Abstract
Plasmodium falciparum gametocytes are not associated with clinical symptoms, but they are responsible for transmitting the pathogen to mosquitoes. Therefore, gametocytocidal interventions are important for malaria control and resistance containment. Currently available drugs and vaccines are not well suited for that purpose. Several dyes have potent antimicrobial activity, but their use against gametocytes has not been investigated systematically. The gametocytocidal activity of nine synthetic dyes and four control compounds was tested against stage V gametocytes of the laboratory strain 3D7 and three clinical isolates of P. falciparum with a bioluminescence assay. Five of the fluorescent dyes had submicromolar 50% inhibitory concentration (IC50) values against mature gametocytes. Three mitochondrial dyes, MitoRed, dihexyloxacarbocyanine iodide (DiOC6), and rhodamine B, were highly active (IC50s < 200 nM). MitoRed showed the highest activity against gametocytes, with IC50s of 70 nM against 3D7 and 120 to 210 nM against clinical isolates. All compounds were more active against the laboratory strain 3D7 than against clinical isolates. In particular, the endoperoxides artesunate and dihydroartemisinin showed a 10-fold higher activity against 3D7 than against clinical isolates. In contrast to all clinically used antimalarials, several fluorescent dyes had surprisingly high in vitro activity against late-stage gametocytes. Since they also act against asexual blood stages, they shall be considered starting points for the development of new antimalarial lead compounds.
INTRODUCTION
Malaria leads to approximately 1 million deaths annually and remains a highly relevant public health problem in tropical and subtropical regions (1). Plasmodium falciparum, the most virulent species, is responsible for most of the morbidity from malaria and accounts for almost all mortality from malaria in sub-Saharan Africa. Gametocytes are the stage responsible for transmission of the parasite from the human host to the mosquito. During the asexual blood stage, a small fraction of parasites differentiates into gametocytes. In P. falciparum, differentiation from sequestered immature stages (stages I to IV) to the free circulating mature stage (stage V) occurs within 8 to 14 days in internal organs, mainly bone marrow (2–4). When a mosquito takes a blood meal, it ingests stage V male and female gametocytes, which can further develop in the mosquito. In the mosquito gut, gametocytes develop into gametes that join to become a zygote, which then matures into the ookinete, followed by the oocyst stage. This stage gives rise to sporozoites, which are released from the oocyst and migrate to the salivary glands of the mosquito, where they can be transmitted during a subsequent blood meal.
Even though gametocytes are essential for the spread of malaria, transmission-blocking agents have not been a priority in the search for antimalarial drugs for a long time. This has recently changed with the ambition to eradicate malaria (5). The development of a new generation of transmission-blocking tools and therapies is a top priority of the Malaria Eradication Research Agenda (malERA) since they are believed to be essential for the elimination and subsequent eradication of malaria (6, 7).
Most current antimalarial drugs show, at most, only modest activity against early gametocytes but have either negligible or no effect on stage V gametocytes, although all are active against the asexual blood-stage parasites (8–10) and some inhibit the growth of preerythrocytic stages. Primaquine, an 8-aminoquinoline, is the only licensed drug that has proven gametocytocidal activity in vivo. Its use is limited due to the risk of hemolysis in people with glucose-6-phosphate dehydrogenase (G6PD) deficiency, a common trait in areas where malaria is endemic. In addition, primaquine is a prodrug that requires activation by cytochrome P450 2D6 (CYP2D6), which is polymorphic and can lead to treatment failures in poor and intermediate metabolizers (11). Other drugs with activity against gametocytes are bulaquine, a compound newly registered in India (12), and tafenoquine, which has been in late-phase clinical development for more than a decade (13). Both drugs are 8-aminoquinolines and are likely to share safety and efficacy problems with primaquine in G6PD-deficient individuals and poor metabolizers of CYP2D6, respectively (14). Dihydroartemisinin (DHA), the active metabolite of all artemisinin derivates, has been reported to be active against stage I to III gametocytes (15, 16), but it is uncertain whether DHA has clinically relevant activity against late-stage gametocytes (8). Evidence available from clinical studies shows that transmission is only partially blocked following treatment with artemisinin-based combination therapies (17, 18), the current mainstay of antimalarial therapy in areas where malaria is endemic.
In conclusion, no licensed drug with a satisfactory profile that can be used to control the transmission of P. falciparum is available. Some synthetic dyes have potent antimicrobial activity and have been successfully used as antimalarials since the late 19th century, when Guttmann and Ehrlich used the thiazine dye methylene blue for the treatment of malaria (19). Since then, several other dyes were tested for their antiplasmodial activity (20, 21). We revived this idea in a previous study by testing a panel of synthetic dyes for their in vitro activity against asexual stages and identified several compounds with high levels of in vitro activity (22). Methylene blue is known to show gametocytocidal activity (15, 23–25); therefore, we evaluated the activity of eight other antimalarial dyes against P. falciparum stage V gametocytes derived from the laboratory strain 3D7 and three clinical isolates from Lambaréné, Gabon, with an established bioluminescence assay (8).
