Abstract
Background:
Lithium, a treatment for bipolar disorder, is not clinically indicated for use in fracture management but has been reported to positively influence bone biology. It is hypothesized that lithium dosing for beneficial effects on bone health may be much lower than the dosing required for psychotropic benefits in patients with bipolar disorder. A preclinical study with a rodent fracture model was utilized to best define the lowest effective dose, best timing of treatment onset, and optimal treatment duration for the use of lithium as a new treatment in fracture care.
Methods:
A design-of-experiments approach was used to assess the parameters of dose, timing of treatment onset, and treatment duration. Closed femoral shaft fractures were generated and analyzed with use of destructive torsional mechanical testing and microcomputed tomography-based image analysis. Eleven different outcome measures were quantified, with maximum yield torque as the primary study outcome, to assess the quality of long-bone fracture-healing.
Results:
Fracture-healing was maximized with a lithium treatment combination of a low dose (twenty milligrams per kilogram of body weight per day), later onset of lithium treatment (seven days after fracture), and longer treatment duration (two weeks), with maximum yield torque displaying a 46% increase compared with nontreated and sham-treated controls (481.1 ± 104.0 N-mm compared with 329.9 ± 135.8 N-mm; p = 0.04). Design-of-experiments analysis determined the timing of treatment onset to be the most influential parameter for improving fracture-healing, with femora treated at a later onset (seven days after fracture) showing a significant (21%) increase in maximum yield torque compared with those treated at an earlier onset (three days after fracture) (p = 0.01).
Conclusions:
A later onset of lithium administration significantly improved femoral fracture-healing. Trends indicated that a lower dose and longer treatment duration also had a positive effect on fracture repair.
Clinical Relevance:
Orally administered low-dose lithium therapy with a large postfracture administration window has the potential to yield a safe, reliable, and cost-effective treatment to enhance bone-healing and restore earlier function and mobility pending appropriate large-animal proof-of-concept models, safety data, and U.S. Food and Drug Administration clinical trials approval.
Approximately one-third of individuals experience a fracture during their lifetime. Fracture union generally occurs after several weeks of immobilization, surgery, or both. Unfortunately, delayed or impaired bone-healing occurs in 5% to 10% of fractures, and this often necessitates extensive surgical interventions to promote union1,2. For elderly patients with osteoporosis who have fragility fractures, improved bone formation and early healing are critical to prevent a decline in health and autonomy3-5. However, progression of effective treatment options to improve fracture-healing has remained relatively static. Current U.S. Food and Drug Administration (FDA)-approved anabolic drug treatments therapeutically aimed at stimulating bone growth include recombinant human bone morphogenetic protein (rhBMP)-2, rhBMP-7, and teriparatide (a recombinant fragment of the parathyroid hormone [PTH] 1-34)2. Of these drug treatments, only rhBMP-2 is indicated for fracture management and is limited to open tibial shaft fractures. The use of these treatments has not gained consensus indication in fracture management because of limitations with respect to required local implantation, systemic injection, and/or high costs. An inexpensive and noninvasive approach to augment fracture repair could decrease the overall time to healing and reduce the occurrence of delayed union.
Lithium, a glycogen synthase kinase (GSK)-3β inhibitor, has a positive impact on bone formation in patients with bipolar disorder who are receiving therapeutic doses of lithium6-8. Although GSK-3β proteins are one of many targets for lithium, its action to inhibit GSK-3β, activating the canonical Wingless (Wnt)/β-catenin signaling pathway, is important in bone biology and as a mechanism of increasing bone formation2,9,10. Lithium’s potential to induce bone growth may have a direct clinical implication for treating fractures. Mesenchymal progenitor cell recruitment occurs during the inflammatory phase of fracture repair. As Wnt signaling is activated during the proliferative phase, mesenchymal progenitor cells are induced to differentiate into osteogenic progenitors for mineralization and bone development. In concert with this process, lithium’s anabolic effects seem to be effective when given after a fracture has occurred11, during Wnt-signaling activation. This concept is particularly advantageous for clinical applications, since patients who sustain a fracture are referred to an orthopaedic surgeon during the early inflammatory and proliferative phases of fracture-healing, which typically occur in the first two weeks after injury.
Prior to consideration of lithium for clinical use, further studies are needed to delineate the precise administration parameters specifically for fracture care. As a psychoactive medication, lithium interacts with neuronal monovalent or divalent cation transport in the central nervous system. Psychopharmacological dosing guidelines for bipolar disorder and mania can yield serum levels approaching toxic levels. Lithium side effects and toxicity generally become more evident and concerning at doses that yield serum levels above the recommended upper limit of 1.2 mEq/L12. Normally, clinical dosing begins at 300 mg/day, increasing to a maintenance serum level of 0.8 mEq/L (less for the elderly and children). The narrow therapeutic-to-toxic ratio of lithium necessitates monitoring for plasma concentrations in this patient population. However, for its effects on bone, we postulate that such monitoring may not be necessary, as the required lithium dose would be substantially lower.
Currently, no studies to our knowledge have explored lithium-administration parameters (dose, treatment onset, and treatment duration) with respect to effects on bone regeneration and potential interactions during fracture repair. This is a void in our present knowledge of a known Wnt pathway modulator that is important in bone formation and is safe, accessible, and cost effective. The objective of this preclinical study was to determine the influence of lithium-administration parameters on the quality of the healing of long bones. Our hypothesis was that lithium dosage would be the most critical parameter affecting fracture repair, followed by duration and onset of treatment after fracture.
