Abstract
We investigated the activity of trimethoprim-sulfamethoxazole (SXT) against Mycobacterium tuberculosis, the pathogen that causes tuberculosis (TB). The MIC distribution of SXT was 0.125/2.4 to 2/38 mg/liter for the 100 isolates tested, including multi- and extensively drug-resistant isolates (MDR/XDR-TB), whereas the intracellular MIC90 of sulfamethoxazole (SMX) for the pansusceptible strain H37Rv was 76 mg/liter. In an exploratory analysis using a ratio of the unbound area under the concentration-time curve from 0 to 24 h over MIC (fAUC0–24/MIC) using ≥25 as a potential target, the cumulative fraction response was ≥90% at doses of ≥2,400 mg of SMX. SXT is a potential treatment option for MDR/XDR-TB.
TEXT
Due to the global increase in multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) (1), already approved drugs, such as trimethoprim-sulfamethoxazole (SXT), have been reinvestigated with favorable results (2, 3). However, very few highly drug-resistant isolates have been tested, despite the main potential of SXT being in the treatment of XDR-TB. The active component of SXT regarding Mycobacterium tuberculosis is sulfamethoxazole (SMX) (3–9), although it is unknown whether its effect is mainly extra- or intracellular. Therefore, the objective of this study was to determine the extra- and intracellular activities of SMX against both MDR- and XDR-TB isolates and to explore a ratio of the unbound area under the concentration-time curve from 0 to 24 h over MIC (fAUC0–24/MIC) using ≥25 as a potential target and the associated cumulative fraction response values (10) at different doses of SMX.
In this study, 100 M. tuberculosis isolates with unique restriction fragment length polymorphism (RFLP) patterns were included, comprising 14 consecutive fully susceptible wild-type isolates, 48 MDR-TB isolates, 13 XDR-TB isolates, and the remainder with mixed resistance patterns, referred to as non-MDR/XDR-TB isolates. The pansusceptible strain H37Rv (ATCC 27294) was used as a control.
A stock solution of trimethoprim (TMP) and SMX diluted in dimethyl sulfoxide (DMSO) and 1 M NaOH, respectively, was prepared in serial two-step dilutions, reaching a final concentration range of 0.008 to 8/0.15 to 152 of TMP and SMX, respectively (ratio of 1:19). The MICs were determined using Middlebrook 7H10 (7H10) medium (n = 84), as previously described (11). In Bactec 960 MGIT (MGIT; Becton, Dickinson, Franklin Lakes, NJ, USA) tubes, 17 isolates were included (14 fully susceptible wild-type isolates, two isoniazid-resistant isolates, and one MDR-TB isolate).
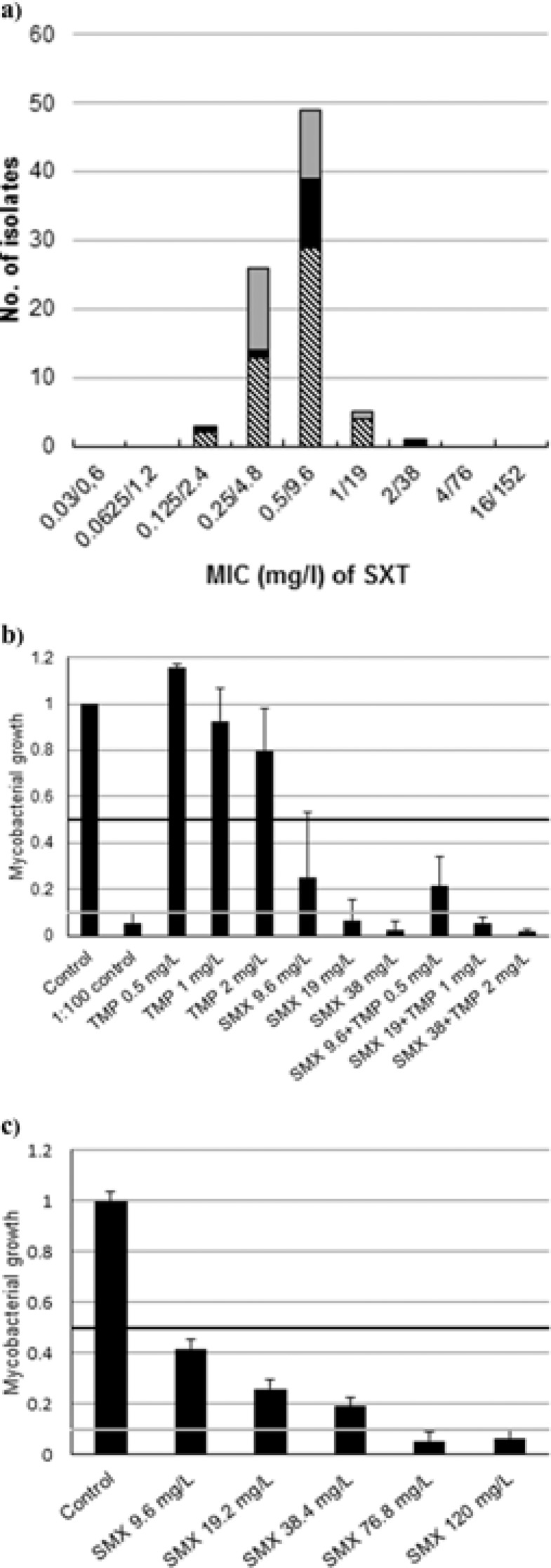