MATERIALS AND METHODS
Cultivation of asexual parasites.
P. falciparum strain 3D7 (Malaria Research and Reference Reagent Resource, ATCC, Manassas, VA, USA) and clinical isolates obtained from Lambaréné, Gabon, were kept in continuous culture as described earlier (26). Clinical isolates were from a study reported previously (27). Blood was sampled directly from patients with P. falciparum monoinfection and cryopreserved in glycerolyte at −150°C until culture for the experiment was initiated. The cultures of both 3D7 and clinical isolates were kept at 5% hematocrit in complete culture medium (RPMI 1640 supplemented with 25 mM HEPES, 28 mM NaHCO3, 50 μg/ml gentamicin, 0.5% [wt/vol] lipid-rich bovine serum albumin [Albumax II], 2.4 mM l-glutamine, and 0.14 mM hypoxanthine at 5% CO2 and 5% O2). For clinical isolates, 5% human serum was added to the complete culture medium. Medium was replaced every 24 h.
Gametocyte culture and purification.
Gametocyte culture was performed as described previously (8), with some modifications. Cultures were started from asexual parasites and kept in continuous culture with sorbitol synchronization twice weekly (28). Culture medium for gametocytes always contained 5% human serum. Initially, the culture was adjusted to 9% hematocrit and 0.5% parasitemia and kept at 5% O2 and 5% CO2 at 37°C with a daily change of medium without parasite dilution for 2 weeks. In the second week, the volume of medium was doubled and cultures were treated with 50 mM N-acetyl-d-glucosamine (MP Biomedicals GmbH, Santa Ana, CA, USA) for 4 days, beginning 11 days after initiation to remove asexual parasites. To enrich stage V gametocytes from approximately 2% to over 90%, a two-step purification was performed on day 15 using a NycoPrep 1.077 cushion density gradient (Axis-Shield PoC AS, Oslo, Norway), followed by magnetic separation with LD-MACS magnetic columns (Miltenyi Biotec, Gladbach, Germany) to remove the remaining erythrocytes. Purification was done at 37°C to keep the gametocytes viable and avoid exflagellation during the procedure.
Viability of mature gametocytes after separation.
The viability of gametocytes after purification was assessed by microscopy (Fig. 1) and the capacity of male gametocytes to exflagellate in exflagellation medium (complete culture medium with 5% human serum and 100 μM xanthurenic acid) for 20 min at room temperature, as described earlier (25, 29).
FIG 1.
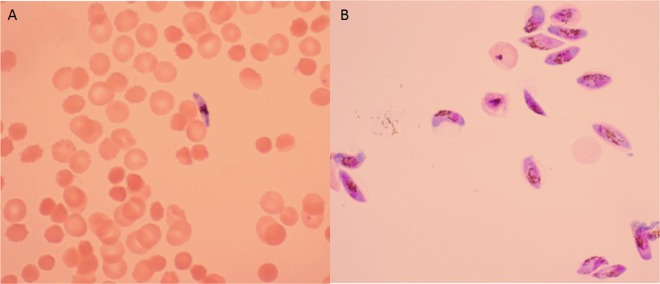
Giemsa-stained mature gametocyte culture on day 15 before (A) and after (B) purification.
Compounds.
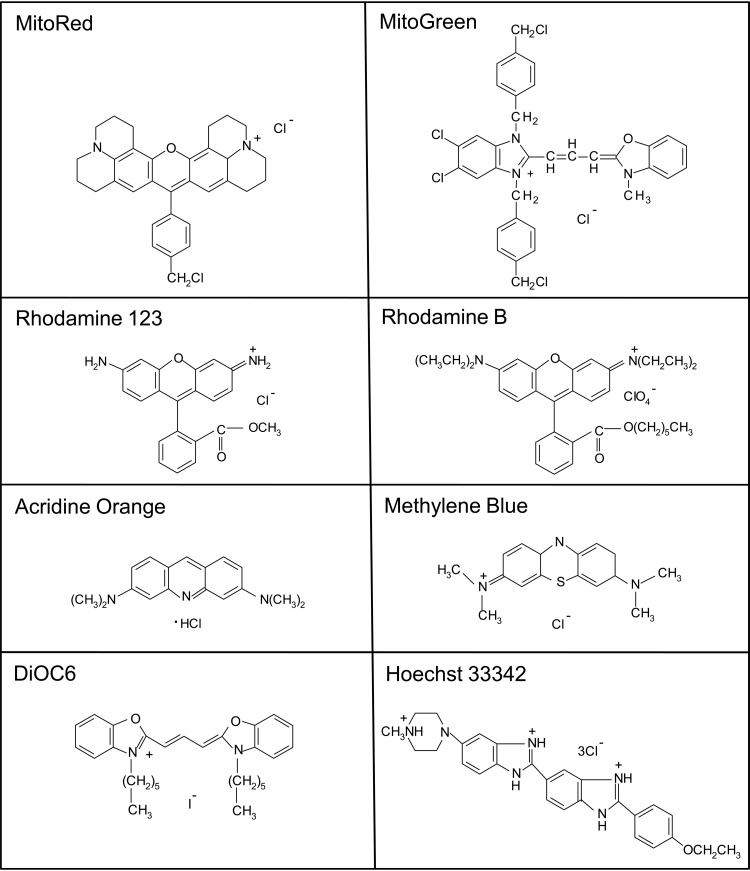
We selected eight fluorescent dyes (see the structures in Fig. 2) on the basis of their activity against asexual parasite stages evaluated previously (22), in addition to methylene blue and control compounds. The panel of fluorescent dyes consisted of three nucleic acid dyes, Hoechst 33342 (CAS number 23491-52-3), acridine orange (CAS number 65-61-2), and SYTO 9 (SYTO 9 green fluorescent nucleic acid stain; the structure is proprietary), and five mitochondrial dyes, MitoRed (MitoTracker Red CMXRos; CAS number 167095-09-2), MitoGreen (MitoTracker Green FM; CAS number 201860-17-5), dihexyloxacarbocyanine iodide (DiOC6; CAS number 53213-82-4), rhodamine 123 (molecular weight [MW], 380.83; CAS number 62669-70-9), and rhodamine B (hexyl ester perchlorate; MW, 627.17; CAS number 877933-92-1). We compared the activities of these dyes to those of methylene blue (MW, 319.86; CAS number 61-73-4), epoxomicin (MW, 554.7; CAS number 134381-21-8), DHA (MW, 284.35; CAS number 71939-50-9), artesunate (MW, 384.4; CAS number 88495-63-0), and primaquine (primaquine phosphate; MW, 455.33; CAS number 63-45-6). Methylene blue was obtained from AppliChem. All other dyes were from Invitrogen. Epoxomicin and DHA were obtained from Calbiochem and Shinpoong, respectively. Artesunate and primaquine were from Santa Cruz Biotechnology.