Materials and Methods
Experimental Design
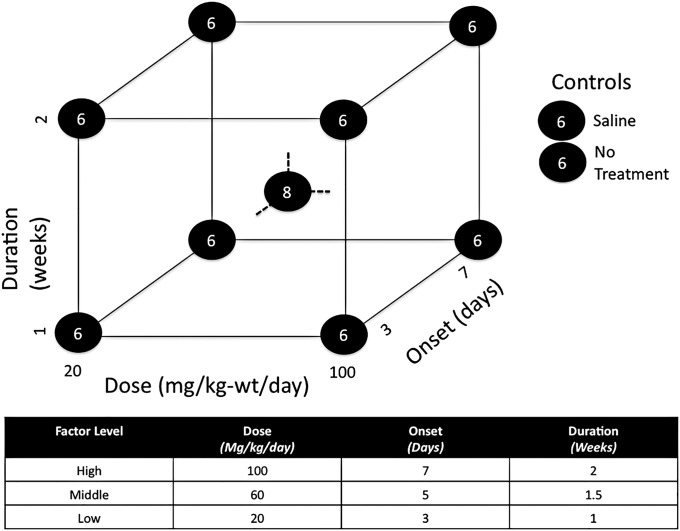
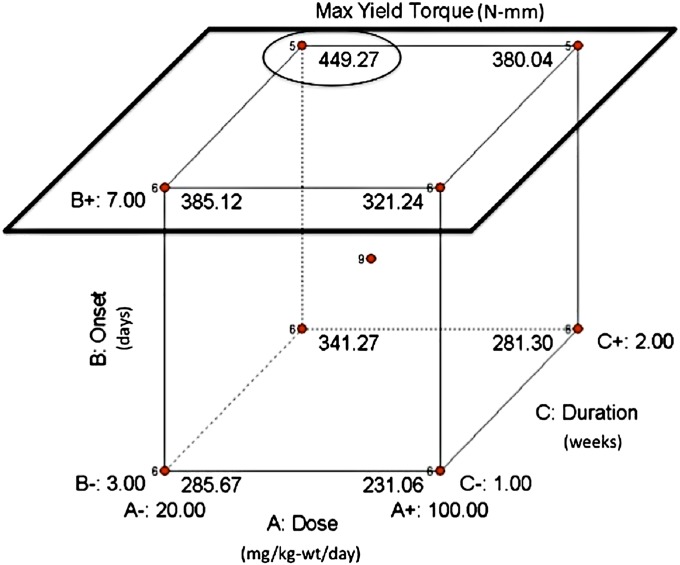
Administration parameters of dose, treatment onset, and treatment duration were investigated with use of a three-factor design-of-experiments approach. Administration parameters were considered at combinations of high and low factor levels, yielding eight experimental groups (Fig. 1). A ninth experimental group was included with all parameters set at middle factor levels (to account for potential nonlinear responses) along with two external control groups (no treatment and sham treatment with saline solution).
Fig. 1.
Diagram showing the high, middle, and low factor levels for lithium dosing, treatment-onset, and treatment-duration parameters explored in this study. In the screening phase, eight experimental groups were tested at the eight different combinations of high and low factor levels. A ninth group was tested at middle factor levels, while two control groups (receiving no treatment or sham treatment with saline solution) were tested outside the cubic design space.
Animal Model
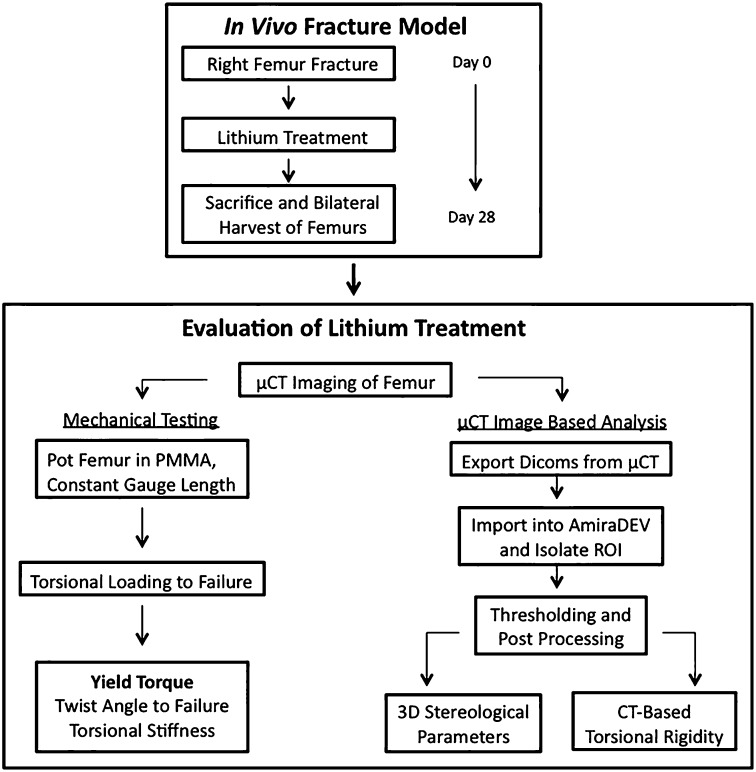
Eighty-four healthy twelve-week old female Sprague Dawley rats (weight, approximately 300 g) were subjected to a closed midshaft unilateral femoral fracture generated with the technique of Bonnarens and Einhorn13, slightly modified by prestabilizing the pin through engaging the proximal cortex and cutting the pin flush distally. The fracture was allowed to heal over twenty-eight days while lithium was administered via daily oral gavage with the rats under light anesthetic14. All animals were euthanized at twenty-eight days after fracture, the femora were harvested bilaterally, and the intramedullary pins were removed (Fig. 2). Biomechanical testing, microcomputed tomography (μCT)-based three-dimensional bone stereology, and torsional rigidity analysis were used to evaluate how variations in lithium treatment affected the overall quality of bone-healing.
Fig. 2.
