Abstract
We used an enzyme induction approach to study the role of detoxification enzymes in the interaction of the anthelmintic compound naphthalophos with Haemonchus contortus larvae. Larvae were treated with the barbiturate phenobarbital, which is known to induce the activity of a number of detoxification enzymes in mammals and insects, including cytochromes P450 (CYPs), UDP-glucuronosyltransferases (UDPGTs), and glutathione (GSH) S-transferases (GSTs). Cotreatment of larvae with phenobarbital and naphthalophos resulted in a significant increase in the naphthalophos 50% inhibitory concentration (IC50) compared to treatment of larvae with the anthelmintic alone (up to a 28-fold increase). The phenobarbital-induced drug tolerance was reversed by cotreatment with the UDPGT inhibitors 5-nitrouracil, 4,6-dihydroxy-5-nitropyrimidine, probenecid, and sulfinpyrazone. Isobologram analysis of the interaction of 5-nitrouracil with naphthalophos in phenobarbital-treated larvae clearly showed the presence of strong synergism. The UDPGT inhibitors 5-nitrouracil, 4,6-dihydroxy-5-nitropyrimidine, and probenecid also showed synergistic effects with non-phenobarbital-treated worms (synergism ratio up to 3.2-fold). This study indicates that H. contortus larvae possess one or more UDPGT enzymes able to detoxify naphthalophos. In highlighting the protective role of this enzyme group, this study reveals the potential for UDPGT enzymes to act as a resistance mechanism that may develop under drug selection pressure in field isolates of this species. In addition, the data indicate the potential for a chemotherapeutic approach utilizing inhibitors of UDPGT enzymes as synergists to increase the activity of naphthalophos against parasitic worms and to combat detoxification-mediated drug resistance if it arises in the field.
INTRODUCTION
The control of gastrointestinal nematode (GIN) parasites of livestock relies largely on the use of anthelmintic drugs. However, resistance to most of the currently available anthelmintic classes threatens our ability to control these parasites in livestock production systems worldwide (1, 2). In Australia, there is widespread resistance to the three most widely used chemical classes: benzimidazoles, macrocyclic lactones, and nicotinic agonists (3). The organophosphate compound naphthalophos (NAP) has also been used for many years to control nematodes; however, it has been used on a much smaller scale than the three other chemical groups. This limited use has been largely due to the fact that it is only a “midspectrum” drench. NAP-based drenches show nearly 100% efficacy against susceptible adult stages of the parasite Haemonchus contortus; however, they are only approximately 70 to 90% effective against immature stages of this species as well as all life stages of susceptible worms of the other two major parasites which affect sheep production systems in Australia (Trichostrongylus colubriformis and Teladorsagia circumcincta) (4). However, as resistance to the major drug groups has rendered them far less effective for controlling all the important worm species, the negative impact of this limited spectrum has diminished, and emphasis on the use of NAP has increased. As well as being marketed in single-active drench products (as it has been for many years), it is now also sold as a combination product, formulated with one or more compounds from the other chemical groups. The low usage rates of NAP in the past may be the main reason why resistance to this compound does not threaten its continued use. Indeed, only two reports have described resistance to the compound (5, 6). The usefulness of the compound was demonstrated recently by Baker et al. (7), who showed that the addition of NAP to a combination of chemicals from the three major drug groups increased efficacy against a population of H. contortus isolated from the field in New South Wales (NSW), Australia, from 40% to 100%. This role for organophosphate compounds in combination drenches to combat resistance to the other chemical groups has also been demonstrated in cattle and sheep in South America (8, 9).
As part of an effort to maintain the usefulness of NAP (that is, to reduce the rate at which resistance may develop), we were interested in developing molecular assay-based diagnostics that could be used to detect NAP resistance in worm populations. We were therefore interested in exploring the potential mechanisms by which H. contortus may develop resistance to NAP. There are several common mechanisms by which insects develop resistance to organophosphate insecticides: increased metabolism by cytochromes P450 (CYPs), glutathione transferases (GSTs), and esterases and target site insensitivity (insensitive acetyl cholinesterase) (10–13). One approach to study the role of enzymatic metabolism in drug detoxification, and hence the potential role in drug resistance, is to induce enzyme activities in organisms and then examine the consequences of this induction in terms of whether it equips the organism with an increased ability to tolerate the presence of a particular drug. Several early insecticide metabolism studies used the barbiturate phenobarbital (PHB) to induce detoxification enzymes in insects and then measured the ability of the insect to subsequently survive exposure to insecticides (14–16). In this way, a role for the induced detoxification enzyme systems in protecting the insects from a specific toxin was demonstrated. The potential usefulness of this induction approach was illustrated in studies with the sheep blowfly: the ability of PHB-treated blowfly larvae to tolerate higher concentrations of diflubenzuron (alongside increased CYP and GST enzyme activities) (17) was followed by measurements of elevated CYP activities in field strains showing tolerance to the compound (18). In this way, the ability of PHB-induced flies to tolerate insecticides simulated the effects of drug selection pressure acting to increase detoxification enzymes in drug-tolerant field strains of this species. PHB is a particularly important agent for the enzyme induction approach to the study of xenobiotic defensive mechanisms, as it is known to induce a number of drug-metabolizing enzymes. While most attention has focused on the induction of CYPs by PHB (19, 20), the compound is also known to induce other detoxification enzymes, including GSTs (21, 22) and UDP glucuronosyltransferases (UDPGTs) (23).
Given the previous demonstration of induction of CYP activity by PHB in H. contortus larvae (24) and the presence of GSTs and UDPGTs in this species (25, 26), which may be expected to be inducible with PHB, it was apparent that PHB induction may be a useful tool to determine whether these enzyme systems could play a role in the detoxification of NAP. The aim of the present study therefore was to examine the consequences of exposure to PHB on the ability of H. contortus larvae to tolerate NAP. In addition, we aimed to utilize chemical inhibitors targeting the principal enzyme groups inducible by PHB in order to indicate the detoxification enzyme systems that may be involved in any observed PHB-induced drug tolerance. Piperonyl butoxide (PBO) is a potent inhibitor of CYP (27) and hence is widely used to indicate the role of CYP enzymes in insecticide resistance (e.g., see reference 28). Diethyl maleate (DEM) can enzymatically conjugate reduced glutathione, thus depleting cells of this tripeptide and, hence, reducing the ability of GST enzymes to utilize the GSH in conjugation reactions with xenobiotics (29). DEM is therefore widely used in this context as a synergist for investigating the role of GST enzymes in insecticide resistance (e.g., see reference 30). Inhibitors of mammalian UDPGT activity include the pyrimidine derivatives 5-nitrouracil (5-NU) and 4,6-dihydroxy-5-nitropyrimidine (4,6-diOHNP) (31), as well as probenecid (32) and sulfinpyrazone (33).
MATERIALS AND METHODS
Chemicals and parasites.
