Abstract
Aminoglycosides exhibit relatively poor activity against intracellular Salmonella enterica serovar Typhimurium due to their low permeativity across eukaryotic cell membranes. Previously, we identified the unique ability of AR-12, a celecoxib-derived small-molecule agent, to eradicate intracellular Salmonella Typhimurium in macrophages by facilitating autophagosome formation and suppressing Akt kinase signaling. In light of this unique mode of antibacterial action, we investigated the ability of AR-12 to sensitize intracellular Salmonella to aminoglycosides in macrophages and in an animal model. The antibacterial activities of AR-12 combined with various aminoglycosides, including streptomycin, kanamycin, gentamicin, and amikacin, against intracellular S. Typhimurium in murine RAW264.7 macrophages were assessed. Cells were infected with S. Typhimurium followed by treatment with AR-12 or individual aminoglycosides or with combinations for 24 h. The in vivo efficacies of AR-12, alone or in combination with gentamicin or amikacin, were also assessed by treating S. Typhimurium-infected BALB/c mice daily for 14 consecutive days. Exposure of S. Typhimurium-infected RAW264.7 cells to a combination of AR-12 with individual aminoglycosides led to a reduction in bacterial survival (P < 0.05), both intracellular and extracellular, that was greater than that seen with the aminoglycosides alone. This sensitizing effect, however, was not associated with increased aminoglycoside penetration into bacteria or macrophages. Moreover, daily intraperitoneal injection of AR-12 at 0.1 mg/kg of body weight significantly increased the in vivo efficacy of gentamicin and amikacin in prolonging the survival of S. Typhimurium-infected mice. These findings indicate that the unique ability of AR-12 to enhance the in vivo efficacy of aminoglycosides might have translational potential for efforts to develop novel strategies for the treatment of salmonellosis.
INTRODUCTION
Aminoglycosides are highly potent, broad-spectrum antibiotics that interfere with protein synthesis by selectively binding to the 30S ribosomes (1). Although aminoglycosides are effective against many bacterial pathogens, they exert poor to no activity in treating intracellular bacterial infections caused by Chlamydia species (2, 3), Staphylococcus aureus (4), Legionella pneumophila (5), and Salmonella enterica (6). To overcome this intrinsic problem, many approaches to increase the entry of aminoglycosides into host cells, including the use of liposomal (7) and chitosan (8) encapsulations, have been proposed.
Salmonella enterica is a Gram-negative, rod-shaped, facultative intracellular pathogen responsible for two major human diseases, gastroenteritis and typhoid fever (9). S. enterica is a leading cause of gastroenteritis, and it is estimated that tens of millions of cases and hundreds of thousands of deaths are related to S. enterica infection worldwide per year (10, 11). Among more than 2,500 S. enterica serovars, S. enterica serovar Typhi (here S. Typhi) causes diseases only in humans, while infection with S. enterica serovar Typhimurium (here S. Typhimurium) often leads to gastroenteritis in humans and a typhoid-like disease in animals (12). In light of this host specificity, S. Typhimurium represents a useful model to evaluate the in vivo efficacy of salmonellosis treatment. During infection, S. Typhimurium crosses the intestinal mucosa and proliferates intracellularly in macrophages at Peyer's patches and lymph nodes. Secondary infection can occur in the liver, spleen, and bone marrow after hematogenous spread, accompanied by typhoid-fever-like syndromes (13). In addition, S. Typhimurium can invade both phagocytic and nonphagocytic cells and proliferate inside an endosome-like vacuole called the Salmonella-containing vacuole (SCV) (14). Thus, the cytoplasmic and SCV membranes protect intracellular Salmonella against host cellular defenses as well as against aminoglycosides and other antibiotics with poor membrane permeativity (15). Moreover, drug resistance has rendered first-line antibiotics, including amoxillin, chloramphenicol, and cotrimoxazole, ineffective against more than two-thirds of Salmonella isolates (16), and the emergence of the nalidixic acid-resistant and ciprofloxacin-resistant isolates further limits antibiotic choice for the treatment of Salmonella infection (17). Thus, development of new therapeutic strategies for Salmonella infections represents an urgent public health issue.
Previously, we demonstrated that a celecoxib-derived chemical agent, AR-12 (also known as OSU-03012), exhibits interesting antibacterial activities against S. Typhimurium and F. tularensis in macrophages (18, 19). Evidence indicated that the ability of AR-12 to eradicate these intracellular pathogens was attributable to two mechanisms: induction of autophagy and inhibition of Akt activation in host cells. In contrast to its effects on intracellular bacteria, AR-12 had no direct microbicidal activity against bacteria residing extracellularly (18). In light of AR-12's unique mode of antibacterial action against intracellular Salmonella, we investigated the potential synergy between AR-12 and aminoglycosides in eradicating S. Typhimurium in infected macrophages and BALB/c mice. Our data indicate that combinations of AR-12 with individual aminoglycosides led to a greater reduction in bacterial survival, relative to that seen with aminoglycosides alone, in macrophages and that daily intraperitoneal (i.p.) injection of AR-12 at 0.1 mg/kg of body weight significantly increased the in vivo efficacy of gentamicin and amikacin in prolonging the survival of bacteria-infected BALB/c mice. This sensitizing effect, however, was not associated with increased aminoglycoside penetration into bacterial or host cells. Together, these findings underscore the translational potential of this combination to foster novel strategies for the treatment of salmonellosis.
MATERIALS AND METHODS
Bacterial strain and macrophage cell lines.