A flowchart highlighting the chronology of the experimental procedures and analysis performed on each sample. μCT = microcomputed tomography, PMMA = polymethylmethacrylate, DICOMs = Digital Imaging and Communications in Medicine images, ROI = region of interest, 3D = three dimensional, and CT = computed tomography.
Stereological Analysis
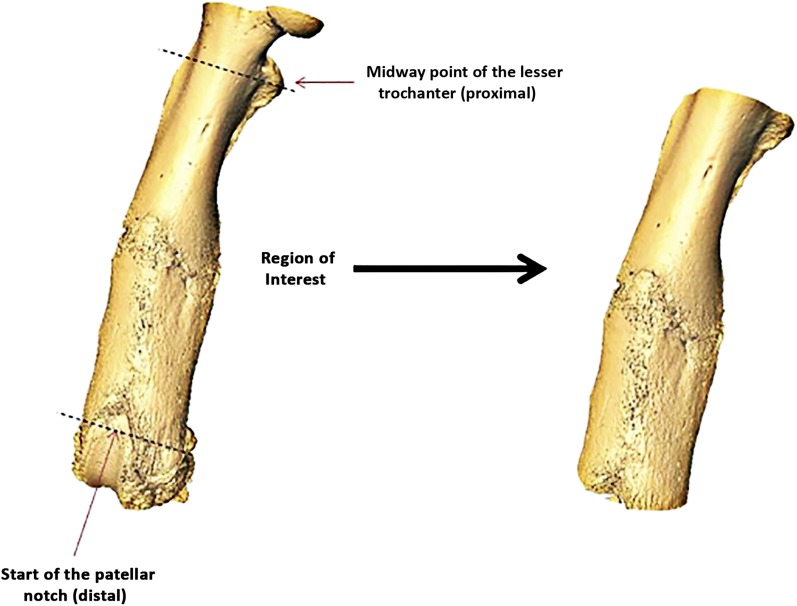
Following harvest, each rat femur was imaged at an isotropic voxel size of 14.8 μm (55 kV, 200 μA, beam-hardening correction factor of 1200 mg hydroxyapatite [HA]/cm3; μCT 100 scanner; Scanco Medical, Brüttisellen, Switzerland). The intensity-bone density relationship was generated via scanner precalibration with use of HA phantoms. Reconstructed scans were exported as Digital Imaging and Communications in Medicine images (DICOMs) into Amira software (version 5.4; Visage Imaging, Carlsbad, California) to crop and align the region of interest. The region of interest was denoted as a fixed distance from the midway point of the lesser trochanter proximally to the start of the patellar notch distally (Fig. 3). A fixed global threshold of 25% of the maximum native grayscale value, corresponding to a mineral density of 365 mg HA/cm3, was used to differentiate mineralized tissue that was included in the analysis from excluded unmineralized or poorly mineralized tissue15. A custom processing module was then used for stereological analysis (CT analyzer software; SkyScan, Kontich, Belgium). Consistent with the results of other papers that have investigated fracture-healing in a rodent closed femoral fracture model15-17, stereological parameters—including bone volume, total volume, bone volume fraction, bone mineral density, tissue mineral density, and bone mineral content—were calculated. Minimum and average computed tomography (CT)-based torsional rigidity were also calculated with the method developed by Nazarian and colleagues18.
Fig. 3.
Illustration showing the region of interest used in the stereological analysis.
Biomechanics
Destructive torsional testing was used to determine the mechanical properties of the healing long bones19. Each femur was aligned longitudinally to the loading axis of a Bionix 858 materials testing system (MTS Systems, Eden Prairie, Minnesota), with its proximal and distal ends potted in bone cement (polymethylmethacrylate) at a constant gauge length of 15 mm20,21. A 1.4-N-m reaction torque transducer (Futek, Irvine, California) measured applied torque. An angular displacement of 1.5°/sec was applied until failure occurred or until a maximum angular displacement of 50° was achieved22,23. Maximum yield torque, twist angle at failure, and experimental torsional stiffness were determined on the basis of the generated load displacement curves. Maximum yield torque was used as the primary study outcome measure to assess the quality of fracture repair.
Design-of-Experiments Modeling and Data Analysis
Eleven different outcome responses were obtained (maximum yield torque, twist angle at failure, experimental torsional stiffness, bone volume, total volume, bone volume fraction, bone mineral density, tissue mineral density, bone mineral content, minimum torsional rigidity, and average torsional rigidity). Data were reported as the mean and standard deviation. Design-of-experiments system modeling was conducted on the primary outcome measure (maximum yield torque) and the ten secondary outcome responses with use of a commercial design-of-experiments-specific statistical package (Design-Ease 7; Stat-Ease, Minneapolis, Minnesota). Raw data were imported into the software in coded matrix notation, and data normality was confirmed. When the data deviated from normality, an appropriate Box-Cox transformation was applied to the output responses to help stabilize the variance. A sum-of-squares chart was used to determine which terms were included in the model space based on a 10% weighted contribution threshold for inclusion. Analysis of variance (ANOVA) was used to determine whether the model itself was significant, which effects and interactions were significant to the model, and whether the model showed significant curvature, indicating a nonlinear or saturated response within the cubic design space. In all cases, a p value of <0.05 was considered significant. To account for the use of multiple and independent estimates made from the same data, a Bonferroni correction24 was applied: the alpha level was divided by the number of estimates. Comparisons of selected treatment groups were conducted with respect to the experimental control groups outside the design-of-experiments space. Additional comparisons were made between these select treatment groups and the experimental controls on the contralateral, nonfractured femora in order to investigate lithium’s potential effect on the off-target limb.
Source of Funding
This work was generously funded by the AO Foundation, the Canadian Institutes of Health Research, the National Institutes of Health, and the Ontario Graduate Scholarship Program.