Naphthalophos (NAP), sodium phenobarbital (PHB), 5-nitrouracil (5-NU), 4,6-dihydroxy-5-nitropyrimidine (4,6-diOHNP), probenecid, sulfinpyrazone, and diethyl maleate (DEM) were purchased from Sigma Chemical Co. (USA). Piperonyl butoxide (PBO) was purchased from ChemService (USA). PHB was prepared as a 25-mg/ml stock solution in water. The other compounds were prepared as stock solutions in dimethyl sulfoxide (DMSO): NAP at 10 mg/ml, 5-NU at 80 mg/ml, 4,6-diOHNP at 50 mg/ml, probenecid at 400 mg/ml, sulfinpyrazone at 400 mg/ml, DEM at 200 mg/ml, and PBO at 10 mg/ml. Each of these stock solutions was diluted serially 2-fold in DMSO to produce a series of working solutions.
The H. contortus organisms were from the drug-susceptible Kirby isolate, which was originally isolated from the field at the University of New England Kirby Research Farm in northern NSW in 1986 (34). This isolate is susceptible to all commercial anthelmintics.
Larval development assays.
Larval development assays were conducted using an agar-based 96-well format modified from the work of Gill et al. (35) as described by Kotze et al. (36). Initial experiments examined dose responses to the chemical synergists and PHB alone, in order to determine concentrations that had low toxicity and weretherefore suitable for use in subsequent experiments in combination with NAP. Subsequent dose response experiments with NAP were performed using duplicate assay wells across a range of 8 or 9 drug concentrations.
Assay plates were prepared by the addition of 2-μl aliquots of NAP and/or synergist solutions in DMSO to each well (DMSO only or synergist only in control wells) and overlaying this with 200 μl of 2% agar. After the contents of the plates had solidified, 10 μl of PHB was added to some wells (water was added to control wells) to give final concentrations of 1 mM, 2 mM, or 4 mM (after addition of eggs and nutrient solutions as described below). The plates were allowed to equilibrate at room temperature for several hours before addition of worm eggs. Nematode eggs were recovered from sheep feces by filtration and sucrose density centrifugation (35) and aliquoted into each well. Plates containing naphthalophos were sealed using 96-well plate sealers (Applied Biosystems), as described previously (37). The next morning, each well of the plate received 10 μl of a nutrient solution containing a fresh culture of E. coli as described by Kotze et al. (36). Fresh plate sealers were applied to the naphthalophos plates. The assays were terminated after 6 days by the addition of 10 μl Lugol's iodine to each well, and the numbers of L3 larvae were counted and compared to the numbers in control (no drug, no PhB, or no synergist) wells.
Control wells contained (i) DMSO, synergists, or PHB alone or (ii) combinations of synergist and PHB. Numbers of developed larvae in wells containing each concentration of NAP (with and without synergist, and with and without PHB) were expressed relative to controls containing the synergist alone or PHB alone (or both), as appropriate, in order for the dose response to represent a measure of the effect of NAP only. Three separate experiments were conducted with each drug-parasite combination.
In some experiments, eggs and larvae were exposed to 5-NU for 24 h prior to exposure to NAP. The preincubation period took place in 6-well plates containing 10-fold-greater volumes of all solutions described above for the 96-well plate assays. Egg solution and 5-NU (at various concentrations) were added, and the plates were incubated for 24 h at 28°C. The larvae were then collected from the wells using a pipette, and 20-μl aliquots of larvae were dispensed into the wells of 96-well plates containing NAP at a range of concentrations (identical to the plates used for nonpretreatment experiments) with and without 5-NU. Nutrient solution was added to each well. The plates were then incubated at 28°C for 5 days more, and larval development was scored as described above. Three separate experiments were performed.
Dose-response analysis.
The larval development data were analyzed using nonlinear regression with GraphPad Prism software (version 5.03) (GraphPad Software Inc., USA) in order to generate dose-response curves. The model used to fit the data was based on a normalized response (dose-response curve from 100% to 0%) and a variable slope. The 50% inhibitory concentrations (IC50s) were calculated for each separate experiment, and hence triplicate IC50s were generated for each drug-synergist-PHB combination from the three separate experiments. The triplicate IC50s were log transformed and analyzed using repeated-measures 1-way analysis of variance (ANOVA) (with pairing of IC50 data within the three separate experiments) in order to determine the effects of PHB and synergists on NAP dose responses. Significant differences were determined using Tukey's multiple comparison test (at a P value of 0.05).
Finally, data from each set of three replicate experiments were pooled and used to generate dose-response curves representing the full study data set. This pooling of data was for illustrative purposes only, not for statistical analysis.
Isobologram analysis.
Isobologram analysis was performed in order to define the interaction of NAP with 5-NU. The analysis was performed using CalcuSyn software (Biosoft, Cambridge, United Kingdom). The analysis is based on the median-effect principle and the median-effect equation, as described by Chou and Talalay (38). We used normalized rather than classical isobolograms, as our data consisted of combinations of one drug over a range of concentrations (NAP) with a second drug (5-NU) at a single concentration within each experiment. The analysis yielded combination index (CI) values for each separate drug combination data point. CI values describe the degree of synergism or antagonism as follows: CIs of <1, 1, and >1 represent synergism, additivity, and antagonism, respectively. Further, the degree of deviation from a value of 1 indicates the degree of synergism or antagonism: e.g., for synergism, a CI of 0.1 to 0.3 indicates strong synergism, a value of 0.3 to 0.7 indicates synergism, a value of 0.7 to 0.85 indicates moderate synergism, and a value of 0.85 to 0.90 indicates slight synergism. We calculated CI values for each experimental data point and then used frequency histograms to describe the distribution of CI values from the three replicate experiments for each NAP–5-NU combination at each PHB level. The interaction of the drugs was illustrated graphically by observing the position of experimental data points relative to the line of additivity on normalized isobolograms (above the line indicates antagonism, on the line indicates additive, and below the line indicates synergism).
RESULTS
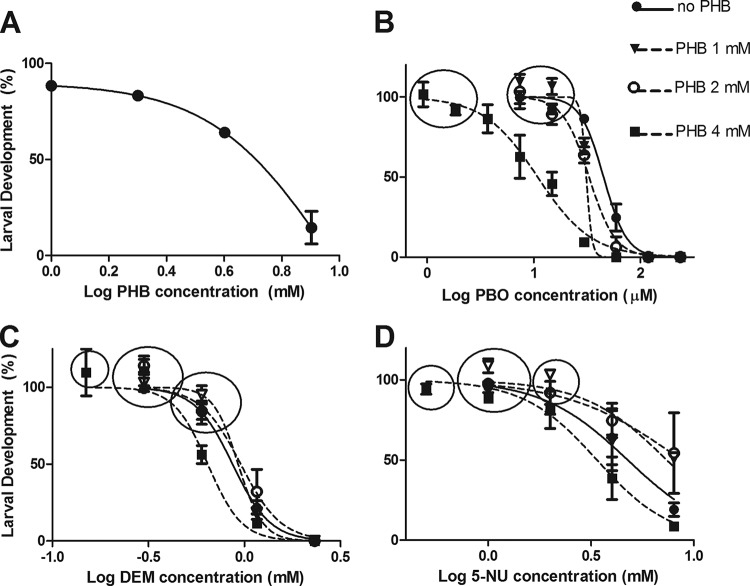
We initially performed dose-response experiments with PHB and all the synergists in order to identify concentrations suitable for use in induction/synergism experiments. Figure 1A shows that PHB had little effect on worm development (development > 85%) at concentrations of 1 and 2 mM, while development decreased to approximately 65% at 4 mM. These three concentrations were chosen for subsequent experiments. Figure 1B and C show the effects of PBO, 5-NU, and DEM on larval development in the absence of PHB and at PHB levels of 1 mM, 2 mM, and 4 mM (data for 4,6-diOHNP, probenecid, and sulfinpyrazone are not shown). The circled points in each panel of Fig. 1 show the concentrations selected for subsequent PHB-NAP-synergist experiments. In each case, the larvae exposed to 4 mM PHB were more susceptible to the chemical synergists. Hence, while subsequent experiments without PHB and with PHB at 1 mM or 2 mM could be conducted in the presence of synergists at the same concentrations, the 4 mM PHB experiments were conducted using PBO, 5-NU, and DEM concentrations either equivalent to or slightly lower than those used for the lower PHB concentrations (for 5-NU and DEM; from Fig. 1C and D) or significantly lower than used for 1 and 2 mM PHB (for PBO; from Fig. 1B).
