Abstract
Adenovirus infections of immunocompromised patients can develop into deadly multiorgan or systemic disease. The virus is especially threatening for pediatric allogeneic hematopoietic stem cell transplant recipients; according to some studies, 10% or more of these patients succumb to disease resulting from adenovirus infection. At present, there is no drug approved for the treatment or prevention of adenovirus infections. Compounds that are approved to treat other virus infections are used off-label to combat adenovirus, but only anecdotal evidence of the efficacy of these drugs exists. Ganciclovir, a drug approved for the treatment of herpesvirus infection, was previously reported to be effective against human adenoviruses in vitro. To model adenovirus infections in immunocompromised humans, we examined ganciclovir's efficacy in immunosuppressed Syrian hamsters intravenously infected with type 5 human adenovirus (Ad5). This animal model is permissive for Ad5 replication, and the animals develop symptoms similar to those seen in humans. We demonstrate that ganciclovir suppresses Ad5 replication in the liver of infected hamsters and that it mitigates the consequences of Ad5 infections in these animals when administered prophylactically or therapeutically. We show that ganciclovir inhibits Ad5 DNA synthesis and late gene expression. The mechanism of action for the drug is not clear; preliminary data suggest that it exerts its antiadenoviral effect by directly inhibiting the adenoviral DNA polymerase. While more extensive studies are required, we believe that ganciclovir is a promising drug candidate to treat adenovirus infections. Brincidofovir, a drug with proven activity against Ad5, was used as a positive control in the prophylactic experiment.
INTRODUCTION
Human adenoviruses (Ads) are double-stranded DNA viruses with a nonenveloped icosahedral capsid. Ads are classified into seven species (A to G) and 60 types (Ad type 1 [Ad1] to Ad60) (for a review of Ad biology, see references 1 and 2). The virus is ubiquitous and causes generally mild, self-limiting infections in immunocompetent adults (for a review of Ad epidemiology and pathology, see reference 3). The symptoms range from respiratory to enteric, ocular, and urinary, depending on which type caused the infection. Infection with a specific type causes long-term immunity to that type. Approximately 60% of patients with Ad infection are under 5 years of age (4).
In some cases, Ads can cause serious illness. Epidemic keratoconjunctivitis (EKC), caused by Ad types 8, 3, 4, 19, 34, 37, 53, 54, and some other types, can result in lasting vision defects and even blindness. Ad types 4, 7, 3, 21, and 14 cause cases of acute respiratory disease in military recruits that frequently require hospitalization, and in some cases, these infections result in death (for Ad pathology, see reference 3). The most significant disease caused by Ads is seen in immunocompromised patients, in which Ad infection can lead to a serious, often life-threatening illness. These patients, unlike immunocompetent ones, cannot clear the virus infection, and thus, it can develop into a multiorgan or generalized disease. Especially at risk are pediatric allogeneic hematopoietic stem cell transplant (HSCT) patients, in which the incidence of Ad infection is between 3 and 47% and the mortality rate is considerable (3, 5–10). In allogeneic HSCT patients with a rising load of Ad in peripheral blood (as determined by quantitative PCR [qPCR]), despite antiviral therapy, mortality can approach 100% (9).
To date, there is no drug approved by regulatory authorities for the treatment of Ad infections. Clinical trials are being conducted with a promising drug candidate, brincidofovir (BCV; hexadecyloxypropyl-cidofovir, formerly known as CMX001) (11), but pivotal trials must be completed before approval of BCV. Cidofovir, which has documented in vitro efficacy against Ads, is often used off-label to treat serious cases of Ad infection. Case reports and retrospective studies suggest that cidofovir is reasonably effective against the virus; however, its use is limited to the most serious cases due to its high risk of nephrotoxicity. Ribavirin and, to a lesser extent, ganciclovir (GCV) have also been used. Unfortunately, the limited clinical data available are unclear as to the efficacy of these drugs (for reviews, see references 3, 5 to 9, and 12).
With our recent development of a Syrian hamster animal model to investigate human Ad infections (reviewed in reference 13), it became possible to carry out controlled in vivo experiments to test the efficacy of antiadenoviral compounds (14). In these experiments, hamsters immunosuppressed by cyclophosphamide (CP) (14) treatment are infected intravenously (i.v.) with Ad5, leading to the replication of Ad5 in most organs, most prominently in the liver. The replication of Ad5 in the liver is very rapid; large quantities of infectious progeny virus can be isolated from this organ 24 h after challenge (15). Depending on the virus dose, a large portion of hepatocytes become infected and die during the course of infection with the original input bolus. The released progeny viruses infect new susceptible cells, thus spreading the infection (14, 16). Hamsters develop quantifiable pathology (mortality, body weight loss, elevation of serum transaminase levels, cellular pathology) and have a high virus burden in the infected organs (17, 18). Thus, this route of infection results in a disease akin to the one seen in immunocompromised patients with advanced disseminated multiorgan Ad infections. The symptoms of Ad5 infection in hamsters can be mitigated or even reversed with the use of BCV (14). Given the seriousness of Ad infections in humans, it is important that the efficacy of available antivirals, as well as ones under development, be tested in this model. Here we present data obtained using GCV.
GCV is an acyclic analogue of guanosine that is primarily used to control herpesvirus infections (19). In herpes simplex virus (HSV)- or human cytomegalovirus (CMV)-infected cells, GCV is first converted to GCV monophosphate by a viral genome-encoded thymidine kinase (TK) or a protein kinase (UL97), respectively, and then cellular enzymes phosphorylate the GCV monophosphate to GCV triphosphate. It is this triphosphate form of the drug which hinders viral DNA replication, by acting as a selective competitive inhibitor and a substrate for the viral DNA polymerase (Pol) (reviewed in references 20 and 21). After incorporating GCV monophosphate into the daughter DNA strand and adding one more nucleotide, the CMV DNA polymerase can stall (21). The first step, i.e., phosphorylation by a viral kinase, is the main factor for its selectivity, inasmuch as cellular kinases have a low affinity to GCV.
The Ad genome is not known to code for a kinase, and therefore, Ad might not be expected to be susceptible to GCV, yet GCV has been shown to inhibit the replication in vitro of many Ad types, including types with ocular tropism, with 50% effective concentrations (EC50) ranging from 26 to 206 μM, depending on the Ad type, the cell line used, and the method of evaluation (22–29). Also, some reports noted that prophylactic treatment of transplant patients with GCV to prevent CMV infection seemed to decrease the incidence of Ad infections (30–32). Several case studies suggested that GCV used therapeutically in transplant patients has anti-Ad activity (33–35). GCV may also be useful against Ad ocular infections (see Discussion).
Here, we demonstrate that GCV suppresses Ad5 replication in the liver and Ad5-induced pathology in immunosuppressed Syrian hamsters. In cell culture, GCV inhibits Ad5 DNA replication and expression of late genes. We also show that the drug is only minimally phosphorylated in Ad-infected cells and is not incorporated into the viral genome. Preliminary data suggest that the mechanism of action of GCV may involve direct inhibition of the Ad DNA polymerase.
MATERIALS AND METHODS
Cells and viruses.
A549 human lung adenocarcinoma cells and HepG2 hepatocellular carcinoma cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA), while HEK293 human embryonic kidney cells were purchased from Microbix (Mississauga, Ontario, Canada). All three cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA) with 10% fetal bovine serum (FBS) at 37°C. A wild-type human Ad5 isolate, named Ad5 wt500, was derived in our laboratory by plaque purification from an Ad5 stock purchased from ATCC. KD3/TK is a replication-competent Ad5-based vector expressing HSV-1 thymidine kinase (TK). The vector was constructed similarly to the KD3-IFN vector (described in reference 36), except that the gene for TK instead of the gene for alpha interferon (α-IFN) was used. The titer of the viruses was determined by plaque assay.
Antiviral compounds.
GCV was purchased from Wako Chemicals USA, Inc. (Richmond, VA), and dissolved in phosphate-buffered saline (PBS) at 10 mg/ml and 3.3 mg/ml for the 100 mg/kg and 33 mg/kg doses, respectively. BCV (hexadecyloxypropyl-cidofovir) was obtained from Chimerix, Inc. (Durham, NC), and was dissolved in water at 1 mg/ml.
Syrian hamsters.
Female (for the prophylactic study) or male (for the therapeutic study) Syrian hamsters (Mesocricetus auratus) were purchased from Harlan Laboratories (Indianapolis, IN) at approximately 100 g body weight. All studies were approved by the Institutional Animal Care and Use Committee of Saint Louis University and were conducted according to federal and institutional regulations.