Salmonella enterica serovar Typhimurium ATCC 14028 was obtained from American Type Culture Collection (Manassas, VA) and cultured in Luria-Bertani (LB) broth (Athena Enzyme Systems, Baltimore, MD) at 37°C. The RAW264.7 murine macrophage cell line was purchased from the Bioresource Collection and Research Centre (Hsinchu, Taiwan) and maintained in Dulbecco's modified Eagle medium (DMEM; Gibco-BRL/Invitrogen Corp., Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco-BRL) and 4.5 g/liter of d-glucose.
Reagents.
AR-12 was synthesized as previously described (20) and dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO) as stock solutions. Gentamicin (USB, Santa Clara, CA), amikacin (Yung-Shin Pharm, Taichung, Taiwan), kanamycin (USB), streptomycin (Bio Basic, Ontario, Canada), and fluorescein isothiocyanate (FITC)-conjugated gentamicin (Bioss, Woburn, MA) were dissolved in sterilized deionized water as stock solutions. Captisol was purchased from Ligand Tech (La Jolla, CA) and dissolved in normal saline solution before use.
MIC assay.
The MIC was determined by the broth microdilution method as recommended by the Clinical and Laboratory Standards Institute (21). Bacteria grown overnight on Luria-Bertani (LB) agar plates were suspended in phosphate-buffered saline to an optical density at 600 nm (OD600) of 0.60, which was equivalent to 5.0 × 108 CFU/ml, and then diluted in Mueller-Hinton broth to a final concentration of 5.0 × 105 CFU/ml. The bacteria were treated with aminoglycosides alone at doses ranging from 0.125 to 64 μg/ml or in combination with 1 μM AR-12 in 96-well plates, and the plates were incubated at 37°C for 24 h. The lowest concentration with no visible bacterial growth was defined as the MIC.
Analysis of bacterial growth in macrophage culture.
After overnight growth, S. Typhimurium bacteria were inoculated in LB broth at a 1:100 dilution ratio and incubated at 37°C for 2 h. Bacteria were then centrifuged at 6,000 × g for 3 min and suspended in phosphate-buffered saline (PBS) to an OD600 of 0.60, which was equivalent to 1.20 × 108 CFU/ml. Cultured RAW264.7 cells were seeded and grown to 5 × 105 cells/well in 6-well plates, after which they were infected by adding S. Typhimurium suspension into the culture medium at a multiplicity of infection (MOI) of 25 for 30 min. After infection, cells were washed with warm PBS and then exposed to 100 μg/ml gentamicin in culture medium for 1 h to eliminate extracellular bacteria. After gentamicin treatment, cells were washed with warmed PBS followed by a combined treatment of 1 μM AR-12 and individual aminoglycosides at concentrations at 4×, 8×, or 16× the MIC in Mueller-Hinton broth. After 24 h of treatment, cell culture media were collected and serially diluted with PBS. Cells were washed with warm PBS, and then intracellular bacteria were harvested by incubation in PBS with 0.1% Triton X-100 for 10 min at 37°C. The cell lysates were collected and serially diluted in PBS. Diluted culture medium and lysates were spread on LB agar plates and incubated at 37°C for 16 h. The bacterial colonies that grew on the plates were enumerated and expressed as CFU.
Analysis of the entry of aminoglycosides into cells.
Cultured RAW264.7 macrophage cells were seeded and were grown to 5 × 105 cells/well in 6-well plates. Cells were replenished with fresh medium and incubated for 30 min, followed by treatment with 100 μg/ml gentamicin for 1 h, and then washed with warm PBS and treated with individual aminoglycosides alone at concentrations equal to five times the respective MICs or in combination with 1 μM AR-12 for different intervals. After treatment, the 6-well plates were placed on ice for 10 min, followed by washing with cold PBS and lysis of cells with 400 μl cell lysis buffer containing 150 mM NaCl, 50 mM Tris (pH 7.5), 5 mM EDTA, and 1% Triton X-100 as described previously (22). The bacterial-growth-inhibitory activity of cell lysates was determined by 2-fold serial dilution in Mueller-Hinton broth containing 5.0 × 105 CFU/ml of bacteria and incubation at 37°C for 24 h. The titer of the dilution without visible bacterial growth was considered the minimum inhibitory titer. Each aminoglycoside was also added into lysates of nontreated cells as a control for the MIC assay.
For the fluorescent aminoglycoside uptake assay, cultured RAW264.7 cells were seeded and were grown to 2 × 104 cells/well in 96-well plates. Cells were replenished with fresh medium and incubated for 30 min followed by treatment with 100 μg/ml gentamicin for 1 h and were then washed with warm PBS and treated with 10 μg/ml of FITC-conjugated gentamicin, alone or in combination with 1 μM AR-12, for different intervals. After treatment, the 96-well plates were placed on ice for 10 min and washed with cold PBS, followed by fixation with 4% formaldehyde (Sigma-Aldrich, St. Louis, MO) at 37°C for 30 min. Afterwards, cells were stained with 5 μg/ml of DAPI (4′,6-diamidino-2-phenylindole; AAT Bioquest, Sunnyvale, CA) for 20 min and examined using a fluorescence microscope (IX71; Olympus, Center Valley, PA) equipped with a digital camera (DP71; Olympus) to capture the images of cell nuclei and gentamicin-FITC.
In vivo efficacy study.
