Abstract
Urinary tract infections (UTIs) due to extended-spectrum-β-lactamase (ESBL)-producing Enterobacteriaceae in children are becoming more frequent, and they are commonly treated initially with a second- or third-generation cephalosporin. We developed a murine model of ascending UTI caused by ESBL-producing Escherichia coli. Using this model, we investigated the renal bacterial burden, interleukin-6 (IL-6) expression, and histopathological alterations caused by ESBL- and non-ESBL-producing bacteria after 1, 2, or 6 days with or without ceftriaxone therapy. The renal bacterial burden, IL-6 concentration, and histological inflammatory lesions were not significantly different between mice infected with ESBL- and non-ESBL-producing bacteria without treatment at any of the time points examined. Following ceftriaxone administration, the bacterial burden was eliminated in the kidneys of mice infected with ESBL- and non-ESBL-producing bacteria on the 6th postinfection day. The histological analysis demonstrated that among mice treated with ceftriaxone, those infected with ESBL-producing bacteria had more profound renal alterations than those infected with non-ESBL-producing bacteria on the 6th day (P < 0.001). In comparison, microbiological outcomes did not differ significantly between mice infected with ESBL- and non-ESBL-producing bacteria at any of the time points examined. The effectiveness of ceftriaxone in mice with UTIs due to ESBL-producing E. coli may have therapeutic implications; it is, however, hampered by limited activity on the histopathological lesions, a finding that needs further investigation.
INTRODUCTION
Extended-spectrum β-lactamases (ESBL) are enzymes that hydrolyze penicillins, cephalosporins of the first, second, and third generations, and aztreonam and are mostly produced by Enterobacteriaceae (1, 2). Since their discovery, ESBL enzymes have spread in many microorganisms and have caused a wide variety of infections in all age groups worldwide (3, 4). Infections caused by ESBL-producing bacteria are difficult to treat, as these bacteria are generally resistant to the empirical therapy commonly used (5–13). Infections caused by such bacteria are believed to have worse outcomes than infections caused by non-ESBL-producing bacteria (5, 6, 9). It is not clear whether the worse outcomes of infections caused by ESBL-producing bacteria are attributable to the greater pathogenicity of the invading bacteria, to inappropriate initial therapy, or to both.
Urinary tract infections (UTIs) are common causes of morbidity in children and adults (14–16). UTIs caused by ESBL-producing bacteria have been spreading in both nosocomial settings and in the community (17–19). It is unclear whether UTIs caused by ESBL-producing bacteria share the unfavorable outcomes of other ESBL infections, such as sepsis or pneumonia. We developed an ascending UTI model in BALB/c mice in order to study the microbiological, inflammatory, and immunological aspects of UTIs caused by ESBL-producing bacteria and the effect of ceftriaxone, a third-generation cephalosporin. Specifically, we quantified the renal bacterial load and searched for proinflammatory cytokine interleukin-6 (IL-6) release from phagocytes at the site of infection and localized inflammatory cells expressing IL-6 immunohistochemically. We further evaluated the extent of the histopathological alterations in the kidneys of mice infected with ESBL- compared to non-ESBL-producing bacteria with and without treatment with ceftriaxone. To our knowledge, this is the first study to investigate such aspects of infections caused by ESBL- versus non-ESBL-producing bacteria and their response to a third-generation cephalosporin.
MATERIALS AND METHODS
Bacteria.
Three isolates of ESBL-producing and three isolates of non-ESBL-producing Escherichia coli strains were obtained from the microbiology laboratories of Hippokration Hospital of Thessaloniki and University Hospital of Larissa, both in Greece. All of the ESBL- and non-ESBL-producing strains originated from patients with febrile UTIs. The genetic characterization of ESBL-producing strains showed that the E10 strain had an SHV5-type ESBL activity, whereas the E16 and E94 strains showed a CTX-M15-type ESBL activity. The antibiotic susceptibility results by the Vitek 2 automated system (bioMérieux, Marcy l'Etoile, France) for all strains used are shown in Table S1 in the supplemental material. As ceftriaxone was not included in the antimicrobial panel of the Vitek 2 system routinely applied in the microbiology lab, in all study strains double-disk synergy tests using ceftriaxone and ceftriaxone MICs by agar dilution were performed according to the CLSI guidelines and breakpoints (20). ESBL-producing strains were resistant to ceftriaxone (E10, 4 μg/ml; E16 and E94, >64 μg/ml) in contrast to non-ESBL-producing strains (9189, 9142, and BRA, each ≤1 μg/ml). The strains were maintained as stocks in 75% glycerol-25% peptone at −80°C. Prior to each experiment, the strains were revived by inoculation on Mueller-Hinton (MH) solid medium (Applichem GmbH, Darmstadt, Germany) at 37°C for 24 h; for ESBL-producing strains, the culture medium contained 60 μg/ml ampicillin. Five single colonies from each strain were then inoculated in 20 ml MH medium and incubated at 37°C for an additional 24 h with shaking. The final inoculum concentration was 2 × 109 bacteria/ml.
Animals.
Female BALB/c mice aged 8 to 10 weeks and weighing 18 to 20 g were obtained from the Alexander Fleming Biomedical Science Research Center (Athens, Greece). Animals were randomly grouped in cages of 6 and housed in the pathogen-free animal facility of the Laboratory of Anatomy, Histology and Embryology of Veterinary Medicine, Aristotle University of Thessaloniki, operating in accordance with the European Union Guidelines (Official Journal of the European Communities No. L 374/11) and Hellenic Government Legislation. Mice were fed with a standard diet and given water without antibiotics, ad libitum, and acclimated in the animal facility 1 week prior to inoculation.
A total of 177 mice were used. Our experimental design consisted of groups of mice infected with either ESBL- or non-ESBL-producing E. coli strains. In the first three experiments, no antibiotics were used. Three distinct ESBL-producing strains were used to infect 23, 18, and 21 mice, and three distinct non-ESBL-producing strains were used to infect 20, 14, and 21 mice in each experiment, respectively. In the fourth experiment, two groups of 22 mice each were infected with one ESBL-producing strain (E94) and one non-ESBL-producing (BRA) strain, respectively, and ceftriaxone treatment was started at 9 h postinfection. Additionally, 4 mice in each of four experiments received normal saline transurethrally and were used as controls.
Inoculation procedure.
A previously described procedure was used with modifications (21–23). Briefly, animals were anesthetized by intraperitoneal injection of a solution containing 0.01 mg fentanyl (Janssen, Beerse, Belgium) and 0.8 mg midazolam (Roche, Basel, Switzerland) dissolved in 0.32 ml sterile water for injection. After 20 min to allow sedation, anesthetized mice were transurethrally catheterized by use of a polyethylene catheter of 0.6-mm outer diameter (26 GA; Becton Dickinson, Helsingborg, Sweden). Fifty microliters of the prepared inoculum was gently injected in the bladders of the mice via a 1-ml insulin syringe. Control animals received 50 μl of normal saline. In order to achieve obstruction, the outer orifice of the urethra was sealed with collodion (Digas, Salonika, Greece). Six hours later, the collodion was removed by gentle massage with acetone.
One, 2, and 6 days later, mice were sacrificed by cervical dislocation; their kidneys were aseptically removed, bisected sagittally, and weighed. Two halves originating from different kidneys of each mouse were used for the bacterial burden analysis and IL-6 determination, while the other two halves were used for histological and IL-6 immunohistochemical studies. In this way, we were able to analyze the kidneys of each mouse simultaneously and eliminate erroneous results that could possibly arise due to potentially different handling conditions.
