Abstract
Background
Few studies have examined protective factors for diabetes distress.
Purpose
To examine the moderating role of social support in the relationship between the burden of diabetes and diabetes distress.
Methods
Adults with type 2 diabetes (N=119; 29% Latino, 61% Black, 25% White) completed validated measures of diabetes distress and social support. Multiple linear regression evaluated the moderating role of social support in the relationship between diabetes burden, indicated by prescription of insulin and presence of complications, and distress.
Results
Greater support satisfaction was significantly associated with lower distress after controlling for burden. Support satisfaction and number of supports significantly moderated the relationship between diabetes burden and distress. Post-hoc probing revealed a consistent pattern: Insulin was significantly associated with more diabetes distress at low levels of support but was not at high levels of support.
Conclusion
Findings support the stress-buffering hypothesis and suggest that social support may protect against diabetes distress.
Keywords: Social support, Buffering hypothesis, Diabetes-related distress, Insulin, Disease burden
Introduction
Diabetes affects 25.8 million people and 8.3 percent of the U.S. population [1]. The prevalence continues to increase, with the number of people with diabetes estimated to reach 330 million by 2030 [2]. Diabetes is a complex, chronic disease that requires burdensome patient self-management, involving daily decisions concerning diet, physical activity, blood glucose monitoring, and consistent medication adherence, including daily insulin injections for some. Diabetes can affect many organs of the body, increasing the risk of complications such as retinopathy, nephropathy, neuropathy, and cardiovascular disease [3,4]. Diabetes complications are a significant cause of increased morbidity and mortality among individuals with diabetes [5]. In addition, the substantial burdens of impaired functioning and the demands of self-management can contribute to significant emotional distress.
Depression is more common in individuals with diabetes than in the general population [6]. Meta-analyses suggest that depression is between 60% and 100% more common in adults living with diabetes [6, 7]. However, data also suggest that depression is elevated only among those with diagnosed diabetes; no increase is observed among individuals with impaired fasting glucose or undiagnosed diabetes [8–11]. Treatment intensity also appears to be associated with risk of depression [9]. For example, a population-based survey found that type 2 diabetes patients prescribed insulin had significantly higher likelihood of depression than those not prescribed insulin [12]. Prescription of insulin may not only indicate increased burden of treatment and higher demands for self-management, but it may also indicate a further progression of illness [13]. Furthermore, research demonstrates that most individuals with diabetes who endorse depressive symptoms on self-report measures are not clinically depressed [6,14,15]. Emotional distress specific to living with the burden of diabetes and its management, or diabetes distress, is more common than depression among patients and is more closely associated with problematic diabetes self-management and glycemic control [13, 16–23]. Change in diabetes distress, but not change in depressive symptoms, has been associated with change in glycemic control following a diabetes education intervention [24, 25]. Thus, diabetes distress is closely linked with diabetes-specific biological and behavioral variables and reflects distress resulting from the burden of illness and treatment.
Diabetes complications have been consistently shown to predict emotional distress in individuals with diabetes and have been characterized as the most important disease-specific determinant of quality of life in diabetes [26]. For example, studies have demonstrated close relationships between diabetic peripheral neuropathy severity, neuropathy symptoms, and functional impairment on the one hand and symptoms of depression on the other, both cross-sectionally and longitudinally [27, 28]. Moreover, diabetes complications have been cross-sectionally associated with increased diabetes distress [25, 29, 30] and predict the onset of significant diabetes distress over time, with the occurrence of negative life events amplifying the strength of this relationship [25]. Type 2 diabetes patients prescribed insulin, who often have greater diabetes severity and more demanding self-management requirements, also report increased diabetes distress as compared to patients on oral medications only or those not prescribed medication [13, 31]. Thus, there is considerable evidence to suggest that the burden of diabetes and its treatment contributes to emotional distress in general, and more specifically, to diabetes distress. However, the extent to which psychosocial resources may protect individuals from the impact of diabetes burden on emotional distress has received little attention.
Social support is a psychosocial resource that has consistent associations with better physical and mental health, throughout a range of populations [32, 33]. Researchers have taken various approaches in defining social support. Some define it as the process through which help is provided to others [34], while others focus on the provision of informational feedback or an exchange of resources, where the intent is to increase the well being of the receiver [35, 36]. Most measures of social support fall into one of following categories: 1) social network characteristics that are determined by the degree to which a person is socially integrated; 2) received support measures that evaluate what support a person has received; and 3) perceived support measures that assess perceptions about the availability and adequacy of support [37].
Whether diabetes-related burden will lead to emotional distress may depend on the quantity and quality of social support. Several studies have shown that medical patients who report more social support also report better adjustment and less emotional distress [38–41]. The buffering model of social support holds that health-related stressors will have deleterious effects on the health of those with little or no social support, while these effects will be lessened or eliminated for those with stronger support [42]. Thus, the role of social support as a protective factor may be most evident in individuals facing stressful life circumstances.