FIG 1.
Effect of phenobarbital (PHB) and synergists on H. contortus larval development. (A) Effect of PHB alone; (B, C, and D) effects of piperonyl butoxide (PBO), diethyl maleate (DEM) and 5-nitrouracil (5-NU), respectively, in the absence or presence of PHB at three different concentrations, on H. contortus larval development. Circles (B, C, and D) indicate synergist concentrations selected for subsequent experiments examining interactions between PHB, synergists, and naphthalophos. Each data point shows the mean ± standard error (SE) (n = 6; pooled data from three separate experiments, each with duplicate assays at each PHB and synergist concentration).
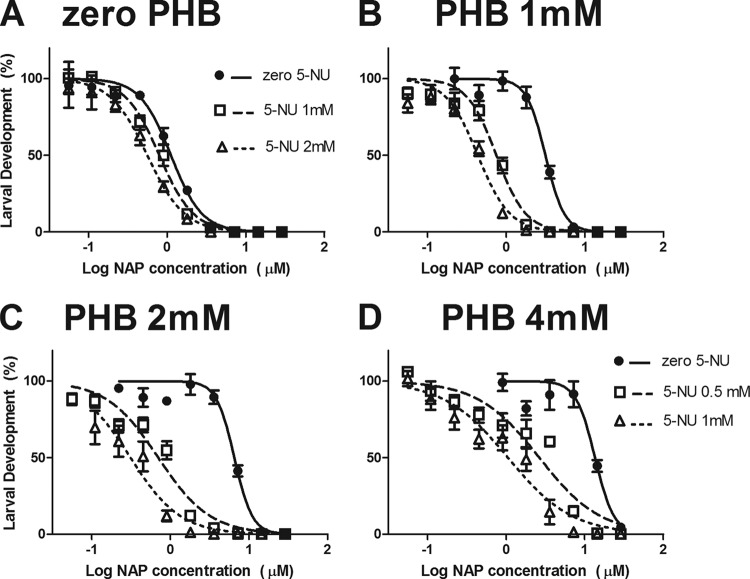
The effects of PHB on the response of larvae to NAP are shown in Fig. 2. The response was shifted to the right as the PHB concentration increased, with significant increases in NAP IC50 across each PHB level. NAP IC50s were increased 4-, 11-, and 28-fold at PHB concentrations of 1, 2, and 4 mM, respectively. Larvae exposed to PHB were clearly less sensitive to the anthelmintic effects of NAP.
FIG 2.
Effect of phenobarbital (PHB) on the sensitivity of H. contortus larvae to naphthalophos (NAP). Each data point is the mean ± SE (n = 6; pooled data from three separate experiments, each with duplicate assays at each NAP concentration).
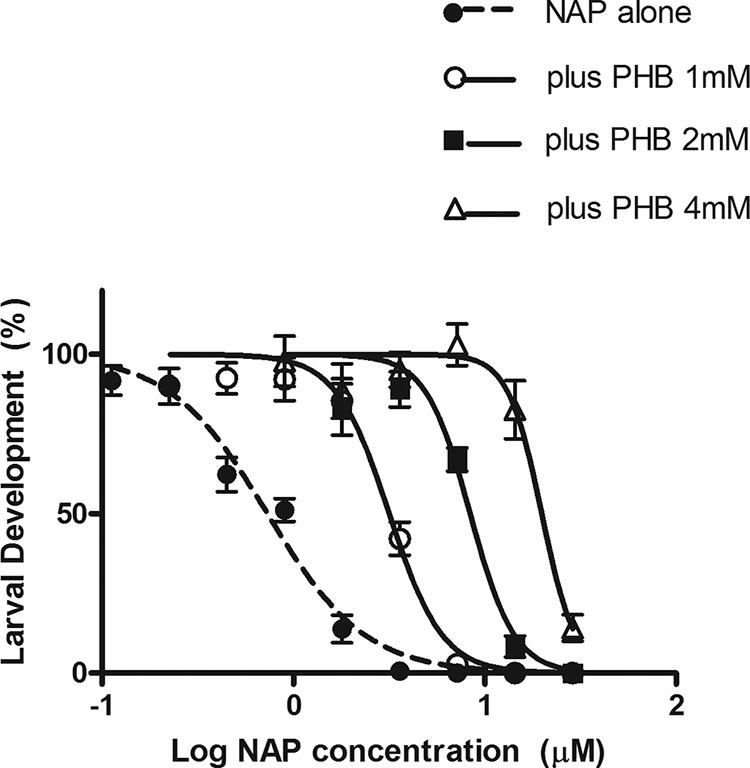
Having demonstrated that PHB-exposed worms were more tolerant of NAP, we next wanted to use chemical synergists to investigate which enzymatic pathways may be involved in the observed tolerance. Dose responses of worms exposed to NAP alone (a range of concentrations), to NAP plus PHB, or to NAP plus PHB plus a synergist are presented, with IC50s and inhibition ratios, in Table 1, while responses for 5-NU are also shown in Fig. 3. As expected from Fig. 1, the dose responses to NAP were shifted to the right as the PHB level increased in the absence of any synergist, as shown by increasing IC50s in the presence of PHB alone (Table 1). Synergistic effects of 5-NU are represented graphically in Fig. 3 by the movement of the dose response back toward, or to the left of, the zero-PHB response, that is, in the ability of 5-NU to reverse the NAP tolerance derived from PHB exposure. In numerical terms, this is described by decreases in NAP IC50s in the presence of 5-NU to levels approaching, or less than, the NAP IC50 of worms in the absence of any additive (no PHB, no 5-NU) (Table 1). The extent of the shift in IC50 in the presence of synergist is described in Table 1, with synergism ratios at each PHB level. The addition of 5-NU to assays resulted in significant reductions in IC50 at each PHB level, with synergism ratios up to 18.2-fold. The dose response of worms in the presence of PHB and 5-NU was similar to that of the worms not exposed to PHB; that is, the 5-NU was able to completely reverse the PHB-induced drug tolerance. Importantly, 5-NU also caused a shift in the dose response in worms not exposed to PHB (Fig. 3A). A concentration of 2 mM 5-NU resulted in a 2-fold decrease in the IC50s of these larvae (Table 1).