Antibiotic administration.
Nine hours postinfection, mice were injected with 2.5 mg ceftriaxone subcutaneously administered every 12 h until the end of each experiment.
Bacterial burden analysis.
Halves of each kidney were weighed and homogenized in 1 ml phosphate-buffered saline (PBS) (pH 7.2). Serial 10-fold dilutions of homogenized kidney tissue were plated on MH medium. The plates were then incubated at 37°C overnight. CFU were counted, and the numbers of colonies obtained were converted to log10 CFU/g of mouse kidney tissue for statistical evaluation.
IL-6 detection and measurement.
The homogenized kidneys were centrifuged at 10,000 rpm for 10 min to remove cell debris. One hundred microliters of supernatant was used to assess production of IL-6 with an enzyme-linked immunosorbent assay (ELISA) (Quantikine; R&D, Minneapolis MN). Each condition was tested in duplicate, and the samples were measured spectrophotometrically at 450 nm with a reference wavelength at 540 nm. Results of IL-6 release are expressed in pg/ml of supernatant and transformed to pg of IL-6 per gram of renal tissue.
Histological analysis.
One-half of each kidney was fixed by immersion in 4% paraformaldehyde dissolved in 0.1 M PBS (pH 7.4) for a week, then transferred, dehydrated, and embedded in paraffin wax. Serial sagittal 8-μm-thick sections were cut and collected on Superfrost Plus slides (Menzel Gläser, Braunschweig, Germany). Systematic random sampling was performed on sections from each kidney. Starting randomly with the first or the second section, every fifth section was used for analysis, yielding 24 to 32 consecutive sections per kidney. These selected sections were stained with hematoxylin and eosin (H&E) and examined with a Nikon Eclipse 80i photomicroscope by two independent researchers, blinded to the identity of the histological preparations. The severity of kidney damage was determined by a semiquantitative scoring of renal inflammatory lesions according to the following categories: grade 0, normal renal tissue; grade 1, mild pyelitis, characterized by infiltration of low to moderate numbers of neutrophils and hemorrhage in the pelvic cavity and intact uroepithelium; grade 2, moderate pyelitis shown as diffuse damage to the uroepithelium with infiltration of moderate numbers of neutrophils in the uroepithelium and in the renal medullar parenchyma adjacent to the pelvic cavity; grade 3, severe pyelitis with moderate inflammation in the parenchyma adjacent to the pelvic cavity and moderate damage to the uroepithelium (either disrupted due to necrosis and ulceration or thickened by regenerative hyperplasia); grade 4, mild pyelonephritis involving <50% of the kidney; and grade 5, severe pyelonephritis with extensive (>50% of the kidney) damage (24). Median scores were used whenever the histopathology of kidneys corresponded to assessments ranging between two successive scores. Both kidneys of each animal were evaluated and scored independently, as differences in the degrees of infection were observed in most cases.
IL-6 detection by immunohistochemistry.
For the detection of IL-6-containing inflammatory cells, selected paraffin sections were first processed for microwave-mediated antigen retrieval in 0.01 M citrate buffer (pH 6.0). Sections were heated at 750 W for 3 min and subsequently at 350 W for 9 min. They were then cooled at room temperature for 35 min, rinsed in 0.1 M PBS (pH 7.4), blocked with 4% bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO) for 1 h, and incubated with the primary antibody to IL-6 (polyclonal goat anti-IL-6, 1:250; Santa Cruz Biotechnology, Inc., Dallas, TX) in PBS-0.5% Triton X-100 solution at 4°C for 24 h. On the next day, sections were transferred in PBS solution containing biotinylated goat anti-mouse IgG antibody (1:200, Vectastain ABC systems; Vector Laboratories, Burlingame, CA) for 2 h, placed in the avidin-biotin-peroxidase complex (ABC) (1:100 in PBS) for 1 h, and incubated in a solution of diaminobenzidine (DAB)-0.01% H2O2 (DAB substrate kit for peroxidase, SK-4100; Vector Laboratories) in PBS for 5 min. Sections were then dehydrated, coverslipped, and finally examined with a Zeiss Axioplan photomicroscope. Control sections, in which the primary antibody or the IgG was omitted, showed no positive staining.
Statistical evaluation.
Microbiological and immunological analyses were performed using InStat version 3 (GraphPad, Inc., San Diego, CA). The nonparametric Mann-Whitney U test was used for bacterial burden and ELISAs. The mean values of the bacterial burden scores for each portion of the kidney and treatment groups were calculated. Prior to statistical analysis, all values of bacterial burdens and IL-6 were transformed to logarithms of 10 and are expressed as means ± standard errors (SE). Logarithms of values of zero were replaced with the minimal value of 0.1. The averages of the duplicate wells from each ELISA were used in the data analysis to calculate the mean ± SE of all the experiments for each treatment tested. Moreover, the histopathological evaluation results were examined by the Kruskal-Wallis test followed by the Mann-Whitney U test to reveal the source of the differences and analyzed by SPSS software version 20. The correlation between the histopathology scores and the microbial burden was evaluated using curve estimation analysis. A two-sided P value of <0.05 was considered significant for all tests.
RESULTS
Microbiology.
The renal bacterial burden of mice infected with ESBL-producing E. coli strains did not significantly differ from that of mice infected with non-ESBL-producing strains throughout the study in each of the three initial experiments, in which no ceftriaxone was administered (Table 1).
TABLE 1.
Bacterial burden from kidneys of mice inoculated with ESBL- and non-ESBL-producing strains without antibiotic treatment
| Expt | Bacterial burden of kidneys (mean ± SE) (log10 CFU/g) or P value on day: |
||
|---|---|---|---|
| 1 | 2 | 6 | |
| 1 | |||
| ESBL (E16,a n = 23) | 3.5 ± 1.1 | 4 ± 0.8 | 2.6 ± 0.9 |
| Non-ESBL (9189,a n = 20) | 5.1 ± 0.3 | 2.3 ± 0.9 | 1.0 ± 1.3 |
| P | 0.18 | 0.18 | 0.63 |
| 2 | |||
| ESBL (E10,a n = 18) | 5.2 ± 1.2 | 3.9 ± 1 | 4.8 ± 0.5 |
| Non-ESBL (9142,a n = 14) | 4 ± 0.8 | 5.4 ± 0.5 | 3.9 ± 1.4 |
| P | 0.45 | 0.23 | 0.50 |
| 3 | |||
| ESBL (E94,a n = 21) | 2.3 ± 1.2 | 2.9 ± 1 | 2 ± 1 |
| Non-ESBL (BRA,a n = 21) | 4.3 ± 1.1 | 3.1 ± 0.9 | 3.5 ± 0.9 |
| P | 0.25 | 0.87 | 0.31 |
Different ESBL and non-ESBL clinical isolates. ESBL-producing strains were resistant to ceftriaxone (E10, 4 μg/ml; E16 and E94, >64 μg/ml) in contrast to non-ESBL-producing strains (all ≤1 μg/ml).