Female BALB/c mice (8 weeks of age) were purchased from the National Laboratory Animal Center (Taipei, Taiwan). Mice were housed as groups under conditions of constant photoperiods (12 h of light and 12 h of dark) and provided with sterilized water and food in an animal biosafety level 2 (ABSL-2) room of the Laboratory Animal Center, College of Medicine, National Taiwan University (Taipei, Taiwan). All experimental procedures with the mice were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the National Taiwan University College of Medicine. For infection, mice were fasted for 5 h and then received by orogastric administration 0.2 ml of 5% sodium hydrogen carbonate–PBS, followed by 105 CFU of S. Typhimurium ATCC 14028–0.2 ml of PBS. Mice were then randomly assigned to six treatment groups (n = 5 for each group) that received the following treatments once daily for 14 consecutive days starting at 72 h postinfection: vehicle (normal saline solution containing 5% DMSO and 5% Captisol), AR-12 (0.1 mg/kg via intraperitoneal [i.p.] injection), gentamicin or amikacin (50 or 100 mg/kg, respectively, in normal saline solution via intramuscular [i.m.] injection), or a combination of AR-12 and individual aminoglycosides. The dose of AR-12 was selected based on a pilot study in S. Typhimurium-infected mice that showed that AR-12 at 0.1 mg/kg already possesses potent antibacterial activity under in vivo conditions (data not shown). After the end of the treatment period, mice were monitored for another 14 days to follow animal survival. The body weight and general health of each mouse were monitored daily, and mice that exhibited significant signs of illness or a weight loss of >20% of initial body weight were euthanized. The survival time was defined as the number of days from infection to when the animal was euthanized.
Statistical analysis.
Data are expressed as means ± standard deviations (SD). Differences between group means were assessed using a two-tailed t test for independent samples and were considered significant at a P value of <0.05. Statistical analyses were performed by using SPSS for Windows (version 16.0; SPSS, Inc., Chicago, IL).
RESULTS
Combined treatments with aminoglycosides and AR-12 reduced both extracellular and intracellular Salmonella levels in infected RAW264.7 macrophages.
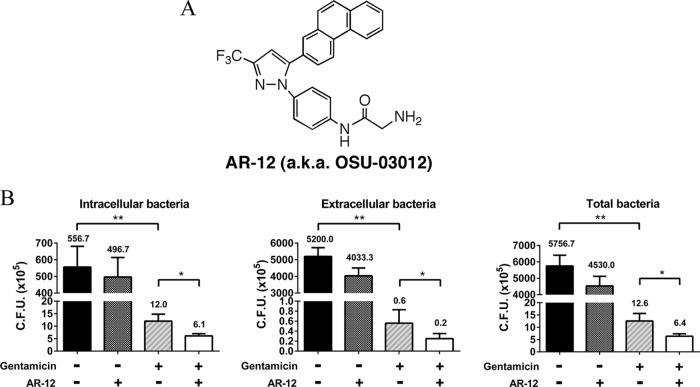
In a previous study, we demonstrated that AR-12 effectively suppressed the intracellular survival of S. Typhimurium inside macrophages at submicromolar concentrations (50% infective concentration [IC50] of 0.2 μM) and had no significant cytotoxic effect on macrophage cells (IC50 of over 10 μM) (18). However, administration of AR-12 had only a modest effect on the survival of S. Typhimurium-infected BALB/c mice (18). This low in vivo efficacy might be attributable to its ineffectiveness with respect to eradication of extracellular pathogens, as the antibacterial activity of AR-12 is associated with its ability to eradicate intracellular bacteria by facilitating autophagosome formation and suppressing Akt kinase signaling in macrophages (18). Pursuant to this premise, we investigated the effect of combining AR-12 (1 μM) with gentamicin (10 μg/ml; 5× the MIC) relative to that of each drug alone on suppressing Salmonella proliferation in S. Typhimurium-infected RAW264.7 cells. In contrast to our previous short-term study (8-h exposure) (18), we conducted the drug treatment for 24 h, as we reasoned that this prolonged exposure allowed intracellular pathogens to escape and reinfect macrophages in the course of drug treatment, thereby recapitulating the process that occurs in vivo. As shown in Fig. 1B, the number of CFU of S. Typhimurium bacteria present in the medium from vehicle-treated cells far exceeded that of intracellular bacteria, suggesting that the bacteria released from infected macrophages were highly proliferative in culture medium. This outgrowth in the extracellular environment, in conjunction with reinvasion, might underlie the very modest suppressive effect (P > 0.05) of AR-12 alone on the proliferation of both extracellular and intracellular bacteria. In contrast, gentamicin treatment suppressed the growth of extracellular bacteria, thereby reducing the number of bacteria available to reinfect macrophages, resulting in substantial reductions in the numbers of intracellular, extracellular, and total bacteria (Fig. 1B). The addition of AR-12 reduced the CFU of intracellular, extracellular, and total bacteria to approximately half of that seen with addition of gentamicin alone (Fig. 1B). This greater antibacterial efficacy might be attributable to the abilities of AR-12 and gentamicin to concomitantly suppress intracellular and extracellular bacterial growth, respectively.
FIG 1.
In vitro evidence that AR-12 potentiates the anti-Salmonella activity of gentamicin. (A) Structure of AR-12. a.k.a., also known as. (B) Salmonella Typhimurium-infected RAW264.7 macrophages were exposed to vehicle, AR-12 (1 μM), gentamicin (10 μg/ml), or the combination of AR-12 and gentamicin for 24 h, and the CFU values of cell lysates (intracellular bacteria; left) and culture medium (extracellular bacteria; middle) and the sum of CFU (total bacteria; right) were determined. Data are presented as means ± SD (n = 3). Statistical significance was examined using a two-tailed t test. *, P < 0.05; **, P < 0.01.