Infection of hamsters with adenovirus and treatment with GCV or BCV.
The hamsters were immunosuppressed using cyclophosphamide (CP) (14). CP was administered intraperitoneally (i.p.) at a dose of 140 mg/kg of body weight and then twice weekly for the duration of the study at a dose of 100 mg/kg. Five to 7 days after the first administration of CP, the animals were anesthetized with a ketamine-xylazine mixture, and Ad5 was injected i.v. (via the jugular vein) at a dose of 1.5 × 1011 or 2 × 1011 PFU/kg for the prophylactic and the therapeutic experiments, respectively (37). Control animals were injected with PBS. For the experiment with the prophylactic administration of GCV, the drug was injected i.p. starting 1 day prior to Ad5 injection and then continuing daily at a single daily dose of 33 mg/kg/day or 100 mg/kg/day. Control animals received vehicle (PBS) injections. In a separate group of hamsters in the prophylactic experiment, BCV was administered through oral (p.o.) gavage at a daily dose of 2.5 mg/kg, starting at 1 day before Ad5 injection. For the experiment in which GCV was administered therapeutically, the drug was injected i.p. starting 1 day before or 1, 2, 3, or 4 days after Ad challenge and then continuing daily at the single daily dose of 60 mg/kg/day.
Treatment groups consisted of 15 animals. Five hamsters of each group were designated to be sacrificed at 5 days (for the prophylactic study) or at 7 days (for the therapeutic study) after Ad5 challenge. At necropsy, the animals were bled out and the liver was collected. Virus was extracted from the liver and was quantified by the 50% tissue culture infectious dose (TCID50) assay in HEK293 cells (14). A portion of the collected tissues was preserved in formalin for histopathology. Serum was assayed for liver transaminase levels. The remaining 10 hamsters in each group were used for the same analyses as the groups sacrificed at 5 days and also for a survival study. All hamsters were observed and weighed daily.
Immunofluorescent staining and Western blotting.
Human A549 cells were plated in 6-well plates on coverslips in DMEM containing 10% FBS. Cells were infected 2 days later with Ad5, Ad6 (species C), Ad7, Ad35 (species B), or Ad4 (species E) at 5 PFU/cell in serum-free DMEM. At 90 min postinfection (p.i.), 500 μM GCV was added to half of the wells. The cells were fixed at 26 h p.i. and were immunostained with a rabbit anti-DNA binding protein (anti-DBP) antibody specific for the C terminus of the Ad genome-encoded DBP (a gift of Maurice Green, Saint Louis University [38]) or a mouse monoclonal antibody specific for the hexon for all human Ads (2Hx-2; ATCC) (39). Secondary antibodies were goat anti-rabbit IgG (Alexa Fluor 488 conjugate; Invitrogen Corp., Carlsbad, CA) and goat anti-mouse IgG (Alexa Fluor 594 conjugate; Invitrogen Corp.). Images were taken on a Nikon Optiphot microscope (Nikon, Melville, NY) with a Nikon DXM1200 digital camera and ACT-1 software (Nikon). All images were taken with the same exposure settings.
For Western blotting, A549 cells were infected and treated as described above (in wells parallel to those used for immunostaining). At 24 h p.i., the cells were lysed, and 20 μg of protein from each sample was electrophoresed on 15% SDS-polyacrylamide gels and electroblotted onto an Immobilon polyvinylidene difluoride membrane (Millipore, Bedford, MA). The membrane was probed with a rabbit polyclonal antiserum against Ad pVIII (generated against a TrpE fusion protein containing amino acids 54 to 227 of the Ad2 pVIII protein) or DBP (the same rabbit polyclonal antiserum used in immunofluorescent staining). pVIII is an Ad capsid protein that is synthesized exclusively at late stages of infection. The secondary antibody used was goat anti-rabbit horseradish peroxidase (Cappel, Durham, NC). The blots were developed using a LumiGlo chemiluminescent substrate system (KPL, Gaithersburg, MD).
Quantification of the effect of GCV on adenovirus replication using qPCR assay.
A549 cells seeded in 24-well plates at 1 × 105 cells per well were mock infected or infected with Ad5 at 5 PFU per cell. After 1.5 h at 37°C, the infection medium was replaced with DMEM containing serial dilutions of GCV. Each dilution was applied in triplicate, and mock-infected cultures receiving only GCV and Ad5-infected cultures receiving no GCV treatment were included as controls in each plate. At 24 h p.i., the cells were washed twice with PBS and lysed in 200 μl of lysis buffer (10 mM Tris-HCl [pH 8.0], 75 mM NaCl, 0.1% SDS, 0.5% NP-40, 0.5% Tween 20, 0.5 mg/ml proteinase K) at 56°C for 4 to 5 h and then boiled for 15 min to inactivate the proteinase K. After clarification of the samples by centrifugation, 5 μl of 10-fold-diluted supernatant was used as the template in TaqMan-based qPCRs to detect viral genome copy numbers. The primers, probe, and reaction conditions were reported previously (18). For absolute quantification, 102 to 107 copies of purified Ad5 genomic DNA were used to generate a standard curve, and the mean copy number per cell was calculated. The EC50 was calculated by extrapolation as the compound concentration at which the number of viral DNA copies was 50% compared to the value obtained from the untreated virus control.
Determining the phosphorylation rate of [3H]GCV in adenovirus-infected cells and [3H]GCV incorporation into adenovirus DNA.
A549 or HepG2 cell monolayers in 6-well plates were mock infected or infected with Ad5 or KD3/TK at 20 PFU/cell for 1 h at 37°C. The cells were incubated in DMEM containing 0.36 μM [3H]GCV (5 μCi/well; Moravek Biochemicals, Brea, CA). At 48 h p.i., the cells were washed 3 times and harvested, and the cell pellet was extracted three times with 60% (vol/vol) cold methanol (−20°C). The extracts were combined, and the volume was reduced to approximately 100 μl using vacuum centrifugation for 4 to 6 h at 30°C. To determine the [3H]GCV phosphates, 20-μl aliquots of the extracts were spotted onto DE81 paper discs (Whatman, GE Healthcare Biosciences, Pittsburgh, PA), and the discs were dried, rinsed once for 5 min with distilled water, rinsed twice with 70% ethanol containing 2 mM ammonium acetate (pH 7.5) for 5 min, and rinsed once for 5 min with 95% ethanol. The discs were then dried, and the amount of radioactivity was determined by counting in an LS 6500 scintillation counter (Beckman Coulter Inc., Brea, CA). Phosphorylated forms of [3H]GCV are negatively charged and bind to the positively charged DE81 paper, while nonphosphorylated [3H]GCV is washed away. Thus, the fraction of [3H]GCV bound to DE81 discs following the washing procedure represents the phosphorylated forms of [3H]GCV. Total [3H]GCV metabolites, including both phosphorylated and nonphosphorylated forms, were determined by spotting the same amount of the extracts onto DE81 discs without washing. The phosphorylation rate was calculated as the percentage of radioactivity bound to the disc after washing.
To determine whether [3H]GCV was incorporated into viral DNA, A549 cells in 6-well plates were mock infected or infected with Ad5 or KD3/TK at 20 PFU/cell in DMEM containing [3H]GCV (5 μCi/well) at 37°C for 2 h. Each infection was done in duplicate. Following incubation for another 46 h, viral DNA was extracted from each well using the Hirt DNA preparation procedure (40). The amount of radioactivity in 5 μg of each DNA preparation was determined in a scintillation counter.
Ad5 DNA polymerase primer extension analysis.
The Ad5 genome-encoded DNA polymerase (Ad5 Pol) with a six-histidine affinity tag at the C terminus was expressed from a baculovirus/Ad5 Pol vector in SF9 cells (41). The Ad5 Pol was purified to near homogeneity (41). Polymerase extension was tested on a substrate containing a 5′ 32P-labeled 20-mer annealed to a template 30-mer as described previously (41, 42). The reactions were set up in a 20-μl reaction volume using 0.25 nM radiolabeled substrate in a reaction buffer containing 50 mM Tris-HCl (pH 7.5), 1 mg/ml bovine serum albumin (BSA), 5 mM MgCl2, 1 mM dithiothreitol (DTT), 60 μM deoxynucleoside triphosphates (dNTPs), and 5% glycerol. Ad5 Pol was added to the reaction mixture in the absence or presence of GCV (50, 100, 200 μM) and incubated with the substrate at 37°C for 10 min. Reactions were stopped with 20 μl of 2× termination dye (90% [vol/vol] formamide, 10 mM EDTA, 0.01% bromophenol blue, 0.01% xylene cyanol), followed by heating for 5 min at 95°C. The reaction products were separated by electrophoresis on a 22.5%, 7 M urea polyacrylamide gel for 1 h 30 min at 80 W. The gel was dried and exposed to a phosphor screen, which was scanned with a GE Healthcare phosphorimager and analyzed using ImageQuant (version 1.2) software. The experiment was performed in triplicate, and a representative gel is shown in Fig. 3B.