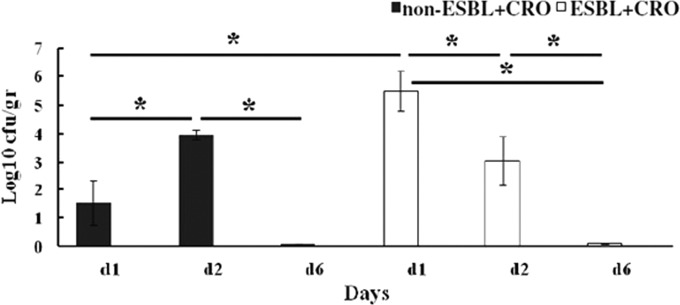
The mice infected with either an ESBL- or a non-ESBL-producing strain and treated with ceftriaxone were bacteria free on the 6th postinfection day (Fig. 1). Specifically, in mice infected with an ESBL-producing strain, the renal bacterial burden was significantly smaller on the 6th postinfection day (mean ± SE, 0.1 ± 0 log10 CFU/g) than on the 1st (5.5 ± 0.7 log10 CFU/g, P < 0.001) or 2nd (3 ± 0.8 log10 CFU/g, P = 0.012) postinfection day. Additionally, in these mice, the renal bacterial burden was significantly smaller on the 2nd than on the 1st (P = 0.046) postinfection day. Among the ceftriaxone-treated mice infected with a non-ESBL-producing strain, the renal bacterial burden was significantly smaller on the 6th (0.1 ± 0 log10 CFU/g) than on the 2nd (3.9 ± 0.1 log10 CFU/g, P < 0.001) (Fig. 1) postinfection day.
FIG 1.
Impact of ceftriaxone (CRO) treatment on the renal bacterial burden of mice infected with ESBL- and non-ESBL-producing bacteria. Mice inoculated with ESBL-producing (E94, n = 22) and non-ESBL-producing (BRA, n = 22) bacteria were treated with 2.5 mg CRO at 9 h postinfection and every 12 h thereafter until the end of the study period. Viable cell counts in the kidneys (CFU/g) are shown for CRO-treated mice infected with non-ESBL- and ESBL-producing bacteria for day 1 (d1), day 2 (d2), and day 6 (d6). Values are expressed as means ± standard errors. The statistical significance between columns is denoted by a bar and an asterisk (P < 0.01).
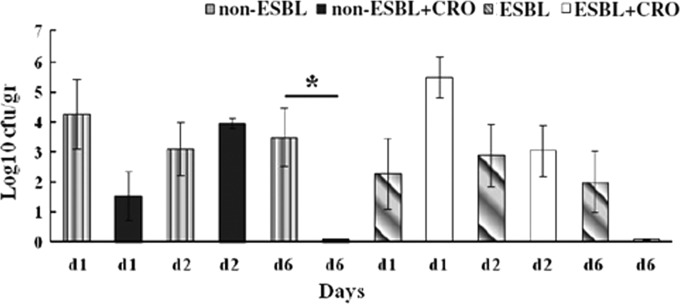
The renal bacterial burden of mice treated with ceftriaxone after infection with non-ESBL-producing strains was significantly smaller than that after infection with ESBL-producing strains on the 1st (mean ± SE, 1.5 ± 0.8 log10 CFU/g versus 5.5 ± 0.7 log10 CFU/g, respectively, P = 0.002) but not on the 2nd or 6th postinfection days (Fig. 1). Among mice infected with non-ESBL-producing strains, those treated with ceftriaxone had a significantly smaller renal bacterial burden than those without antibiotic treatment on the 6th (0.1 ± 0 log10 CFU/g versus 3.5 ± 0.1 log10 CFU/g, P = 0.011) postinfection day (Fig. 2).
FIG 2.
Comparison of the effect of ceftriaxone (CRO) treatment versus no antibiotic on the renal bacterial burden of mice infected with ESBL- and non-ESBL-producing bacteria. Mice inoculated with ESBL-producing (E94, n = 22) and non-ESBL-producing (BRA, n = 22) bacteria were treated with ceftriaxone, and 21 mice received no antibiotic. Viable cell counts in the kidneys (CFU/g) are shown for untreated and CRO-treated mice infected with non-ESBL- and ESBL-producing bacteria for day 1, day 2, and day 6. Values are expressed as means ± standard errors. The statistical significance between columns is denoted by a bar and an asterisk (P < 0.01).
Determination of IL-6 levels in the kidneys.
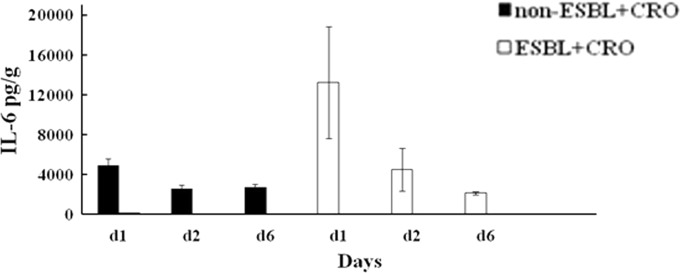
Without antibiotic treatment, the renal IL-6 levels did not significantly differ between mice infected with ESBL-producing strains (mean ± SE, during the 1st day, 2,705.2 ± 1,204.3 pg/g; 2nd day, 1,509.5 ± 75.9 pg/g; 6th day, 1,586 ± 285.7 pg/g) or non-ESBL-producing strains (1st day, 4,242.4 ± 1,588 pg/g; 2nd day, 1,835 ± 628 pg/g; 6th day, 3,079.6 ± 984.3 pg/g). Likewise, among the ceftriaxone-treated mice infected with ESBL-producing strains, IL-6 did not differ significantly between the 1st, 2nd, or 6th postinfection day (during 1st day, 13,231 ± 5,632.6 pg/g; 2nd day, 4,477.4 ± 2,164.3 pg/g; 6th day, 2,098.5 ± 150.5 pg/g) (Fig. 3). In contrast, among the ceftriaxone-treated mice infected with non-ESBL-producing strains, IL-6 levels were significantly higher on the 1st (4,852.5 ± 692 pg/g) than on the 2nd (mean ± SE, 2,546.5 ± 355.6 pg/g, P = 0.018) or the 6th (2,672.3 ± 365.3 pg/g, P = 0.024) postinfection day (Fig. 3).
FIG 3.
Impact of ceftriaxone (CRO) treatment on renal IL-6 concentration in mice infected with ESBL- and non-ESBL-producing bacteria. The supernatants of homogenized kidneys explanted from CRO-treated mice infected with ESBL-producing (E94, n = 22) and non-ESBL-producing (BRA, n = 22) bacteria were assessed for the production of IL-6 using ELISAs. IL-6 production in CRO-treated mice infected with non-ESBL- and ESBL-producing bacteria for day 1, day 2, and day 6 are shown. Levels of IL-6 release are expressed as means ± standard errors in pg/g. The statistical significance between columns is denoted by a bar and an asterisk (P < 0.01).
Histological analysis. (i) Without antibiotic treatment.
The kidneys of 21 mice infected with ESBL-producing bacteria or 21 mice infected with non-ESBL-producing bacteria that received no ceftriaxone were first studied by histological examination. The kidneys of 4 noninfected mice were used as controls. The control mice exhibited normal renal morphology under microscopic investigation (grade 0) at all time points examined.