FIG 2.
Chemical structures of the tested synthetic dyes. The structure of SYTO 9 is proprietary.
Methylene blue and acridine orange were dissolved in water, while all other tested dyes were dissolved in dimethyl sulfoxide (DMSO). Further dilutions were done with complete culture medium. Final DMSO concentrations were less than 0.5%, a concentration that does not interfere with gametocyte viability.
Epoxomicin was used as a positive control, as it is a potent inhibitor of all clinically relevant parasite stages, including gametocytes, in vitro.
ATP-based bioluminescence assay.
We used a bioluminescence assay based on the luciferin-luciferase ATP-dependent reaction to assess the activity of the different compounds against mature gametocytes.
The assay was performed as previously described with some modifications (8, 30). In brief, compounds were 3-fold serially diluted in a 96-well plate. After purification, gametocytes were counted microscopically using an improved Neubauer cell-counting chamber, and 5 × 104 purified stage V gametocytes were added to each well in a final volume of 100 μl. In order to validate the test protocol and determine the linear relationship between the ATP content and the number of gametocytes in culture medium, we generated a standard curve using a 2-fold serial dilution starting with 5 × 104 purified mature gametocytes. On every plate, a serial dilution of viable stage V gametocytes was added as a control.
ATP measurements were done using equal volumes (50 μl) of gametocyte culture and BacTiterGlo reagent (Promega, Mannheim, Germany) in white opaque-walled 96-well plates, which were incubated at room temperature in the dark for 5 min to allow cell lysis and stabilization of the luminescent signal. Subsequently, the luminescence signal was recorded with a luminometer (LUMO; Anthos Mikrosysteme, Krefeld, Germany).
Stage V gametocytes were exposed to serial dilutions of the test compounds for 24 h or 48 h to assess the effect of the incubation time on the activity. Experiments using 3D7 and a 48-h incubation time were performed at least three times independently.
Statistical analysis.
We used the drc package (version 2.3.7) of R (version 2.15.1) to calculate the inhibitory concentrations of compounds by nonlinear regression analysis of log concentration-response curves. The 50% inhibitory concentrations (IC50s) were determined for each compound. If more than two experiments were performed, the mean and standard deviation are given. The coefficient of determination (R2) was calculated to assess correlations.
Ethical approval.
We used three clinical isolates described in a previous study (27). The study was approved by the ethics committee of the International Foundation for the Albert Schweitzer Hospital in Lambaréné and followed the principles of the Declaration of Helsinki (6th revision). The participating children and legal representatives gave assent and informed consent, respectively.
RESULTS
Viability of mature gametocytes and validation of luminescence.
As a first step, we evaluated the viability of gametocytes after purification and validated the ATP readout. Gametocytes appeared noncompromised after purification with intact cytoplasm (Fig. 1), male gametocytes showed exflagellation, and stage V gametocytes consistently remained viable for approximately 3 weeks. ATP levels and the number of gametocytes in the assay correlated closely (R2 = 0.99).
Activity of fluorescent dyes against mature stage V gametocytes.
