Abstract
In the male zebra finch, highly variable juvenile song and stereotyped adult song induce mRNA expression of the immediate early gene zenk in telencephalon. However, the functional consequences of this behavior-driven gene expression remain unknown. Here we characterize the developmental expression of zenk mRNA and protein in two forebrain song regions (HVC, the higher vocal center, and RA, the robust nucleus of the archistriatum). In HVC, singing results in similar percentages of cells producing zenk mRNA and zenk protein at different stages of vocal development. Similarly, song behavior at all stages of vocal development induces a comparable percentage of RA cells expressing zenk mRNA. However, the percentage of RA zenk immunoreactive cells is low during early vocal learning, increasing only as the vocal pattern matures. Early induction of a stereotyped vocal pattern in juvenile birds is associated with increased zenk immunoreactivity in RA, indicating that it is the form of the behavior (and not the age of the bird) that correlates with changes in zenk immunoreactivity. Together, our findings reveal a previously unrecognized relationship between behavioral development and post-transcriptional gene regulation.
Keywords: Immediate early genes, zif-268, egr-1, NGFI-A, krox-24, Vocal learning
1. Introduction
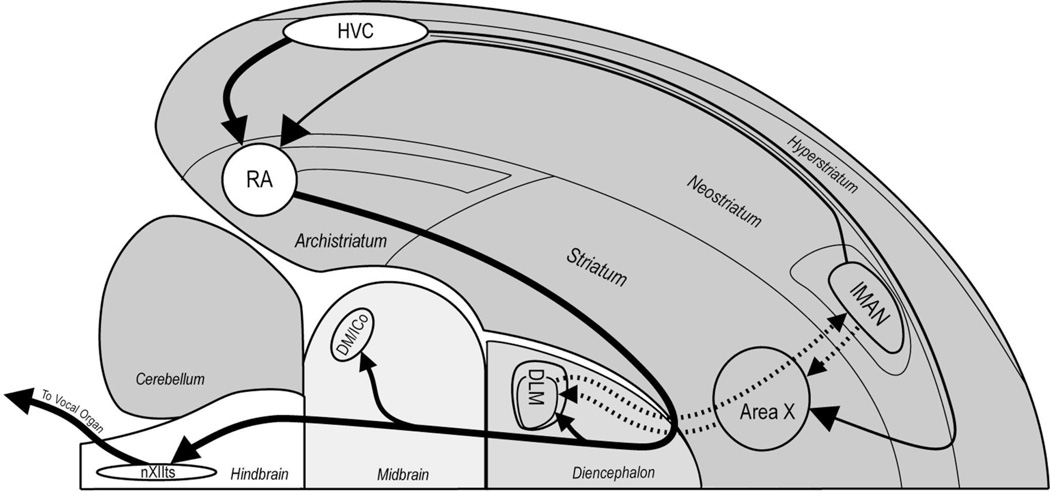
Like human language, birdsong is learned progressively during early development and is controlled by neural activity within a complex telencephalic network. Two neural pathways in the songbird forebrain control song behavior and song development (see Fig. 1). HVC and RA are part of a posterior motor pathway that leads to the syrinx and confers the ability to produce song [27,33]. The anterior forebrain circuit includes Area X, DLM and lMAN and plays a major role in the development of vocal behavior [3,32,34].
Fig. 1.
Schematic sagittal view of zebra finch CNS shows relative positions of major song nuclei and their axonal connections. Two nuclei in the caudal telencephalon (HVC and RA) are important during song learning and adult vocal behavior, whereas song regions in the anterior forebrain (lMAN, DLM, Area X) play an important role in song learning but are not necessary for adult vocal behavior [3,27,32–34]. Abbreviations: Area X, Area X of striatum; DLM, medial portion of the dorsolateral nucleus of the thalamus; DM/ICo, dorsomedial nucleus of the intercollicular complex; HVC, higher vocal center; lMAN, lateral magnocellular nucleus of the anterior neostriatum; RA, robust nucleus of the archistriatum; nXIIts, hypoglossal nucleus, tracheosyringeal portion.
Zenk is also known as Zif268, Egr-1, NGFI-A, Krox-24, and tis8. Zenk is a zinc-finger DNA-binding transcription factor that can function as an activator or repressor of target gene transcription [2,5,19,21,24,25,35]. Song behavior (in hearing or deafened birds) induces zenk mRNA and protein expression within both anterior and posterior forebrain pathways [12–14,23]. In contrast to motor-driven expression in forebrain regions for song-control, song playback induces zenk mRNA and protein expression within the caudomedial neostriatum, but not within song control nuclei [22–24].
To determine whether motor-driven zenk expression differed as a function of vocal learning, Jin and Clayton [14] measured singing-induced zenk mRNA in juvenile and adult male zebra finches. Whereas a robust, uniform induction of zenk was observed in RA of juvenile birds that were learning to sing, there was little zenk mRNA induction in RA following the production of already-learned song by adults. These data raised the possibility that increased zenk mRNA expression in RA might contribute to neuronal plasticity necessary for song learning in juveniles. However, Jarvis et al. [13] subsequently reported that zenk mRNA expression in RA shows a much stronger relationship to social context rather than stage of song learning. That is, adult birds singing female-directed song showed little zenk mRNA expression in RA, while undirected adult song (song produced in a solo context, or in the presence of other males) significantly increased zenk mRNA expression in RA.
Because previous developmental studies have measured the expression of zenk at the level of mRNA, but not protein [12–14], we have reevaluated the role of zenk during song development by measuring its expression at both mRNA and protein levels. Here we report measurement of zenk mRNA and zenk protein in two song regions of the caudal telencephalon (HVC and RA, see Fig. 1) during three distinct stages of vocal development (subsong, plastic song, adult song, see Fig. 2). Our findings suggest that zenk may be subject to post-transcriptional regulation during early vocal learning and that expression of zenk protein is associated with the onset and maintenance of vocal stereotypy.
Fig. 2.