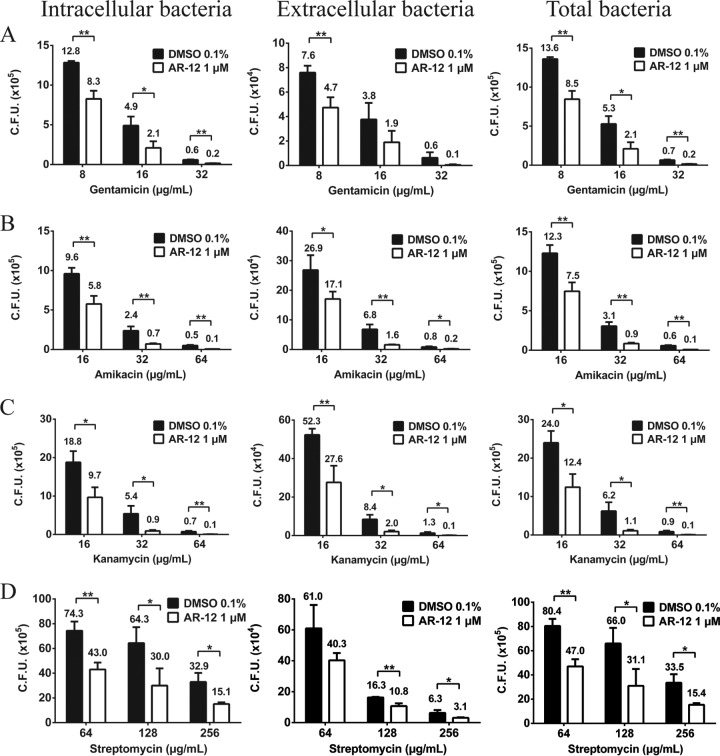
To investigate whether the sensitizing effect of AR-12 was gentamicin specific, we compared the combination of AR-12 (1 μM) and gentamicin with combinations of AR-12 and other aminoglycosides, specifically, amikacin, kanamycin, and streptomycin, at concentrations corresponding to 4×, 8×, and 16× their respective MICs obtained in Mueller-Hinton broth (Table 1) under the aforementioned experimental conditions. For all aminoglycosides tested, the addition of AR-12 increased the suppression of intracellular, extracellular, and total bacterial growth over that seen with each antibiotic alone at each dose tested (Fig. 2). Together, these findings suggest the complementary effect of AR-12 and aminoglycosides on the growth of intracellular and extracellular S. Typhimurium, resulting in greater antibacterial activity.
TABLE 1.
AR-12 does not enhance the antibacterial activity of aminoglycosides against Salmonella Typhimurium in different assay media
| Drug treatment | MIC (μg/ml) |
|||||
|---|---|---|---|---|---|---|
| MH broth |
DMEM |
DMEM + 10% FBS |
||||
| DMSO | AR-12 | DMSO | AR-12 | DMSO | AR-12 | |
| Gentamicin | 2 | 2 | 2 | 2 | 2 | 2 |
| Amikacin | 2 | 2 | 4 | 4 | 4 | 4 |
| Kanamycin | 4 | 4 | 8 | 8 | 8 | 8 |
| Streptomycin | 16 | 16 | 16 | 16 | 16 | 16 |
FIG 2.
Dose-dependent anti-Salmonella effects of gentamicin (A), amikacin (B), kanamycin (C), and streptomycin (D), alone or in combination with AR-12, in Salmonella Typhimurium-infected RAW264.7 macrophages. Bacterially infected macrophages were treated with individual aminoglycosides at concentrations corresponding to 4×, 8×, and 16× MICs, alone or in combination with AR-12 (1 μM), and the CFU values of cell lysates (intracellular bacteria; left panels) and culture medium (extracellular bacteria; center panels) and the combination of the values corresponding to panels A and B (total bacteria; right panels) were determined. Data represent means ± SD (n = 3). Statistical significance was examined using a two-tailed t test. *, P < 0.05; **, P < 0.01.
The sensitizing effect of AR-12 is not attributable to its ability to potentiate the antibacterial activity of aminoglycosides.
Although AR-12 exhibits no direct effect on the growth of S. Typhimurium in either LB broth or acidic magnesium minimal medium (18), we could not rule out the possibility that the aforementioned sensitizing effect was due to the ability of AR-12 to potentiate the anti-Salmonella activity of aminoglycosides. Pursuant to this issue, we assessed the MICs of gentamicin, amikacin, kanamycin, and streptomycin against S. Typhimurium in the presence of AR-12 (1 μM) or a DMSO vehicle control in Mueller-Hinton broth, as well as the cell culture medium DMEM alone or containing 10% FBS. As shown, AR-12 did not affect the antibacterial activity of individual aminoglycosides under any of these assay conditions (Table 1). These results clearly indicate that the ability of AR-12 to increase the anti-Salmonella activity of aminoglycosides was not mediated through modulation of the antibacterial activity of the aminoglycosides.
AR-12 does not facilitate the accumulation of aminoglycosides in macrophages.
Despite their reported poor membrane permeativity, recent evidence indicates that aminoglycosides can be internalized into phagocytes and macrophages via endocytosis/pinocytosis (15, 23–25). This finding raised a possibility that the sensitizing effect of AR-12 might be associated with its ability to increase uptake/accumulation of aminoglycosides in macrophages. However, we obtained two lines of evidence to refute this possibility.
First, the effect of AR-12 on aminoglycoside uptake was examined by assessing the accumulation of FITC-conjugated gentamicin into RAW264.7 macrophages at 24, 72, and 120 h by fluorescence microscopy (Fig. 3). In line with previous reports (25, 26), our data showed a time-dependent increase in the level of intracellular gentamicin-FITC, which, however, was not affected by AR-12. Second, we assessed the anti-Salmonella activities of RAW264.7 cell lysates after exposing intact cells to individual aminoglycosides (gentamicin, amikacin, kanamycin, and streptomycin) at concentrations corresponding to 5 times the respective MICs (DMEM; Table 1) in the presence or absence of AR-12 (1 μM) for different time intervals (Table 2). In addition, individual aminoglycosides were added to lysates of mock-treated cells (mock lysates) or Mueller-Hinton (MH) broth as positive controls for testing MICs (lower panel). Although no appreciable anti-Salmonella activity was noted in any of the lysates from cells exposed for 24 h (i.e., a minimal inhibitory titer of >1/2), the 72- and 120-h lysates showed increases in antibacterial activities indicative of time-dependent intracellular accumulations of aminoglycosides. Again, cotreatment with AR-12 had no appreciable effect on the antibacterial activities of these lysates, indicating that AR-12 did not alter aminoglycoside uptake and accumulation in macrophages.