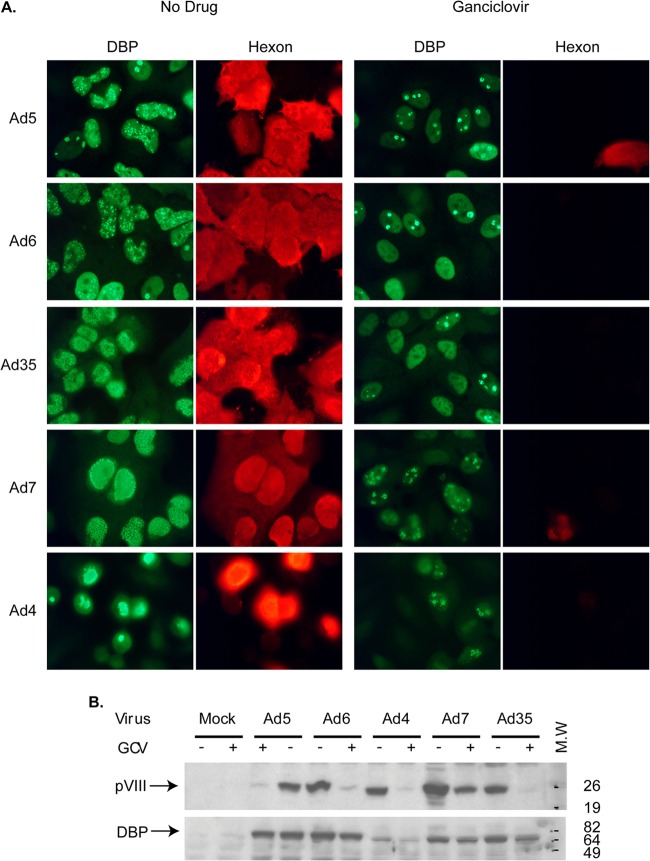
FIG 3.
GCV prevents the progression of adenoviral infection into the late phase. (A) Immunofluorescent staining for an early (DBP) and a late (hexon) Ad protein in GCV-treated or untreated A549 cells infected with species C (Ad5, Ad6), B (Ad7, Ad35), or E (Ad4) Ads. Uninfected cells did not stain with either antibody (data not shown). All images were taken with the same exposure settings. (B) Immunoblot staining for DBP and pVIII (another late Ad protein) in cells infected with the same types of Ads used for the assay whose results are shown in panel A. M.W., molecular weight markers (numbers on the right are in thousands).
Statistical analysis.
Statistical analysis was performed using GraphPad Prism (version 4) software (GraphPad Software). Two-way analysis of variance (ANOVA) was used to compare body weight changes. For serum transaminase levels, the virus burden in the liver, GCV phosphorylation levels, and quantitative PCR, the overall effect was calculated using the Kruskal-Wallis test, and comparison between groups was performed using the Mann-Whitney U test. P values of ≤0.05 were considered significant.
RESULTS
Prophylactically administered GCV or BCV protects immunosuppressed Syrian hamsters from adenovirus replication and pathogenicity. (i) Experimental design.
In this pilot study, we aimed at determining the working dose range of GCV in CP-treated, Ad5-infected Syrian hamsters, i.e., a dose (if any) that has anti-Ad activity and that is not toxic to the animals. As a positive control, we used BCV, a compound with excellent activity against Ad5 in our model (14). To facilitate the detection of antiviral activity, we started administering GCV or BCV 1 day before Ad5 challenge.
There were seven groups of animals in this experiment, three groups of uninfected animals and four groups of Ad5-infected animals. The uninfected groups received the following: (i) virus vehicle i.v. and drug vehicle i.p. (vehicle-vehicle), (ii) virus vehicle i.v. plus 33 mg/kg GCV i.p. (vehicle-GCV low), and (iii) virus vehicle i.v. plus 100 mg/kg GCV i.p. (vehicle-GCV high). Animals in the infected groups received 1.5 × 1011 PFU/kg of Ad5 i.v. There were four such groups, in which the animals received (i) Ad5 i.v. and drug vehicle i.p. (Ad5-vehicle), (ii) Ad5 i.v. plus 33 mg/kg GCV i.p. (Ad5-GCV low), (iii) Ad5 i.v. plus 100 mg/kg GCV i.p. (Ad5-GCV high), or (iv) Ad5 i.v. plus BCV at 2.5 mg/kg p.o. (Ad5-BCV). For the GCV- and BCV-treated groups, the drug was administered every day for the duration of the experiment, starting a day before virus challenge.
(ii) In-life observations.
Two hamsters died while on study due to anesthetic overdose during the administration of Ad5. No significant clinical findings were noted during the in-life phase. The Ad5-infected, vehicle-treated (Ad5-vehicle) animals lost weight starting from after virus challenge to ca. 5 days postchallenge, and then they slowly started to gain weight, but their weights never caught up to the weights of their vehicle-challenged counterparts. Treatment of the Ad5-infected hamsters with either dose of GCV resulted in a significantly smaller initial weight loss (Fig. 1A). The kinetics of the weight gain/loss were similar for the two GCV groups up to ca. 7 days postchallenge. At this time, the high-dose GCV-treated Ad5-infected hamsters started losing weight again, while the low-dose GCV-treated ones continued gaining weight and their weights eventually caught up to the weights of the vehicle-challenged animals. Delayed weight loss was also observed with vehicle-challenged animals that were treated with the high dose of GCV; these hamsters started losing weight at 6 to 7 days postchallenge and continued deteriorating throughout the study. Thus, the weight loss seen after 7 days postchallenge with the Ad5-infected, high-dose GCV-treated hamsters is probably due to the toxic effect of high-dose GCV. The Ad5-challenged, BCV-treated hamsters did not lose weight during the experiment (Fig. 1A). This result is in accord with our previous observations with BCV (14).
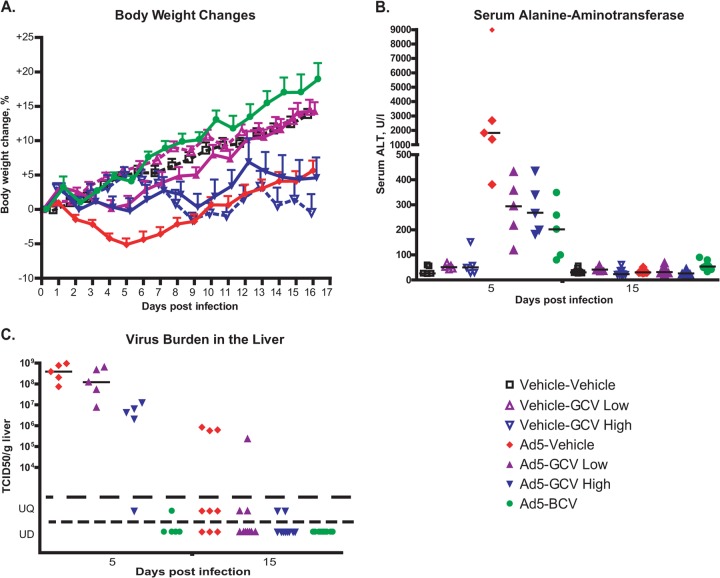
FIG 1.