The MIC distribution of the 84 drug-resistant M. tuberculosis isolates in 7H10 ranged from 2.4 to 38 mg/liter of sulfamethoxazole (0.125/2.4 to 2/38 mg/liter of trimethoprim-sulfamethoxazole, i.e., SXT) (Fig. 1a). No significant differences in the MIC distributions between MDR-TB, XDR-TB, or isolates with other resistance patterns were observed. All isolates had MICs of ≤38 mg/liter of SMX, including the susceptible consecutive wild-type strains (n = 14) tested by MGIT only (MIC range, 0.5/9.6 to 2/38 mg/liter of SXT) and the 84 strains tested with the 7H10 method. The MIC of H37Rv was 0.25/4.8 mg/liter of SXT in 7H10 in duplicates, and the MICs in MGIT for H37Rv tested on three different occasions were 0.5/9.6 to 1/19 mg/liter.
FIG 1.
Effects of SXT on different M. tuberculosis isolates. (a) MIC distribution of SXT (trimethoprim-sulfamethoxazole) for the M. tuberculosis isolates (n = 84) tested using Middlebrook 7H10 medium, shown by bars shaded as follows: non-MDR/XDR-TB (gray), XDR-TB (black), and MDR-TB (hatched). (b) Extracellular growth inhibition of M. tuberculosis H37Rv-lux. Mycobacterial growth inhibition following antibiotic exposure in concentration gradients of trimethoprim (TMP), sulfamethoxazole (SMX), and TMP and SMX in combination (mg/liter). The black horizontal line indicates MIC50, and the gray line indicates MIC90. (c) Intracellular effects of different concentrations of sulfamethoxazole against M. tuberculosis H37Rv-lux in a THP-1 macrophage model. The black horizontal line indicates MIC50, and the gray line indicates MIC90. Error bars show standard deviations.
To evaluate extracellular and intracellular growth inhibition of SXT, the M. tuberculosis strain H37Rv (ATCC 27294) carrying the gene for Vibrio harveyi luciferase (H37Rv-lux) (12) was used. For the extracellular evaluation, bacterial inocula of H37rv-lux were exposed to SXT and to TMP and SMX separately and compared to an undiluted growth control, as well as a 1:100-diluted control, by measurement of luminescence. There was no synergistic effect of the combination of TMP and SMX. The extracellular MIC of SMX for H37Rv-lux was 19 mg/liter (Fig. 1b).
To evaluate the intracellular growth inhibition of SMX, a THP-1 (Sigma-Aldrich, Stockholm, Sweden) macrophage model in 96-well plates was used. Following phagocytosis for 1 h with H37Rv-lux and extensive washing (12), the intracellular fraction was measured by luminescence reading after 5 days. Occasional bacilli might still be attached to the cell surface after phagocytosis, but as previously investigated by microscopy (12), these bacilli are either phagocytized or removed due to the extensive washing before exposure to the antibiotics. In order to evaluate the growth inhibition, the median value of the intracellular lysate fraction in the triplicates was normalized against the value for infected macrophages without antibiotic exposure within each experiment. The intracellular MIC90 of SMX for H37Rv-lux was 76 mg/liter. A small number of viable bacilli was also found at 120 mg/liter, which may indicate intracellular survival even at high levels of SMX (Fig. 1c).