FIG 3.
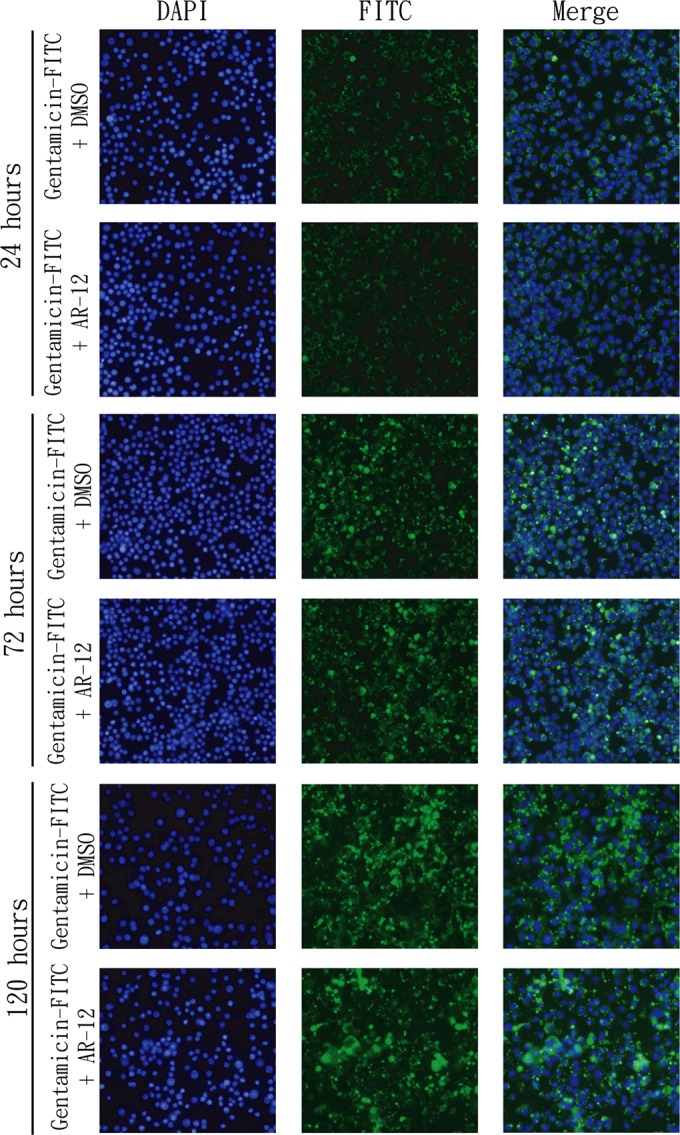
AR-12 has no effect on the accumulation of gentamicin-FITC in RAW264.7 macrophages. Cells were treated with gentamicin-FITC (10 μg/ml), alone or in combination with AR-12 (1 μM), for 24, 72, or 120 h. After treatment, cellular accumulations of gentamicin-FITC were examined with fluorescence microscopy.
TABLE 2.
AR-12 does not enhance the accumulation of aminoglycosides in macrophages
| Treatment | Minimal inhibitory titer |
MIC (μg/ml) |
||||
|---|---|---|---|---|---|---|
| 24 h | 72 h | 120 h | 24 h | 72 h | 120 h | |
| Mock | >1/2 | >1/2 | >1/2 | |||
| Gentamicin (10 μg/ml) | >1/2 | 1/2 | 1/4 | |||
| Gentamicin (10 μg/ml) + AR-12 (1 μM) | >1/2 | 1/2 | 1/4 | |||
| Amikacin (20 μg/ml) | >1/2 | 1/2 | 1/8 | |||
| Amikacin (20 μg/ml) + AR-12 (1 μM) | >1/2 | 1/2 | 1/8 | |||
| Kanamycin (40 μg/ml) | >1/2 | >1/2 | 1/4 | |||
| Kanamycin (40 μg/ml) + AR-12 (1 μM) | >1/2 | >1/2 | 1/4 | |||
| Streptomycin (80 μg/ml) | >1/2 | >1/2 | 1/2 | |||
| Streptomycin (80 μg/ml) + AR-12 (1 μM) | >1/2 | >1/2 | 1/2 | |||
| Mock lysate + gentamicin | 2 | 2 | 2 | |||
| Mock lysate + amikacin | 2 | 2 | 2 | |||
| Mock lysate + kanamycin | 4 | 4 | 4 | |||
| Mock lysate + streptomycin | 16 | 16 | 16 | |||
| MH broth + gentamicin | 2 | 2 | 2 | |||
| MH broth + amikacin | 2 | 2 | 2 | |||
| MH broth + kanamycin | 4 | 4 | 4 | |||
| MH broth + streptomycin | 16 | 16 | 16 | |||
Combinations of aminoglycosides with AR-12 improve the survival of Salmonella-infected BALB/c mice.