GCV treatment reduces Ad5 replication and pathogenicity after intravenous challenge with the virus in immunosuppressed Syrian hamsters. (A) GCV reduces weight loss. Hamsters immunosuppressed with CP were injected with 1.5 × 1010 PFU of Ad5 i.v. on day 0 and treated with 33 mg/kg of GCV (GCV low) or 100 mg/kg of GCV (GCV high) daily. BCV was administered at a daily dose of 2.5 mg/kg. Drug treatment started at 12 h before virus challenge. Each symbol represents the group mean (calculated for the animals designated for the survival study; n = 10); the whiskers signify the standard errors of the means. For Ad5-vehicle versus Ad5-GCV low, P < 0.0001; for Ad5-vehicle versus Ad5-GCV high, P = 0.1650; and for Ad5-vehicle versus Ad5-BCV, P < 0.0001 (two-way ANOVA). (B) GCV treatment reduces liver pathogenicity. Each symbol represents an individual animal (n = 5 for day 5 and n = 9 or 10 for day 15); the horizontal bars signify the medians. For samples collected at 5 days postchallenge, for Ad-vehicle versus Ad5-GCV low, P = 0.0159; for Ad-vehicle versus Ad5-GCV high, P = 0.0159; and for Ad-vehicle versus Ad5-BCV, P = 0.0079 (Mann-Whitney U test). (C) GCV inhibits the replication of Ad5 in the liver. Each symbol represents the value from an individual animal (n = 5 for day 5 and n = 9 or 10 for day 15); the horizontal bars signify the means. For the day 5 samples, for Ad vehicle versus Ad5-GCV low, P = 0.3095; for Ad vehicle versus Ad5-GCV high, P < 0.01; and for Ad vehicle versus Ad5-BCV, P < 0.01 (Mann-Whitney U test). UQ, unquantifiable; UD, undetectable.
(iii) Necropsy and histopathology.
At 5 days postchallenge, 5 animals from each group were sacrificed and necropsied. The remaining animals were sacrificed and necropsied at 15 days postchallenge. No significant findings were noted for the uninfected and vehicle-, GCV-, or BCV-treated hamsters necropsied at 5 days postchallenge. At the sacrifice at 15 days postchallenge, several animals treated with the high dose of GCV had pale, pitted kidneys.
Microscopic pathology was scored on a scale ranging from 0 to 4. Each animal was assigned one of the following values, depending on the severity of the lesions: 0 for no significant microscopic findings, 1 for minimal microscopic findings, 2 for mild microscopic findings, 3 for moderate microscopic findings, and 4 for marked microscopic findings. A composite score for a group was calculated by averaging the individual severity scores. For the Ad5-challenged, vehicle-treated hamsters, microscopic examination of the liver samples collected at 5 days postchallenge revealed decreased hepatocellular vacuolization and increased hepatocellular necrosis accompanied by neutrophil granulocytic infiltration (Table 1). Intranuclear inclusion bodies were present, as is typical of Ad-infected cells (3). For all three Ad5-challenged, drug-treated (low-dose GCV, high-dose GCV, BCV) groups, the character and severity of the pathology were substantially lower at 5 days postchallenge than those observed with the Ad5-vehicle group (Table 1).
TABLE 1.
At 5 days postchallenge, prophylactic administration of GCV reduces the liver pathology induced by i.v. Ad5 challenge in immunosuppressed hamsters
| Group | Lesion | No. of animals affected/total no. tested | Mean (range) severitya | Distribution |
|---|---|---|---|---|
| Vehicle-vehicle | 0/5 | NAb | NA | |
| Vehicle-GCV low | 0/5 | NA | NA | |
| Vehicle-GCV high | 0/5 | NA | NA | |
| Ad5-vehicle | Inflammation | 4/5 | 2.5 (1–3) | Multifocal |
| Ad5-vehicle | Decreased hepatocellular vacuolization | 5/5 | 4 (4) | Diffuse |
| Ad5-vehicle | Hepatocellular necrosis | 1/5 | 0.6 (0–3) | Multifocal |
| Ad5-GCV low | Inflammation | 4/5 | 2.2 (2–3) | Multifocal |
| Ad5-GCV low | Decreased hepatocellular vacuolization | 0/5 | NA | NA |
| Ad5-GCV low | Hepatocellular necrosis | 0/5 | NA | NA |
| Ad5-GCV high | Inflammation | 1/5 | 0.4 (0–2) | Multifocal |
| Ad5-GCV high | Decreased hepatocellular vacuolization | 1/5 | 0.4 (0–2) | Diffuse |
| Hepatocellular necrosis | 0/5 | NA | NA | |
| Ad5-BCV | Inflammation | 0/5 | NA | NA |
| Decreased hepatocellular vacuolization | 2/5 | 0.8 (0–2) | Multifocal | |
| Hepatocellular necrosis | 0/5 | NA | NA |
Severity scores were as follows: 0, no specific microscopic findings; 1, minimal microscopic findings; 2, mild microscopic findings; 3, moderate microscopic findings; 4, marked microscopic findings.
NA, not applicable.
With the samples collected at 15 days postchallenge, multifocal to diffuse mixed mononuclear and neutrophilic inflammation was observed with the hamsters in the Ad5-vehicle group, which in one animal was accompanied by hepatocellular degeneration/necrosis. At this time, the majority of the pathology had resolved for most of the drug-treated animals; no significant microscopic lesions were noted in 6 out of 10, 8 out of 10, or 5 out of 10 animals in the Ad5-GCV low, Ad5-GCV high, and Ad5-BCV groups, respectively, and the remaining animals in these groups presented with minimal pathology (data not shown).
(iv) Serum transaminase levels.
Serum was collected at necropsy and was analyzed for transaminase levels. As expected, i.v. injection of Ad5 caused elevation of alanine aminotransferase (ALT) (Fig. 1B) and aspartate aminotransferase (data not shown) levels at 5 days postchallenge. This elevation was significantly mitigated by GCV treatment (for the Ad5-vehicle versus Ad5-GCV low and Ad5-vehicle versus Ad5-GCV high groups, P < 0.05) (Fig. 1B). The serum alanine transaminase level was significantly reduced by BCV (for the Ad5-vehicle versus Ad5-BCV groups, P < 0.01).
(v) Virus burden in the liver.
At 5 days postchallenge, the GCV-treated animals tended to have less virus burden in the liver than vehicle-treated ones, and this difference was significant for the high-dose group (P < 0.01) (Fig. 1C). At 15 days postchallenge, the virus burden was lower in all drug-treated hamsters than that in the Ad5-infected, vehicle-treated animals, but this difference was not statistically significant (Fig. 1C). BCV was very effective in reducing the virus burden in the liver (Fig. 1C).
Therapeutically administered GCV protects immunosuppressed Syrian hamsters from adenovirus replication and pathogenicity.
To follow up on the study described above, we determined the therapeutic window of GCV efficacy. Besides the dosing schedule of GCV, we changed other parameters as well: male hamsters were used to determine if there were any gender differences, we increased the Ad dose (to ca. the 90% lethal dose) to achieve more marked pathology, and we used an intermediate drug dose (60 mg/kg daily) to avoid toxicity but achieve efficacy.
(i) Experimental design.
There were eight groups of animals in this experiment, two uninfected groups of animals and six Ad5-infected groups of animals. The uninfected groups were animals treated with virus vehicle i.v. and drug vehicle i.p. (vehicle-vehicle) and virus vehicle i.v. plus 60 mg/kg GCV i.p. (vehicle-GCV). Animals in the infected groups received 2 × 1011 PFU/kg of Ad5 i.v. and either drug vehicle (Ad5-vehicle) or 60 mg/kg GCV i.p. starting at 1 day before (Ad5-GCV day −1) or 1 day (Ad5-GCV day +1), 2 days (Ad5-GCV day +2), 3 days (Ad5-GCV day +3), or 4 days (Ad5-GCV day +4) after Ad5 challenge and then every day for the duration of the experiment.
(ii) In-life observations.
GCV treatment significantly (P < 0.001) reduced mortality, even when the administration of the drug started 2 days after virus challenge (Fig. 2A). GCV also markedly reduced the Ad5-induced body weight loss when it was initiated 1 day before or 1 day after Ad5 challenge (Fig. 2B). No body weight loss or mortality was observed in the group treated with drug only.
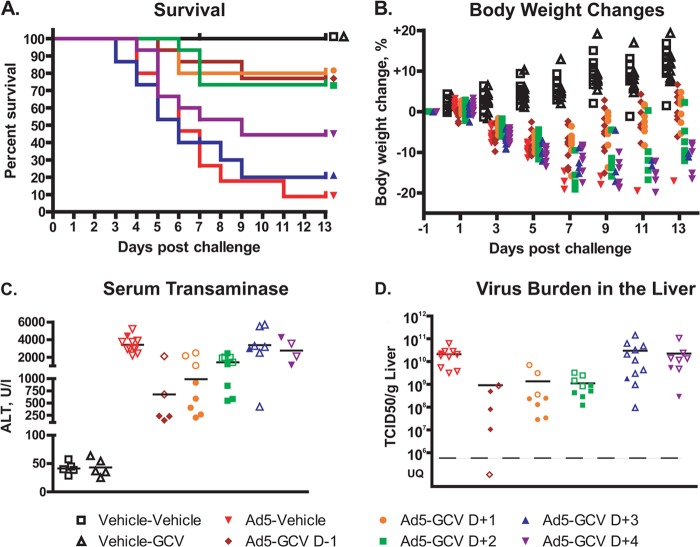
FIG 2.