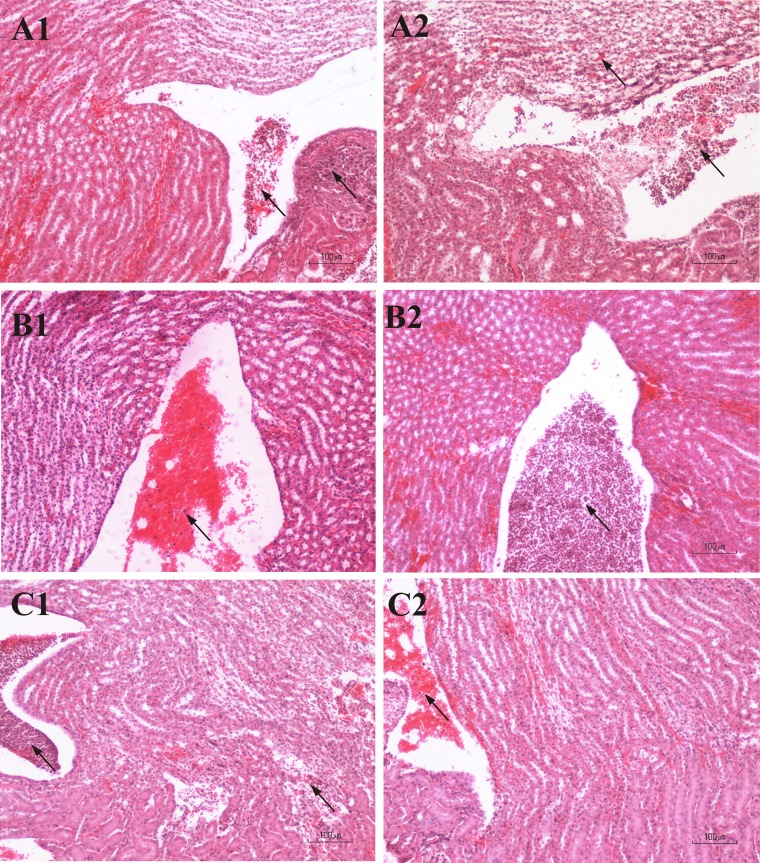
In mice infected with ESBL- and non-ESBL-producing bacteria, renal histopathological alterations, in most cases bilateral, were noted at all time points (Fig. 4). On the 1st postinfection day, the histopathological alterations in mice infected with ESBL-producing bacteria had mostly inflammatory lesion scores of grade 1. The lesions were characterized by a moderate inflammatory infiltration consisting of neutrophils that extended from the pelvic cavity to the renal medulla, especially at the poles of kidneys. The majority of mice infected with non-ESBL-producing bacteria were scored with grades of 2 and 3. They displayed hemorrhage and exudates within the pelvic cavity, moderate inflammatory cellular infiltration in the surrounding medullar parenchyma, and disruption of the uroepithelium. Mild pyelonephritis (grade 4) occurred in rare cases in mice infected with either the ESBL- or the non-ESBL-producing strains. In these animals, the medullar parenchyma showed hyperemia, extensive interstitial invasion of inflammatory cells, tubular dilation or destruction, and disruption of the uroepithelium by a fibrinous inflammatory infiltrate. The renal damage also involved cortical glomeruli (see Table S2 in the supplemental material).
FIG 4.
Photomicrographs of H&E-stained renal sections of mice infected with ESBL-producing (A1, B1, and C1) and non-ESBL-producing (A2, B2, and C2) bacteria, at different time points, without antibiotic treatment. (A1 and A2) First day postinfection. (A1) Inflammatory lesion score 1. Neutrophils within the renal pelvic cavity (left arrow) and medulla (right arrow) are shown. (A2) Inflammatory lesion score 3. Numerous neutrophils within the pelvic cavity (right arrow) and extensive inflammatory cellular infiltration of the medullar parenchyma (left arrow) are shown. (B1 and B2) Second day postinfection. (B1) Inflammatory lesion score 1. Hemorrhage is shown with moderate numbers of neutrophils within the renal pelvic cavity (arrow). (B2) Inflammatory lesion score 2. Numerous neutrophils within the renal pelvic cavity (arrow) are shown. (C1 and C2) Sixth day postinfection. (C1) Inflammatory lesion score 3. Congestion of neutrophils within the renal pelvic cavity (left arrow) is shown. Extensive inflammatory cellular infiltration and tubular destruction in the medulla (right arrow) are shown. (C2) Inflammatory lesion score 1. Hemorrhage is shown with neutrophils within the renal pelvic cavity (arrow) and disrupted uroepithelium.
On the 2nd postinfection day, the histopathological alterations in mice infected with ESBL- and non-ESBL-producing bacteria had mainly scores of grades 1 and 2. On the 6th postinfection day, the histopathological alterations in mice infected with ESBL-producing bacteria had scores of grades 1 to 3. In mice infected with non-ESBL-producing bacteria, scores ranged from grades 1 to 5. At no time point were the scores significantly different between mice infected with ESBL- or non-ESBL-producing bacteria. Moreover, there were no differences in scores among the 1st, 2nd, and 6th days postinfection in mice infected with ESBL- or non-ESBL-producing bacteria. Of note, at the early postinfection time points (1st and 2nd days), the majority of inflammatory cells were neutrophils and macrophages, whereas at the late postinfection time point (6th day) monocytes, lymphocytes, and fibroblasts were also abundant in mice infected with ESBL- and non-ESBL-producing bacteria.
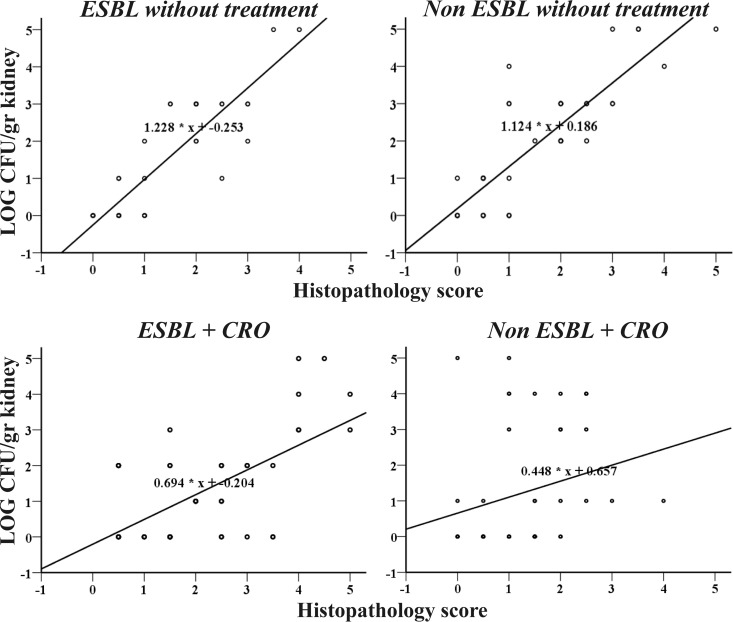
Curve fit analysis showed a strong positive linear correlation between histopathological scores and microbial burdens of mice infected with ESBL- and non-ESBL-producing bacteria either overall (R2 = 0.781 and R2 = 0.722, respectively, P < 0.0001) or at each postinfection time point (for ESBL: day 1, R2 = 0.902; day 2, R2 = 0.655; day 6, R2 = 0.841, all P < 0.0001; for non-ESBL: day 1, R2 = 0.647; day 2, R2 = 0.590; day 6, R2 = 0.859, all P ≤ 0.004). The equations describing the linear correlations of ESBL- and non-ESBL-producing strains without treatment were almost the same (Fig. 5), indicating that for a given microbial burden the histopathological scores for either ESBL- or non-ESBL-producing strains are approximately equal.
FIG 5.
Linear correlation between the microbial burden and the histopathology score of ESBL- and non-ESBL-producing strains with and without ceftriaxone (CRO).