Five of the eight fluorescent dyes in our assay showed IC50s below 1 μM (Table 1). Among the dyes tested, the three mitochondrial dyes, MitoRed, DiOC6, and rhodamine B, showed the best activity, with IC50s being below 200 nM, which is even lower than the IC50 of the control dye, methylene blue (770 nM). Among all dyes, MitoRed was the most active compound against laboratory strain 3D7 as well as against clinical isolates. The activities of the different dyes as well as the control drugs were consistently higher against the laboratory strain 3D7 than against clinical isolates (2- to 10-fold). Particularly, artesunate and DHA had considerably higher IC50s against clinical isolates than against 3D7. As expected for a prodrug, primaquine did not inhibit gametocytes at concentrations achievable in vivo. Epoxomicin served as our internal positive control and was highly active against the 3D7 laboratory strain and clinical isolates. The IC50s of the single compounds against asexual blood stages are given for comparison (Table 1). All compounds except epoxomicin were less active against gametocytes than against asexual blood stages.
TABLE 1.
In vitro activities of standard antimalarial drugs and dyes against stage V gametocytes of the P. falciparum 3D7 laboratory strain and clinical isolates after 48 h of incubationa
| Compound or dye | Mean IC50 ± SD (μM) |
||||
|---|---|---|---|---|---|
| 3D7 gametocytes | Clinical isolates (gametocytes) |
3D7 asexual blood stages | |||
| Isolate 1 | Isolate 2 | Isolate 3 | |||
| Standard compounds | |||||
| Epoxomicin | 0.0016 ± 0.0007 | 0.0021 | 0.0024 | 0.0015 | 0.005 |
| DHA | 0.20 ± 0.07 | 2.81 | 2.66 | 1.80 | 0.002 |
| Artesunate | 0.38 ± 0.12 | 4.39 | 0.99 | 2.91 | 0.001 |
| Primaquine | 20.05 ± 5.67 | 4.88 | 7.11 | 10.55 | |
| Methylene blue | 0.77 ± 0.68 | 1.05 | 1.49 | 1.05 | 0.008b |
| Dyes with IC50s of <300 nM for 3D7 gametocytes | |||||
| MitoRed | 0.07 ± 0.04 | 0.21 | 0.18 | 0.12 | 0.008b |
| DiOC6 | 0.14 ± 0.03 | 0.11 | 0.72 | 0.58 | 0.011b |
| Rhodamine B | 0.18 ± 0.08 | 0.23 | 0.99 | 0.34 | 0.026b |
| Hoechst 33342 | 0.23 ± 0.06 | 0.25 | 0.31 | 0.44 | 0.007b |
| SYTO 9 | 0.26 ± 0.07 | 1.34 | 1.13 | 1.24 | 0.021b |
| Dyes with IC50s of >1 μM for 3D7 gametocytes | |||||
| MitoGreen | 1.02 ± 0.23 | 1.66 | 1.24 | 1.45 | 0.116b |
| Acridine orange | 3.04 ± 1.07 | 36.56 | 2.63 | 0.466b | |
| Rhodamine 123 | 7.13 ± 2.97 | 13.83 | 10.39 | 0.388b | |
The activities of the evaluated compounds against mature gametocytes of 3D7 and three different clinical isolates of P. falciparum were measured after 48 h of incubation in serum-supplemented medium. Results for 3D7 gametocytes are the means of three independent experiments.
For comparison, the activity of dyes against asexual blood stages evaluated by a histidine-rich protein 2 enzyme-linked immunosorbent assay is given (results are from Joanny et al. [22]). The activities of epoxomicin, artesunate, and DHA were evaluated with the same method in our laboratory.
Activities of compounds after different incubation times (24 h and 48 h) with mature gametocytes.
In vitro gametocyte assays are poorly standardized and use different incubation times to test gametocytocidal compounds (8, 30). To assess the influence of the incubation time on the antiparasitic activity, we incubated fluorescent dyes and standard antimalarial drugs for either 24 h or 48 h with stage V gametocytes of 3D7 parasites and clinical isolates. For most compounds the incubation time had no significant effect (Table 2). However, MitoRed was 3-fold more active when used over 48 h than when used over 24 h. This effect was even more pronounced for epoxomicin and artesunate incubations, with a 10-fold increase in activity when parasites were exposed for 48 h.
TABLE 2.
In vitro activities of compounds against mature gametocytes of 3D7 and clinical isolates of P. falciparum after 24 h or 48 h of incubationa
| Compound, P. falciparum strain | IC50 (μM) after incubation for: |
|
|---|---|---|
| 24 h | 48 h | |
| Standard compounds | ||
| Artesunate | ||
| 3D7 | 31.38 | 0.38b |
| Clinical isolate 1 | 24.04 | 4.39 |
| Primaquine | ||
| 3D7 | 44.92 | 20.05b |
| Clinical isolate 1 | 4.64 | 4.88 |
| Epoxomicin, clinical isolate 1 | 0.015 | 0.002 |
| DHA, clinical isolate 1 | 2.86 | 2.81 |
| Dyes | ||
| MitoRed, 3D7 | 0.20 | 0.07b |
| Acridine orange | ||
| 3D7 | 3.19 | 3.04b |
| Clinical isolate 3 | 1.90 | 2.63 |
| MitoGreen, 3D7 | 2.30 | 1.02b |
| DiOC6, clinical isolate 1 | 0.47 | 0.11 |
| Rhodamine B | ||
| Clinical isolate 1 | 0.18 | 0.23 |
| Clinical isolate 3 | 1.41 | 0.34 |
| Hoechst 33342, clinical isolate 1 | 0.25 | 0.24 |
| Methylene blue, clinical isolate 1 | 2.04 | 1.05 |
| Rhodamine 123 | ||
| Clinical isolate 1 | 14.71 | 13.83 |
| Clinical isolate 3 | 14.85 | 10.39 |
| SYTO 9 | ||
| Clinical isolate 1 | 0.48 | 1.34 |
| Clinical isolate 3 | 1.03 | 1.13 |
Individual values are presented unless indicated otherwise.