TABLE 1.
Effect of co-treatment with phenobarbital and synergists on the sensitivity of H. contortus larvae to naphthalophos
| Synergist | PHB concn (mM) | Synergist concn (mM or μM)a | Naphthalophos IC50 (μM)b | Synergism ratioc |
|---|---|---|---|---|
| 5-NU | 0 | 0 | 1.16 ± 0.07 A | |
| 1 | 0.85 ± 0.11 AB | 1.4 | ||
| 2 | 0.57 ± 0.07 B | 2.0 | ||
| 1 | 0 | 3.17 ± 0.33 A | ||
| 1 | 0.74 ± 0.06 B | 4.3 | ||
| 2 | 0.44 ± 0.07 B | 7.2 | ||
| 2 | 0 | 6.56 ± 0.40 A | ||
| 1 | 0.75 ± 0.14 B | 8.8 | ||
| 2 | 0.36 ± 0.16 B | 18.2 | ||
| 4 | 0 | 12.88 ± 1.94 A | ||
| 0.5 | 3.02 ± 0.89 B | 4.3 | ||
| 1 | 1.16 ± 0.26 B | 11.1 | ||
| PBO | 0 | 0 | 0.73 ± 0.03 A | |
| 7.5 | 0.67 ± 0.05 A | 1.1 | ||
| 15 | 0.64 ± 0.08 A | 1.1 | ||
| 1 | 0 | 2.81 ± 0.79 A | ||
| 7.5 | 1.61 ± 0.02 AB | 1.7 | ||
| 15 | 0.94 ± 0.08 B | 3.0 | ||
| 2 | 0 | 5.62 ± 1.90 A | ||
| 7.5 | 3.19 ± 0.51 AB | 1.8 | ||
| 15 | 2.47 ± 0.33 B | 2.3 | ||
| 4 | 0 | 12.39 ± 5.35 A | ||
| 1.0 | 11.92 ± 4.38 A | 1.0 | ||
| 2.0 | 10.14 ± 4.30 A | 1.2 | ||
| DEM | 0 | 0 | 0.51 ± 0.03 A | |
| 0.15 | 0.48 ± 0.03 A | 1.1 | ||
| 0.30 | 0.50 ± 0.04 A | 1.0 | ||
| 0.60 | 0.50 ± 0.02 A | 1.0 | ||
| 1 | 0 | 1.26 ± 0.10 A | ||
| 0.30 | 1.31 ± 0.13 A | 1.0 | ||
| 0.60 | 1.24 ± 0.08 A | 1.0 | ||
| 2 | 0 | 2.39 ± 0.34 A | ||
| 0.30 | 2.67 ± 0.27 A | 0.9 | ||
| 0.60 | 2.51 ± 0.33 A | 0.9 | ||
| 4 | 0 | 6.25 ± 1.26 A | ||
| 0.15 | 6.11 ± 0.88 A | 1.0 | ||
| 0.30 | 7.50 ± 0.84 A | 0.8 |
5-NU and DEM concentrations are in mM units; PBO concentrations are in μM units.
Values are means ± SE (n = 3) from separate experiments with duplicate assay wells at each of a range of naphthalophos concentrations. Within a synergist, and within a PHB concentration level, naphthalophos IC50s followed by the same letter are not significantly different (P = 0.05).
Naphthalophos IC50 in the absence of synergist/naphthalophos IC50 in the presence of synergist. The ratio was calculated separately for each PHB concentration level.
FIG 3.
Effect of 5-nitrouracil (5-NU) on the sensitivity of H. contortus larvae to naphthalophos (NAP). Larvae were treated with 5-NU (0.5, 1, or 2 mM) in the absence of phenobarbital (PHB) or in the presence of PHB at three concentrations (1, 2 or 4 mM). (A) No PHB; (B, C, and D) PHB at various concentrations. The key in panel A also applies to panels B and C. Each data point is the mean ± SE (n = 6; pooled data from three separate experiments, each with duplicate assays at each NAP concentration).
PBO also had some effects in partially reversing the PHB-induced NAP tolerance; however, these effects were not as great as those seen with 5-NU (Table 1). Synergism ratios of up to 3-fold were observed with PBO. No synergism was observed at the highest PHB level of 4 mM; however, the PBO concentrations used here were lower than those used with 1 mM and 2 mM PHB (from Fig. 1B). PBO did not have any synergistic effect with worms that had not been exposed to PHB (Table 1). No synergism was observed with DEM at any of the PHB levels (Table 1).
The other UDPGT inhibitors, 4,6-diOHNP, probenecid, and sulfinpyrazone, also showed an ability to synergize the toxicity of NAP (Table 2). The former two compounds had significant effects on the toxicity of NAP to worms that had not been exposed to PHB, while all three compounds had significant effects on the NAP IC50 for worms exposed to 4 mM PHB.
TABLE 2.
Effect of cotreatment with phenobarbital and synergists on the sensitivity of H. contortus larvae to naphthalophos
| PHB concn (mM) | Synergist (concn [mM]) | Naphthalophos IC50 (μM)a | Synergism ratiob |
|---|---|---|---|
| 0 | None | 0.96 ± 0.13 A | |
| 4,6-Di-OHNP (0.64) | 0.46 ± 0.14 B | 2.1 | |
| Probenecid (1.4) | 0.41 ± 0.02 B | 2.3 | |
| Sulfinpyrazone (1.0) | 0.56 ± 0.01 AB | 1.7 | |
| 4 | None | 9.76 ± 2.78 A | |
| 4,6-diOHNP (0.64) | 2.76 ± 0.25 B | 3.5 | |
| Probenecid (1.4) | 1.80 ± 0.36 B | 5.4 | |
| Sulfinpyrazone (1.0) | 1.72 ± 0.13 B | 5.7 |
Values are means ± SE (n = 3) from separate experiments with duplicate assay wells at each of a range of naphthalophos concentrations. Within a PHB concentration level, naphthalophos IC50s followed by the same letter are not significantly different (P = 0.05).
Naphthalophos IC50 in the absence of synergist/naphthalophos IC50 in the presence of synergist.
The interactions of NAP and 5-NU at each level of PHB were analyzed using normalized isobolograms. The interaction of the two compounds was described by this analysis in terms of the combination index (CI) values for each separate combination of the 2 drugs. Hence, as an example, dose-response experiments consisting of 6 data points (5-NU at a constant concentration, at 6 separate NAP concentrations) resulted in the calculation of 6 separate CI values for that experiment. Figure 4 shows a frequency histogram describing the numbers of separate data points (derived from three separate experiments) whose CI values fell within specific ranges indicative of different degrees of synergism or antagonism. In the absence of PHB, many data points showed an absence of synergism; however, there were also a number of points showing the presence of synergism (CI < 0.90). Several data points showed slight antagonism. For each PHB level, the histograms were dominated by CI values indicating the presence of synergism (CI, 0.3 to 0.7) or strong synergism (0.1 to 0.3). Representative normalized isobolograms for single dose-response experiments are shown in Fig. 5 to further illustrate the interaction between the two compounds. Figure 5A shows the presence and absence of synergism in separate data points derived from an experiment in the absence of PHB, while Fig. 5B shows the strong synergism evident in an experiment at 4 mM PHB.