Results
In our study, exclusion criteria for an unacceptable fracture based on radiographs made immediately after fracture were extramedullary malpositioning of the pin, a nondiaphyseal fracture, and a complex unstable fracture with substantial femoral shortening that was not amenable to immediate weight-bearing on the affected limb. Rats with open fractures and postoperative wound infections were also excluded. Of the eighty-four animals that underwent surgery, fourteen were excluded for the following reasons: wound infection (two), extramedullary pin placement (five), nondiaphyseal fractures (three), and unstable and/or complex fractures (four).
Significantly Positive for Bone-Healing: Later Onset of Treatment
The design-of-experiments analysis found only four response models to be statistically significant (maximum yield torque, tissue mineral density, minimum torsional rigidity, and average torsional rigidity). A summary of the biomechanical and stereological data on these four responses is provided (Table I) along with the results from the design-of-experiments models (see Appendix). In each model, treatment onset was the only significant input parameter. In the maximum yield torque (p = 0.0125) and both torsional rigidity models (p = 0.0035 and p = 0.0114), increasing the time to treatment onset was significantly positive for the response, whereas in the tissue mineral density model (p = 0.005), increasing the time to treatment onset was significantly negative for the response. In three other secondary models (experimental torsional stiffness, bone volume, and total volume), treatment onset was the input parameter with the largest (albeit nonsignificant) effect on the output response. In the maximum yield torque response, defined as the primary study outcome measure used to quantify the quality of bone-healing, ANOVA showed that the model was significant (p = 0.02) and that onset of treatment was the only significant term in the model space, with later onset (seven days after fracture) showing a 21% increase in maximum yield torque compared with those treated at an earlier onset (three days after fracture) (p = 0.01).
TABLE I.
Summary Design-of-Experiments Modeling Results*
| Outcome Response | Applied Transformation | Model Terms | Effect | P Value | Coefficient of Determination (r2) |
| Maximum yield torque (N-mm) | Square root | Model | 0.0168† | 0.183 | |
| Dose | Negative | 0.1109 | |||
| Onset | Positive | 0.0125† | |||
| Duration | Positive | 0.1409 | |||
| Curvature | Nonsignificant | 0.7692 | |||
| Tissue mineral density (mg HA/cm3) | None | Model | 0.0391† | 0.211 | |
| Dose | Positive | 0.9499 | |||
| Onset | Negative | 0.0050† | |||
| Duration | Positive | 0.8046 | |||
| Dose and onset | Negative | 0.2006 | |||
| Dose and duration | Positive | 0.1695 | |||
| Curvature | Significant | 0.0252† | |||
| Minimum torsional rigidity (kN-mm2) | Logarithmic | Model | 0.0080† | 0.172 | |
| Dose | Negative | 0.2729 | |||
| Onset | Positive | 0.0035† | |||
| Curvature | Nonsignificant | 0.9125 | |||
| Average torsional rigidity (kN-mm2) | Logarithmic | Model | 0.0111† | 0.118 | |
| Onset | Positive | 0.0114† | |||
| Curvature | Nonsignificant | 0.5544 |
In each model, five outcomes are reported: the applied transformation (if needed) to help stabilize the variance, the terms included in the model that satisfied the 10% weighted contribution threshold, the direction of each effect, the p value denoting the significance of each effect, and the coefficient of determination for the model. Onset = treatment onset, duration = treatment duration, and HA = hydroxyapatite.
Values with a dagger are significant (p < 0.05).
Positive for Bone-Healing: Lower Dose and Longer Treatment Duration
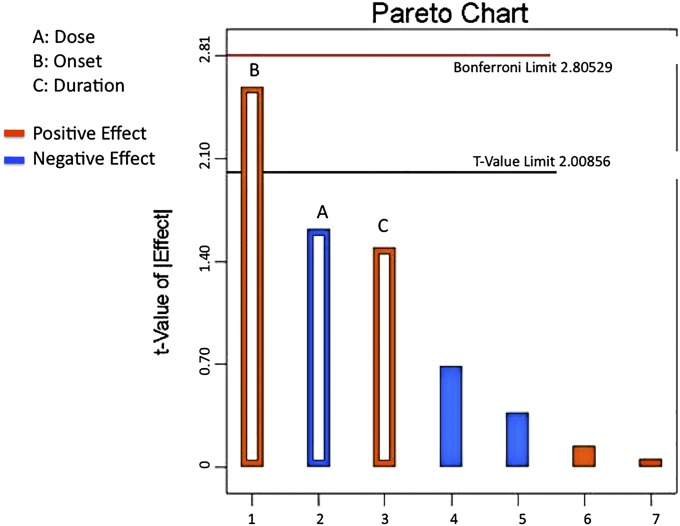
The next largest contributions to the model space were dose (effect = −1.70, 22% contribution; p = 0.11) and treatment duration (effect = 1.57, 18% contribution; p = 0.14). The direction of each input effect indicated that increasing time to treatment onset and increasing treatment duration had a positive influence on the maximum yield torque output response (positive effect), whereas increasing dose negatively influenced this outcome (negative effect). No interaction effect exceeded the 10% weighted threshold and, thus, all interaction terms were applied as model error (Fig. 4). In addition, the model had no significant curvature (p = 0.77), implying that the output responses displayed linear behavior, with no indication of saturation within the cubic design space.
Fig. 4.
A Pareto chart showing both the size and direction of each input parameter as related to the output response. Treatment onset, dose, and treatment duration, as indicated by the hollow bars, were the terms included in the model space, whereas the four solid bars, representing the three two-factor interactions and the one three-factor interaction, were excluded from the model and applied to the error. The top seven most influential factors or interactions in decreasing order are labeled 1 through 7. A, B, and C are labeled, as these are the most important factors. Treatment onset showed the largest effect, followed by dose and then treatment duration. The tip of the onset bar extends beyond the t-value limit of 2.00856, implying that this effect was significant to the model space. Orange bars depict positive effects, whereas blue bars represent negative effects.