(ii) With antibiotic treatment.
In the kidneys of 22 mice infected with ESBL-producing bacteria and 22 mice infected with non-ESBL-producing bacteria that received ceftriaxone, histopathological alterations, in most cases bilateral, were noted at all time points (Fig. 6). On the 1st postinfection day, the renal lesions in mice infected with ESBL-producing bacteria had scores that ranged between 1 and 5, with the majority being 2 or 3, while mice infected with non-ESBL-producing bacteria had mostly scores of 1 to 2 (see Table S2 in the supplemental material).
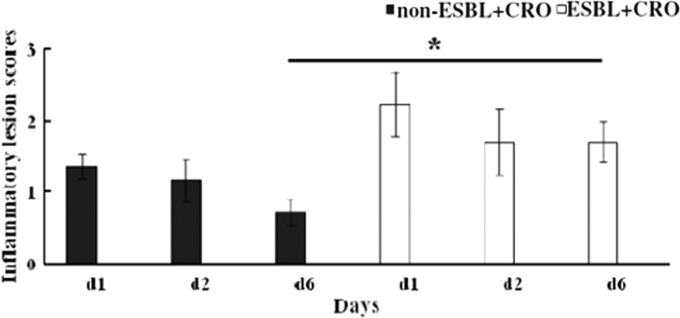
FIG 6.
Histopathological alterations defined as inflammatory lesion scores induced by ESBL-producing or non-ESBL-producing E. coli strains at the 1st, 2nd, and 6th postinfection day. All mice infected with ESBL- and non-ESBL-producing strains were treated with ceftriaxone. Lesion scores of histopathological alterations are defined in Materials and Methods. An asterisk denotes a significant difference between non-ESBL and ESBL producers at day 6.
On the 2nd postinfection day, the lesions in mice infected with ESBL- or non-ESBL-producing bacteria had scores of 1 to 3 and rarely 4 or 5. However, on the 6th postinfection day, renal lesions in mice infected with ESBL-producing bacteria had scores ranging between 1 and 4, while mice infected with non-ESBL-producing bacteria had limited histopathological findings (mainly of grade 1; P = 0.0001; Fig. 6 and Table S2).
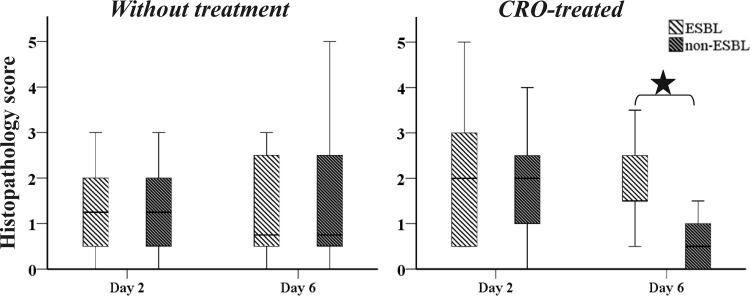
Scores significantly differed between mice infected with ESBL- and non-ESBL-producing bacteria and treated with ceftriaxone on the 6th postinfection day, but not on the 2nd postinfection day (Fig. 7). The Kruskal-Wallis variance analysis showed that the degree of inflammation was significantly different on different postinfection days among the groups of mice infected with non-ESBL- but not ESBL-producing bacteria. The Mann-Whitney U test demonstrated that the histopathological findings were significantly milder on day 6 than on day 2 postinfection in mice infected with non-ESBL-producing bacteria (P = 0.002). Moreover, in mice infected with ESBL-producing bacteria, the inflammatory cells prevailing at the early and late postinfection periods were neutrophils and monocytes, indicating a persistent infection, whereas in mice infected with non-ESBL-producing bacteria at the late postinfection period (6th day) neutrophils were almost absent.
FIG 7.
Box plots of histopathology scores for the 2nd and 6th postinfection day of ESBL-producing strain E94 and non-ESBL-producing strain BRA with and without ceftriaxone (CRO). By Mann-Whitney U test significant difference between the scores of mice infected with ESBL- and non-ESBL-producing bacteria and treated with CRO on the 6th postinfection day is indicated by an asterisk.
Curve fit analysis showed a strong positive linear correlation between the histopathological scores and microbial burdens of kidneys of mice infected with ESBL- and non-ESBL-producing bacteria without treatment. A milder positive linear correlation was found in the case of kidneys from mice infected with ESBL-producing bacteria that received ceftriaxone in the early postinfection period. However, for kidneys from mice infected with non-ESBL-producing bacteria that received ceftriaxone, no correlation was detected, indicating that elimination of the microbial burden precedes the resolution of inflammation.
Indeed, there was a positive linear correlation between the histopathological scores and microbial burdens of kidneys from mice infected with ESBL-producing bacteria either overall (R2 = 0.381, P < 0.0001) or at each time point except day 6 (day 1, R2 = 0.32, P = 0.035; day 2, R2 = 0.766, P < 0.0001). In kidneys from mice infected with non-ESBL-producing bacteria, no correlation was detected between the histopathological scores and microbial burdens (R2 = 0.061, P = 0.107) (Fig. 5).
Immunohistochemistry.
Renal sections from the normal control animals showed no immunoreactivity for IL-6. In kidney sections from mice infected with ESBL- or non-ESBL-producing bacteria, with or without ceftriaxone treatment, immunoreactive cells for IL-6 were identified at all time points (Fig. 8). In kidneys with scores of 1 to 3, moderate numbers of IL-6-positive cells were scattered throughout the renal medulla and accumulated within the lumen of the pelvis. In kidneys with higher scores, massive infiltration of the medulla and uroepithelium by IL-6-immunoreactive cells was noted. In addition, an abundance of labeled cells was detected in the pelvic cavity. In all sections examined, the areas displaying intense IL-6 immunoreactivity corresponded to the areas that showed inflammatory lesions stained with H&E.
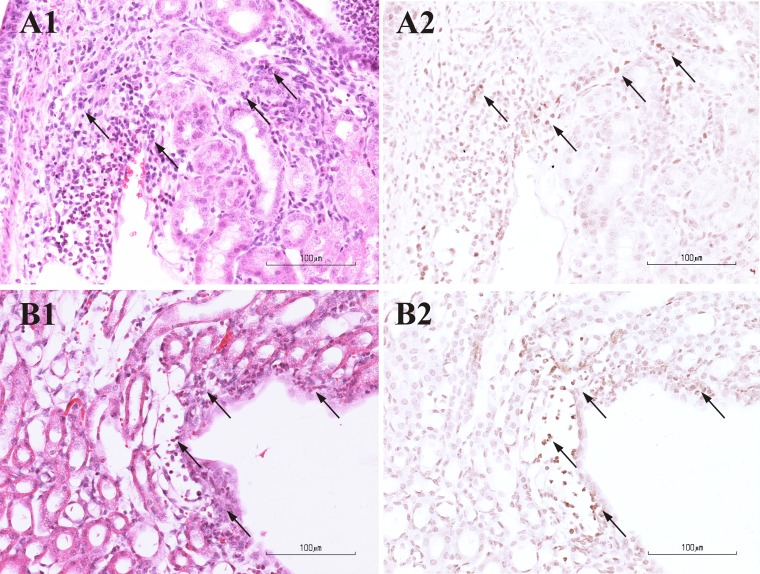
FIG 8.