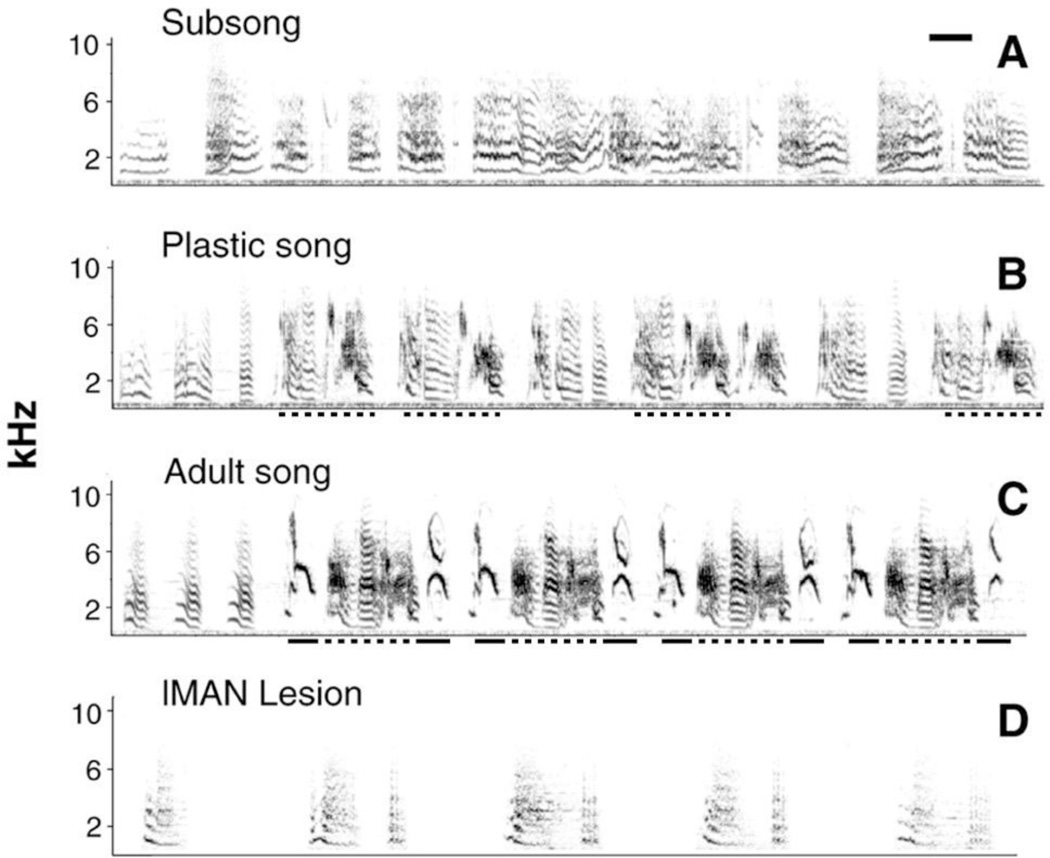
Song stereotypy develops progressively, but can be induced prematurely with bilateral lMAN lesions. Audiospectrograms (A,B,C) show example song bouts from a male zebra finch advancing from subsong to plastic song to adult song. This bird was not used in the present study, but is shown to demonstrate stages of zebra finch song development. (A) Bouts of subsong show little structure in terms of note types and note sequence. (B) During bouts of plastic song, specific note types and a conserved note sequence begin to emerge (dashed underscores). However, note production is unstable and song notes are sometimes produced out of sequence. (C) Fully stereotyped adult song begins with a series of introductory notes followed by a conserved note sequence. The conserved note sequences produced during plastic song (dashed underscores), along with additional notes that emerged later in plastic song (solid underscores), have been incorporated into an adult song phrase that is repeated four times. (D) Unlike normal subsong birds (see A), juveniles with bilateral lMAN lesions produce a limited set of notes in a stereotyped fashion. Scale bar in (A) is 100 ms.
2. Materials and methods
2.1. Subjects
All experimental procedures were conducted in accordance with the Florida State University Animal Care and Use Committee Guidelines. Male zebra finches were obtained from our breeding aviary and housed with food and water freely available on a 14:10 light / dark cycle. Four groups of juvenile and adult birds (subsong, lMAN lesion, plastic song, adult song) were used in three different experiments. At the time of sacrifice, the mean age for subsong, lMAN lesion, and plastic song birds was 45, 44, and 63 days, respectively. All adult birds were >120 days old.
2.2. Song behavior
Zebra finch vocal learning, like human language acquisition, is developmentally regulated and involves multiple cognitive processes. During an early stage of vocal development (termed sensory-motor learning), juvenile males practice imitating a previously memorized adult male song [11]. Sensory-motor learning begins with subsong, characterized by the production of highly variable individual song notes and note sequences (Fig. 2A). With practice and auditory feedback, birds develop plastic song, where individual song notes and note sequences become less variable and more stereotyped (Fig. 2D). Finally, as zebra finches reach adulthood, individual notes and note sequences become stereotyped (Fig. 2G). Once learned the adult vocal pattern does not change.
Subsong and plastic song birds were removed from the breeding aviary at 35–40 days of age and housed singly in a sound recording chamber, visually (but not acoustically) isolated from other birds. All song produced under these conditions was undirected. Adult birds were removed from the colony at >120 days of age and housed under identical conditions. For all birds, digital audio recordings and spectrograms of song bouts (defined as 2–5 s burst of song elements produced in rapid succession) were made using a PC equipped with Avisoft Recorder and Avisoft SASlab software (www.avisoft.de).
Stereotypy scores were used to quantify the stage of vocal behavior (subsong, plastic song, adult song, see Fig. 2) of each bird. Following the procedure of Scharff and Nottebohm [32], song bouts from each bird (n = 10 per bird) were analyzed using the following equations to generate a stereotypy score:
Sstereotypy = (Slinearity + Sconsistency)/2
Slinearity = # different song notes /# transition types
Sconsistency = ∑typical transitions /∑total transitions
Slinearity measures note order whereas Sconsistency measures how often the sequence pattern is followed. Males singing subsong were assigned stereotypy scores of 0.0 since their vocal patterns had no repeated note types or note transitions. Mean stereotypy scores for males singing plastic song and adult song were 0.59 and 0.86, respectively. Subsong-age males with bilateral lMAN lesions had a mean stereotypy score of 0.77.
The behavioral parameters we used for measuring singing-induced zenk mRNA and protein expression were guided by previous reports. While Jin and Clayton [14] measured zenk expression as a function of total time spent singing, Jarvis et al. [13] compared total time spent singing with total number of song bouts produced and found that zenk expression in song nuclei correlated most strongly with the number of song bouts produced. Jarvis and Nottebohm [12] and Mello and Rubiero [23] have demonstrated robust expression of zenk mRNA and protein within 15 and 30 min, respectively. Once induced, zenk mRNA persists for 30–60 min [12], whereas zenk protein persists for 2–4 h [23]. Thus, based on the combined data of Jarvis and Nottebohm [12], Jin and Clayton [14], Jarvis et al. [13], and Mello and Rubiero [23], we sacrificed birds from each stage of vocal behavior (subsong, plastic song, and adult song) 30–60 min after the onset of morning singing behavior. Birds were sacrificed only if they produced a minimum number of song bouts (30). The 30–60 min recording period was necessary because birds vary in their rate of vocal production. Note that the mean time of sacrifice relative to the onset of singing was comparable for each stage of song development (subsong 42 min, s.d. 9 min; plastic song 41 min, s.d. 15 min; adult song 47 min, s.d. 12 min) and that sacrifice after 30 min of singing was sufficient to induce robust zenk immunoreactivity in RA (e.g., Fig. 3B) and HVC (e.g., Fig. 4C).
Fig. 3.