Ganciclovir reduces mortality and morbidity even when administered 2 days after virus challenge. (A) Survival (n = 15). For Ad5-vehicle versus Ad5-GCV on day −1 (D−1), day +1 (D+1), and day +2 (D+2), P < 0.001; for Ad5-vehicle versus Ad5-GCV on day +3 (D+3), P = 0.802; and for Ad5-vehicle versus Ad5-GCV on day +4 (D+4), P = 0.083 (log rank test). Symbols placed at the end of each line of this graph help identify the groups that the lines represent. (B) Body weight loss (n = 10). Significance was not computed because of the low number of surviving animals in the Ad5-vehicle group. (C) Serum transaminase levels (n = 5 to 9). For Ad5-vehicle versus Ad5-GCV on day −1, day +1, and day +2, P < 0.01; for Ad5-vehicle versus Ad5-GCV on day +3, P = 0.856; and for Ad5-vehicle versus Ad5-GCV on day +4, P = 0.396 (Mann-Whitney U test). (D) Virus burden in the liver (n = 5 to 9). For Ad5-vehicle versus Ad5-GCV on day −1, day +1, and day +2, P < 0.001; for Ad5-vehicle versus Ad5-GCV on day +3, P = 0.647; and for Ad5-vehicle versus Ad5-GCV on day +4, P = 0.408 (Mann-Whitney U test). For panels B, C, and D, symbols represent data for individual animals. For the Ad5-infected animals, empty symbols represent data collected from an animal sacrificed moribund ahead of schedule. UQ, unquantifiable.
(iii) Necropsy.
For the purposes of gross pathology evaluation, animals sacrificed moribund before the day 7 time point were grouped with animals that were scheduled to be sacrificed at that time. All eight hamsters in the Ad5-vehicle group had yellow, mottled, friable livers, as is characteristic for immunosuppressed hamsters infected with high doses of Ad5. Some of these hamsters also showed evidence of internal bleeding. Only 2 out of 6, 3 out of 8, and 4 out of 9 animals presented such pathology in the Ad5-GCV day −1, Ad5-GCV day +1, and Ad5-GCV day +2 groups, respectively. In these three groups, the animals with Ad-specific pathology were all sacrificed moribund ahead of schedule. Nine out of 11 and 6 out of 8 hamsters in the Ad5-GCV day +3 and Ad5-GCV day +4 groups, respectively, had badly damaged livers. No significant findings were noted for the uninfected and vehicle- or GCV-treated hamsters necropsied at 7 days postchallenge. The animals that were sacrificed moribund at various time points after the day 7 scheduled sacrifice presented with a pathology characteristic of systemic Ad5 infection.
At the conclusion of the study (day 13), the sole surviving hamster in the Ad5-vehicle group showed a liver pathology similar in grade and characteristics to that seen in animals sacrificed moribund. Necrotic foci were observed in the liver of the hamsters in the Ad5-GCV day +2, +3, and +4 groups. The size and number of these lesions increased from 2 to 3 pinhead-sized foci in the Ad5-GCV day +2 group to numerous 2- to 3-mm-diameter foci in the Ad5-GCV day +4 group. No pathology was observed in the hamsters in the vehicle-vehicle, vehicle-GCV, Ad5-GCV day −1, and Ad5-GCV day +1 groups.
(iv) Serum transaminase levels.
For the purposes of evaluation, animals sacrificed moribund before the day 7 time point were grouped with animals that were scheduled to be sacrificed at that time. Consistent with the liver pathology observed at necropsy, animals in the Ad5-vehicle group had highly elevated serum transaminase levels (Fig. 2C). GCV treatment significantly (P < 0.01) reduced this elevation, even when administration of the drug started 2 days after Ad5 challenge. Notably, almost exclusively, only hamsters in the Ad5-GCV day −1 and day +1 groups that were sacrificed moribund ahead of schedule had highly elevated transaminase levels (Fig. 2C).
(v) Virus burden in the liver.
At 7 days postchallenge, animals in groups in which GCV treatment started 1 day before or 1 or 2 days after Ad5 challenge had a significantly (P < 0.001) lower virus burden in the liver (Fig. 2D). No significant decrease in liver virus load was detected in the Ad5-GCV day +3 and day +4 groups.
GCV does not inhibit Ad early gene expression, but it prevents adenovirus infection from progressing into the late stage.
Ad infection can be divided into an early phase, which precedes viral DNA replication and in which about 20 early genes are expressed, and a late phase, which requires the initiation of synthesis of Ad genomic DNA and is characterized by the expression of Ad late (mostly structural) proteins (43). To investigate the stage in infection at which GCV exerts its effects, we carried out immunoassays to determine if Ad early and late proteins are produced in Ad-infected, GCV-treated cells. As a representative early protein, we examined the DNA binding protein (DBP; a protein involved in Ad DNA replication), which is expressed prior to the onset of Ad DNA replication and whose expression increases in the late phase of infection. As representative late proteins, we selected hexon and protein pVIII, both virion structural proteins. As shown in Fig. 3A, both DBP and the hexon protein were synthesized abundantly in cells not treated with GCV. In GCV-treated cells, DBP expression was observed, but the expression of the Ad hexon protein was dramatically decreased. Further, subtle changes in the localization of DBP were detected. In untreated cells, DBP localized in large numbers of replication centers; in some cells, the DBP staining was throughout the nucleus (Fig. 3A). These large replication centers, which are seen at late stages of infection, are sites for Ad DNA replication and late gene transcription. In GCV-treated cells, DBP was mostly confined to the original replication centers associated with the input virus (this is an early DBP localization pattern) or showed uniform diffuse nuclear staining (a pattern seen in very early infection) (Fig. 3A) (44, 45), confirming that GCV inhibits the progression of the viral infection into the late phase. These findings hold true for multiple Ad types belonging to species C (Ad5, Ad6), species B (Ad7, Ad35), and species E (Ad4), ascertaining that the mechanism of action is probably the same for all Ad species. These results were corroborated by immunoblotting, in which we demonstrated that the expression of protein pVIII was greatly reduced in GCV-treated Ad-infected cultures (Fig. 3B). Conversely, no significant changes in the expression of DBP were seen (Fig. 3B). These results indicate that the blockade of Ad replication by GCV happens after the expression of Ad early proteins, such as DBP, and before the expression of Ad late proteins, such as hexon and pVIII.
GCV inhibits Ad5 DNA replication.
For Ad infections, the signal to change from the early to the late transcription pattern is the initiation of viral DNA replication (43). To determine directly whether Ad5 DNA replication is inhibited by GCV, we established a qPCR assay that allows direct quantification of the viral DNA copies in Ad5-infected A549 cells. Treatment with 200 μM GCV reduced the level of viral DNA replication 4-fold compared to that in the untreated control, while GCV treatment at 2 mM knocked down viral DNA replication 2 to 3 log units (Fig. 4A). The EC50 calculated on the basis of the inhibition of viral DNA replication was 100 μM (95% confidence interval, 76.4 to 128.8 μM).
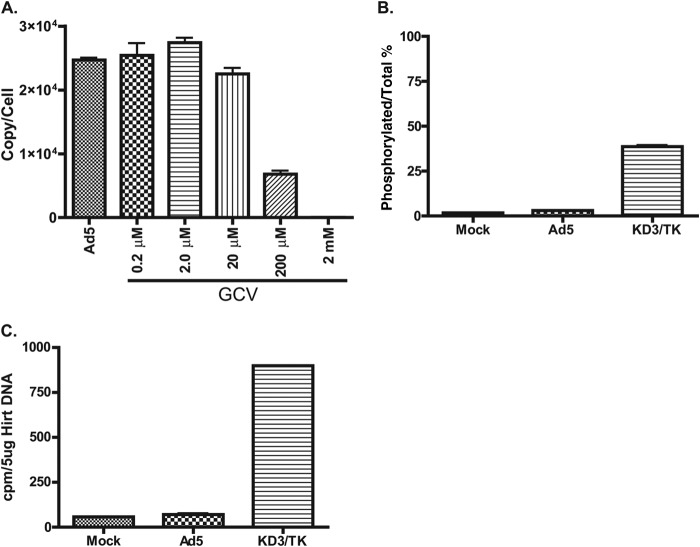
FIG 4.