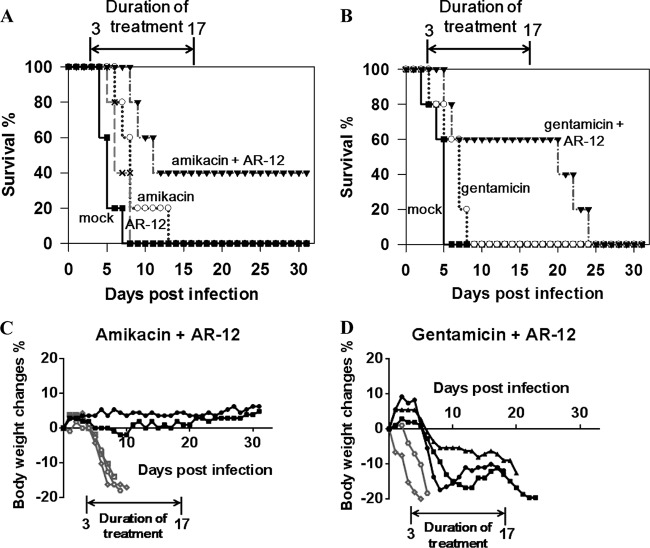
Pursuant to the above findings, we examined the in vivo efficacy of the combination of AR-12 and gentamicin or amikacin in prolonging the survival of Salmonella-infected BALB/c mice. Female BALB/c mice were infected with a lethal dose of S. Typhimurium (105 CFU) and, at 72 h postinfection, were randomly assigned to six groups that received one of the following treatments once daily for 14 consecutive days (n = 5 for each group): vehicle, AR-12 (0.1 mg/kg administered i.p.), gentamicin or amikacin (50 or 100 mg/kg, respectively, administered i.m.), or a combination of AR-12 and individual aminoglycosides. In the vehicle and single-agent treatment groups, mice quickly reached criteria for euthanasia, i.e., signs of severe illness or ≥20% body weight loss, with the following average survival times: vehicle, 4.8 ± 0.8 days; AR-12, 6.6 ± 1.3 days; gentamicin, 6.0 ± 2.0 days; amikacin, 8.4 ± 2.7 days (Fig. 4A and B). In contrast, 40% and 60% of mice in the AR-12-plus-amikacin and AR-12-plus-gentamicin groups, respectively, survived to the end of the 14-day treatment period. Although the body weights of the remaining three mice in the AR-12-plus-gentamicin group initially decreased, these weights either leveled off or rebounded at day 8 or 13 postinfection before decreasing further after the combination therapy was stopped, and the mice thereafter reached sacrifice criteria. In contrast, the two surviving mice in the AR-12-plus-amikacin group survived the infection and showed no signs of illness or body weight loss for an extended period of time after the end of treatment (Fig. 4C).
FIG 4.
The combination of aminoglycosides with AR-12 improves the survival of Salmonella-infected BALB/c mice. Three days after intragastric inoculation of Salmonella Typhimurium (105 CFU), mice were treated once daily with 0.1 mg/kg of AR-12 or vehicle (mock) by i.p. injection and/or with 100 mg/kg of amikacin or 50 mg/kg of gentamicin by i.m. injection (n = 5 for each treatment group). (A and B) Survival rates, as represented by Kaplan-Meier curves, for the amikacin and/or AR-12 treatment groups (A) and the gentamicin and/or AR-12 treatment groups (B). (C and D) Percentages of body weight changes of infected mice treated with the combination of AR-12 and amikacin (C) or gentamicin (D). Each line represents an individual mouse. The black lines indicate mice which survived till the end of treatment; the gray lines indicate mice which were euthanized during the treatment.
DISCUSSION
In light of the rapid emergence of antibiotic resistance, host-directed therapeutic strategies against intracellular bacteria, including activation of innate immunity and blockade of host factors hijacked by bacteria, have received much attention due to their abilities to clear intracellular bacteria without exerting selective pressure (27–29). For instance, adjunct host-directed immunotherapy has been shown to improve outcomes of the antibiotic therapy of tuberculosis (30). Here, we evaluated the therapeutic effect of aminoglycosides in combination with a host-directed drug, AR-12, on Salmonella infection in macrophages and BALB/c mice. These in vitro and in vivo results support our hypothesis that AR-12 sensitizes intracellular bacteria to aminoglycosides via a host-targeted mechanism. The in vivo efficacy of combining AR-12 at a low dose with gentamicin or amikacin in prolonging the survival of Salmonella-infected BALB/c mice is particularly noteworthy.
Among the four aminoglycosides assessed for combination with AR-12, gentamicin and amikacin exhibited better antibacterial activities than kanamycin and streptomycin (Table 1). Despite having the same MIC in Mueller-Hinton broth, the activity of amikacin against extracellular Salmonella of infected macrophages was inferior to that of gentamicin (Fig. 2A and B). This discrepancy is likely due to the lower antibacterial activity of amikacin in DMEM (Table 1), which might be associated with the cation content (Ca2+/Mg2+) being higher in DMEM than in cation-adjusted Mueller-Hinton broth (298 versus 37.5 mg/liter) (31). Higher cation content is known to reduce the antibacterial activity of aminoglycosides (32). However, from a clinical perspective, higher doses of amikacin can be tolerated due to its lesser nephrotoxicity compared to gentamicin (33).
Although aminoglycosides are able to penetrate into macrophages through pinocytosis/endocytosis and are later retained in phagosomes after fusion with endosomes (15), they show poor or no activity against intracellular bacteria. This partly results from the accumulation of aminoglycosides in phagosomes, which become acidified after fusion with lysosomes. As the antibacterial activity of aminoglycosides decreases in a low-pH environment (25), the intracellular aminoglycosides are ineffective in inhibiting intracellular bacteria. Therefore, combining aminoglycosides with a host-directed antimicrobial agent, such as AR-12, represents a feasible strategy to eradicate intracellular bacteria.