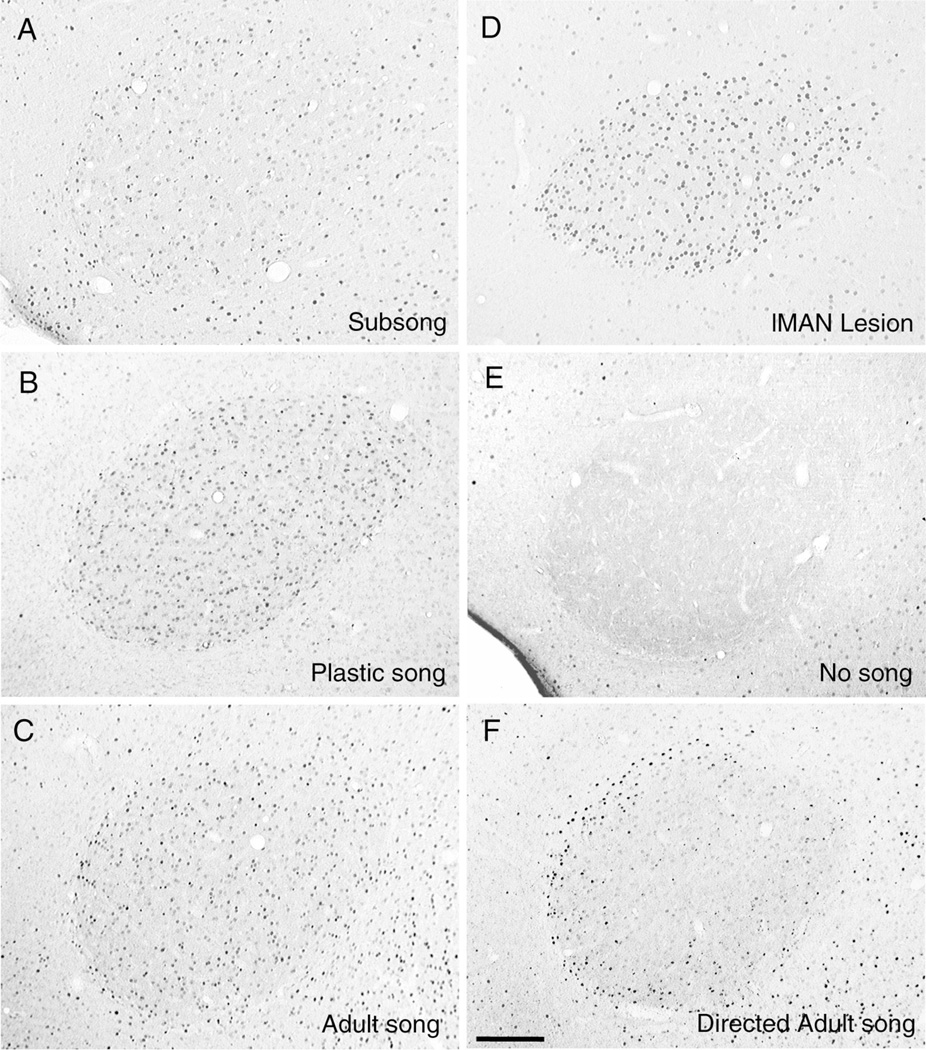
Song-driven zenk immunoreactivity in the motor song nucleus RA is regulated as a function of behavioral development. Digital photomicrographs show zenk immunoreactivity in RA for birds singing a similar number of undirected song bouts at different stages of vocal development (compare to HVC labeling in Fig. 4). Because birds vary in the rate at which song bouts are produced, birds were perfused 30–60 min following the onset of singing (see Materials and methods). Zenk immunoreactivity in RA was low following subsong (A, this bird sang 65 bouts in 60 min), but increased following plastic song (B, this bird sang 61 bouts in 30 min) or adult song (C, this bird sang 76 bouts in 45 min). Subsong-age birds with bilateral lMAN lesions sing a stereotyped song prematurely and show robust zenk immunoreactivity in RA (D, this bird sang 44 bouts in 50 min, compare to staining in A). Non-singing birds show no zenk immunoreactivity in RA (E). A reduced and heterogeneous pattern of zenk immunoreactivity in RA was observed following female-directed song (F, this bird sang 84 bouts in 50 min, compare to undirected adult song in C). Coronal plane of section, medial is left. All photomicrographs (including those in Figs. 4 and 6) were taken with a Sony DKC-5000 and imported to Adobe Photoshop 4.0. Scale bar 150 µm.
Fig. 4.
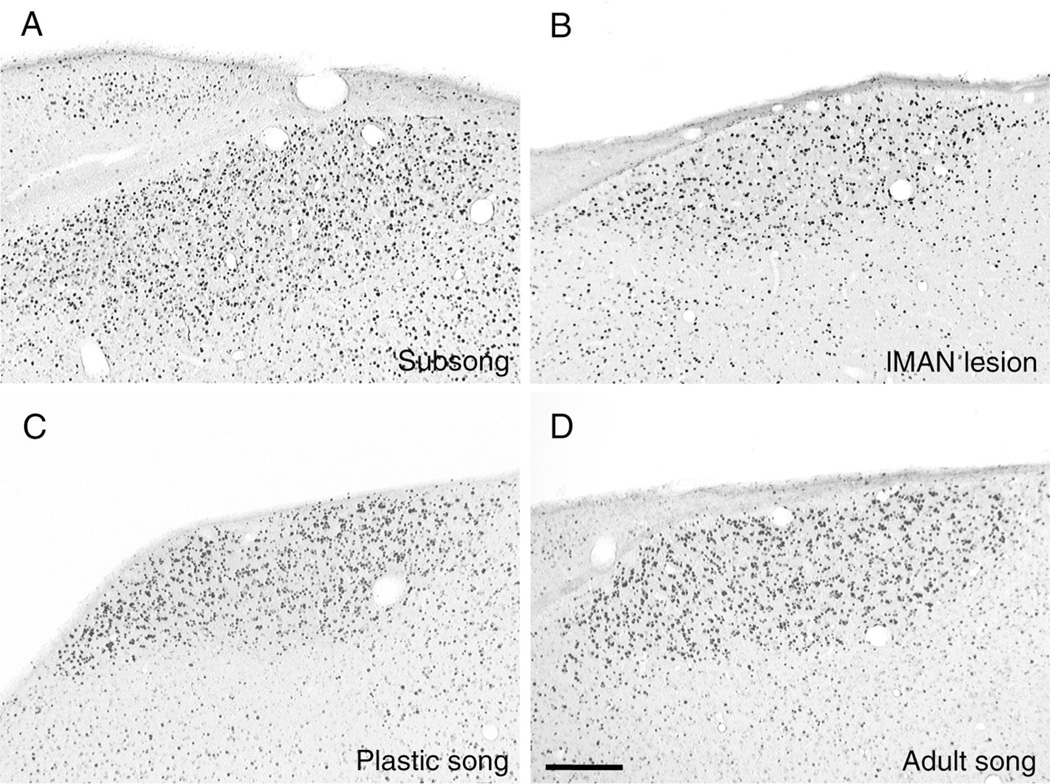
Song-driven zenk immunoreactivity in HVC is not influenced by stage of vocal development. Digital photomicrographs (from the same birds shown in Fig. 3A–D) show the induction of zenk protein in HVC. Immunoreactive staining in HVC was comparable following subsong (A), prematurely stereotyped song induced by bilateral lMAN lesions (B), plastic song (C), and adult song (D). Coronal plane of section, medial is left. Scale bar 150 µm.