In cell culture, unphosphorylated GCV inhibits adenoviral DNA replication. (A) GCV inhibits Ad5 DNA replication. A549 cells were infected with Ad5 at 5 PFU/cell. The infection was treated with different concentrations of GCV for 24 h. The viral DNA was quantified by qPCR; the result is presented as the viral genome copy number per cell. The means of three biological replicates ± standard deviations are shown. For Ad5 versus Ad5 plus GCV at 0.2 μM, P = 0.4510; for Ad5 versus Ad5 plus GCV at 2 μM, P < 0.01; for Ad5 versus Ad5 plus GCV at 20 μM, P < 0.01; for Ad5 versus Ad5 plus GCV at 200 μM, P < 0.01; and for Ad5 versus Ad5 plus GCV at 2 mM, P < 0.01 (Mann-Whitney U test). (B) GCV is minimally phosphorylated in Ad5-infected cells. A549 cells were mock infected or infected with Ad5 or KD3/TK at 20 PFU/cell. The infected cells were incubated with 0.36 μM [3H]GCV for 48 h. The levels of radioactivity from the phosphorylated forms of [3H]GCV and total [3H]GCV were determined by the DE81 disc assay. The phosphorylation rate was calculated as the percentage of radioactivity in the phosphorylated fraction compared to the total amount of [3H]GCV radioactivity. The means from two independent experiments (two biological replicates each) ± standard errors of the means are shown. For mock infection versus Ad5 infection, P < 0.05; for mock infection versus KD3/TK infection, P < 0.05; and for Ad5 infection versus KD3/TK infection, P < 0.05 (Mann-Whitney U test). (C) GCV is not incorporated into viral DNA. A549 cells were mock infected or infected with Ad5 or KD3/TK in the presence of 0.36 μM [3H]GCV for 48 h. Viral DNA was extracted by the Hirt DNA procedure. The amount of radioactivity in 5 μg of a DNA preparation was determined by scintillation counting. The value represents the average of duplicate experiments.
GCV is minimally phosphorylated in adenovirus-infected cells, and it is not incorporated into the viral genome.
Phosphorylation of GCV is the necessary first step for the drug to inhibit the replication of HSV or CMV DNA. To explore the mechanism of action of GCV in inhibiting Ad replication, we examined whether [3H]GCV is effectively phosphorylated in Ad-infected cells using a DE81 paper disc assay. As shown in Fig. 4B, a very slight increase in [3H]GCV phosphorylation was observed in Ad5-infected cells compared to mock-infected cells (2.8% versus 1.7%, respectively). In contrast, in A549 cells infected with the KD3/TK virus, which expresses the HSV-1 TK, phosphorylated forms of [3H]GCV accounted for approximately 39% of the total [3H]GCV present in the cell extract (Fig. 4B). The same results were seen in Ad5- or KD3/TK-infected HepG2 cells (data not shown).
In a related experiment, we examined the incorporation of GCV into viral DNA by labeling Ad5- or KD3/TK-infected A549 cells with [3H]GCV and then determining the radioactivity of the viral DNA preparation. The level of [3H]GCV incorporation into Ad5 DNA was barely above the level of [3H]GCV incorporation into cellular DNA in the mock-infected control (Fig. 4C). In contrast, a significantly higher level of [3H]GCV incorporation was detected in KD3/TK viral DNA (Fig. 4C). Altogether, these results indicate that GCV is a poor substrate for cellular TK (or other cellular kinases) and that GCV does not get incorporated into the replicating viral genome to a significant extent.
Unphosphorylated GCV directly inhibits Ad5 DNA polymerase.
In searching for another mechanism of action for GCV, we investigated whether GCV can directly inhibit the Ad5 DNA polymerase (Ad5 Pol). Purified Ad5 Pol enzyme can utilize dNTPs in a primer extension assay in vitro. As shown in Fig. S1 in the supplemental material, addition of increasing concentrations of GCV to the reaction mixture reduced the extension efficacy of Ad5 Pol. This result suggests that direct inhibition of the Ad DNA polymerase might be one of the mechanisms by which GCV inhibits Ad5 replication.
DISCUSSION
Treatment of Ad infections using GCV in humans has been a controversial practice, as the scientific rationale behind it is tenuous, inasmuch as for herpesviruses, GCV needs to be phosphorylated by a viral kinase to the active monophosphate form and the genomes of Ads are not known to encode a kinase. However, GCV can inhibit Ad replication in vitro, and there is anecdotal evidence as to its clinical efficacy against Ad infection and pathogenesis in transplant patients (see the introduction).
Further, there is interest in the possible use of GCV to treat Ad infections of the eye. Ad is the most common cause of acute external ocular viral infections worldwide, e.g., with more than 1 million cases per year in Japan (reviewed in references 3 and 46 to 48). Ads cause EKC, follicular conjunctivitis, acute hemorrhagic conjunctivitis, and pharyngeal conjunctival fever. Topical GCV as a 0.15% ophthalmic gel has been approved for use to treat acute herpetic keratitis in many countries since 1995 (as Virgan) and in the United States since 2009 (as Zirgan). Topical GCV has also been explored off-label for Ad keratoconjunctivitis. As noted above, GCV inhibits the replication of Ads that cause EKC and other eye infections in vitro. One research group working in the cotton rat eye model reported that 3% GCV reduced Ad5 replication and pathology (23). In a published clinical study, treatment with 0.15% GCV ophthalmic gel improved the outcome of Ad conjunctivitis (49). There are three other clinical trials evaluating 0.15% GCV ophthalmic gel: (i) ClinicalTrials.gov trial NCT01156025 (Efficacy and Safety of GV 550 in Acute Adenovirus Keratoconjunctivitis; study completed), (ii) ClinicalTrials.gov trial NCT1533480 (A Placebo Controlled Comparison of Topical Zirgan Versus Genteal for the Treatment of Adenovirus Conjunctivitis; currently recruiting participants), and (iii) a Clinicaltrialsregister.eu trial (Efficacy and Safety of GV 550 in Acute Adenoviral Keratoconjunctivitis; study completed).
In the present report, we have demonstrated that systemically administered GCV mitigates the effects of generalized Ad5 infection in immunosuppressed Syrian hamsters (Fig. 1 and 2). The drug, at a daily dose of 60 mg/kg, significantly reduced mortality and body weight loss and decreased the liver pathology caused by the virus, even when the administration of the drug started at 2 days after challenge. The 60 mg/kg hamster dose corresponds to an 8.1 mg/kg human equivalent dose (50). This dose is clinically relevant; the recommended dose of GCV for the prevention of CMV retinitis in transplant patients is 10 mg/kg i.v. daily for 14 to 21 days and then 5 mg/kg i.v. daily for 100 to 120 days after transplantation (51).
As to the mechanism of action of GCV, we have shown by qPCR that the drug inhibits viral DNA replication (Fig. 4A). Further, GCV treatment blocked the advancement of viral infection into the late phase (Fig. 3). As progression to the late phase of infection is an event dependent on Ad DNA replication, this suggests that GCV targets the latter process. Inhibition of Ad DNA synthesis by GCV was also reported in earlier studies by labeling Ad DNA with 32P (29) and by qPCR (22). Thus, we argue that the major means by which the drug inhibits Ad replication is by counteracting viral DNA replication.
How GCV affects Ad DNA replication is unclear, inasmuch as we found the drug to be only minimally phosphorylated in Ad-infected cells. While there is no evidence that the Ad genome encodes a TK, early studies reported that cellular TK activity increased 4- to 6-fold in Ad-infected cells (52, 53). The slight increase in GCV phosphorylation in Ad-infected cells that we observed (Fig. 4B) may be the result of this elevated cellular TK activity. However, it is unlikely that this would be responsible for the anti-Ad efficacy of the drug, especially since there was little or no incorporation of [3H]GCV into the Ad genome. Still, it is possible that the anti-Ad effect that we have obtained with GCV in vivo could involve the induction of cellular TK by Ad to a greater extent than what is observed in vitro. We note that the Ad DNA polymerase is capable of using GCV triphosphate as a substrate, as shown in studies of Ad infection of cells expressing HSV TK and treatment with GCV (8, 22). Also, many different replication-competent Ad vectors that express HSV TK as a suicide gene or an anticancer therapeutic gene have been constructed; these vectors are very sensitive to GCV; see, for example, references 54 and 55.