FIG 4.
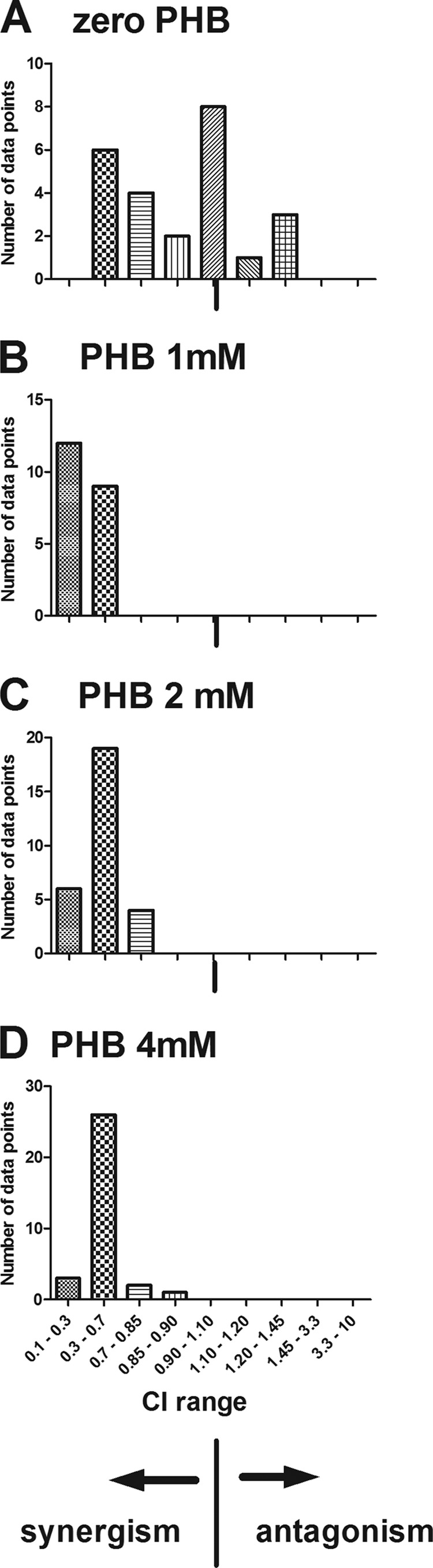
Frequency histograms of combination index (CI) values derived from normalized isobolograms describing the interaction of naphthalophos and 5-nitrouracil in the absence and presence of phenobarbital (PHB) at three concentrations (1, 2, and 4 mM).
FIG 5.
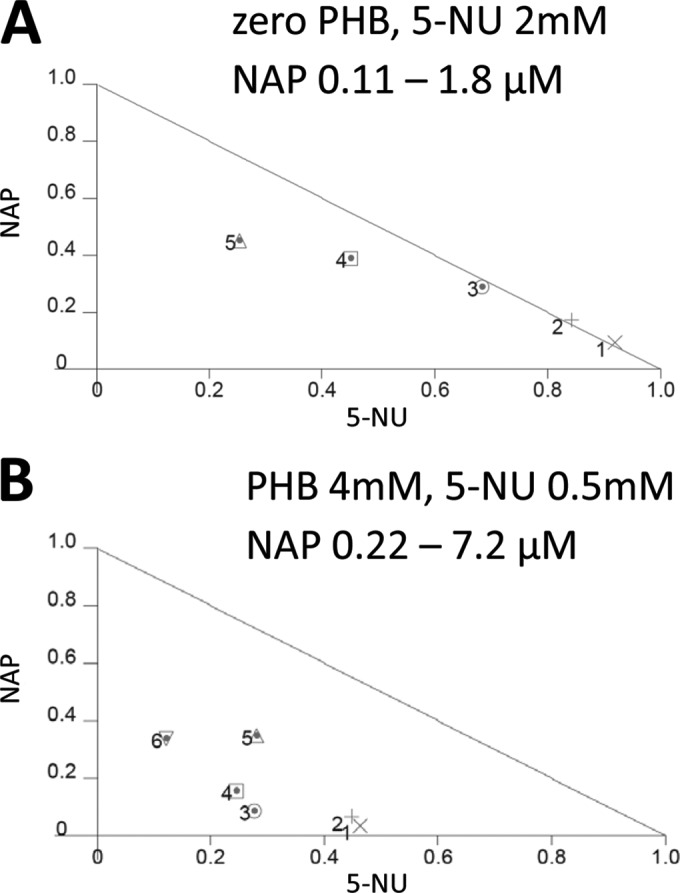
Representative normalized isobolograms generated by CalcuSyn software describing interactions of naphthalophos (NAP) and 5-nitrouracil (5-NU). (A) Data from an experiment in the absence of PHB, with 5 separate concentrations of NAP and with 5-NU constant at 2 mM. (B) Data from an experiment in the presence of 4 mM PHB, with 6 separate concentrations of NAP and with 5-NU constant at 0.5 mM. The diagonal line is the line of additivity. Experimental data points on, above, and below the line represent additivity, antagonism, and synergism, respectively.
Finally, we were interested in whether the synergistic effects observed with 5-NU and NAP in the absence of PHB (from Fig. 3A and Table 1) could be increased if the worms were exposed to the synergist for a period prior to exposure to NAP. Table 3 shows that preexposure to 5-NU concentrations of 0.5 mM, 1 mM, and 2 mM resulted in significant decreases in the NAP IC50 compared to values obtained with worms not exposed to 5-NU. ANOVA comparing IC50s from nonpretreated larvae (from Table 1) and pretreated larvae (from Table 3) in the absence of PHB showed that pretreatment significantly (P = 0.05) increased the synergistic effects for 1 mM 5-NU (synergism ratio of 1.4 compared to 3.2); however, pretreatment had no effect at 2 mM 5-NU (ratio of 2.0 compared to 2.9).
TABLE 3.
Effect of pretreatment with 5-nitrouracil on the sensitivity of H. contortus larvae to naphthalophos
| 5-NU concn (mM) | Naphthalophos IC50 (μM)a | Synergism ratiob |
|---|---|---|
| 0 | 0.98 ± 0.14 A | |
| 0.125 | 0.72 ± 0.05 AB | 1.4 |
| 0.25 | 0.72 ± 0.06 AB | 1.4 |
| 0.5 | 0.49 ± 0.20 B | 2.0 |
| 1.0 | 0.31 ± 0.07 B | 3.2 |
| 2.0 | 0.34 ± 0.04 B | 2.9 |
Values are means ± SE (n = 3) from separate experiments with triplicate assay wells at each of a range of naphthalophos concentrations. Naphthalophos IC50s followed by the same letter are not significantly different (P = 0.05).
Naphthalophos IC50 in the absence of 5-NU/naphthalophos IC50 in the presence of 5-NU.
DISCUSSION
The present study shows that treatment of H. contortus larvae with PHB confers a significant degree of tolerance to the anthelmintic drug NAP. This suggests that the induction of xenobiotic detoxification enzymes by PHB equips the larvae with an ability to detoxify significantly greater amounts of the anthelmintic drug than noninduced worms. The effects of 5-NU, 4,6-diOHNP, probenecid, and sulfinpyrazone in reversing the PHB-induced NAP tolerance indicates that the observed tolerance is largely due to the activity of UDPGT enzymes within the nematode.