Several studies have provided support for the buffering hypothesis. One study reported that the relationship between chronic disease and depressive symptoms weakened in the presence of higher levels of instrumental social support in an older adult population [43]. Another found a moderating effect of perceived social support in the relationship between chronic physical illness and depression and anxiety disorders among low-income primary care patients [44]. Similarly, a study of type 1 diabetes indicated that depressive symptoms increased significantly more for individuals with lower perceived social support than for individuals with higher social support in the face of greater physical impairment, supporting the buffering model of social support [45]. To our knowledge, buffering effects of social support on diabetes distress in type 2 diabetes have not been examined. Also, although prior studies of the buffering hypothesis have compared those with and without chronic illness, we are unaware of tests of the buffering hypothesis that examine different levels of illness burden among individuals with diabetes. Given the evidence for associations between illness burden and emotional distress in type 2 diabetes, we believe this is an important area for further investigation.
The present study examines the association between diabetes burden and diabetes-related emotional distress, and the moderating role of social support in this relationship. We evaluate evidence for the buffering hypothesis at two levels of analysis. First, we conceptualize diagnosed type 2 diabetes as a significant stressor and examine whether social support is associated with reduced distress among adults treated for type 2 diabetes. We hypothesize that the quantity and quality of social support will be associated with significantly lower diabetes distress overall. At the second level of analysis, we conceptualize the occurrence of diabetes complications and the prescription of insulin therapy as indicators of increased disease- and treatment-related burden, and hypothesize that each of these indicators will be associated with increased diabetes distress. We also hypothesize that the strength of these relationships will be moderated by social support. As social support increases, we expect an attenuation of the effects of disease burden on distress.
Method
Participants and Procedures
Adults with type 2 diabetes were recruited via direct clinician referrals, clinic screenings, fliers and mailings to participate in a larger study of distress and treatment adherence. Participants were recruited from diabetes specialty care clinics and primary care practices affiliated with an academic medical center serving a predominantly ethnic minority and socio-economically disadvantaged urban population. Eligible participants were required to be taking either oral medication or insulin to treat diabetes. Participants (N=119) provided informed consent; completed self-report questionnaires and a blood draw; and received $50 compensation. The Institutional Review Board at the Albert Einstein College of Medicine approved all study procedures.
Measures
Diabetes Burden: complications and insulin use
The presence of diabetes complications was assessed using a 7-item self-report questionnaire inquiring whether the patient had ever received a diagnosis or had a medical procedure (e.g., laser surgery of the eyes) to indicate the presence of diabetes complications [46]. Dichotomous responses (yes/no) were used to assess presence of retinopathy (2 items), nephropathy (1item), neuropathy (1 item), and cardiovascular complications (3 items). The complication variable was coded as 1 if at least one of the complications was present and 0 if none was reported. We treated complications as a dichotomous variable, with approximately half of our sample reporting at least one of the four diabetes complications. We chose a dichotomous approach to complications because we conceptualize the diagnosis of any complication of diabetes to be a major stressor in the course of type 2 diabetes, indicating advancing illness and possible functional impairment. Prescription of insulin was assessed by self-report and details of the prescription (name, dose, frequency) were obtained from participants.
Social Support Questionnaire (SSQ)
The 6-item Social Support Questionnaire (SSQ) assesses perceptions of the number and quality of available social supports [47]. For each item, respondents first list the number of individuals available for support in specific circumstances (e.g., “Whom can you really count on to be dependable when you need help?”), and then indicate how satisfied they are with that support using a 6-point Likert-type scale ranging from very dissatisfied (1) to very satisfied (6). The means of each of the two parts are summed separately to create two scores: average number of social supports (sample range = 0 – 51.67) and average satisfaction with social support (range = 1–6). Cronbach’s alpha (α) for number of social supports and satisfaction with social support were .80 and .95, respectively.
Diabetes Distress Scale (DDS)
The Diabetes Distress Scale (DDS) is a validated 17-item self-report measure with each item scored on a Likert scale from 1 (no distress) to 6 (serious distress) concerning distress experienced over the last month [48]. The scale yields four reliable subscales via item mean scores: emotional burden (e.g., “feeling angry, scared, and/or depressed when I think about living with diabetes,” “feeling overwhelmed by the demands of living with diabetes;” α = .92), physician-related distress (e.g., “feeling that my doctor doesn’t take my concerns seriously enough,” “feeling that my doctor doesn’t give me clear enough directions on how to manage my diabetes;” α = .89), regimen-related distress (e.g., “feeling that I am often failing with my diabetes routine,” “not feeling confident in my day-to-day ability to manage diabetes;” α= .91), and interpersonal distress (e.g., “feeling that friends or family don’t appreciate how difficult living with diabetes can be,” “feeling that friends or family are not supportive enough of self-care efforts;” α = .89). The regimen distress scale assesses perceived problems with diabetes self-management and is thus somewhat distinct in content from the other subscales. The total score is derived as the mean of all 17 items. Internal reliability of the total scale was excellent (α = .95). All scales are treated as continuous variables. Clinical validation of the DDS suggests that the following thresholds of severity should be applied when interpreting scores: little or no distress < 2.0, moderate distress = 2.0–2.9, and high distress ≥ 3.0 [49].