Photomicrographs from pairs of consecutive renal sections of mice infected with ESBL-producing bacteria with (B1 and B2) or without (A1 and A2) ceftriaxone (CRO) treatment. In the left panels, sections are stained with H&E (A1 and B1). In the right panels, sections are immunohistochemically labeled for IL-6 (A2 and B2). Numerous inflammatory cells (mainly neutrophils) infiltrating the tubules of renal medulla (arrows in A1) are positive for IL-6 (arrows in A2). Inflammatory cells located within the renal medulla and uroepithelium, adjacent to the pelvis (arrows in B1), are immunoreactive for IL-6 (arrows in B2).
DISCUSSION
In this study, we developed an ascending UTI model, in which we found that ESBL-producing bacteria do not have greater pathogenicity than non-ESBL-producing bacteria. Ceftriaxone treatment eliminates the renal bacterial burden on the 6th postinfection day in mice infected with either ESBL- or non-ESBL-producing bacteria, although it permits more profound inflammatory lesions with the ESBL-producing bacteria. To our knowledge, this is the first study investigating the pathogenicity of ESBL-producing bacteria in terms of the bacterial load, a detailed histological analysis, and IL-6 expression and the effect of a cephalosporin on it.
Infections caused by ESBL-producing bacteria are thought to have worse outcomes than infections caused by non-ESBL-producing bacteria (5, 6, 9). It is unclear whether the unfavorable outcomes of infections caused by ESBL-producing bacteria are attributable to potential greater pathogenicity. Our study demonstrates that the natural history of UTI caused by ESBL-producing strains does not significantly differ from that of UTI caused by non-ESBL-producing strains. This has been confirmed for each of the three pairs of ESBL- and non-ESBL-producing bacteria used. We have chosen to compare three different pairs of ESBL- and non-ESBL-producing strains in order to avoid misleading results due to a randomly selected single pair of strains.
There is a strong positive linear correlation of the microbial burden of the kidneys of untreated mice infected with ESBL- and non-ESBL-producing bacteria with their histopathological scores. Moreover, the equations describing these linear correlations are almost the same, indicating that for a given microbial burden the histopathological scores for ESBL- and non-ESBL-producing strains are approximately equal. However, the correlation coefficients are dramatically altered when ceftriaxone is administered to mice infected with non-ESBL- but not ESBL-producing bacteria, suggesting its effectiveness on non-ESBL-producing bacteria. These results and other findings (6, 7, 11–13) provide evidence that the unfavorable outcomes of such infections may be due to inappropriate empirical therapy and not to enhanced pathogenicity.
Renal bacterial loads gradually decline in mice infected by either ESBL- or non-ESBL-producing bacteria with ceftriaxone treatment and are eliminated on the 6th postinfection day in both groups of mice. ESBL-producing bacteria are resistant in vitro to third-generation cephalosporins such as ceftriaxone (1). Inappropriate empirical therapy was related to unfavorable outcomes of bloodstream infections caused by ESBL-producing bacteria in several clinical studies (6, 9, 25). On the other hand, bacteremia secondary to UTI that does not have an unfavorable outcome compared to bacteremia of another origin despite inappropriate empirical therapy has been shown (11). There is evidence that UTI caused by ESBL-producing bacteria can be successfully treated despite administration of inappropriate therapy in vitro (7, 8, 25, 26). In a previous study, we have shown that hospitalized children with UTI caused by ESBL-producing bacteria had favorable clinical and microbiological outcomes despite inappropriate empirical therapy compared to children with UTI caused by non-ESBL-producing bacteria who were receiving appropriate therapy (27). Furthermore, two of the children with UTI caused by ESBL-producing bacteria showed clinical and microbiological responses on the 1st day of the inappropriate therapy administration (27).
It is noteworthy that despite inappropriate treatment, mice infected with ESBL-producing bacteria had negative cultures on the 6th postinfection day in our study. Ceftriaxone might cause elimination of ESBL-producing bacteria in the host urinary tract due to specific pharmacokinetic (PK) and pharmacodynamic (PD) properties. To our knowledge, there has been no PK/PD study on ceftriaxone urine concentrations in mice. Nevertheless, while CLSI susceptibility breakpoints for ceftriaxone are ≤1 μg/ml, ceftriaxone urine concentrations may reach 526 or 2,692 μg/ml following single 0.5 or 2 g intravenous doses in adult humans (28). However, the inhibition of microbial growth of ESBL-producing E. coli after a 6-day exposure to ceftriaxone in vivo does not necessarily imply entire renal parenchymal microbial clearance.
IL-6 is a proinflammatory cytokine related to the host immune response (29). Several clinical and experimental studies have investigated the role of IL-6 in various localized or systemic infections (30–36). Through a complex cascade of interactions between IL-6 and its corresponding receptors on the surface of various cell types, IL-6 provokes local and systemic immune responses (37–40). During a UTI, IL-6 is produced by inflammatory cells in the urinary tract (41–45), and depending on the severity of infection, it may diffuse to the systemic circulation (46, 47). Inflammatory responses related to local IL-6 secretion are pyuria, proteinuria, and hematuria (47, 48), while fever is a systemic inflammatory response related to the circulating IL-6 in the case of a severe UTI (47).
In our study, increased renal IL-6 levels were observed but did not differ significantly between untreated mice infected with ESBL- and non-ESBL-producing strains throughout the study period. This result is in agreement with our microbiological results. Rugo et al. found no detectable levels of IL-6 mRNA on the 5th postinfection day of a murine UTI model (45). A possible explanation might be that the kinetics of IL-6 protein and mRNA differs in the course of a UTI. de Man et al. showed that there is a strong correlation between the persistence of a UTI and a high renal IL-6 concentration (44). Among mice treated with ceftriaxone, IL-6 levels did not significantly differ between mice infected with ESBL- and non-ESBL-producing strains throughout the study period. We and others have shown significant decreases in urine IL-6 levels during or at the end of UTI treatment (46, 47, 49–51). In contrast, despite the negative cultures of mice treated with ceftriaxone in our study, renal IL-6 levels are strongly detectable on the 6th postinfection day in mice infected with either ESBL- or non-ESBL-producing bacteria. A possible explanation for this discrepancy is that the renal parenchymal but not urine IL-6 levels were investigated. Mice infected with non-ESBL-producing bacteria and treated with ceftriaxone showed greater declines in renal IL-6 concentrations than mice infected with ESBL-producing bacteria, possibly due to the appropriateness of the antibiotic administered.
Histopathological alterations caused by UTI sometimes lead to scar formation, which may have dismal long-term sequelae, such as hypertension or renal failure later in life (14, 16). Without antibiotic treatment, the histopathological alterations were not significantly different between mice infected with ESBL- or non-ESBL-producing bacteria mice throughout the study. This result is in agreement with the microbiological and immunological results of our study. With ceftriaxone treatment, mice infected with ESBL-producing bacteria still had profound histopathological alterations compared to those of mice infected with non-ESBL-producing bacteria on the 6th postinfection day, despite the negative cultures of the mice infected with either ESBL- or non-ESBL-producing bacteria. Thus, the apparent elimination of the microbial burden of ESBL-producing bacteria was not followed by a histological recovery in infected kidneys. Our findings suggest that although negative cultures are obtained after empirical ceftriaxone therapy of UTI caused by ESBL-producing E. coli, the resolution of kidney inflammation maybe a prolonged process.
It is plausible that the differences in histopathology noted on day 6 are due to the greater bacterial burden on day 1 of strain E94, which is probably due to experimental variability and not to greater virulence or to a theoretical ceftriaxone (CRO) + E94 response dynamic.