Optimal Parameters for Dose, Treatment Onset, and Treatment Duration
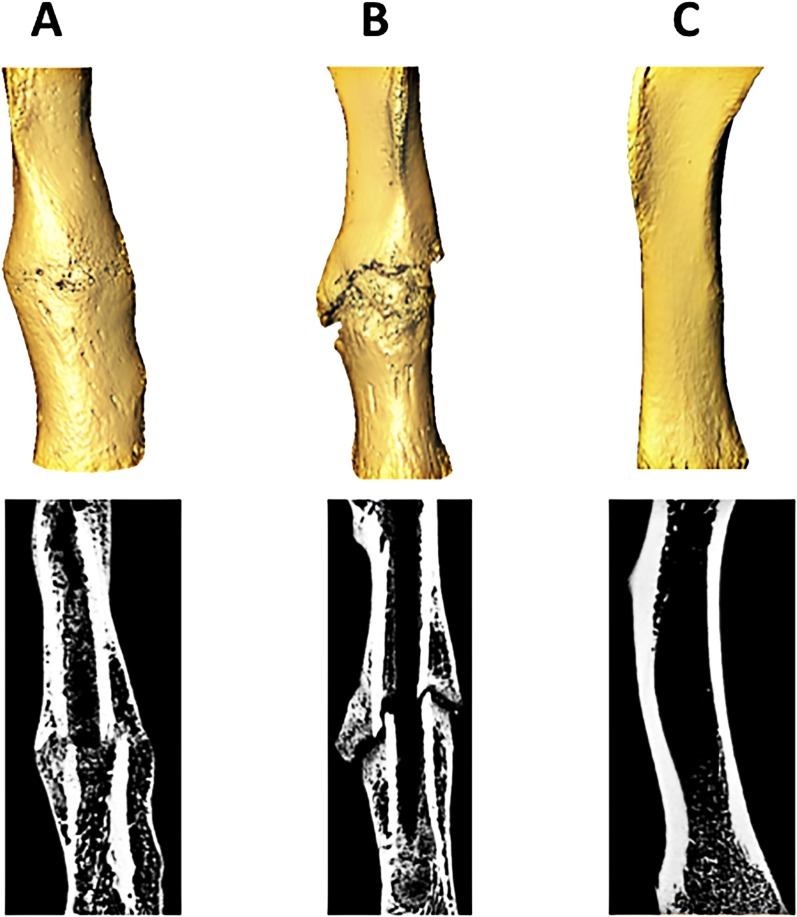
Collectively, the design-of-experiments analysis suggested that the best treatment combination occurred when input factors were set to lower dose (twenty milligrams per kilogram of body weight per day), later treatment onset (seven days after fracture), and longer treatment duration (two weeks) (Group 10, low/high/high). The worst treatment combination occurred when input factors were set to the opposite extremes: a higher dose (100 milligrams per kilogram of body weight per day), earlier treatment onset (three days after fracture), and shorter treatment duration (one week) (Group 5, high/low/low) (Fig. 5). Representative three-dimensional models of healing femoral fractures from Groups 10 and 5 are shown in Figure 6.
Fig. 5.
The predicted design space for maximum yield torque based on the design-of-experiments analysis. A later treatment onset (the upper cubic face, outlined by the black square) displays four of the five largest responses, while an earlier treatment onset (situated on the direct opposite cubic face) displays four of the five smallest responses. A = dose, B = treatment onset, and C = treatment duration. As indicated by the black circle, the best location in the design space is at a low-dose, later-treatment-onset, longer-treatment-duration (A−, B+, C+) combination situated in the upper back-left corner of the cube. Interestingly, the worst treatment combination (A+, B−, C−) is situated in the cubic corner directly opposite the best location.
Fig. 6.
Figs. 6-A, 6-B, and 6-C Three-dimensional isosurface models and microcomputed tomography image slices of three rat femora used in the study at twenty-eight days after fracture. Fig. 6-A depicts a healing femur from a rat that was in experimental Group 10, which received the combination of lithium treatment that was determined to be the best (lower dose, later treatment onset, and longer treatment duration). Fig. 6-B depicts a healing femur from a rat that was in experimental Group 5, which received the combination of lithium treatment that was determined to be the worst (higher dose, earlier treatment onset, and shorter treatment duration). Fig. 6-C depicts an intact contralateral femur from a control rat that was in experimental Group 6.
The treatment combination of lower dose, later onset, and longer duration maximized the primary outcome response of maximum yield torque, demonstrating a significant (46%) increase over pooled controls (481.1 ± 104.0 N-mm compared with 329.9 ± 135.8 N-mm; p = 0.04). The opposite combination of treatment parameters (higher dose, earlier onset, and shorter duration) demonstrated a 22% reduction in maximum yield torque compared with pooled controls; however, this was not significant (255.8 ± 89.1 N-mm compared with 329.9 ± 135.8 N-mm; p = 0.246). There were no significant differences between the group of untreated controls and the group receiving sham treatment with oral administration of saline solution (p ≥ 0.05). Thus, multiple sedations for oral gavage had no effect. No significant differences were observed in the contralateral limbs between these groups in any mechanical or stereological outcome parameters, except for a small increase in tissue mineral density in comparing Group 10 with Group 5 (3.1%; p = 0.03; see Appendix).