One possible mechanism by which GCV may inhibit viral DNA replication is the direct inhibition of the Ad DNA polymerase. We have preliminary data showing that unphosphorylated GCV reduces the extension efficacy of the purified enzyme in a dose-dependent manner. The drug inhibited Ad5 Pol activity over a concentration range of 50 to 200 μM, which is similar to the EC50 (26 to 206 μM). The 50% cytotoxic concentration (CC50) of GCV is ca. 2 mM (22); thus, it is likely that cellular DNA polymerases are not affected by GCV over this concentration range. Further studies are warranted to confirm these data and determine the exact mechanism of this inhibition.
Taken together, we have demonstrated that GCV is effective against Ad5 infection both in vitro and in immunosuppressed hamsters. This is the first report showing that GCV is effective against Ad5 infection, replication, and pathogenicity in an Ad5 i.v. challenge in an animal model that is permissive for Ad5 replication. Our data implicate that a direct inhibition of the Ad DNA polymerase may be one of the mechanisms of action of GCV; however, the possibility of other means of inhibition by the drug cannot be excluded.
In the current study, we have confirmed previous observations that BCV is very effective in inhibiting Ad5 pathogenicity and replication in the liver (14). Furthermore, we believe that GCV should be considered an option for the treatment of Ad infections in immunocompromised humans. GCV is approved for prophylaxis of CMV in transplant patients, and in such treatments, GCV may have suppressed disseminated Ad infection (30, 31). Our data also support exploration of the use of GCV to treat Ad ocular infections.
Supplementary Material
ACKNOWLEDGMENTS
This project has been funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, U.S. Department of Health and Human Services, under contract no. HHSN272201000021I.
We thank Dawn Schwartz for assistance.
We declare no conflict of interest.
Footnotes
Published ahead of print 15 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03860-14.
REFERENCES
- 1.Berk AJ. 2013. Adenoviridae, p 1704–1731 In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B. (ed), Fields virology, 6th ed (electronic) Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Russell WC. 2009. Adenoviruses: update on structure and function. J. Gen. Virol. 90:1–20. 10.1099/vir.0.003087-0. [DOI] [PubMed] [Google Scholar]
- 3.Wold WSM, Ison MG. 2013. Adenoviruses, p 1732 In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B. (ed), Fields virology, 6th ed (electronic) Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 4.Collins RH, Jr, Goldstein S, Giralt S, Levine J, Porter D, Drobyski W, Barrett J, Johnson M, Kirk A, Horowitz M, Parker P. 2000. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant. 26:511–516. 10.1038/sj.bmt.1702555. [DOI] [PubMed] [Google Scholar]
- 5.Ison MG. 2006. Adenovirus infections in transplant recipients. Clin. Infect. Dis. 43:331–339. 10.1086/505498. [DOI] [PubMed] [Google Scholar]
- 6.Echavarria M. 2008. Adenoviruses in immunocompromised hosts. Clin. Microbiol. Rev. 21:704–715. 10.1128/CMR.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindemans CA, Leen AM, Boelens JJ. 2010. How I treat adenovirus in hematopoietic stem cell transplant recipients. Blood 116:5476–5485. 10.1182/blood-2010-04-259291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenaerts L, De Clercq E, Naesens L. 2008. Clinical features and treatment of adenovirus infections. Rev. Med. Virol. 18:357–374. 10.1002/rmv.589. [DOI] [PubMed] [Google Scholar]
- 9.Matthes-Martin S, Boztug H, Lion T. 2013. Diagnosis and treatment of adenovirus infection in immunocompromised patients. Expert Rev. Anti Infect. Ther. 11:1017–1028. 10.1586/14787210.2013.836964. [DOI] [PubMed] [Google Scholar]
- 10.Stercz B, Nagy K, Ongradi J. 2012. Adenovirus infections in immunocompromised patients. Orv. Hetil. 153:1896–1904 (In Hungarian.) 10.1556/OH.2012.29496. [DOI] [PubMed] [Google Scholar]
- 11.Florescu DF, Pergam SA, Neely MN, Qiu F, Johnston C, Way S, Sande J, Lewinsohn DA, Guzman-Cottrill JA, Graham ML, Papanicolaou G, Kurtzberg J, Rigdon J, Painter W, Mommeja-Marin H, Lanier R, Anderson M, van der Horst C. 2012. Safety and efficacy of CMX001 as salvage therapy for severe adenovirus infections in immunocompromised patients. Biol. Blood Marrow Transplant. 18:731–738. 10.1016/j.bbmt.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenaerts L, Naesens L. 2006. Antiviral therapy for adenovirus infections. Antiviral Res. 71:172–180. 10.1016/j.antiviral.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Wold WS, Toth K. 2012. Syrian hamster as an animal model to study oncolytic adenoviruses and to evaluate the efficacy of antiviral compounds. Adv. Cancer Res. 115:69–92. 10.1016/B978-0-12-398342-8.00003-3. [DOI] [PubMed] [Google Scholar]
- 14.Toth K, Spencer JF, Dhar D, Sagartz JE, Buller RM, Painter GR, Wold WSM. 2008. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc. Natl. Acad. Sci. U. S. A. 105:7293–7297. 10.1073/pnas.0800200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas MA, Spencer JF, La Regina MC, Dhar D, Tollefson AE, Toth K, Wold WS. 2006. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 66:1270–1276. 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- 16.Dhar D, Spencer JF, Toth K, Wold WSM. 2009. Pre-existing immunity and passive immunity to adenovirus 5 prevents toxicity caused by an oncolytic adenovirus vector in the Syrian hamster model. Mol. Ther. 17:1724–1732. 10.1038/mt.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichtenstein DL, Spencer JF, Doronin K, Patra D, Meyer J, Shashkova EV, Kuppuswamy M, Dhar D, Thomas MA, Tollefson AE, Zumstein LA, Wold WSM, Toth K. 2009. An acute toxicology study with INGN 007, an oncolytic adenovirus vector, in mice and permissive Syrian hamsters; comparisons with wild-type Ad5 and a replication-defective adenovirus vector. Cancer Gene Ther. 16:644–654. 10.1038/cgt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying B, Toth K, Spencer JF, Meyer J, Tollefson AE, Patra D, Dhar D, Shashkova EV, Kuppuswamy M, Doronin K, Thomas MA, Zumstein LA, Wold WSM, Lichtenstein DL. 2009. INGN 007, an oncolytic adenovirus vector, replicates in Syrian hamsters but not mice: comparison of biodistribution studies. Cancer Gene Ther. 16:625–637. 10.1038/cgt.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans CM, Kudesia G, McKendrick M. 2013. Management of herpesvirus infections. Int. J. Antimicrob. Agents 42:119–128. 10.1016/j.ijantimicag.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 20.Matthews T, Boehme R. 1988. Antiviral activity and mechanism of action of ganciclovir. Rev. Infect. Dis. 10:S490–S494. 10.1093/clinids/10.Supplement_3.S490. [DOI] [PubMed] [Google Scholar]
- 21.Coen DM, Richman DD. 2013. Antiviral agents, p 338–373 In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B. (ed), Fields virology, 6th ed, vol 1 (electronic) Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 22.Naesens L, Lenaerts L, Andrei G, Snoeck R, Van Beers D, Holy A, Balzarini J, De Clercq E. 2005. Antiadenovirus activities of several classes of nucleoside and nucleotide analogues. Antimicrob. Agents Chemother. 49:1010–1016. 10.1128/AAC.49.3.1010-1016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trousdale MD, Goldschmidt PL, Nobrega R. 1994. Activity of ganciclovir against human adenovirus type-5 infection in cell culture and cotton rat eyes. Cornea 13:435–439. 10.1097/00003226-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Kodama E, Shigeta S, Suzuki T, De Clercq E. 1996. Application of a gastric cancer cell line (MKN-28) for anti-adenovirus screening using the MTT method. Antiviral Res. 31:159–164. 10.1016/0166-3542(96)06966-5. [DOI] [PubMed] [Google Scholar]