Values are the means of three independent experiments (48-h assay of 3D7 laboratory strains).
DISCUSSION
The primary aim of antimalarial treatment is to remove asexual blood-stage parasites in order to cure the disease and prevent or treat severe forms of malaria. In contrast, late-stage gametocytes do not cause significant harm to the infected individual and appear in the circulation after a lag period due to sequestration. Most of the current antimalarials act against asexual blood stages but have no significant effect on sexual stages, which remain in the circulation for extended periods after treatment and could therefore be transmitted (31, 32). To achieve sustained malaria control and contain resistance development, an efficient gametocytocidal drug is fundamental. We identified five synthetic dyes with good gametocytocidal activity. These five dyes, particularly the three mitochondrial dyes MitoRed, DiOC6, and rhodamine B, were more active than methylene blue, a drug with known gametocytocidal activity (15, 23–25). The cytotoxic concentration of the mitochondrial dyes was higher than 2.5 μM when evaluated against HeLa cells in our previous study (22). MitoRed was the most active compound against gametocytes. Interestingly, we had previously selected it as a promising candidate for development as an antimalarial due to its high activity against asexual blood-stage parasites and its favorable selectivity index when tested against HeLa cells. It belongs to the rhodamine dyes. Some of its derivatives are used in the cosmetic industry, where toxicity has been more extensively studied (33, 34). However, no further toxicity data on MitoRed are available. The results from the current gametocyte assay underline the interesting properties of mitochondrial dyes as starting points for new antimalarials, and further toxicity studies of the most promising compounds should be performed. Interestingly, all compounds except epoxomicin showed higher activity against asexual blood stages than against gametocytes. This is likely due to the less active metabolism during the late gametocyte stage. Dyes selectively target certain organelles in the organism, presumably inhibiting the targeted organelle. Interestingly, atovaquone, a potent inhibitor of the cytochrome bc1 complex of the mitochondrion, does not affect gametocyte development (8). Nevertheless, mitochondrial dyes are highly active, which is most likely because they do not share the same mechanism of action.
In addition to P. falciparum 3D7 gametocytes, we used three clinical isolates from Lambaréné, Gabon, an area of high drug pressure and widespread chloroquine and folate inhibitor resistance (35, 36). Gametocytes derived from the laboratory strain 3D7 seemed to be more sensitive to most compounds than the tested clinical isolates. This finding has important implications for larger screening programs, since hits achieved in laboratory isolates should be considered to be validated in clinical isolates. Primaquine presented one exception to this rule because it is more active against clinical isolates than against 3D7. However, the in vitro activity is likely not a good surrogate for the relevant activity of primaquine in vivo, as it has to be metabolized by CYP2D6 (37, 38) and monoamine oxidase A (38) to be active. Therefore, primaquine shows rather unspecific activity in vitro and can be used only as a comparator between assays, showing that our results are in line with results obtained by others (8, 30, 39).
The difference in activities between clinical isolates and 3D7 was particularly high for artemisinin derivatives. Whether or not this is due to drug pressure may only be hypothesized. Artemisinin combination therapy has been the recommended first-line therapy since 2003 in Gabon (40). Since then, local parasite populations may have adapted to the presence of artemisinin derivatives. It would be very interesting to test parasite isolates obtained before 2003. Unfortunately, such isolates were not available.
A prolonged incubation time influenced the activity of the drugs. In particular, the most active compounds and artemisinin derivatives were more active when incubated for 48 h than when incubated for 24 h. There are two possible explanations for this: (i) the drug needs a longer time to exert its effect, or (ii) the drug acts early but the readout detects only late changes in the metabolism of the gametocytes. Since artemisinin derivatives have a very short half-life (41), scenario (i) would result in very low in vivo activity, whereas scenario (ii) is more likely. When we compare the results to those of our previous assays in which we used medium supplemented with Albumax instead of human serum (42), we can also see an approximately 10-fold increase of the IC50 for artesunate and DHA for the Albumax-supplemented medium. The in vitro activity of artemisinin derivatives against late-stage gametocytes is highly variable and depends on the assay used. Commonly used methods differ in the numbers of gametocytes, parasite strains, incubation times, serum supplementation, and readouts used. These differences result in inhibitory concentrations between 3 nM and 11 μM for the same compound (8, 30, 43–45). These differences demonstrate that better standardization and validation of assays is required in order to compare results and identify compounds with promising activities. One method of independent validation would be the use of standard membrane feeding assays. Interestingly, most reports show modest effects (24, 44) or no effect (46) of artemisinins on oocyst counts following membrane feeding.