2.3. Experiment 1: immunocytochemical measurement of singing-driven zenk following subsong, plastic song, and adult song
Final recording sessions began at the onset of the light phase, a time when birds sing spontaneously. Subjects for this experiment were birds producing undirected subsong (n = 2), plastic song (n = 3), and adult song (n = 3). For each bird, song bouts were recorded during a 30–60 min period that began with the onset of morning singing. Birds producing 30 or more song bouts were immediately euthanized with 0.08 cc of Equithesin and transcardially perfused with 20 ml of PBS followed by 60 ml of ice-cold 4% paraformaldehyde in 0.02 M phosphate buffered saline (PBS). No more than 10 min elapsed between a bird’s final song bout and perfusion of the brain with fixative. Since it was previously determined that light fixation can result in high levels of background avidin reactivity within song regions of juvenile males [16], extracted brains were post-fixed in 4% paraformaldehyde for 24 h to allow for denaturation of endogenous biotinylated proteins. The caudal telencephalon of each bird was then sectioned coronally on a vibrating microtome (section thickness 20 µm) with the cutting angle set so that single tissue sections from each bird contained both HVC and RA. In this way, antibody labeling in one brain region served as a positive control for an absence of mRNA labeling in the other.
Zenk protein was detected with a rabbit polyclonal antibody (Santa Cruz Biotech) raised against the 19 amino acid carboxy terminus of mouse egr-1. This antibody provides specific labeling of zebra finch zenk as determined by Western blot [23]. We further evaluated the specificity of this antibody in tissue sections from the caudal telencephalon of adult birds singing female-directed (n = 2) vs. undirected (n = 2) song. Consistent with a previous report that birds singing female-directed song show reduced levels of zenk transcript in RA [13], birds singing female-directed song showed reduced levels of zenk immunoreactivity in RA relative to birds singing undirected song (compare Fig. 3C and F).
All immunocytochemical reagents were from Vector Laboratories (Burlingame, CA, USA). Initially, tissue was quenched for endogenous peroxidase with 1% H2O2. Sections were rinsed in PBS and nonspecific labeling was blocked with 5% normal goat serum (NGS) for 60 min. Next, sections were rinsed in PBS and incubated overnight in an anti-egr-1 solution (1:1000 dilution) containing 1% NGS, 0.01% NaN3, and 0.3% Tri-X in PBS. The next day, tissue was rinsed with PBS and incubated in a biotinylated anti-rabbit solution for 60 min. This was followed by a rinse with PBS and then a 1-h incubation in an avidin–biotin peroxidase complex solution. Antibody labeling was visualized with a 3,3′-diaminobenzidine solution. Sections were then rinsed in PBS, mounted on gelatin-coated slides, and coverslipped with Permount (Fisher). A series of control sections (primary antibody omitted) showed no specific immunoreactivity. Similarly, non-singing controls (n = 2) showed no zenk immunoreactive labeling in HVC or RA (see Fig. 3E).
2.4. Experiment 2: immunocytochemistry and in situ hybridization for singing-driven zenk following subsong, plastic song, and adult song
Experiment 1 revealed reduced zenk immunoreactive labeling in RA of birds producing subsong (relative to birds producing plastic and adult song). This was surprising to us given reports that zenk mRNA is expressed at high levels in RA of birds producing subsong [13,14]. Therefore, in Experiment 2 we combined in situ hybridization with immunocytochemistry to directly compare levels of zenk mRNA and zenk protein in birds producing undirected subsong (n = 6), plastic song (n = 4) and adult song (n = 4). Following perfusion and post-fixation, brains were cryo-protected overnight by immersion in 20% sucrose (in PBS) and the caudal telencephalon was then frozen-sectioned in the coronal plane on a cryostat (section thickness 20 µm) with the cutting angle set so that single tissue sections from each bird contained both HVC and RA. Three series of alternate tissue sections were collected. One series of sections was mounted onto gelatin-coated slides, stained with thionin, and used to estimate neuron density in HVC and RA. The second series was collected and processed for zenk immunocytochemistry as described above for Experiment 1. The third series was collected into 20 ml scintillation vials containing ice-cold 2× SSC for fixed-tissue in situ hybridization [9].
Zenk mRNA was detected autoradiographically using a [32P]-labeled cDNA probe. The template used for Klenow-catalyzed, random hexamer-primed probe synthesis was cDNA encoding a fragment of zebra finch zenk. This fragment was PCR amplified from zebra finch brain cDNA and subcloned into a Stratagene Bluescript SK+ cloning vector using a standard protocol. PCR primers were designed based on the published partial coding sequence for zebra finch zenk (Genbank accession # AF026084). The sense primer was 5′-ATGCTGCAGTGTGACCGGAGGTTTTC-3′ and the antisense primer was 5′-ATGCTCGAGGCCACATGTGAGTGTCTCATCA-3′ (both primers incorporate 3-base spacers and restriction sites at their 5′ ends).
The Florida State University Core Sequencing Facility (under the direction of Dr. Chris Bacot) determined the sequence of our zebra finch zenk PCR fragment using cycle sequencing with the BigDye TM chemistry and the Applied Biosystems Model 373A automated DNA sequencer (the sequencing reaction was initiated at the T7 primer site present within the plasmid). Sequencing results indicated that the nucleotide sequence of our PCR product is identical to the partial zebra finch zenk cDNA sequence available from GenBank (accession # AF026084).
The specificity of our [32P]-labeled zebra finch zenk cDNA probe was evaluated and confirmed in the following ways: (1) Northern blotting (which produced the expected single band at ~3.5 kb [22]). (2) Elimination of specific labeling following the addition of RNAse A to a series of control sections (200 µg/ml of hybridization buffer). (3) Absence of zenk mRNA labeling in HVC and RA in non-singing controls (n = 2), although basal zenk mRNA labeling persisted in surrounding brain regions [14]. (4) Zenk mRNA labeling in adult birds was found in expected song regions (HVC, RA, Area X) following undirected song (n = 2) and reduced in RA and Area X (but not HVC) following female-directed song (n = 2) [13]. Based on these four criteria, our [32P]-labeled zebra finch zenk cDNA probe labels zenk mRNA in zebra finch CNS with specificity comparable to the [35S]-labeled canary zenk riboprobe used in previous studies of zebra finch vocal development [13,14].
Tissue sections were prehybridized at 48°C for 60 min in a solution containing 50% formamide, 10% dextran sulfate, 2× SSC, 1× Denhardts solution, 50 mM DTT, and 0.05 mg/ml denatured herring sperm DNA. Denatured [32P]-labeled zebra finch zenk cDNA probe was added to the prehybridization solution and allowed to hybridize overnight at 48°C. The following day, sections were washed for 15-min intervals at 48°C in decreasing concentrations of SSC (2, 2, 1, 0.5, 0.25, 0.125×) and then mounted onto gelatin-coated slides. Slides were processed for emulsion autoradiography (Kodak NTB2) and developed after exposure times of 7–10 days (Kodak D19). Slides were then counterstained with thionin and coverslipped with Permount.
All quantification for individual birds was done without knowledge of experimental group. Neuron density in HVC and RA was estimated using established cell quantification techniques for these brain regions [15,39]. Briefly, using transmitted-light microscope fitted with a 100× objective, an ocular grid was used to define a high-power field (volume 0.0002 mm3) randomly placed within the central portion of HVC and RA. Neuronal nucleoli were the unit of count since they are small relative to section thickness and morphologically distinct from glial nucleoli (however, neurons with two nucleoli were only counted once). In each bird, two high-power fields were counted per HVC or RA in five different tissue sections (a total of 10 grids per HVC or RA). The total number of neurons counted (in HVC or RA) was then divided by the volume of the 10 grids to estimate neuron density.