The therapeutic combination of aminoglycosides and AR-12 led to prolonged survival of Salmonella-infected mice. Three of five mice receiving the drug combination that included gentamicin survived for the entire 14-day treatment period (Fig. 4B). Although these three mice showed some improvement in their body weights toward the end of the treatment period, they were not able to maintain this recovery after the withdrawal of drug treatment (Fig. 4B and D), suggesting that the bacteria within these mice were not fully cleared during the treatment. Although we also assessed the combination using a higher dose of AR-12 (0.3 mg/kg), no significant improvement in the therapeutic effect was noted in infected mice (data not shown). This finding suggests that the peak plasma concentration in mice receiving 50 mg/kg of gentamicin might be insufficient to clear extracellular bacteria. In contrast to the results seen with gentamicin, two of five mice receiving the combination of amikacin and AR-12 survived to the study endpoint (>30 days postinfection), were able to maintain a consistent body weight, and exhibited no sign of illness after drug withdrawal, suggesting that these two mice were cured of Salmonella infection. As amikacin has been shown to possess lower nephrotoxicity and a wider therapeutic window than gentamicin, it is possible that administration of higher doses of amikacin together with AR-12 might further improve the survival of Salmonella-infected mice. In addition to the in vivo efficacy, a lower drug resistance rate of amikacin has been observed according to the antibiograms of Salmonella worldwide. Surveys of Salmonella clinical isolates conducted in Ghana, Kuwait, United Arab Emirates, India, and Spain showed that the incidence of resistance is lowest for amikacin among all aminoglycosides (34–37). Thus, amikacin in combination with AR-12 might be considered a potential candidate for the development of a new therapeutic strategy for treatment of salmonellosis.
ACKNOWLEDGMENTS
This work was supported by grant numbers NSC 101-2320-B-002-032-MY3 and NSC 102-2321-B-002-090 from National Science Council, Taiwan, to H.-C.C.
Footnotes
Published ahead of print 29 September 2014
REFERENCES
- 1.Magnet S, Blanchard JS. 2005. Molecular insights into aminoglycoside action and resistance. Chem. Rev. 105:477–498. 10.1021/cr0301088. [DOI] [PubMed] [Google Scholar]
- 2.Wentworth BB. 1973. Use of gentamicin in isolation of subgroup-a Chlamydia. Antimicrob. Agents Chemother. 3:698–702. 10.1128/AAC.3.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White LA, Hall HE, Tzianabos T, Chappell WA. 1976. Effect of gentamicin on growth of viral, chlamydial, and rickettsial agents in mice and embryonated eggs. Antimicrob. Agents Chemother. 10:344–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seral C, Van Bambeke F, Tulkens PM. 2003. Quantitative analysis of gentamicin, azithromycin, telithromycin, ciprofloxacin, moxifloxacin, and oritavancin (LY333328) activities against intracellular Staphylococcus aureus in mouse J774 macrophages. Antimicrob. Agents Chemother. 47:2283–2292. 10.1128/AAC.47.7.2283-2292.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodgers FG, Tzianabos AO, Elliott TS. 1990. The effect of antibiotics that inhibit cell-wall, protein, and DNA synthesis on the growth and morphology of Legionella pneumophila. J. Med. Microbiol. 31:37–44. 10.1099/00222615-31-1-37. [DOI] [PubMed] [Google Scholar]
- 6.Kihlström E, Andaker L. 1985. Inability of gentamicin and fosfomycin to eliminate intracellular Enterobacteriaceae. J. Antimicrob. Chemother. 15:723–728. 10.1093/jac/15.6.723. [DOI] [PubMed] [Google Scholar]
- 7.Lutwyche P, Cordeiro C, Wiseman DJ, St-Louis M, Uh M, Hope MJ, Webb MS, Finlay BB. 1998. Intracellular delivery and antibacterial activity of gentamicin encapsulated in pH-sensitive liposomes. Antimicrob. Agents Chemother. 42:2511–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gnanadhas DP, Ben Thomas M, Elango M, Raichur AM, Chakravortty D. 2013. Chitosan-dextran sulphate nanocapsule drug delivery system as an effective therapeutic against intraphagosomal pathogen Salmonella. J. Antimicrob. Chemother. 68:2576–2586. 10.1093/jac/dkt252. [DOI] [PubMed] [Google Scholar]
- 9.Coburn B, Grassl GA, Finlay BB. 2007. Salmonella, the host and disease: a brief review. Immunol. Cell Biol. 85:112–118. 10.1038/sj.icb.7100007. [DOI] [PubMed] [Google Scholar]
- 10.Crump JA, Luby SP, Mintz ED. 2004. The global burden of typhoid fever. Bull. World Health Organ. 82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 11.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ, Jones TF, Fazil A, Hoekstra RM, International Collaboration on Enteric Disease ‘Burden of Illness' Studies 2010. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 50:882–889. 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 12.Haraga A, Ohlson MB, Miller SI. 2008. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 6:53–66. 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 13.Watson KG, Holden DW. 2010. Dynamics of growth and dissemination of Salmonella in vivo. Cell. Microbiol. 12:1389–1397. 10.1111/j.1462-5822.2010.01511.x. [DOI] [PubMed] [Google Scholar]