PBO was also able to partially reverse the PHB-induced drug tolerance. However, this needs to be interpreted with caution given that 5-NU was able to completely reverse the PHB-induced drug tolerance to levels measured in non-PHB-treated worms. That is, it is difficult to reconcile the ideas that an inhibitor of one enzyme pathway could cause complete reversion of induced tolerance and that an inhibitor of an alternative pathway could also cause some reversion. The issue of the specificity of the enzyme inhibitors is most likely important here. The only xenobiotic metabolism pathway reported to be inhibited by 5-NU in the scientific literature, as far as we are aware, is conjugation mediated by UDPGTs. On the other hand, while PBO is most often linked to acting as a synergist through the inhibition of CYPs, it has also been reported to inhibit mammalian UDPGT enzyme activity (39), as well as synergizing insecticides through inhibition of esterase enzymes (40). Hence, given the effectiveness of 5-NU in completely reversing the PHB-induced NAP tolerance, it is most likely that the synergism observed with PBO in the present study is due to the effect of the compound on worm UDPGT enzymes rather than CYPs. GST enzymes appear to play no role in NAP detoxification in H. contortus larvae.
5-NU, 4,6-diOHNP, and probenecid were able to synergize NAP in the absence of any PHB treatment. This highlights the potential of a chemotherapeutic approach utilizing UDPGT inhibitors as synergists to increase the activity of NAP against parasitic nematodes. As indicated by our induction/synergism experiments, this strategy would become even more useful if UDPGT-mediated metabolism should emerge as a resistance mechanism in field populations. The ability to synergize the action of NAP against worms also raises the possibility of being able to maintain anthelmintic efficacy while reducing the concentration of NAP itself in commercial drench products. This potential ability to utilize lower concentrations of NAP may ease concerns over toxicity and environmental residues associated with the use of organophosphate compounds.
Little is known about the role of enzymatic detoxification systems in anthelmintic efficacy and resistance in parasitic nematodes. While the recent H. contortus genome paper by Laing et al. (26) showed that this species possesses 42 CYP genes, only a limited number of studies have actually reported the presence of CYP-mediated enzyme activities in parasitic worms (24, 41). There is, however, some evidence that these enzymes may play a direct role in drug detoxification in H. contortus: Alvinerie et al. (42) detected a metabolite produced from moxidectin by adult worms, and the inhibition of the reaction by carbon monoxide suggested the involvement of CYP, while Kotze et al. (43) showed that worm larvae and adults treated in vitro with PBO were more susceptible to rotenone, thereby implicating CYP enzymes in detoxification of the compound. Glutathione transferase enzymes have been known to be present in parasitic helminths (cestodes, trematodes, and nematodes) for many years (44, 45). The enzymes are known to act in detoxifying xenobiotics by two pathways: conjugation with glutathione and the binding of the enzyme itself to the xenobiotic. However, while they are known to be involved in the detoxification of some anthelmintics (e.g., dichlorvos [46]) and to function in detoxification of endogenous products of lipid peroxidation and in binding various endogenous ligands (e.g., bile acids and steroids), they have been linked to drug resistance in only a single study, which found slightly increased activities in an isolate of H. contortus showing resistance to cambendazole (25). UDPGT enzymes are also known to exist in nematodes, with the H. contortus genome possessing 34 UDPGT genes (26) and C. elegans known to possess 72 genes (47). While there are some reports of conjugation of glucose to benzimidazole anthelmintics in C. elegans (48) and H. contortus (49, 50), there have, to our knowledge, been no reports of the conjugation of glucuronic acid, mediated by UDPGT enzymes, as a pathway of xenobiotic detoxification in parasitic worms prior to the present study.
While the present study suggests an important role for UDPGT enzymes in detoxification of NAP, the results represent an examination of only a component of the detoxification enzyme armory of the worm. It is known from mammalian studies that the PHB induces only a subset of the many enzymes within the major detoxification enzyme groups (20, 23). The H. contortus genome study by Laing et al. (26) revealed that this species possesses 42, 34, and 28 CYP, UDPGT, and GST genes, respectively. It is likely that PHB induces only a subset of the detoxification enzymes within these three groups. Hence, while induced NAP tolerance in the present study indicates that H. contortus UDPGTs are able to detoxify NAP, a lack of tolerance is not a definitive indication of the absence of an enzyme able to detoxify the anthelmintic. It remains possible that H. contortus larvae possess non-PHB-inducible CYPs and GSTs able to detoxify the drug and also have other non-PHB-inducible UDPGTs able to contribute to NAP detoxification. However, the failure of PBO or DEM to synergize NAP in the non-PHB-treated worms suggests that any such metabolism by CYPs and GSTs occurs at only a very low level. The sole ability of the UDPGT inhibitors to synergize NAP in non-PHB-treated worms indicates the primary role for UDPGT enzymes in “normal” (i.e., noninduced) worms.
Given the protective role of UDPGTs in PHB-exposed worm larvae, the question arises as to the potential role that this enzyme system could play in anthelmintic resistance. That is, could the level of UDPGT-mediated NAP-detoxification demonstrated in noninduced worms in the present study be increased following drug selection pressure in the field to provide the type of protection for the worm against the drug seen following PHB exposure? Several factors will impact the relationship between the present study and the issue of resistance to NAP in gastrointestinal nematodes. First, the experiments conducted here utilized the free-living larval life stage of the parasite, whereas resistance in gastrointestinal nematodes is of importance only when it occurs in parasitic late-larval and adult worm stages targeted by chemical drench treatments. The relationship between UDPGT levels in free-living larval stages and parasitic stages of gastrointestinal worms is unknown. Second, there is a distinct difference between temporary enzyme induction in response to a barbiturate and drug selection pressure over multiple generations leading to resistance. While induction in this study suggests that H. contortus possesses an enzyme system capable of metabolizing NAP, there is no evidence that selection pressure with NAP in the field would lead to increased expression of UDPGTs as a resistance mechanism. Only the potential for such enzymes to play a role has been illustrated. It is apparent from the present study that detoxification by nematode UDPGT enzymes should be considered a potential resistance mechanism applicable to NAP. Further work should be aimed at developing sensitive biochemical or molecular tests to allow UDPGT enzyme activities/gene expression levels to be measured in worm isolates suspected of being resistant to the drug.