Zenk mRNA labeling in autoradiograms was quantified using a 99% Poisson criterion [1,7]. High-power images, using the same sampling procedure as described above for neuron counting, were captured using a digital camera (Sony DKC 5000) attached to a transmitted light microscope. An image analysis program (Scion Image, www.scioncorp.com) was then used to determine whether silver grain labeling over individual cells exceeded a 99% confidence interval based on a Poisson distribution of background silver grain labeling [1]. The total number of zenk mRNA labeled cells counted was then divided by the total sampled volume to estimate the density of zenk mRNA labeled cells in HVC and RA. Visual inspection of HVC and RA of all birds indicated that cells labeled with silver grains were distributed evenly throughout both nuclei, the expected pattern in birds producing undirected song [13]. Note that a gradient of zenk mRNA labeling in RA of adult males would be expected had our birds been producing directed song (compare Refs. [13,14]).
Zenk immunolabeled cells were quantified using the same procedure described above for neuron counting, except that immunoreactive nuclei were the unit of count. Immunoreactive cells in HVC and RA varied somewhat in their staining intensity, and we initially quantified cells as being either lightly or darkly stained, but experimental groups did not differ in the percentage of cells that were lightly vs. darkly stained and we therefore combined lightly and darkly stained cells into a single category (the same procedure used by Mello and Ribiero [23]). The total numbers of zenk immunoreactive cells counted were then divided by the total sampled volume to estimate the density of zenk immunoreactive cells in HVC and RA.
Neuron density values were then divided by the density of zenk mRNA and zenk antibody labeled cells to determine the percentage of HVC and RA neurons that expressed zenk mRNA and zenk protein, respectively. Since the percentage of HVC and RA neurons expressing zenk mRNA per song bout did not differ as a function of stage of vocal development (ANOVA, F < 1), we took the ratio of zenk protein and mRNA values (percentage of antibody labeled cells / percentage of nucleotide labeled cells) to measure the relative efficiency of zenk translation in HVC and RA at each stage of vocal development. Data were analyzed using ANOVA followed by multiple comparison tests (Student–Newman–Keuls). All analyses passed normality and equal variance tests.
2.5. Experiment 3: immunocytochemical measurement of singing-driven zenk following premature induction of stereotyped vocal patterns
The combined data from Experiments 1 and 2 revealed a positive correlation between zenk immunoreactivity in RA and the development and maintenance of stereotyped song. To further investigate this relationship, juvenile males (30 d/o) were given a sham surgery (n = 4) or bilateral electrolytic lesions of lMAN (n = 5) to induce a stereotyped vocal pattern prematurely [3]. Surgery control birds producing subsong and age-matched lMAN lesion birds producing prematurely stereotyped song were compared for zenk immunoreactive labeling in HVC and RA.
In preparation for surgery, all birds were deeply anesthetized with Equithesin and then placed in a stereotaxic instrument. Bilateral electrolytic lesions of lMAN were made using the following stereotaxic coordinates: AP = 3.6, ML = ±1.8, D = 2.7. An insulated tungsten wire passed 0.1 mA of current to lMAN for 3 min. Surgery control birds received identical treatment, save the placement of the lesioning electrode into lMAN. Birds were returned to their home aviaries until 35 days of age, when they were removed and housed as described above. At 41–46 days of age, lMAN lesion and surgery control birds were recorded, perfused, and their brains sectioned on a vibrating microtome as described above. Two series of sections from the caudal telencephalon were collected, one for zenk immunocytochemistry (as described in Experiment 1) and a second series for thionin staining. Sections from the rostral telencephalon were also collected and stained with thionin to confirm the accuracy of lMAN lesions. Neuron density and zenk immunocytochemical labeling in HVC and RA were measured as described in Experiment 2. Because zenk expression shows a linear relationship to number of song bouts [12,13], we normalized the percentage of HVC and RA cells labeled with the zenk antibody to the number of song bouts each bird produced during its final recording. Data were analyzed for statistical significance using t-tests. Non-singing lMAN lesion birds (n = 2) demonstrated that lMAN lesions alone did not increase zenk immunoreactivity in RA.
3. Results
Experiment 1 revealed reduced zenk immunoreactivity in RA of birds producing subsong relative to birds producing plastic song or adult song (compare Fig. 3A–C). In contrast, similar levels of zenk immunoreactivity were observed in HVC following subsong, plastic song, and adult song (see Fig. 4A, C, and D). To determine if the reduced zenk immunoreactivity that we observed in RA of subsong birds coincided with a reduced number of cells expressing the zenk transcript, in Experiment 2 we quantified both zenk mRNA and zenk protein expression in HVC and RA of individual birds that sang subsong, plastic song, or adult song.
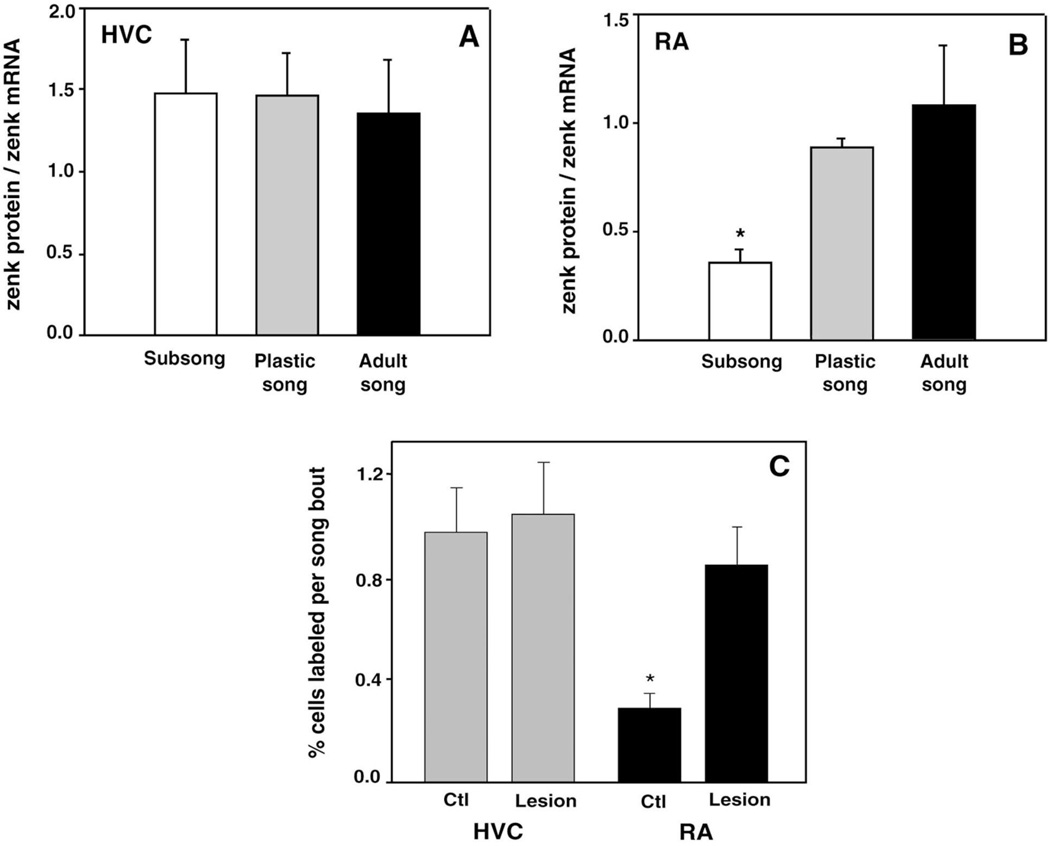
Singing induced zenk mRNA and zenk immunoreactivity in similar percentages of HVC cells, regardless of stage of vocal development (Fig. 5A). Mean zenk protein/mRNA ratios in HVC of birds singing subsong, plastic song, and adult song were not significantly different (F < 1), indicating that song behavior drives zenk mRNA transcription and subsequent zenk protein translation in HVC cells at similar rates during subsong, plastic song, and adult song. The higher percentage of HVC cells expressing zenk protein compared to zenk mRNA may be attributable to high efficiency translation or to differential stability of the molecules.