- 25.Smith KO, Galloway KS, Kennell WL, Ogilvie KK, Radatus BK. 1982. A new nucleoside analog, 9-[[2-hydroxy-1-(hydroxymethyl)ethoxyl]methyl]guanine, highly active in vitro against herpes simplex virus types 1 and 2. Antimicrob. Agents Chemother. 22:55–61. 10.1128/AAC.22.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wreghitt TG, Gray JJ, Ward KN, Salt A, Taylor DL, Alp NJ, Tyms AS. 1989. Disseminated adenovirus infection after liver transplantation and its possible treatment with ganciclovir. J. Infect. 19:88–89. 10.1016/S0163-4453(89)95214-6. [DOI] [PubMed] [Google Scholar]
- 27.Tabbara KF, Omar N, Hammouda E, Akanuma M, Ohguchi T, Ariga T, Tagawa Y, Kitaichi N, Ishida S, Aoki K, Ishiko H, Ohno S. 2010. Molecular epidemiology of adenoviral keratoconjunctivitis in Saudi Arabia. Mol. Vis. 16:2132–2136. [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon YJ, Romanowski E, Araullo-Cruz T, Seaberg L, Erzurum S, Tolman R, De Clercq E. 1991. Inhibitory effect of (S)-HPMPC, (S)-HPMPA, and 2′-nor-cyclic GMP on clinical ocular adenoviral isolates is serotype-dependent in vitro. Antiviral Res. 16:11–16. 10.1016/0166-3542(91)90054-U. [DOI] [PubMed] [Google Scholar]
- 29.Taylor DL, Jeffries DJ, Taylor-Robinson D, Parkin JM, Tyms AS. 1988. The susceptibility of adenovirus infection to the anti-cytomegalovirus drug, ganciclovir (CHPG). FEMS Microbiol. Lett. 49:337–341. 10.1111/j.1574-6968.1988.tb02753.x. [DOI] [Google Scholar]
- 30.Avivi I, Chakrabarti S, Milligan DW, Waldmann H, Hale G, Osman H, Ward KN, Fegan CD, Yong K, Goldstone AH, Linch DC, Mackinnon S. 2004. Incidence and outcome of adenovirus disease in transplant recipients after reduced-intensity conditioning with alemtuzumab. Biol. Blood Marrow Transplant. 10:186–194. 10.1016/j.bbmt.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Bruno B, Gooley T, Hackman RC, Davis C, Corey L, Boeckh M. 2003. Adenovirus infection in hematopoietic stem cell transplantation: effect of ganciclovir and impact on survival. Biol. Blood Marrow Transplant. 9:341–352. 10.1016/S1083-8791(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 32.Lenaerts L, Kelchtermans H, Geboes L, Matthys P, Verbeken E, De Clercq E, Naesens L. 2008. Recovery of humoral immunity is critical for successful antiviral therapy in disseminated mouse adenovirus type 1 infection. Antimicrob. Agents Chemother. 52:1462–1471. 10.1128/AAC.01311-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen FE, Liang RH, Lo JY, Yuen KY, Chan TK, Peiris M. 1997. Treatment of adenovirus-associated haemorrhagic cystitis with ganciclovir. Bone Marrow Transplant. 20:997–999. 10.1038/sj.bmt.1700997. [DOI] [PubMed] [Google Scholar]
- 34.Blohme I, Nyberg G, Jeansson S, Svalander C. 1992. Adenovirus infection in a renal transplant patient. Transplant. Proc. 24:295. [PubMed] [Google Scholar]
- 35.Duggan JM, Farrehi J, Duderstadt S, Turner NJ, Fekety R. 1997. Treatment with ganciclovir of adenovirus pneumonia in a cardiac transplant patient. Am. J. Med. 103:439–440. 10.1016/S0002-9343(97)85997-9. [DOI] [PubMed] [Google Scholar]
- 36.Shashkova EV, Spencer JF, Wold WSM, Doronin K. 2007. Targeting interferon-alpha increases antitumor efficacy and reduces hepatotoxicity of E1A-mutated spread-enhanced oncolytic adenovirus. Mol. Ther. 15:598–607. 10.1038/sj.mt.6300064. [DOI] [PubMed] [Google Scholar]
- 37.Thomas MA, Spencer JF, Wold WSM. 2007. Use of the Syrian hamster as an animal model for oncolytic adenovirus vectors, p 169–183 In Tollefson AE, Wold WSM. (ed), Methods in molecular medicine, 2nd ed, vol 1 Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 38.Lillie JW, Loewenstein PM, Green MR, Green M. 1987. Functional domains of adenovirus type 5 E1a proteins. Cell 50:1091–1100. 10.1016/0092-8674(87)90175-9. [DOI] [PubMed] [Google Scholar]
- 39.Cepko CL, Whetstone CA, Sharp PA. 1983. Adenovirus hexon monoclonal antibody that is group specific and potentially useful as a diagnostic reagent. J. Clin. Microbiol. 17:360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cauthen AN, Welton AR, Spindler KR. 2007. Construction of mouse adenovirus type 1 mutants. Methods Mol. Med. 130:41–59. 10.1385/1-59745-166-5:41. [DOI] [PubMed] [Google Scholar]
- 41.Capella C, Beltejar MJ, Brown C, Fong V, Daddacha W, Kim B, Dewhurst S. 2012. Selective modification of adenovirus replication can be achieved through rational mutagenesis of the adenovirus type 5 DNA polymerase. J. Virol. 86:10484–10493. 10.1128/JVI.00739-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pike JE, Henry RA, Burgers PM, Campbell JL, Bambara RA. 2010. An alternative pathway for Okazaki fragment processing: resolution of fold-back flaps by Pif1 helicase. J. Biol. Chem. 285:41712–41723. 10.1074/jbc.M110.146894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas GP, Mathews MB. 1980. DNA replication and the early to late transition in adenovirus infection. Cell 22:523–533. 10.1016/0092-8674(80)90362-1. [DOI] [PubMed] [Google Scholar]
- 44.Sugawara K, Gilead Z, Wold WSM, Green M. 1977. Immunofluorescence study of the adenovirus type 2 single-stranded DNA binding protein in infected and transformed cells. J. Virol. 22:527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voelkerding K, Klessig DF. 1986. Identification of two nuclear subclasses of the adenovirus type 5-encoded DNA-binding protein. J. Virol. 60:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaufman HE. 2011. Adenovirus advances: new diagnostic and therapeutic options. Curr. Opin. Ophthalmol. 22:290–293. 10.1097/ICU.0b013e3283477cb5. [DOI] [PubMed] [Google Scholar]
- 47.Colin J. 2007. Ganciclovir ophthalmic gel, 0.15%: a valuable tool for treating ocular herpes. Clin. Ophthalmol. 1:441–453. [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer-Rusenberg B, Loderstadt U, Richard G, Kaulfers PM, Gesser C. 2011. Epidemic keratoconjunctivitis: the current situation and recommendations for prevention and treatment. Dtsch. Arztebl. Int. 108:475–480. 10.3238/arztebl.2011.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yabiku ST, Yabiku MM, Bottos KM, Araujo AL, Freitas D, Belfort R., Jr 2011. Ganciclovir 0.15% ophthalmic gel in the treatment of adenovirus keratoconjunctivitis. Arq. Bras. Oftalmol. 74:417–421 (In Portuguese.) 10.1590/S0004-27492011000600007. [DOI] [PubMed] [Google Scholar]
- 50.Center for Drug Evaluation and Research. 2005, posting date Guidance for industry estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Center for Drug Evaluation and Research Food and Drug Administration, Rockville, MD. [Google Scholar]
- 51.Holland GN, Sidikaro Y, Kreiger AE, Hardy D, Sakamoto MJ, Frenkel LM, Winston DJ, Gottlieb MS, Bryson YJ, Champlin RE, Ho WG, Winters RE, Wolfe PR, Cherry JD. 1987. Treatment of cytomegalovirus retinopathy with ganciclovir. Ophthalmology 94:815–823. 10.1016/S0161-6420(87)33534-1. [DOI] [PubMed] [Google Scholar]
- 52.Kit S, Nakajima K, Dubbs DR. 1970. Origin of thymidine kinase in adenovirus-infected human cell lines. J. Virol. 5:446–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheetham BF, Shaw DC, Bellett AJ. 1982. Adenovirus type 5 induces progression of quiescent rat cells into S phase without polyamine accumulation. Mol. Cell. Biol. 2:1295–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wildner O, Hoffmann D, Jogler C, Uberla K. 2003. Comparison of HSV-1 thymidine kinase-dependent and -independent inhibition of replication-competent adenoviral vectors by a panel of drugs. Cancer Gene Ther. 10:791–802. 10.1038/sj.cgt.7700638. [DOI] [PubMed] [Google Scholar]
- 55.Ibrisimovic M, Nagl U, Kneidinger D, Rauch M, Lion T, Klein R. 2012. Targeted expression of herpes simplex virus thymidine kinase in adenovirus-infected cells reduces virus titers upon treatment with ganciclovir in vitro. J. Gene Med. 14:3–19. 10.1002/jgm.1638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.