We also performed an exploratory analysis of potential targets for the fAUC0–24/MIC ratio (25, 50, and 75) for SMX. The probability of target attainment and cumulative fraction response values for different doses up to 7,200 mg of SMX with Monte Carlo simulations, using previously published pharmacokinetic results (13), were determined as previously described (10). The results are shown in Fig. S1a to c and Table S1 in the supplemental material. A cumulative fraction response of ≥90%, including MDR and XDR isolates, was reached at doses of ≥2,400 mg, ≥3,600 mg, and ≥7,200 mg of SMX using target indices of 25, 50, and 75, respectively (See Table S1). A fAUC0–24/MIC ratio for SMX of >25 has been suggested for melioidosis (14) and TB (13), but there are no defined target values and our analysis should be interpreted with caution in relation to dose recommendations. Nevertheless, this analysis may aid dosing when more information about the appropriate pharmacodynamic targets for SMX in TB becomes available. There are a few case reports supporting the efficacy of SMX in TB treatment (2, 15), but clinical outcome data are very limited.
In this study, we show that both drug-sensitive and MDR/XDR-TB strains were inhibited by SXT, on both solid (Middlebrook 7H10) and liquid (MGIT) media. Moreover, SMX was more effective against extracellular than intracellular M. tuberculosis. The in vitro effect of SXT on M. tuberculosis has been shown in earlier studies, including 181 fully susceptible and 165 drug-resistant isolates (102 MDR-TB and 6 XDR-TB) (2, 3, 5, 6, 9, 13, 15–17). The majority of both drug-sensitive and drug-resistant M. tuberculosis isolates were susceptible to SXT below 2/38 mg/liter, with no difference between the groups. Hence, our results support a tentative breakpoint for SXT against M. tuberculosis of 2/38 mg/liter, as suggested by others (17). A limitation of our MIC analysis is that we only analyzed a small number of wild-type and drug-resistant M. tuberculosis isolates in MGIT, as this method is highly labor intensive. Nevertheless, MGIT has the advantage of accessibility in the clinical routine.
The high MIC90 of 76 mg/liter in the intracellular model indicates that intracellular M. tuberculosis may not be accessible for treatment with SMX in therapeutic dosages. This implies that SMX should preferably be used during the first months of treatment, when the bacteria are mainly located extracellularly.
In conclusion, SXT was active against M. tuberculosis, including highly drug-resistant isolates, whereas it was less active inside macrophages. As SXT is an affordable and well-tolerated drug, it could be a treatment option in selected MDR- and XDR-TB cases in the initial phase.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Swedish Society of Medicine (grant number SLS 169241; K.Ä.), the Marianne and Marcus Wallenberg Foundation (T.S.), the Swedish Heart and Lung Foundation (Oscar II Jubilée Foundation; T.S.), the Swedish Society of Antimicrobial Chemotherapy (T.S.), the Research Council of Southeast Sweden (FORSS; T.S.), and the Department of Infectious Diseases and the Department of Clinical Microbiology, Karolinska University Hospital Solna, Stockholm, Sweden.
We thank Brian Davies for language correction.
Footnotes
Published ahead of print 22 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02995-14.