- 14.Steele-Mortimer O. 2008. The Salmonella-containing vacuole: moving with the times. Curr. Opin. Microbiol. 11:38–45. 10.1016/j.mib.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menashe O, Kaganskaya E, Baasov T, Yaron S. 2008. Aminoglycosides affect intracellular Salmonella enterica serovars typhimurium and virchow. Antimicrob. Agents Chemother. 52:920–926. 10.1128/AAC.00382-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler T. 2011. Treatment of typhoid fever in the 21st century: promises and shortcomings. Clin. Microbiol. Infect. 17:959–963. 10.1111/j.1469-0691.2011.03552.x. [DOI] [PubMed] [Google Scholar]
- 17.Crump JA, Mintz ED. 2010. Global trends in typhoid and paratyphoid fever. Clin. Infect. Dis. 50:241–246. 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu HC, Kulp SK, Soni S, Wang D, Gunn JS, Schlesinger LS, Chen CS. 2009. Eradication of intracellular Salmonella enterica serovar Typhimurium with a small-molecule, host cell-directed agent. Antimicrob. Agents Chemother. 53:5236–5244. 10.1128/AAC.00555-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu HC, Soni S, Kulp SK, Curry H, Wang D, Gunn JS, Schlesinger LS, Chen CS. 2009. Eradication of intracellular Francisella tularensis in THP-1 human macrophages with a novel autophagy inducing agent. J. Biomed. Sci. 16:110. 10.1186/1423-0127-16-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu J, Huang JW, Tseng PH, Yang YT, Fowble J, Shiau CW, Shaw YJ, Kulp SK, Chen CS. 2004. From the cyclooxygenase-2 inhibitor celecoxib to a novel class of 3-phosphoinositide-dependent protein kinase-1 inhibitors. Cancer Res. 64:4309–4318. 10.1158/0008-5472.CAN-03-4063. [DOI] [PubMed] [Google Scholar]
- 21.Wikler MA. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 22.Karasawa T, Wang Q, David LL, Steyger PS. 2010. CLIMP-63 is a gentamicin-binding protein that is involved in drug-induced cytotoxicity. Cell Death Dis. 1:e102. 10.1038/cddis.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barcia-Macay M, Seral C, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. 2006. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob. Agents Chemother. 50:841–851. 10.1128/AAC.50.3.841-851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drevets DA, Canono BP, Leenen PJ, Campbell PA. 1994. Gentamicin kills intracellular Listeria monocytogenes. Infect. Immun. 62:2222–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maurin M, Raoult D. 2001. Use of aminoglycosides in treatment of infections due to intracellular bacteria. Antimicrob. Agents Chemother. 45:2977–2986. 10.1128/AAC.45.11.2977-2986.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonventre PF, Imhoff JG. 1970. Uptake of h-dihydrostreptomycin by macrophages in culture. Infect. Immun. 2:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finlay BB, Hancock RE. 2004. Can. innate immunity be enhanced to treat microbial infections? Nat. Rev. Microbiol. 2:497–504. 10.1038/nrmicro908. [DOI] [PubMed] [Google Scholar]
- 28.Kuijl C, Savage NDL, Marsman M, Tuin AW, Janssen L, Egan DA, Ketema M, van den Nieuwendijk R, van den Eeden SJF, Geluk A, Poot A, van der Marel G, Beijersbergen RL, Overkleeft H, Ottenhoff THM, Neefjes J. 2007. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature 450:725–730. 10.1038/nature06345. [DOI] [PubMed] [Google Scholar]
- 29.Schwegmann A, Brombacher F. 2008. Host-directed drug targeting of factors hijacked by pathogens. Sci. Signal. 1:re8. 10.1126/scisignal.129re8. [DOI] [PubMed] [Google Scholar]
- 30.Zumla A, Nahid P, Cole ST. 2013. Advances in the development of new tuberculosis drugs and treatment regimens. Nat. Rev. Drug Discov. 12:388–404. 10.1038/nrd4001. [DOI] [PubMed] [Google Scholar]
- 31.Wu X, Lin M, Li Y, Zhao X, Yan F. 2009. Effects of DMEM and RPMI 1640 on the biological behavior of dog periosteum-derived cells. Cytotechnology 59:103–111. 10.1007/s10616-009-9200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barry AL, Reller LB, Miller GH, Washington JA, Schoenknect FD, Peterson LR, Hare RS, Knapp C. 1992. Revision of standards for adjusting the cation content of Mueller-Hinton broth for testing susceptibility of Pseudomonas aeruginosa to aminoglycosides. J. Clin. Microbiol. 30:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweileh WM. 2009. A prospective comparative study of gentamicin- and amikacin-induced nephrotoxicity in patients with normal baseline renal function. Fundam. Clin. Pharmacol. 23:515–520. 10.1111/j.1472-8206.2009.00702.x. [DOI] [PubMed] [Google Scholar]
- 34.Newman MJ, Frimpong E, Donkor ES, Opintan JA, Asamoah-Adu A. 2011. Resistance to antimicrobial drugs in Ghana. Infect. Drug Resist. 4:215–220. 10.2147/IDR.S21769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rotimi VO, Jamal W, Pal T, Sonnevend A, Dimitrov TS, Albert MJ. 2008. Emergence of multidrug-resistant Salmonella spp. and isolates with reduced susceptibility to ciprofloxacin in Kuwait and the United Arab Emirates. Diagn. Microbiol. Infect. Dis. 60:71–77. 10.1016/j.diagmicrobio.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Manchanda V, Bhalla P, Sethi M, Sharma VK. 2006. Treatment of enteric fever in children on the basis of current trends of antimicrobial susceptibility of Salmonella enterica serovar typhi and paratyphi A. Indian J. Med. Microbiol. 24:101–106. 10.4103/0255-0857.25182. [DOI] [PubMed] [Google Scholar]
- 37.Morosini MI, Garcia-Castillo M, Coque TM, Valverde A, Novais A, Loza E, Baquero F, Canton R. 2006. Antibiotic coresistance in extended-spectrum-beta-lactamase-producing Enterobacteriaceae and in vitro activity of tigecycline. Antimicrob. Agents Chemother. 50:2695–2699. 10.1128/AAC.00155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]