Footnotes
Published ahead of print 6 October 2014
REFERENCES
- 1.Kaplan RM. 2004. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 20:477–481. 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Sutherland IA, Leathwick DM. 2011. Anthelmintic resistance in nematode parasites of cattle: a global issue? Trends Parasitol. 27:176–181. 10.1016/j.pt.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Besier RB, Love SCJ. 2003. Anthelmintic resistance in sheep nematodes in Australia: the need for new approaches. Aus. J. Exp. Agric. 43:1383–1391. 10.1071/EA02229. [DOI] [Google Scholar]
- 4.Prichard RK. 1978. Sheep anthelmintics, p 75–107 In Donald AD, Southcott WH, Dineen JK. (ed), The epidemiology and control of gastrointestinal parasites of sheep in Australia. Academic Press, Sydney, Australia. [Google Scholar]
- 5.Green PE, Forsyth BA, Rowan KJ, Payne G. 1981. The isolation of a field strain of Haemonchus contortus in Queensland showing multiple anthelmintic resistance. Aust. Vet. J. 57:79–84. 10.1111/j.1751-0813.1981.tb00451.x. [DOI] [PubMed] [Google Scholar]
- 6.Le Jambre LF, Geoghegan J, Lyndal-Murphy M. 2005. Characterization of moxidectin resistant Trichostrongylus colubriformis and Haemonchus contortus. Vet. Parasitol. 128:83–90. 10.1016/j.vetpar.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Baker KE, George SD, Stein PA, Seewald W, Rolfe PF, Hosking BC. 2012. Efficacy of monepantel and anthelmintic combinations against multiple-resistant Haemonchus contortus in sheep, including characterisation of the nematode isolate. Vet. Parasitol. 186:513–517. 10.1016/j.vetpar.2011.11.060. [DOI] [PubMed] [Google Scholar]
- 8.Fiel C, Guzmán M, Steffan P, Prieto O, Bhushan C. 2011. Comparative efficacy of trichlorphon and trichlorphon/ivermectin combination treatment against anthelmintic-resistant cattle nematodes in Argentina. Parasitol. Res. 109(Suppl 1):S105–S112. 10.1007/s00436-011-2407-3. [DOI] [PubMed] [Google Scholar]
- 9.Fiel C, Guzmán M, Steffan P, Rodriguez E, Prieto O, Bhushan C. 2011. The efficacy of trichlorphon and naphthalophos against multiple anthelmintic-resistant nematodes of naturally infected sheep in Argentina. Parasitol. Res. 109(Suppl 1):S139–S148. 10.1007/s00436-011-2410-8. [DOI] [PubMed] [Google Scholar]
- 10.Dauterman WC. 1983. Role of hydrolases and glutathione S-transferases in insecticide resistance, p 229–247 In Georghiou GP, Saito T. (ed), Pest resistance to pesticides. Plenum Press, New York, NY. [Google Scholar]
- 11.Kulkarni AP, Hodgson E. 1984. The metabolism of insecticides: the role of monooxygenase enzymes. Annu. Rev. Pharmacol. Toxicol. 24:19–42. 10.1146/annurev.pa.24.040184.000315. [DOI] [PubMed] [Google Scholar]
- 12.Oppenoorth FJ. 1985. Biochemistry and genetics of insecticide resistance, p 731–770 In Kerkut GA, Gilbert LJ. (ed), Comprehensive insect physiology, biochemistry and pharmacology, vol. 12 Insect control. Pergamon, New York, NY. [Google Scholar]
- 13.Scott JG. 1999. Cytochromes P450 and insecticide resistance. Insect Biochem. Mol. Biol. 29:757–777. 10.1016/S0965-1748(99)00038-7. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad N, Brindley WA. 1969. Modification of parathion toxicity to wax moth larvae by chlorcyclizine, aminopyrine or phenobarbital. Toxicol. Appl. Pharmacol. 15:433–440. 10.1016/0041-008X(69)90041-6. [DOI] [PubMed] [Google Scholar]
- 15.Yu SJ, Teriere LC. 1973. Phenobarbital induction of detoxifying enzymes in resistant and susceptible houseflies. Pestic. Biochem. Physiol. 3:141–148. 10.1016/0048-3575(73)90098-9. [DOI] [Google Scholar]
- 16.Hayaoka T, Dauterman WC. 1982. Induction of glutathione S-transferase by phenobarbital and pesticides in various housefly strains and its effect on toxicity. Pestic. Biochem. Physiol. 17:113–119. 10.1016/0048-3575(82)90015-3. [DOI] [Google Scholar]
- 17.Kotze AC. 1995. Induced insecticide tolerance in larvae of Lucilia cuprina (Wiedemann) (Diptera: Calliphoridea) following dietary phenobarbital treatment. J. Aust. Ent. Soc. 34:205–209. 10.1111/j.1440-6055.1995.tb01319.x. [DOI] [Google Scholar]
- 18.Kotze AC, Sales N, Barchia IM. 1997. Diflubenzuron tolerance associated with monooxygenase activity in field strain larvae of the Australian sheep blowfly (Diptera:Calliphoridae). J. Econ. Entomol. 90:15–20. [DOI] [PubMed] [Google Scholar]
- 19.Bresnick E, Foldes R, Hines RN. 1984. Induction of cytochrome P450 by xenobiotics. Pharmacol. Rev. 36(2 Suppl):43S–51S. [PubMed] [Google Scholar]
- 20.Waxman DJ, Azaroff L. 1992. Phenobarbital induction of cytochrome P-450 gene expression. Biochem. J. 281:577–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terriere LC. 1984. Induction of detoxification enzymes in insects. Annu. Rev. Entomol. 29:71–88. 10.1146/annurev.en.29.010184.000443. [DOI] [PubMed] [Google Scholar]
- 22.Mannervik B. 1985. The isoenzymes of glutathione transferase. Adv. Enzymol. 57:357–417. [DOI] [PubMed] [Google Scholar]
- 23.Bock KW, Lipp HP, Bock-Henning BS. 1990. Induction of drug-metabolizing enzymes by xenobiotics. Xenobiotica 20:1101–1111. 10.3109/00498259009046831. [DOI] [PubMed] [Google Scholar]
- 24.Kotze AC. 1997. Cytochrome P450 monooxygenase activity in Haemonchus contortus (Nematoda). Int. J. Parasitol. 27:33–40. 10.1016/S0020-7519(96)00161-0. [DOI] [PubMed] [Google Scholar]
- 25.Kawalek JC, Rew RS, Heavner J. 1984. Glutathione-S-transferase, a possible drug-metabolizing enzyme, in Haemonchus contortus: comparative activity of a cambendazole-resistant and a susceptible strain. Int. J. Parasitol. 14:173–175. 10.1016/0020-7519(84)90045-6. [DOI] [PubMed] [Google Scholar]
- 26.Laing R, Kikuchi T, Martinelli A, Tsai IJ, Beech RN, Redman E, Holroyd N, Bartley DJ, Beasley H, Britton C, Curran D, Devaney E, Gilabert A, Hunt M, Jackson F, Johnston SL, Kryukov I, Li K, Morrison AA, Reid AJ, Sargison N, Saunders GI, Wasmuth JD, Wolstenholme A, Berriman M, Gilleard JS, Cotton JA. 2013. The genome and transcriptome of Haemonchus contortus, a key model parasite for drug and vaccine discovery. Genome Biol. 14:R88. 10.1186/gb-2013-14-8-r88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodgson E, Levi PE. 1998. Interactions of piperonyl butoxide with cytochrome P450, p 41–54 In Jones DG. (ed), Piperonyl butoxide. Academic Press, London, United Kingdom. [Google Scholar]