In conclusion, ESBL-producing bacteria do not have greater pathogenicity in terms of renal bacterial burden, histopathological alterations, and local IL-6 production and thus worse outcome than non-ESBL-producing bacteria in a murine ascending pyelonephritis model. The microbiological effectiveness of ceftriaxone in UTI due to ESBL-producing E. coli may have clinical implications; this effectiveness might, however, be hampered by a possible delay in the resolution of the kidney inflammation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Danai Sofianou for the donation of the non-ESBL E. coli clinical isolates and the extensive susceptibility testing and genotyping. We also thank Elpiniki Georgiadou and Georgia Kythreotou for their technical help.
This work was supported by the European Society of Pediatric Infectious Diseases (contract 33880/12-5-2008) and Procter & Gamble (contract 49665/12-9-2009).
Footnotes
Published ahead of print 15 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03974-14.
REFERENCES
- 1.Giamarellou H. 2005. Multidrug resistance in Gram-negative bacteria that produce extended-spectrum beta-lactamases (ESBLs). Clin. Microbiol. Infect. 11(Suppl 4):1–16. 10.1111/j.1469-0691.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- 2.Biehl LM, Schmidt-Hieber M, Liss B, Cornely OA, Vehreschild MJ. Colonization and infection with extended spectrum beta-lactamase-producing Enterobacteriaceae in high-risk patients-Review of the literature from a clinical perspective. Crit. Rev. Microbiol., in press. 10.3109/1040841X.2013.875515. [DOI] [PubMed] [Google Scholar]
- 3.Bradford PA. 2001. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933–951, table of contents. 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kassakian SZ, Mermel LA. 2014. Changing epidemiology of infections due to extended spectrum beta-lactamase-producing bacteria. Antimicrob. Resist. Infect. Control 3:9 10.1186/2047-2994-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaoutis TE, Goyal M, Chu JH, Coffin SE, Bell LM, Nachamkin I, McGowan KL, Bilker WB, Lautenbach E. 2005. Risk factors for and outcomes of bloodstream infection caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species in children. Pediatrics 115:942–949. 10.1542/peds.2004-1289. [DOI] [PubMed] [Google Scholar]
- 6.Tumbarello M, Spanu T, Sanguinetti M, Citton R, Montuori E, Leone F, Fadda G, Cauda R. 2006. Bloodstream infections caused by extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae: risk factors, molecular epidemiology, and clinical outcome. Antimicrob. Agents Chemother. 50:498–504. 10.1128/AAC.50.2.498-504.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang CI, Kim SH, Kim DM, Park WB, Lee KD, Kim HB, Oh MD, Kim EC, Choe KW. 2004. Risk factors for and clinical outcomes of bloodstream infections caused by extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. Infect. Control Hosp. Epidemiol. 25:860–867. 10.1086/502310. [DOI] [PubMed] [Google Scholar]
- 8.Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO. 2001. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin. Infect. Dis. 32:1162–1171. 10.1086/319757. [DOI] [PubMed] [Google Scholar]
- 9.Kim YK, Pai H, Lee HJ, Park SE, Choi EH, Kim J, Kim JH, Kim EC. 2002. Bloodstream infections by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob. Agents Chemother. 46:1481–1491. 10.1128/AAC.46.5.1481-1491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhavnani SM, Ambrose PG, Craig WA, Dudley MN, Jones RN. 2006. Outcomes evaluation of patients with ESBL- and non-ESBL-producing Escherichia coli and Klebsiella species as defined by CLSI reference methods: report from the SENTRY Antimicrobial Surveillance Program. Diagn. Microbiol. Infect. Dis. 54:231–236. 10.1016/j.diagmicrobio.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Hyle EP, Lipworth AD, Zaoutis TE, Nachamkin I, Bilker WB, Lautenbach E. 2005. Impact of inadequate initial antimicrobial therapy on mortality in infections due to extended-spectrum beta-lactamase-producing enterobacteriaceae: variability by site of infection. Arch. Intern. Med. 165:1375–1380. 10.1001/archinte.165.12.1375. [DOI] [PubMed] [Google Scholar]
- 12.Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW. 2004. Bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for mortality and treatment outcome, with special emphasis on antimicrobial therapy. Antimicrob. Agents Chemother. 48:4574–4581. 10.1128/AAC.48.12.4574-4581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, Bonomo RA, Rice LB, Wagener MM, McCormack JG, Yu VL. 2004. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin. Infect. Dis. 39:31–37. 10.1086/420816. [DOI] [PubMed] [Google Scholar]
- 14.Larcombe J. 2010. Urinary tract infection in children. Am. Fam. Physician 82:1252–1256. [PubMed] [Google Scholar]
- 15.Nicolle LE. 2008. Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urol. Clin. North Am. 35:1–12. 10.1016/j.ucl.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Chang SL, Shortliffe LD. 2006. Pediatric urinary tract infections. Pediatr. Clin. North Am. 53:379–400, vi. 10.1016/j.pcl.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Colodner R, Rock W, Chazan B, Keller N, Guy N, Sakran W, Raz R. 2004. Risk factors for the development of extended-spectrum beta-lactamase-producing bacteria in nonhospitalized patients. Eur. J. Clin. Microbiol. Infect. Dis. 23:163–167. 10.1007/s10096-003-1084-2. [DOI] [PubMed] [Google Scholar]
- 18.Calbo E, Romani V, Xercavins M, Gomez L, Vidal CG, Quintana S, Vila J, Garau J. 2006. Risk factors for community-onset urinary tract infections due to Escherichia coli harbouring extended-spectrum beta-lactamases. J. Antimicrob. Chemother. 57:780–783. 10.1093/jac/dkl035. [DOI] [PubMed] [Google Scholar]
- 19.Megged O. 2014. Extended-spectrum β-lactamase-producing bacteria causing community-acquired urinary tract infections in children. Pediatr. Nephrol. 29:1583–1587. 10.1007/s00467-014-2810-y. [DOI] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing, 24th informational supplement. M100-S24. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 21.Hagberg L, Engberg I, Freter R, Lam J, Olling S, Svanborg Eden C. 1983. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect. Immun. 40:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hvidberg H, Struve C, Krogfelt KA, Christensen N, Rasmussen SN, Frimodt-Moller N. 2000. Development of a long-term ascending urinary tract infection mouse model for antibiotic treatment studies. Antimicrob. Agents Chemother. 44:156–163. 10.1128/AAC.44.1.156-163.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svensson M, Irjala H, Alm P, Holmqvist B, Lundstedt AC, Svanborg C. 2005. Natural history of renal scarring in susceptible mIL-8Rh−/− mice. Kidney Int. 67:103–110. 10.1111/j.1523-1755.2005.00060.x. [DOI] [PubMed] [Google Scholar]