Discussion
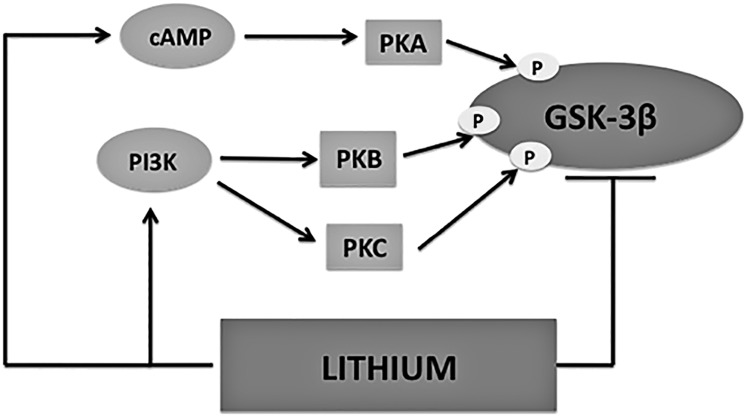
The canonical Wnt/β-catenin pathway has emerged as a promising therapeutic target to modulate bone growth2. This pathway influences the osteoblast lineage, stimulating mesenchymal progenitor precursors to differentiate into mature osteoblasts25,26. In the early stages of fracture repair, β-catenin is needed to direct mesenchymal precursors into their respective chondrocyte and osteoblast lineages. Once mesenchymal precursors have committed to the osteoblast lineage, increasing β-catenin levels improve osteogenesis26. Thus, to positively influence fracture-healing, stimulation of Wnt/β-catenin signaling must occur after mesenchymal precursors have been committed to the osteoblast lineage. Therapeutic strategies aimed to enhance Wnt/β-catenin pathway activity during this critical period have tremendous potential as anabolic interventions for bone growth and possible new treatments for fracture repair. Lithium has various mechanisms of action, many of which are interconnected and relate back to Wnt/β-catenin activation via downstream GSK-3β inhibition27,28 (Fig. 7). Lower doses of lithium could result in signal amplification or, conversely, higher doses could saturate signaling to interfere with proper bone-healing28. Clearly, proper regulation of GSK-3β is imperative29.
Fig. 7.
Lithium’s mechanism of action as it relates to glycogen synthase kinase (GSK)-3β. Lithium has the ability to both directly and indirectly inhibit GSK-3β activity, making it an appealing therapeutic strategy to stimulate Wnt/β-catenin signaling. Direct inhibition occurs via competition with Mg2+ at the GSK-3β active site. Indirect inhibition occurs via serine 9 phosphorylation at the GSK-3β N-terminus. cAMP = cyclic adenosine monophosphate, PKA = protein kinase A, P = phosphorylation site, PI3K = phosphatidylinositol 3-kinase, PKB = protein kinase B, and PKC = protein kinase C.
The administration of lithium stimulated bone growth during fracture-healing. Our results suggest that a combination of a lower dose (twenty milligrams per kilogram of body weight per day), later treatment onset (seven days after fracture), and longer treatment duration (two weeks) improved bone-healing, with a significant (46%) increase in maximum yield torque over pooled controls. The timing of treatment onset was the most influential parameter, suggesting that the exact timing of administration is the most crucial factor in optimizing the treatment regimen. Delayed administration with use of other anabolic treatments, such as BMP-2, at five and ten days postoperatively for critical-sized femoral bone defects has also demonstrated enhanced mineralization and greater mechanical strength in rabbit and rat models30. These findings align with our knowledge of bone-healing as a highly coordinated and tightly regulated temporal physiological process. Our results suggest that short-term systemic lithium treatment successfully targets the healing femoral fracture and does not influence the mechanical properties or microstructure of the intact contralateral limbs.
The ideal therapeutic influence of lithium must target the timing when mesenchymal precursors become committed to the osteoblast lineage11, a cellular transition closely linked to the physiological shift from soft to hard callus during endochondral fracture repair. While the exact timing is not conclusive, evidence suggests that this soft-to-hard callus transition peaks between seven and fifteen days after fracture in the rodent31-33. Therefore, lithium therapy aimed at enhancing fracture-healing should target this time range to be most effective. Typically, the rate of union for simple fractures in Sprague Dawley rats is four to six weeks34 compared with healing in adult humans, which is usually six weeks or more. In our study, we chose a treatment onset of seven days after fracture as our high factor boundary to target the beginning of this physiologic transition. Our results demonstrate a nonsignificant curvature in treatment onset, implying that the outputs displayed an increasing linear trend with no indication of saturation within the factor range tested. As such, the optimal timing for the onset of treatment may lie outside the current design space tested. Further work targeting a treatment onset between seven and fifteen days after fracture (the range for primary callus turnover) is needed to determine whether further delaying treatment onset would benefit the mechanical properties of the healing fracture.
No benefit of higher lithium dose was found. Contrary to expectations, the lower dose of lithium showed a trend toward superior fracture-healing (twenty compared with 100 milligrams per kilogram of body weight per day, maximum yield torque increase of 19%; p = 0.11). Clinically, this finding is very encouraging, as it suggests that patients could benefit from taking very low dose lithium to maximize its positive influence on fracture-healing and substantially reduce its potential adverse systemic effects, as lithium toxicity is dose dependent. Lithium’s major side effects are primarily acute, occurring when levels exceed recommended therapeutic levels. The lower dose of lithium used in our study is nearly four times lower than the regular maintenance dose for lithium’s psychotropic indication in the treatment of bipolar disorder35, which may suggest that the lithium levels needed to improve bone-healing come with minimal risk of acute toxicity. Furthermore, while there are clinically major long-term concerns—including hyperparathyroidism, hypothyroidism, and nephrogenic diabetes insipidus—in patients managed with lithium36-38, these long-term side effects may be less relevant in short-term lithium use for bone-healing.