In conclusion, fluorescent dyes, especially MitoRed, show high in vitro gametocytocidal activities and should be considered starting points for the development of new antimalarial compounds with transmission-blocking potential. In addition to laboratory strains, gametocytes of clinical isolates should be considered for the screening of new compounds, as they have a different genetic background and might show a resistance profile different from that of laboratory strains.
ACKNOWLEDGMENTS
Serum for parasite culture was kindly provided by Torsten J. Schulze, Blood Donation Centre, Institute of Transfusion Medicine and Immunology, Mannheim, Germany.
T.G. received financial support from the Deutscher Akademischer Austausch Dienst (DAAD).
Footnotes
Published ahead of print 29 September 2014
REFERENCES
- 1.WHO. 2013. World malaria report. WHO Global Malaria Programme, Geneva, Switzerland: http://www.who.int/iris/bitstream/10665/97008/1/9789241564694_eng.pdf Accessed 20 March 2014. [Google Scholar]
- 2.Abdulsalam AH, Sabeeh N, Bain BJ. 2010. Immature Plasmodium falciparum gametocytes in bone marrow. Am. J. Hematol. 85:943. 10.1002/ajh.21796. [DOI] [PubMed] [Google Scholar]
- 3.Smalley ME, Abdalla S, Brown J. 1981. The distribution of Plasmodium falciparum in the peripheral blood and bone marrow of Gambian children. Trans. R. Soc. Trop. Med. Hyg. 75:103–105. 10.1016/0035-9203(81)90019-5. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar R, Magallon-Tejada A, Achtman AH, Moraleda C, Joice R, Cistero P, Li Wai Suen CS, Nhabomba A, Macete E, Mueller I, Marti M, Alonso PL, Menendez C, Schofield L, Mayor A. 2014. Molecular evidence for the localization of Plasmodium falciparum immature gametocytes in bone marrow. Blood 123:959–966. 10.1182/blood-2013-08-520767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gates B. 2007. Malaria Forum keynote address. http://www.gatesfoundation.org/media-center/speeches/2007/10/melinda-french-gates-malaria-forum Accessed 20 March 2014.
- 6.Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, Collins F, Doumbo OK, Greenwood B, Hall BF, Levine MM, Mendis K, Newman RD, Plowe CV, Rodriguez MH, Sinden R, Slutsker L, Tanner M. 2011. A research agenda to underpin malaria eradication. PLoS Med. 8:e1000406. 10.1371/journal.pmed.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Modrek S, Gosling RD, Feachem RG. 2013. Malaria eradication: is it possible? Is it worth it? Should we do it? Lancet Global Health 1:e2–e3. 10.1016/S2214-109X(13)70002-0. [DOI] [PubMed] [Google Scholar]
- 8.Lelievre J, Almela MJ, Lozano S, Miguel C, Franco V, Leroy D, Herreros E. 2012. Activity of clinically relevant antimalarial drugs on Plasmodium falciparum mature gametocytes in an ATP bioluminescence “transmission blocking” assay. PLoS One 7:e35019. 10.1371/journal.pone.0035019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butterworth AS, Skinner-Adams TS, Gardiner DL, Trenholme KR. 2013. Plasmodium falciparum gametocytes: with a view to a kill. Parasitology 140:1718–1734. 10.1017/S0031182013001236. [DOI] [PubMed] [Google Scholar]
- 10.White NJ. 2008. The role of anti-malarial drugs in eliminating malaria. Malar. J. 7(Suppl 1):S8. 10.1186/1475-2875-7-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett JW, Pybus BS, Yadava A, Tosh D, Sousa JC, McCarthy WF, Deye G, Melendez V, Ockenhouse CF. 2013. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N. Engl. J. Med. 369:1381–1382. 10.1056/NEJMc1301936. [DOI] [PubMed] [Google Scholar]
- 12.Gogtay NJ, Kamtekar KD, Dalvi SS, Mehta SS, Chogle AR, Aigal U, Kshirsagar NA. 2006. A randomized, parallel study of the safety and efficacy of 45 mg primaquine versus 75 mg bulaquine as gametocytocidal agents in adults with blood schizonticide-responsive uncomplicated falciparum malaria [ISCRTN50134587]. BMC Infect. Dis. 6:16. 10.1186/1471-2334-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lell B, Faucher JF, Missinou MA, Borrmann S, Dangelmaier O, Horton J, Kremsner PG. 2000. Malaria chemoprophylaxis with tafenoquine: a randomised study. Lancet 355:2041–2045. 10.1016/S0140-6736(00)02352-7. [DOI] [PubMed] [Google Scholar]