- 24.Johnson DE, Russell RG, Lockatell CV, Zulty JC, Warren JW, Mobley HL. 1993. Contribution of Proteus mirabilis urease to persistence, urolithiasis, and acute pyelonephritis in a mouse model of ascending urinary tract infection. Infect. Immun. 61:2748–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanafani ZA, Mehio-Sibai A, Araj GF, Kanaan M, Kanj SS. 2005. Epidemiology and risk factors for extended-spectrum beta-lactamase-producing organisms: a case control study at a tertiary care center in Lebanon. Am. J. Infect. Control 33:326–332. 10.1016/j.ajic.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Peña C, Gudiol C, Tubau F, Saballs M, Pujol M, Dominguez MA, Calatayud L, Ariza J, Gudiol F. 2006. Risk-factors for acquisition of extended-spectrum beta-lactamase-producing Escherichia coli among hospitalised patients. Clin. Microbiol. Infect. 12:279–284. 10.1111/j.1469-0691.2005.01358.x. [DOI] [PubMed] [Google Scholar]
- 27.Tratselas A, Iosifidis E, Ioannidou M, Saoulidis S, Kollios K, Antachopoulos C, Sofianou D, Roilides EJ. 2011. Outcome of urinary tract infections caused by extended spectrum beta-lactamase-producing Enterobacteriaceae in children. Pediatr. Infect. Dis. J. 30:707–710. 10.1097/INF.0b013e31820eae6a. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Hu F, Xiong Z, Ye X, Zhu D, Wang YF, Wang M. 2011. Susceptibility of extended-spectrum-beta-lactamase-producing Enterobacteriaceae according to the new CLSI breakpoints. J. Clin. Microbiol. 49:3127–3131. 10.1128/JCM.00222-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishimoto N, Kishimoto T. 2006. Interleukin 6: from bench to bedside. Nat. Clin. Pract. Rheumatol. 2:619–626. 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 30.Rusconi F, Parizzi F, Garlaschi L, Assael BM, Sironi M, Ghezzi P, Mantovani A. 1991. Interleukin 6 activity in infants and children with bacterial meningitis. The Collaborative Study on Meningitis. Pediatr. Infect. Dis. J. 10:117–121. 10.1097/00006454-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Waage A, Halstensen A, Shalaby R, Brandtzaeg P, Kierulf P, Espevik T. 1989. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J. Exp. Med. 170:1859–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groeneveld AB, Tacx AN, Bossink AW, van Mierlo GJ, Hack CE. 2003. Circulating inflammatory mediators predict shock and mortality in febrile patients with microbial infection. Clin. Immunol. 106:106–115. 10.1016/S1521-6616(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 33.Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, Fine J, Krichevsky A, Delude RL, Angus DC, GenIMS Investigators 2007. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch. Intern. Med. 167:1655–1663. 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yende S, D'Angelo G, Kellum JA, Weissfeld L, Fine J, Welch RD, Kong L, Carter M, Angus DC, GenIMS Investigators 2008. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am. J. Respir. Crit. Care Med. 177:1242–1247. 10.1164/rccm.200712-1777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gómez-Cambronero LG, Sabater L, Pereda J, Cassinello N, Camps B, Vina J, Sastre J. 2002. Role of cytokines and oxidative stress in the pathophysiology of acute pancreatitis: therapeutical implications. Curr. Drug Targets Inflamm. Allergy 1:393–403. 10.2174/1568010023344544. [DOI] [PubMed] [Google Scholar]
- 36.Yoshii T, Magara S, Miyai D, Nishimura H, Kuroki E, Furudoi S, Komori T, Ohbayashi C. 2002. Local levels of interleukin-1beta, -4, -6 and tumor necrosis factor alpha in an experimental model of murine osteomyelitis due to Staphylococcus aureus. Cytokine 19:59–65. 10.1006/cyto.2002.1039. [DOI] [PubMed] [Google Scholar]
- 37.Davis S, Aldrich TH, Valenzuela DM, Wong VV, Furth ME, Squinto SP, Yancopoulos GD. 1991. The receptor for ciliary neurotrophic factor. Science 253:59–63. 10.1126/science.1648265. [DOI] [PubMed] [Google Scholar]
- 38.Moritz RL, Ward LD, Tu GF, Fabri LJ, Ji H, Yasukawa K, Simpson RJ. 1999. The N terminus of gp130 is critical for the formation of the high-affinity interleukin-6 receptor complex. Growth Factors 16:265–278. 10.3109/08977199909069145. [DOI] [PubMed] [Google Scholar]
- 39.Chow D, He X, Snow AL, Rose-John S, Garcia KC. 2001. Structure of an extracellular gp130 cytokine receptor signaling complex. Science 291:2150–2155. 10.1126/science.1058308. [DOI] [PubMed] [Google Scholar]
- 40.Castell JV, Gomez-Lechon MJ, David M, Hirano T, Kishimoto T, Heinrich PC. 1988. Recombinant human interleukin-6 (IL-6/BSF-2/HSF) regulates the synthesis of acute phase proteins in human hepatocytes. FEBS Lett. 232:347–350. 10.1016/0014-5793(88)80766-X. [DOI] [PubMed] [Google Scholar]
- 41.Hedges S, Anderson P, Lidin-Janson G, de Man P, Svanborg C. 1991. Interleukin-6 response to deliberate colonization of the human urinary tract with gram-negative bacteria. Infect. Immun. 59:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hedges S, Agace W, Svensson M, Sjogren AC, Ceska M, Svanborg C. 1994. Uroepithelial cells are part of a mucosal cytokine network. Infect. Immun. 62:2315–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agace W, Hedges S, Andersson U, Andersson J, Ceska M, Svanborg C. 1993. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect. Immun. 61:602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Man P, van Kooten C, Aarden L, Engberg I, Linder H, Svanborg Eden C. 1989. Interleukin-6 induced at mucosal surfaces by gram-negative bacterial infection. Infect. Immun. 57:3383–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rugo HS, O'Hanley P, Bishop AG, Pearce MK, Abrams JS, Howard M, O'Garra A. 1992. Local cytokine production in a murine model of Escherichia coli pyelonephritis. J. Clin. Invest. 89:1032–1039. 10.1172/JCI115644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hedges S, Stenqvist K, Lidin-Janson G, Martinell J, Sandberg T, Svanborg C. 1992. Comparison of urine and serum concentrations of interleukin-6 in women with acute pyelonephritis or asymptomatic bacteriuria. J. Infect. Dis. 166:653–656. 10.1093/infdis/166.3.653. [DOI] [PubMed] [Google Scholar]
- 47.Sheu JN, Chen MC, Lue KH, Cheng SL, Lee IC, Chen SM, Tsay GJ. 2006. Serum and urine levels of interleukin-6 and interleukin-8 in children with acute pyelonephritis. Cytokine 36:276–282. 10.1016/j.cyto.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Benson M, Jodal U, Andreasson A, Karlsson A, Rydberg J, Svanborg C. 1994. Interleukin 6 response to urinary tract infection in childhood. Pediatr. Infect. Dis. J. 13:612–616. 10.1097/00006454-199407000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Jantausch BA, O'Donnell R, Wiedermann BL. 2000. Urinary interleukin-6 and interleukin-8 in children with urinary tract infection. Pediatr. Nephrol. 15:236–240. 10.1007/s004670000456. [DOI] [PubMed] [Google Scholar]
- 50.Rodríguez LM, Robles B, Marugan JM, Suarez A, Santos F. 2008. Urinary interleukin-6 is useful in distinguishing between upper and lower urinary tract infections. Pediatr. Nephrol. 23:429–433. 10.1007/s00467-007-0670-4. [DOI] [PubMed] [Google Scholar]
- 51.Roilides E, Papachristou F, Gioulekas E, Tsaparidou S, Karatzas N, Sotiriou J, Tsiouris J. 1999. Increased urine interleukin-6 concentrations correlate with pyelonephritic changes on 99mTc-dimercaptosuccinic acid scans in neonates with urinary tract infections. J. Infect. Dis. 180:904–907. 10.1086/314960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.