Duration of lithium treatment was the least influential treatment parameter, with a nonsignificant trend toward a longer duration showing improved bone-healing. With two weeks of lithium treatment, the period of endochondral ossification in the rat approached completion, at which time mineralization predominated as the cartilage template diminished. This transition may mark the boundary of when lithium therapy may no longer be needed or even be effective. Consequently, the duration of treatment may be reduced if an even later onset of treatment (beyond seven days after fracture) is found to be equivalent or more beneficial.
A strength of this study is the application of the design-of-experiments methodology. A one-factor-at-a-time approach is commonly utilized in preclinical study design, whereby each factor is varied independently while all other factors remain fixed. One-factor-at-a-time approaches require a large number of samples to achieve adequate power to compare multiple variables, and they are unable to elucidate interactions that may occur among factors. More importantly, when multiple parameters are evaluated for the purpose of selecting a treatment protocol, one-factor-at-a-time designs may miss the individual combination that represents the optimal outcome. For example, it is possible that the optimal parameters of dose, treatment onset, and duration for lithium administration in fracture-healing will not be tested with use of this type of experimental method. Design of experiments is a statistically more powerful and efficient methodology for studying two or more parameters that may interact and have nonlinear effects. A well-established technique widely used in engineering analyses, it has been developed specifically with the intention of efficiently identifying the relative influences of factors and the combination of them producing an optimal response and reducing variability. Design of experiments has been successfully implemented in a few biological studies pertaining to microarray protocol optimization39,40 and assay parameter selection41. However, to our knowledge, there have been no documented studies in which the design-of-experiments approach has been used in an in vivo, preclinical, translational drug study focused on exploring optimized treatment regimens.
Overall, while later treatment onset was the only significant parameter shown to improve bone-healing, nonsignificant trends indicated that a lower dose and longer duration also had positive effects on the healing response. These findings are encouraging from a clinical perspective, as they support the administration of lithium to manage patients receiving orthopaedic care after a fracture has occurred.
Lithium therapy is emerging as a very promising option in bone repair, and the results of our study form a critical foundation for future translational studies. As this optimization study is limited by its use of a rodent model, appropriate large-animal proof-of-concept models, safety data, and FDA clinical trials approval are needed.
Appendix
Tables showing the results of the design-of-experiments models (including the experimental and control groups of fractured limbs) and the outcomes in the intact contralateral limbs are available with the online version of this article as a data supplement at jbjs.org.
Footnotes
Investigation performed at Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. None of the authors, or their institution(s), have had any financial relationship, in the thirty-six months prior to submission of this work, with any entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. Also, no author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.MacKenzie EJ, Bosse MJ, Kellam JF, Pollak AN, Webb LX, Swiontkowski MF, Smith DG, Sanders RW, Jones AL, Starr AJ, McAndrew MP, Patterson BM, Burgess AR, Travison T, Castillo RC. Early predictors of long-term work disability after major limb trauma. J Trauma. 2006September;61(3):688-94. [DOI] [PubMed] [Google Scholar]
- 2.Hoeppner LH, Secreto FJ, Westendorf JJ. Wnt signaling as a therapeutic target for bone diseases. Expert Opin Ther Targets. 2009April;13(4):485-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goeree R, O’Brien B, Pettitt D, Cuddy L, Ferraz M, Adachi J. An assessment of the burden of illness due to osteoporosis in Canada. J Soc Obstet Gynaecol Can. 1996July;18(Suppl A):15-24. [Google Scholar]
- 4.Cooper C, Campion G, Melton LJ 3rd. Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992November;2(6):285-9. [DOI] [PubMed] [Google Scholar]
- 5.Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011January25;108(4):1585-90 Epub 2011 Jan 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zamani A, Omrani GR, Nasab MM. Lithium’s effect on bone mineral density. Bone. 2009February;44(2):331-4 Epub 2008 Oct 18. [DOI] [PubMed] [Google Scholar]
- 7.Vestergaard P, Rejnmark L, Mosekilde L. Reduced relative risk of fractures among users of lithium. Calcif Tissue Int. 2005July;77(1):1-8 Epub 2005 Jul 14. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad MA, Kuhanendran D, Kamande IW, Charalambides C. Accelerated tibial fracture union in the third trimester of pregnancy: a case report. J Med Case Rep. 2008;2(44):44 Epub 2008 Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clément-Lacroix P, Ai M, Morvan F, Roman-Roman S, Vayssière B, Belleville C, Estrera K, Warman ML, Baron R, Rawadi G. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005November29;102(48):17406-11 Epub 2005 Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni NH, Onyia JE, Zeng Q, Tian X, Liu M, Halladay DL, Frolik CA, Engler T, Wei T, Kriauciunas A, Martin TJ, Sato M, Bryant HU, Ma YL. Orally bioavailable GSK-3alpha/beta dual inhibitor increases markers of cellular differentiation in vitro and bone mass in vivo. J Bone Miner Res. 2006June;21(6):910-20. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Whetstone HC, Lin AC, Nadesan P, Wei Q, Poon R, Alman BA. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med. 2007July31;4(7):e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenox RH, Manji H. Lithium. In: Schatzberg AF, Nemeroff CB. American psychiatric press textbook of psychopharmacology. 2nd ed.Washington, DC: American Psychiatric Press; 1998. p 379. [Google Scholar]