- 28.Romero A, Potter MF, Haynes KF. 2009. Evaluation of piperonyl butoxide as a deltamethrin synergist for pyrethroid-resistant bed bugs. J. Econ. Entomol. 102:2310–2315. 10.1603/029.102.0637. [DOI] [PubMed] [Google Scholar]
- 29.Plummer JL, Smith BR, Sies H, Bend JR. 1981. Chemical depletion of glutathione in vivo. Methods Enzymol. 77:50–59. 10.1016/S0076-6879(81)77010-1. [DOI] [PubMed] [Google Scholar]
- 30.Ma W, Li X, Dennehy TJ, Lei C, Wang M, Degain BA, Nichols RL. 2010. Pyriproxyfen resistance of Bemisia tabaci (Homoptera: Aleyrodidae) biotype B: metabolic mechanism. J. Econ. Entomol. 103:158–165. 10.1603/EC09122. [DOI] [PubMed] [Google Scholar]
- 31.Naydenova Z, Grancharov K, Shopova M, Golovinsky E. 1995. Inhibition of UDP-glucuronyltransferase activity in rat liver microsomes by pyrimidine derivatives. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 112:321–325. 10.1016/0742-8413(95)02027-6. [DOI] [PubMed] [Google Scholar]
- 32.Uchaipichat V, Mackenzie PI, Guo XH, Gardner-Stephen D, Galetin A, Houston JB, Miners JO. 2004. Human UDP-glucuronosyltransferases: isoform selectivity and kinetics of 4-methylumbelliferone and 1-naphthol glucuronidation, effects of organic solvents, and inhibition by diclofenac and probenecid. Drug Metab. Dispos. 32:413–423. 10.1124/dmd.32.4.413. [DOI] [PubMed] [Google Scholar]
- 33.Uchaipichat V, Mackenzie PI, Elliot DJ, Miners JO. 2006. Selectivity of substrate (trifluoperazine) and inhibitor (amitriptyline, androsterone, canrenoic acid, hecogenin, phenylbutazone, quinidine, quinine, and sulfinpyrazone) “probes” for human UDP-glucuronosyltransferases. Drug Metab. Dispos. 34:449–456. 10.1124/dmd.105.007369. [DOI] [PubMed] [Google Scholar]
- 34.Albers GAA, Burgess SK. 1988. Serial passage of Haemonchus contortus in resistant and susceptible sheep. Vet. Parasitol. 28:303–306. 10.1016/0304-4017(88)90077-5. [DOI] [PubMed] [Google Scholar]
- 35.Gill JH, Redwin JM, van Wyk JA, Lacey E. 1995. Avermectin inhibition of larval development in Haemonchus contortus—effects of ivermectin resistance. Int. J. Parasitol. 25:463–470. 10.1016/0020-7519(94)00087-5. [DOI] [PubMed] [Google Scholar]
- 36.Kotze AC, O'Grady J, Emms J, Toovey AF, Hughes S, Jessop P, Bennell M, Vercoe PE, Revell DK. 2009. Exploring the anthelmintic properties of Australian native shrubs with respect to their potential role in livestock grazing systems. Parasitol. 136:1065–1080. 10.1017/S0031182009006386. [DOI] [PubMed] [Google Scholar]
- 37.Kotze AC, Stein PA, Dobson RJ. 1999. Investigation of intestinal nematode responses to naphthalophos and pyrantel using a larval development assay. Int. J. Parasitol. 29:1093–1099. 10.1016/S0020-7519(99)00064-8. [DOI] [PubMed] [Google Scholar]
- 38.Chou TC, Talalay P. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22:27–55. 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 39.Lucier GW, McDaniel OS, Matthews HB. 1971. Microsomal rat liver UDP glucuronyltransferase: effects of piperonyl butoxide and other factors on enzyme activity. Arch. Biochem. Biophys. 145:520–530. 10.1016/S0003-9861(71)80012-7. [DOI] [PubMed] [Google Scholar]
- 40.Young SJ, Gunning RV, Moores GD. 2005. The effect of piperonyl butoxide on pyrethroid-resistance-associated esterases in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Pest Manag. Sci. 61:397–401. 10.1002/ps.996. [DOI] [PubMed] [Google Scholar]
- 41.Kerboeuf D, Soubieux D, Guilluy R, Brazier JL, Rivière JL. 1995. In vivo metabolism of aminopyrine by the larvae of the helminth Heligmosomoides polygyrus. Parasitol. Res. 81:302–304. 10.1007/BF00931534. [DOI] [PubMed] [Google Scholar]
- 42.Alvinerie M, Dupuy J, Eeckhoutte C, Sutra JF, Kerboeuf D. 2001. In vitro metabolism of moxidectin in Haemonchus contortus adult stages. Parasitol. Res. 87:702–704. 10.1007/s004360100408. [DOI] [PubMed] [Google Scholar]
- 43.Kotze AC, Dobson RJ, Chandler D. 2006. Synergism of rotenone by piperonyl butoxide in Haemonchus contortus and Trichostrongylus colubriformis in vitro: potential for drug-synergism through inhibition of nematode oxidative detoxification pathways. Vet. Parasitol. 136:275–282. 10.1016/j.vetpar.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Brophy PM, Barrett J. 1990. Glutathione transferase in helminths. Parasitol. 100:345–349. 10.1017/S0031182000061369. [DOI] [PubMed] [Google Scholar]
- 45.Brophy PM, Pritchard DI. 1994. Parasitic helminth glutathione S-transferases: an update on their potential as targets for immuno- and chemotherapy. Exp. Parasitol. 79:89–96. 10.1006/expr.1994.1067. [DOI] [PubMed] [Google Scholar]
- 46.O'Leary KA, Tracy JW. 1991. Schistosoma mansoni: glutathione S-transferase-catalyzed detoxication of dichlorvos. Exp. Parasitol. 72:355–361. 10.1016/0014-4894(91)90081-7. [DOI] [PubMed] [Google Scholar]
- 47.Lindblom TH, Dodd AK. 2006. Xenobiotic detoxification in the nematode Caenorhabditis elegans. J. Exp. Zool. A Comp. Exp. Biol. 305:720–730. 10.1002/jez.a.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laing ST, Ivens A, Laing R, Ravikumar S, Butler V, Woods DJ, Gilleard JS. 2010. Characterization of the xenobiotic response of Caenorhabditis elegans to the anthelmintic drug albendazole and the identification of novel drug glucoside metabolites. Biochem. J. 432:505–514. 10.1042/BJ20101346. [DOI] [PubMed] [Google Scholar]
- 49.Vokřál I, Bártíková H, Prchal L, Stuchlíková L, Skálová L, Szotáková B, Lamka J, Várady M, Kubíček V. 2012. The metabolism of flubendazole and the activities of selected biotransformation enzymes in Haemonchus contortus strains susceptible and resistant to anthelmintics. Parasitology 139:1309–1316. 10.1017/S0031182012000595. [DOI] [PubMed] [Google Scholar]
- 50.Vokřál I, Jirásko R, Stuchlíková L, Bártíková H, Szotáková B, Lamka J, Várady M, Skálová L. 2013. Biotransformation of albendazole and activities of selected detoxification enzymes in Haemonchus contortus strains susceptible and resistant to anthelmintics. Vet. Parasitol. 196:373–381. 10.1016/j.vetpar.2013.03.018. [DOI] [PubMed] [Google Scholar]