Fig. 5.
Post-transcriptional regulation of zenk expression within RA, but not HVC, as a function of behavioral development. (A,B) Percentages of HVC and RA cells labeled with antibody and nucleotide probe were expressed as ratios of zenk protein/mRNA. (A) Within HVC, similar percentages of cells expressing zenk mRNA and zenk protein were driven by song behavior at all stages of vocal development. (B) In RA of birds singing subsong, the percentage of cells expressing zenk protein was significantly lower than the percentage of cells expressing zenk mRNA (see Fig. 6A and B), while birds singing plastic song and adult song had similar percentages of RA cells expressing zenk mRNA and zenk protein (see Fig. 6C–F). (C) Juvenile birds were given bilateral IMAN lesions to induce stereotyped song behavior (see Fig. 2D). Compared to a non-lesioned control (ctl) group, such birds show no difference in song-driven zenk immunoreactivity in HVC (see Fig. 4A and B), but a significant increase in song-driven zenk immunoreactivity in RA. Histogram bars are mean ± S.E.M.
In contrast to our findings in HVC, subsong birds had a lower percentage of RA cells expressing zenk immunoreactivity than zenk mRNA (Fig. 5B). Mean zenk protein/mRNA ratios in RA for birds singing subsong, plastic song, and adult song were significantly different (F2,11 = 7.06, P = 0.01) and pairwise comparisons revealed that subsong birds had significantly lower zenk protein/mRNA ratios than birds singing plastic song or adult song (subsong vs. plastic song, P = 0.02; subsong vs. adult song, P = 0.01). Zenk protein/mRNA ratios did not differ between birds singing plastic song and adult song (P = 0.39). Thus, RA of birds singing plastic song and adult song showed similar numbers of cells expressing zenk mRNA and protein (Fig. 6C–F), whereas RA of subsong birds contained many cells expressing zenk mRNA, but relatively few cells expressing zenk protein (compare Fig. 6A and B).
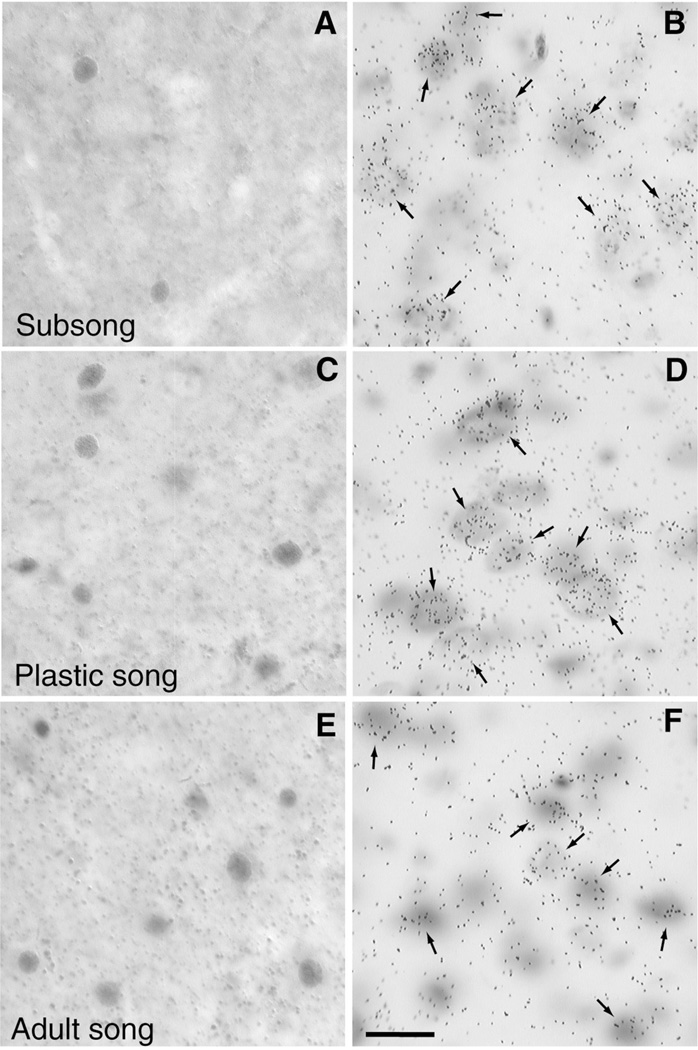
Fig. 6.
High-power photomicrographs show examples of zenk protein and zenk mRNA labeling in RA during sub, plastic and adult song. Immunocytochemistry for zenk protein produced labeling of cell nuclei (A,C,E), whereas autoradiographic labeling was used to detect cells expressing zenk mRNA (arrows in B,D,F). During subsong few RA cells show zenk immunoreactivity (A), even though many RA cells show labeling for zenk mRNA (B). However, during the development and maintenance of stereotyped song (i.e., plastic song, adult song, refer to Fig. 2.) comparable numbers of RA cells show zenk immunoreactivity (C,E) and zenk mRNA labeling (D,F). Scale bar in (F) is 10 µm.
Zenk mRNA labeling in RA was further analyzed to determine whether the density of silver grains over labeled cells differed as a function of stage of vocal development. This was done because although birds singing subsong, plastic song, and adult song did not differ in the overall percentage of zenk mRNA labeled cells in RA (F < 1), perhaps zenk mRNA was less abundant in labeled cells of subsong birds. Reduced levels of zenk transcription in RA of subsong birds might account for the observed reduction in zenk immunoreactivity. Therefore, for each subject the total number of silver grains over labeled cells (minus background) was normalized to the number of song bouts produced during each bird’s final recording.
Subsong, plastic song, and adult song differentially influenced the density of zenk mRNA labeling in RA (F2,11 = 4.51, P = 0.03), with birds producing subsong showing higher levels of zenk mRNA per song bout than birds producing adult song (P = 0.03, compare Fig. 6B and F). A similar trend was observed for plastic song vs. adult song (P = 0.06). Thus, birds producing subsong show reduced levels of zenk immunoreactivity despite high levels of zenk transcript per song bout. Note that our zenk mRNA data are consistent with previous reports that song-driven levels of the zenk transcript in RA decline as birds progress through the stages of vocal development [13,14].