REFERENCES
- 1.Zignol M, Dara M, Dean AS, Falzon D, Dadu A, Kremer K, Hoffmann H, Hoffner S, Floyd K. 2013. Drug-resistant tuberculosis in the WHO European Region: an analysis of surveillance data. Drug Resist. Updat. 16:108–115. 10.1016/j.drup.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Forgacs P, Wengenack NL, Hall L, Zimmerman SK, Silverman ML, Roberts GD. 2009. Tuberculosis and trimethoprim-sulfamethoxazole. Antimicrob. Agents Chemother. 53:4789–4793. 10.1128/AAC.01658-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong W, Sievers A, Leslie DE. 2010. Mycobacterium tuberculosis and sulfamethoxazole susceptibility. Antimicrob. Agents Chemother. 54:2748–2749. 10.1128/AAC.00029-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suling WJ, Reynolds RC, Barrow EW, Wilson LN, Piper JR, Barrow WW. 1998. Susceptibilities of Mycobacterium tuberculosis and Mycobacterium avium complex to lipophilic deazapteridine derivatives, inhibitors of dihydrofolate reductase. J. Antimicrob. Chemother. 42:811–815. 10.1093/jac/42.6.811. [DOI] [PubMed] [Google Scholar]
- 5.Huang TS, Kunin CM, Yan BS, Chen YS, Lee SS, Syu W., Jr 2012. Susceptibility of Mycobacterium tuberculosis to sulfamethoxazole, trimethoprim and their combination over a 12 year period in Taiwan. J. Antimicrob. Chemother. 67:633–637. 10.1093/jac/dkr501. [DOI] [PubMed] [Google Scholar]
- 6.Vilcheze C, Jacobs WR., Jr 2012. The combination of sulfamethoxazole, trimethoprim, and isoniazid or rifampin is bactericidal and prevents the emergence of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 56:5142–5148. 10.1128/AAC.00832-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macingwana L, Baker B, Ngwane AH, Harper C, Cotton MF, Hesseling A, Diacon AH, van Helden P, Wiid I. 2012. Sulfamethoxazole enhances the antimycobacterial activity of rifampicin. J. Antimicrob. Chemother. 67:2908–2911. 10.1093/jac/dks306. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs WR., Jr 2010. Author's Reply to Role of the dihydrofolate reductase DfrA (Rv2763c) in trimethoprim-sulfamethoxazole (co-trimoxazole) resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 54:4951–4952. 10.1128/AAC.00876-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ameen SM, Drancourt M. 2013. In vitro susceptibility of Mycobacterium tuberculosis to trimethoprim and sulfonamides in France. Antimicrob. Agents Chemother. 57:6370–6371. 10.1128/AAC.01683-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. 2005. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J. Antimicrob. Chemother. 55:601–607. 10.1093/jac/dki079. [DOI] [PubMed] [Google Scholar]
- 11.Schon T, Jureen P, Giske CG, Chryssanthou E, Sturegard E, Werngren J, Kahlmeter G, Hoffner SE, Angeby KA. 2009. Evaluation of wild-type MIC distributions as a tool for determination of clinical breakpoints for Mycobacterium tuberculosis. J. Antimicrob. Chemother. 64:786–793. 10.1093/jac/dkp262. [DOI] [PubMed] [Google Scholar]
- 12.Eklund D, Welin A, Schon T, Stendahl O, Huygen K, Lerm M. 2010. Validation of a medium-throughput method for evaluation of intracellular growth of Mycobacterium tuberculosis. Clin. Vaccine Immunol. 17:513–517. 10.1128/CVI.00446-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsaad N, van Altena R, Pranger AD, van Soolingen D, de Lange WC, van der Werf TS, Kosterink JG, Alffenaar JW. 2013. Evaluation of co-trimoxazole in the treatment of multidrug-resistant tuberculosis. Eur. Respir. J. 42:504–512. 10.1183/09031936.00114812. [DOI] [PubMed] [Google Scholar]
- 14.Cheng AC, McBryde ES, Wuthiekanun V, Chierakul W, Amornchai P, Day NP, White NJ, Peacock SJ. 2009. Dosing regimens of cotrimoxazole (trimethoprim-sulfamethoxazole) for melioidosis. Antimicrob. Agents Chemother. 53:4193–4199. 10.1128/AAC.01301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen-Bacrie S, Ben Kahla I, Botelho-Nevers E, Million M, Parola P, Brouqui P, Drancourt M. 2011. Imported extensively drug-resistant Mycobacterium tuberculosis Beijing genotype, Marseilles, France, 2011. Euro Surveill. 16(16):pii=19846 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19846. [PubMed] [Google Scholar]
- 16.Wallace RJ, Jr, Nash DR, Steele LC, Steingrube V. 1986. Susceptibility testing of slowly growing mycobacteria by a microdilution MIC method with 7H9 broth. J. Clin. Microbiol. 24:976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alsaad N, van der Laan T, van Altena R, Wilting KR, van der Werf TS, Stienstra Y, van Soolingen D, Alffenaar JW. 2013. Trimethoprim/sulfamethoxazole susceptibility of Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 42:472–474. 10.1016/j.ijantimicag.2013.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.