- 14.Marcsisin SR, Sousa JC, Reichard GA, Caridha D, Zeng Q, Roncal N, McNulty R, Careagabarja J, Sciotti RJ, Bennett JW, Zottig VE, Deye G, Li Q, Read L, Hickman M, Dhammika Nanayakkara NP, Walker LA, Smith B, Melendez V, Pybus BS. 2014. Tafenoquine and NPC-1161B require CYP 2D metabolism for anti-malarial activity: implications for the 8-aminoquinoline class of anti-malarial compounds. Malar. J. 13:2. 10.1186/1475-2875-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adjalley SH, Johnston GL, Li T, Eastman RT, Ekland EH, Eappen AG, Richman A, Sim BK, Lee MC, Hoffman SL, Fidock DA. 2011. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc. Natl. Acad. Sci. U. S. A. 108:E1214–E1223. 10.1073/pnas.1112037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchholz K, Burke TA, Williamson KC, Wiegand RC, Wirth DF, Marti M. 2011. A high-throughput screen targeting malaria transmission stages opens new avenues for drug development. J. Infect. Dis. 203:1445–1453. 10.1093/infdis/jir037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Targett G, Drakeley C, Jawara M, von Seidlein L, Coleman R, Deen J, Pinder M, Doherty T, Sutherland C, Walraven G, Milligan P. 2001. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J. Infect. Dis. 183:1254–1259. 10.1086/319689. [DOI] [PubMed] [Google Scholar]
- 18.Beshir KB, Sutherland CJ, Sawa P, Drakeley CJ, Okell L, Mweresa CK, Omar SA, Shekalaghe SA, Kaur H, Ndaro A, Chilongola J, Schallig HD, Sauerwein RW, Hallett RL, Bousema T. 2013. Residual Plasmodium falciparum parasitemia in Kenyan children after artemisinin-combination therapy is associated with increased transmission to mosquitoes and parasite recurrence. J. Infect. Dis. 208:2017–2024. 10.1093/infdis/jit431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guttmann P, Ehrlich P. 1891. Ueber die Wirkung des Methylenblau bei Malaria. Ber. Klin. Wochenschr. 39:953–956. [Google Scholar]
- 20.Massimine KM, McIntosh MT, Doan LT, Atreya CE, Gromer S, Sirawaraporn W, Elliott DA, Joiner KA, Schirmer RH, Anderson KS. 2006. Eosin B as a novel antimalarial agent for drug-resistant Plasmodium falciparum. Antimicrob. Agents Chemother. 50:3132–3141. 10.1128/AAC.00621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanabe K. 1984. Inhibitory effect of rhodamine 123 on the growth of the rodent malaria parasite, Plasmodium yoelii. J. Protozool. 31:310–313. 10.1111/j.1550-7408.1984.tb02968.x. [DOI] [PubMed] [Google Scholar]
- 22.Joanny F, Held J, Mordmuller B. 2012. In vitro activity of fluorescent dyes against asexual blood stages of Plasmodium falciparum. Antimicrob. Agents Chemother. 56:5982–5985. 10.1128/AAC.00709-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coulibaly B, Zoungrana A, Mockenhaupt FP, Schirmer RH, Klose C, Mansmann U, Meissner PE, Muller O. 2009. Strong gametocytocidal effect of methylene blue-based combination therapy against falciparum malaria: a randomised controlled trial. PLoS One 4:e5318. 10.1371/journal.pone.0005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Alessandro S, Silvestrini F, Dechering K, Corbett Y, Parapini S, Timmerman M, Galastri L, Basilico N, Sauerwein R, Alano P, Taramelli D. 2013. A Plasmodium falciparum screening assay for anti-gametocyte drugs based on parasite lactate dehydrogenase detection. J. Antimicrob. Chemother. 68:2048–2058. 10.1093/jac/dkt165. [DOI] [PubMed] [Google Scholar]
- 25.Delves MJ, Ruecker A, Straschil U, Lelievre J, Marques S, Lopez-Barragan MJ, Herreros E, Sinden RE. 2013. Male and female Plasmodium falciparum mature gametocytes show different responses to antimalarial drugs. Antimicrob. Agents Chemother. 57:3268–3274. 10.1128/AAC.00325-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ifediba T, Vanderberg JP. 1981. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature 294:364–366. 10.1038/294364a0. [DOI] [PubMed] [Google Scholar]
- 27.Held J, Westerman R, Kremsner PG, Mordmuller B. 2010. In vitro activity of mirincamycin (U24729A) against Plasmodium falciparum isolates from Gabon. Antimicrob. Agents Chemother. 54:540–542. 10.1128/AAC.01090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambros C, Vanderberg JP. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418–420. 10.2307/3280287. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh AK, Dinglasan RR, Ikadai H, Jacobs-Lorena M. 2010. An improved method for the in vitro differentiation of Plasmodium falciparum gametocytes into ookinetes. Malar. J. 9:194. 10.1186/1475-2875-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peatey CL, Leroy D, Gardiner DL, Trenholme KR. 2012. Anti-malarial drugs: how effective are they against Plasmodium falciparum gametocytes? Malar. J. 11:34. 10.1186/1475-2875-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eichner M, Diebner HH, Molineaux L, Collins WE, Jeffery GM, Dietz K. 2001. Genesis, sequestration and survival of Plasmodium falciparum gametocytes: parameter estimates from fitting a model to malaria therapy data. Trans. R. Soc. Trop. Med. Hyg. 95:497–501. 10.1016/S0035-9203(01)90016-1. [DOI] [PubMed] [Google Scholar]