Since the behavioral transition from subsong to plastic song to adult song involves increasing levels of vocal stereotypy, the results of Experiment 2 suggest that zenk protein expression within RA may be related to the development and maintenance of stereotyped song. However, another possibility involves the age of the bird. On average, subsong birds are younger than those producing plastic song, so zenk protein expression within RA could simply be regulated as a function of age. To rule out this possibility, in Experiment 3 we gave juvenile birds a sham surgery or bilateral electrolytic lesions of lMAN to induce a stereotyped vocal pattern prematurely [3].
Subsong-age birds with bilateral lMAN lesions produced prematurely stereotyped vocal patterns (see example in Fig. 2D), whereas all surgery control birds produced normal subsong (comparable to that seen in Fig. 2A). Singing induced the zenk protein in a similar percentage of HVC cells in lMAN lesion and surgery control birds (t = 0.276, P = 0.9, see Fig. 5C and Fig. 4A and B). However, the prematurely stereotyped song behavior of lMAN lesion birds was associated with significantly greater expression of zenk protein within RA (t = 3.21, P = 0.01, see Fig. 5C and compare Fig. 3A and D).
4. Discussion
During the emergence of stereotyped vocal behavior in juveniles through its maintenance in adults, we observed a correlated increase in singing-driven zenk immunoreactivity in RA. In contrast, zenk immunoreactivity in HVC was present at similar levels regardless of stage of vocal development. This led us to compare the expression levels of both zenk mRNA and protein at different stages of vocal development (subsong, plastic song, adult song). This comparison revealed a robust induction of zenk mRNA in HVC and RA, and zenk immunoreactivity in HVC, at all developmental stages. However, zenk immunoreactivity in RA was low during subsong, increasing only as the song pattern crystallized. The relationship between vocal stereotypy and zenk protein expression was further tested by early induction of a stereotyped vocal pattern in juvenile birds. Relative to age-matched subsong controls, lMAN-lesioned juveniles showed dramatically increased zenk immunoreactivity in RA, demonstrating that the relationship between behavioral development and zenk protein expression in RA is independent of the age of the bird. A striking implication of these data is that levels of zenk protein in RA, but not HVC, change as a function of the form of vocal behavior (variable vs. stereotyped), and not age. Together, our findings reveal a previously unrecognized role for zenk expression in the formation and maintenance of behavioral stereotypy, and suggest the existence of developmental mechanisms that are involved in the post-transcriptional regulation of zenk.
4.1. Post-transcriptional regulation of zenk
The zenk mRNA and protein expression differences observed in RA during subsong may be due to a variety of post-transcriptional mechanisms including stability of zenk mRNA, zenk protein sequestration, or increased zenk protein degradation. In the case of mammalian zenk, mRNA secondary structure appears to be an important post-transcriptional control. For example, the 5′ noncoding regions of zif268 [5] and krox-24 [20] have multiple initiation codons that are predicted to impart distinct secondary structure. In other systems, these types of secondary structure differences are related to differences in protein expression levels (for review, see Ref. [18]). Knowledge of the complete zebra finch zenk mRNA sequence will be necessary to determine structural features that may be involved in its post-transcriptional control.
Sequestration of zenk as a post-transcriptional control is plausible as two repressor proteins have recently been identified (NAB1 and NAB2), both of which interact directly with the mammalian zenk homologue, NGFI-A [31,36]. NAB1 and NAB2 transcripts are expressed in both mammalian brain and peripheral tissues, and an interesting truncated splice variant of NAB1 is expressed exclusively in brain. Several in vitro experiments have demonstrated the ability of NAB proteins to repress the transcriptional activity of NGFI-A through a direct protein–protein interaction [30,36,37]. Interaction between zenk and proteins such as NAB1 may result in diminished epitope access, possibly contributing to the observed reduction of zenk immunoreactivity in RA of subsong birds. Our recent isolation and sequencing of a fragment of cDNA encoding zebra finch NAB1 (GenBank accession # AF219136) will allow us to begin to test this hypothesis.
4.2. lMAN and zenk protein regulation
Our data also suggest a regulatory mechanism that may be involved in the inhibition of zenk expression in birds producing subsong, namely lMAN afferent input to RA. Such a role for lMAN is reinforced by data showing that lMAN synaptic input to RA is necessary for vocal variability, during song learning in juveniles (present results and Refs. [3,32]) and song deterioration in deafened adults [4]. Moreover, there is a naturally occurring loss of lMAN synaptic input to RA during the developmental period that corresponds to the emergence of vocal stereotypy in juvenile birds [10]. Thus, the developmental loss of lMAN synaptic input to RA may release post-transcriptional inhibition of zenk expression in RA neurons, thereby leading to stability of the vocal pattern. Interestingly, both the naturally occurring and lesion-induced loss of lMAN synaptic input to RA are paralleled by increased synaptic efficacy between HVC and RA neurons [17]. Since zenk homologues have been shown to promote transcription of gene products associated with synaptic activity [2], one possibility is that expression of zenk protein in RA influences the efficacy of synapses received from HVC.
Another potential source of regulation could come from auditory input to the song control system; auditory feedback is required for song learning in juveniles and maintenance of stereotyped adult song [26,29] and RA neurons show selective auditory responses to playback of song [8]. We emphasize, however, we do not yet know whether it is changes in the form of behavior that drive changes in zenk expression, or whether it is changes in zenk expression that lead to changes in the form of vocal behavior. We also do not know whether the interaction between vocal stereotypy and zenk expression is a direct one or involves intermediary steps [28].
5. Conclusion
To the extent that vocal stereotypy is dependent on processes of learning and memory, our findings suggest a novel association between post-transcriptional regulation of gene expression and cognitive development. While we have characterized the potential role of the zenk protein in strictly behavioral terms, note that the development and maintenance of vocal stereotypy implies a role in processes of memory consolidation [38]. Moreover, differential transcriptional vs. post-transcriptional control raises the possibility that motor-driven gene expression could function as an associative mechanism, where behavioral events that induce transcriptional activity could be linked to stimulus events with the ability to regulate post-transcriptional processes.
Understanding relationships and interactions between environment, behavior, development, and gene expression will be a major goal for future research, and will be important for a better appreciation of the complex interplay between nature and nurture in the determination of behavior. In addition to interactions with the environment [6], the present results suggest that when attempting to isolate genetic influences on behavior it may be necessary to consider interactions with the development of the behavior itself.
Acknowledgements
O.W. is an American Psychological Association MFP Fellow in Neuroscience and K.S. is supported by an NRSA grant (DA05986-01). This research was supported by an NIH grant (DC02035-07) to F.J.
Footnotes
Published on the World Wide Web on 10 August 2000.
A portion of this research was presented at the 29th Annual Meeting of the Society for Neuroscience, October 1999.
