized, controlled, open-label trial of a single day of mebendazole versus a single dose of tinidazole in the treatment of giardiasis in children. Curr. Med. Res. Opin. 22:2131–2136. 10.1185/030079906X132497. [DOI] [PubMed] [Google Scholar]
- 39.Escobedo AA, Alvarez G, González ME, Almirall P, Cañete R, Cimerman S, Ruiz A, Pérez R. 2008. The treatment of giardiasis in children: single-dose tinidazole compared with 3 days of nitazoxanide. Ann. Trop. Med. Parasitol. 102:199–207. 10.1179/136485908X267894. [DOI] [PubMed] [Google Scholar]
- 40.Lemée V, Zaharia I, Nevez G, Rabodonirina M, Brasseur P, Ballet JJ, Favennec L. 2000. Metronidazole and albendazole susceptibility of 11 clinical isolates of Giardia duodenalis from France. J. Antimicrob. Chemother. 46:819–821. 10.1093/jac/46.5.819. [DOI] [PubMed] [Google Scholar]
- 41.Upcroft P, Upcroft JA. 2001. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin. Microbiol. Rev. 14:150–164. 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wensaas KA, Langeland N, Rortveit G. 2009. Prevalence of recurring symptoms after infection with Giardia lamblia in a non-endemic area. Scand. J. Prim. Health Care 27:12–17. 10.1080/02813430802602393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Argüello-García R, Cruz-Soto M, Romero-Montoya L, Ortega-Pierres G. 2009. In vitro resistance to 5-nitroimidazoles and benzimidazoles in Giardia duodenalis: variability and variation in gene expression. Infect. Genet. Evol. 9:1057–1064. 10.1016/j.meegid.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 44.Alum A, Sbai B, Asaad H, Rubino JR, Khalid Ijaz M. 2012. ECC-RT-PCR: a new method to determine the viability and infectivity of Giardia cysts. Int. J. Infect. Dis. 16:e350–e353. 10.1016/j.ijid.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Paz-Maldonado MT, Argüello-García R, Cruz-Soto M, Mendoza-Hernández G, Ortega-Pierres G. 2013. Proteomic and transcriptional analyses of genes differentially expressed in Giardia duodenalis clones resistant to albendazole. Infect. Genet. Evol. 15:10–17. 10.1016/j.meegid.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 46.Ellis JE, Wingfield JM, Cole D, Boreham PF, Lloyd D. 1993. Oxygen affinities of metronidazole-resistant and -sensitive stocks of Giardia intestinalis. Int. J. Parasitol. 23:35–39. 10.1016/0020-7519(93)90095-G. [DOI] [PubMed] [Google Scholar]
- 47.Olivares-Illana V, Rodríguez-Romero A, Becker I, Berzunza M, García J, Pérez-Montfort R, Cabrera N, López-Calahorra F, de Gómez-Puyou MT, Gómez-Puyou A. 2007. Perturbation of the dimer interface of triosephosphate isomerase and its effect on Trypanosoma cruzi. PLoS Negl. Trop. Dis. 1:e1. 10.1371/journal.pntd.0000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cortés-Figueroa AA, Pérez-Torres A, Salaiza N, Cabrera N, Escalona-Montaño A, Rondán A, Aguirre-García M, Gómez-Puyou A, Pérez-Montfort R, Becker I. 2008. A monoclonal antibody that inhibits Trypanosoma cruzi growth in vitro and its reaction with intracellular triosephosphate isomerase. Parasitol. Res. 4:635–643. 10.1007/s00436-007-0803-5. [DOI] [PubMed] [Google Scholar]
- 49.Álvarez G, Aguirre-López B, Varela J, Cabrera M, Merlino A, López GV, Lavaggi ML, Porcal W, Di Maio R, González M, Cerecetto H, Cabrera N, Pérez-Montfort R, de Gómez-Puyou MT, Gómez-Puyou A. 2010. Massive screening yields novel and selective Trypanosoma cruzi triosephosphate isomerase dimer-interface-irreversible inhibitors with anti-trypanosomal activity. Eur. J. Med. Chem. 12:5767–5772. 10.1016/j.ejmech.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 50.Hernández-Alcántara G, Torres-Larios A, Enríquez-Flores S, García-Torres I, Castillo-Villanueva A, Méndez ST, de la Mora-de la Mora I, Gómez-Manzo S, Torres-Arroyo A, López-Velázquez G, Reyes-Vivas H, Oria-Hernández J. 2013. Structural and functional perturbation of Giardia lamblia triosephosphate isomerase by modification of a non-catalytic, non-conserved region. PLoS One 8:e69031. 10.1371/journal.pone.0069031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puscas I, Coltau M, Baican M, Domuta G. 1999. Omeprazole has a dual mechanism of action: it inhibits both H+ K+ ATPase and gastric mucosa carbonic anhydrase enzyme in humans (in vitro and in vivo experiments). J. Pharmacol. Exp. Ther. 290:530–534. [PubMed] [Google Scholar]
- 52.Reynaert H, Fernandes E, Bourgain C, Smekens L, Devis G. 1995. Proton-pump inhibition and gastric giardiasis: a causal or casual association? J. Gastroenterol. 30:775–778. 10.1007/BF02349646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.