- 32.Bousema T, Okell L, Shekalaghe S, Griffin JT, Omar S, Sawa P, Sutherland C, Sauerwein R, Ghani AC, Drakeley C. 2010. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar. J. 9:136. 10.1186/1475-2875-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Combes RD, Haveland-Smith RB. 1982. A review of the genotoxicity of food, drug and cosmetic colours and other azo, triphenylmethane and xanthene dyes. Mutat. Res. 98:101–248. 10.1016/0165-1110(82)90015-X. [DOI] [PubMed] [Google Scholar]
- 34.Webb JM, Hansen WH, Desmond A, Fitzhugh OG. 1961. Biochemical and toxicologic studies of rhodamine B and 3,6-diaminofluroan. Toxicol. Appl. Pharmacol. 3:696–706. 10.1016/0041-008X(61)90033-3. [DOI] [PubMed] [Google Scholar]
- 35.Deloron P, Mayombo J, Le Cardinal A, Mezui-Me-Ndong J, Bruzi-Baert C, Lekoulou F, Elissa N. 2000. Sulfadoxine-pyrimethamine for the treatment of Plasmodium falciparum malaria in Gabonese children. Trans. R. Soc. Trop. Med. Hyg. 94:188–190. 10.1016/S0035-9203(00)90272-4. [DOI] [PubMed] [Google Scholar]
- 36.Pradines B, Mabika MM, Keundjian A, Lebeau C, Fusai T, Owono MM, Rogier C, Parzy D, Kombila M. 1999. In vitro sensitivity of Plasmodium falciparum isolates from Gabon to chloroquine and cycloguanil. Bull. Soc. Pathol. Exot. 92:91–94 (In French.) [PubMed] [Google Scholar]
- 37.Pybus BS, Marcsisin SR, Jin X, Deye G, Sousa JC, Li Q, Caridha D, Zeng Q, Reichard GA, Ockenhouse C, Bennett J, Walker LA, Ohrt C, Melendez V. 2013. The metabolism of primaquine to its active metabolite is dependent on CYP 2D6. Malar. J. 12:212. 10.1186/1475-2875-12-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pybus BS, Sousa JC, Jin X, Ferguson JA, Christian RE, Barnhart R, Vuong C, Sciotti RJ, Reichard GA, Kozar MP, Walker LA, Ohrt C, Melendez V. 2012. CYP450 phenotyping and accurate mass identification of metabolites of the 8-aminoquinoline, anti-malarial drug primaquine. Malar. J. 11:259. 10.1186/1475-2875-11-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chevalley S, Coste A, Lopez A, Pipy B, Valentin A. 2010. Flow cytometry for the evaluation of anti-plasmodial activity of drugs on Plasmodium falciparum gametocytes. Malar. J. 9:49. 10.1186/1475-2875-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nsimba B, Guiyedi V, Mabika-Mamfoumbi M, Mourou-Mbina JR, Ngoungou E, Bouyou-Akotet M, Loembet R, Durand R, Le Bras J, Kombila M. 2008. Sulphadoxine/pyrimethamine versus amodiaquine for treating uncomplicated childhood malaria in Gabon: a randomized trial to guide national policy. Malar. J. 7:31. 10.1186/1475-2875-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Vries PJ, Dien TK. 1996. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs 52:818–836. 10.2165/00003495-199652060-00004. [DOI] [PubMed] [Google Scholar]
- 42.Held J, Gebru T, Kalesse M, Jansen R, Gerth K, Muller R, Mordmuller B. Antimalarial activity of the myxobacterial macrolide chlorotonil A. Antimicrob. Agents Chemother., in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z, Liu M, Liang X, Siriwat S, Li X, Chen X, Parker DM, Miao J, Cui L. 2014. A flow cytometry-based quantitative drug sensitivity assay for all Plasmodium falciparum gametocyte stages. PLoS One 9:e93825. 10.1371/journal.pone.0093825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delves M, Plouffe D, Scheurer C, Meister S, Wittlin S, Winzeler EA, Sinden RE, Leroy D. 2012. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med. 9:e1001169. 10.1371/journal.pmed.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duffy S, Avery VM. 2013. Identification of inhibitors of Plasmodium falciparum gametocyte development. Malar. J. 12:408. 10.1186/1475-2875-12-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Pelt-Koops JC, Pett HE, Graumans W, van der Vegte-Bolmer M, van Gemert GJ, Rottmann M, Yeung BK, Diagana TT, Sauerwein RW. 2012. The spiroindolone drug candidate NITD609 potently inhibits gametocytogenesis and blocks Plasmodium falciparum transmission to Anopheles mosquito vector. Antimicrob. Agents Chemother. 56:3544–3548. 10.1128/AAC.06377-11. [DOI] [PMC free article] [PubMed] [Google Scholar]