- 13.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2(1):97-101. [DOI] [PubMed] [Google Scholar]
- 14.Murphy SJ, Smith P, Shaivitz AB, Rossberg MI, Hurn PD. The effect of brief halothane anesthesia during daily gavage on complications and body weight in rats. Contemp Top Lab Anim Sci. 2001March;40(2):9-12. [PubMed] [Google Scholar]
- 15.Morgan EF, Mason ZD, Chien KB, Pfeiffer AJ, Barnes GL, Einhorn TA, Gerstenfeld LC. Micro-computed tomography assessment of fracture healing: relationships among callus structure, composition, and mechanical function. Bone. 2009February;44(2):335-44 Epub 2008 Oct 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toben D, Schroeder I, El Khassawna T, Mehta M, Hoffmann JE, Frisch JT, Schell H, Lienau J, Serra A, Radbruch A, Duda GN. Fracture healing is accelerated in the absence of the adaptive immune system. J Bone Miner Res. 2011January;26(1):113-24. [DOI] [PubMed] [Google Scholar]
- 17.Nyman JS, Munoz S, Jadhav S, Mansour A, Yoshii T, Mundy GR, Gutierrez GE. Quantitative measures of femoral fracture repair in rats derived by micro-computed tomography. J Biomech. 2009May11;42(7):891-7 Epub 2009 Mar 17. [DOI] [PubMed] [Google Scholar]
- 18.Nazarian A, Pezzella L, Tseng A, Baldassarri S, Zurakowski D, Evans CH, Snyder BD. Application of structural rigidity analysis to assess fidelity of healed fractures in rat femurs with critical defects. Calcif Tissue Int. 2010May;86(5):397-403 Epub 2010 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burstein AH, Frankel VH. A standard test for laboratory animal bone. J Biomech. 1971March;4(2):155-8. [DOI] [PubMed] [Google Scholar]
- 20.Huddleston PM, Steckelberg JM, Hanssen AD, Rouse MS, Bolander ME, Patel R. Ciprofloxacin inhibition of experimental fracture healing. J Bone Joint Surg Am. 2000February;82(2):161-73. [DOI] [PubMed] [Google Scholar]
- 21.Kasra M, Vanin CM, MacLusky NJ, Casper RF, Grynpas MD. Effects of different estrogen and progestin regimens on the mechanical properties of rat femur. J Orthop Res. 1997January;15(1):118-23. [DOI] [PubMed] [Google Scholar]
- 22.Sardone LD, Renlund R, Willett TL, Fantus IG, Grynpas MD. Effect of rosiglitazone on bone quality in a rat model of insulin resistance and osteoporosis. Diabetes. 2011December;60(12):3271-8 Epub 2011 Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azuma Y, Ito M, Harada Y, Takagi H, Ohta T, Jingushi S. Low-intensity pulsed ultrasound accelerates rat femoral fracture healing by acting on the various cellular reactions in the fracture callus. J Bone Miner Res. 2001April;16(4):671-80. [DOI] [PubMed] [Google Scholar]
- 24.Anderson MJ, Whitcomb PJ. DOE simplified: Practical tools for effective experimentation. New York: Productivity Press; 2000. p 256. [Google Scholar]
- 25.Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci. 2006April1;119(Pt 7):1283-96 Epub 2006 Mar 7. [DOI] [PubMed] [Google Scholar]
- 26.Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005May;8(5):727-38. [DOI] [PubMed] [Google Scholar]
- 27.Quiroz JA, Machado-Vieira R, Zarate CA Jr, Manji HK. Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010;62(1):50-60 Epub 2010 May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002December;43(7):1158-64. [DOI] [PubMed] [Google Scholar]
- 29.Sutherland C. What are the bona fide GSK3 substrates. Int J Alzheimers Dis. 2011May;2011:1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Betz OB, Betz VM, Nazarian A, Egermann M, Gerstenfeld LC, Einhorn TA, Vrahas MS, Bouxsein ML, Evans CH. Delayed administration of adenoviral BMP-2 vector improves the formation of bone in osseous defects. Gene Ther. 2007July;14(13):1039-44 Epub 2007 Apr 26. [DOI] [PubMed] [Google Scholar]
- 31.Strohbach CA, Strong DD, Rundle CH. Gene therapy applications for fracture repair. In: Kang C, editor. Gene therapy applications. Shanghai: Intech; 2011. p 27. [Google Scholar]
- 32.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011June;42(6):551-5 Epub 2011 Apr 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadjiargyrou M, Lombardo F, Zhao S, Ahrens W, Joo J, Ahn H, Jurman M, White DW, Rubin CT. Transcriptional profiling of bone regeneration. Insight into the molecular complexity of wound repair. J Biol Chem. 2002August16;277(33):30177-82 Epub 2002 Jun 7. [DOI] [PubMed] [Google Scholar]
- 34.Mills LA, Simpson AH. In vivo models of bone repair. J Bone Joint Surg Br. 2012July;94(7):865-74. [DOI] [PubMed] [Google Scholar]
- 35.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008March;22(3):659-61 Epub 2007 Oct 17. [DOI] [PubMed] [Google Scholar]
- 36.Shorter E. The history of lithium therapy. Bipolar Disord. 2009June;11(Suppl 2):4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malhi GS, Tanious M, Das P, Berk M. The science and practice of lithium therapy. Aust N Z J Psychiatry. 2012March;46(3):192-211. [DOI] [PubMed] [Google Scholar]
- 38.Livingstone C, Rampes H. Lithium: a review of its metabolic adverse effects. J Psychopharmacol. 2006May;20(3):347-55 Epub 2005 Sep 20. [DOI] [PubMed] [Google Scholar]
- 39.Wrobel G, Schlingemann J, Hummerich L, Kramer H, Lichter P, Hahn M. Optimization of high-density cDNA-microarray protocols by ‘design of experiments’. Nucleic Acids Res. 2003June15;31(12):e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wildsmith SE, Archer GE, Winkley AJ, Lane PW, Bugelski PJ. Maximization of signal derived from cDNA microarrays. Biotechniques. 2001January;30(1):202-6: 208. [DOI] [PubMed] [Google Scholar]
- 41.Coffey T, Grevenkamp M, Wilson A, Hu M. Biological assay qualification using design of experiments. Bioprocess Int. 2013June;11(6):42-9. [Google Scholar]