References
- 1.Arnold AP. Quantitative analysis of sex differences in hormone accumulation in the zebra finch brain: methodological and theoretical issues. J. Comp. Neurol. 1980;189:421–426. doi: 10.1002/cne.901890302. [DOI] [PubMed] [Google Scholar]
- 2.Beckmann AM, Wilce PA. Egr transcription factors in the nervous system. Neurochem. Int. 1997;4:447–510. doi: 10.1016/s0197-0186(96)00136-2. [DOI] [PubMed] [Google Scholar]
- 3.Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- 4.Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- 5.Christy BA, Lau LF, Nathans D. A gene activated in mouse 3T3 cells by serum growth factors encodes a protein with ‘zinc finger’ sequences. Proc. Natl. Acad. Sci. USA. 1988;85:7857–7861. doi: 10.1073/pnas.85.21.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 7.Dittrich F, Feng Y, Metzdorf R, Gahr M. Estrogen-inducible, sex-specific expression of brain-derived neurotrophic factor mRNA in a forebrain song control nucleus of the juvenile zebra finch. Proc. Natl. Acad. Sci. USA. 1999;96:8241–8246. doi: 10.1073/pnas.96.14.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doupe AJ, Konishi M. Song selective auditory circuits in the vocal control system of the zebra finch. Proc. Natl. Acad. Sci. USA. 1991;88:11339–11343. doi: 10.1073/pnas.88.24.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guthrie KM, Nguyen T, Gall CM. Insulin-like growth factor-1 mRNA is increased in deafferented hippocampus: spatiotemporal correspondence of a trophic event with axon sprouting. J. Comp. Neurol. 1995;352:147–160. doi: 10.1002/cne.903520111. [DOI] [PubMed] [Google Scholar]
- 10.Herrmann K, Arnold AP. The development of afferent projections to the robust archistriatal nucleus in male zebra finches: a quantitative electron microscopic study. J. Neurosci. 1991;11:2063–2074. doi: 10.1523/JNEUROSCI.11-07-02063.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Immelmann K. Song development in the zebra finch and other Estrildid finches. In: Hinde RA, editor. Bird Vocalizations. Cambridge: Cambridge University Press; 1969. pp. 61–74. [Google Scholar]
- 12.Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc. Natl. Acad. Sci. USA. 1997;94:4097–4102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron. 1998;21:775–788. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- 14.Jin H, Clayton DF. Localized changes in immediate early gene regulation during sensory and motor learning in zebra finches. Neuron. 1997;19:1049–1059. doi: 10.1016/s0896-6273(00)80396-7. [DOI] [PubMed] [Google Scholar]
- 15.Johnson F, Bottjer SW. Afferent influences on cell death and birth during development of a cortical nucleus necessary for learned vocal behavior in zebra finches. Development. 1994;120:13–24. doi: 10.1242/dev.120.1.13. [DOI] [PubMed] [Google Scholar]
- 16.Johnson F, Norstrom E, Soderstrom K. Increased expression of endogenous biotin, but not BDNF, in telencephalic song regions during zebra finch vocal learning. Brain Res. Interactive /Dev. Brain Res. 2000;120:113–123. doi: 10.1016/s0165-3806(00)00002-x. [DOI] [PubMed] [Google Scholar]
- 17.Kittelberger JM, Mooney R. Lesions of an avian forebrain nucleus that disrupt song development alter synaptic connectivity and transmission in the vocal premotor pathway. J. Neurosci. 1999;19:9385–9398. doi: 10.1523/JNEUROSCI.19-21-09385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J. Cell Biol. 1991;115:887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemaire P, Revelant O, Bravo R, Charnay P. Two mouse genes encoding potential transcription factors with identical DNA-binding domains are activated by growth factors in cultured cells. Proc. Natl. Acad. Sci. USA. 1988;85:4691–4695. doi: 10.1073/pnas.85.13.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemaire P, Vesque C, Schmitt J, Stunnenberg H, Frank R, Charnay P. The serum-inducible mouse gene Krox-24 encodes a sequence-specific transcriptional activator. Mol. Cell. Biol. 1990;10:3456–3467. doi: 10.1128/mcb.10.7.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim RW, Varnum BC, Herschman HR. Cloning of tetradecanoyl phorbolester-induced ‘primary response’ sequences and their expression in density-arrested Swiss 3T3 cells and a TPA non-proliferative variant. Oncogene. 1987;1:263–270. [PubMed] [Google Scholar]
- 22.Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J. Neurosci. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mello CV, Rubiero S. Zenk protein regulation by song in the brain of songbirds. J. Comp. Neurol. 1998;393:426–438. doi: 10.1002/(sici)1096-9861(19980420)393:4<426::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc. Natl. Acad. Sci. USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987;238:797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- 26.Nordeen KW, Nordeen EJ. Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behav. Neural Biol. 1992;57:58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- 27.Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary. J. Comp. Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 28.Pfaff DW. Hormones, genes, and behavior. Proc. Natl. Acad. Sci. USA. 1997;94:14213–14216. doi: 10.1073/pnas.94.26.14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price PH. Developmental determinants of structure in zebra finch song. J. Comp. Physiol. Psychol. 1979;93:260–277. [Google Scholar]
- 30.Qu Z, Wolfraim LA, Svaren J, Ehrengruber MU, Davidson N, Milbrandt J. The transcriptional corepressor NAB2 inhibits NGF-induced differentiation of PC12 cells. J. Cell Biol. 1998;142:1075–1082. doi: 10.1083/jcb.142.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo MW, Sevetson BR, Milbrandt J. Identification of NAB1, a repressor of NGFI-A and Krox20-mediated transcription. Proc. Natl. Acad. Sci. USA. 1995;92:6873–6877. doi: 10.1073/pnas.92.15.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finchsong system: implication for vocal learning. J. Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson HB, Vicario DS. Brain pathways for learned and unlearned vocalizations differ in zebra finches. J. Neurosci. 1990;10:1541–1556. doi: 10.1523/JNEUROSCI.10-05-01541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav. Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- 35.Sukhatme VP, Cao XM, Chang LC, Tsai-Morris CH, Stamenkovich D, Ferreira PC, Cohen DR, Edwards SA, Shows TB, Curran T, LeBeau MM, Adamson ED. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988;53:37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- 36.Svaren J, Sevetson BR, Apel ED, Zimonjic DB, Popescu NC, Milbrandt J. NAB2, a corepressor of NGFI-A (Egr-1) and Krox20, is induced by proliferative and differentiative stimuli. Mol. Cell. Biol. 1996;6:3545–3553. doi: 10.1128/mcb.16.7.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swirnoff AH, Apel ED, Svaren J, Sevetson BR, Zimonjic DB, Popescu NC, Milbrandt J. Nab1, a corepressor of NGFI-A (Egr-1), contains an active transcriptional repression domain. Mol. Cell. Biol. 1998;18:512–524. doi: 10.1128/mcb.18.1.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tischmeyer W, Grimm R. Activation of immediate early genes and memory formation. Cell. Mol. Life Sci. 1999;55:564–574. doi: 10.1007/s000180050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tramontin AD, Smith GT, Breuner CW, Brenowitz EA. Seasonal plasticity and sexual dimorphism in the avian song control system: stereological measurement of neuron density and number. J. Comp. Neurol. 1998;396:186–192. doi: 10.1002/(sici)1096-9861(19980629)396:2<186::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]