Statistical analyses
In the first set of analyses, descriptive statistics were used to assess frequency of responses for demographic variables and study variables. Distributions of all variables used in the analysis were examined for normality. Based on this examination, the number of reported social supports from the SSQ was log-transformed to improve substantial kurtosis and positive skew. Second, bivariate relationships among social support, diabetes complications, insulin and diabetes distress were examined. The four DDS subscales were also included to assess different aspects of diabetes distress that might explain significant effects on the total score. Finally, hierarchical multiple linear regression models were used to test the moderating effect of social support on the effects of complications and insulin use on diabetes distress. Covariates of age and gender were included in all models, based on previously reported relationships to diabetes distress in the literature (e.g., [13]). Two sets of analyses were performed using two different moderator variables: 1) the average number of supports and 2) the perceived satisfaction with social support. In each model, covariates of age and gender were entered in Step 1, along with the two indicators of diabetes burden and the relevant social support variable (each in separate models). The interaction effect between each of the indicators of burden (in separate models) and social support was tested in Step 2. Finally, the independence of the interaction effects was examined by entering support interaction effects for complications and insulin (together) in Step 3. To interpret a significant interaction, post-hoc probing was conducted using SPSS 20.0 [50] and the PROCESS macro was used to estimate effects of burden at Mean and +/−1 SD values of the social support moderators; predicted scores were also plotted for each level of burden for mean and +/−1SD values of support to aid in visualization of the moderation effects [51, 52].
Results
Sample characteristics
One hundred and nineteen ethnically diverse adults with type 2 diabetes completed the study (Table 1). On average, participants had been living with diabetes for 13 years. The average HbA1c was nearly a full point higher than the recommended level of 7.0% [53]. Approximately 41% were taking insulin and a little over half reported at least one diabetes complication. Of those with complications, 30% reported retinopathy, 15% nephropathy, 24% cardiovascular disease, and 27% reported neuropathy. The average DDS score indicated “moderate” distress. Participants’ average number of social supports was 3 and average level of satisfaction was “fairly satisfied.”
Table 1.
Participant Characteristics (N = 119)
| Age, M (SD) | 56.3 (9.69) |
| Sex, % (n) | |
| Female (n) | 63.9 (76) |
| Male (n) | 36.1 (43) |
| Race, (n=114), % (n) | |
| Black or African American | 61.4 (70) |
| White | 25.4 (29) |
| Other | 13.2 (15) |
| Ethnicity, (n = 108) % (n) | |
| Hispanic | 28.7 (31) |
| Education Level, (n=118), % (n) | |
| Less than high school diploma | 17.8 (21) |
| High school diploma | 15.3 (18) |
| Some college | 32.2 (38) |
| College degree | 21.2 (25) |
| Some graduate school or degree | 13.6 (16) |
| Yearly Family Income, (n = 113), % (n) | |
| Less than 10,000 | 18.6 (21) |
| Between $10,000–$14,999 | 15.9 (18) |
| $15,000–$24,999 | 18.6 (21) |
| $25,000–$49,999 | 29.2 (33) |
| $50,000–$99,999 | 15.9 (18) |
| $100,000–$149,999 | 1.8 (2) |
| Years since diagnosis, (n=114) M (SD) | 13.3 (9.4) |
| HbA1c, (n=119), M (SD) | 7.9 (1.9) |
| Prescribed insulin, % (n) | 41.2 (49) |
| Diabetes complications, % (n) | |
| No complications | 48.7 (58) |
| At least one complication | 51.3 (61) |
| Total Diabetes Distress, M (SD) | 2.3 (1.2) |
| Emotional Burden | 2.5 (1.5) |
| Regimen Distress | 2.7 (1.5) |
| Interpersonal Distress | 2.0 (1.4) |
| Physician-related Distress | 1.8 (1.3) |
| Number of supports, (n=115), Mdn | 3.3 |
| Support satisfaction, M (SD) | 4.8 (1.4) |
Bivariate relationships among social support, complications, insulin and diabetes distress
Insulin use and diabetes complications shared significant overlap (ϕ = .33, p < .001). Approximately 71% of those taking insulin had diabetes complications whereas 38% of those not prescribed insulin reported complications (χ2(1) = 12.95, p < .001). Correlations also showed that those with complications were significantly more likely than those without to report higher levels of emotional burden. As compared to patients taking oral medications only, those prescribed insulin had more diabetes distress, emotional burden, and greater perceived difficulty with diabetes self-management (i.e., regimen distress). There was a significant negative correlation between social support satisfaction and interpersonal distress. No other significant relationships between indicators of diabetes burden and social support were found. See Table 2.
Table 2.
Correlation Matrix
| DDS | Emotional Burden | Regimen Distress | Physician related distress | Interpersonal distress | Complications (yes/no) | Insulin (yes/no) | |
|---|---|---|---|---|---|---|---|
| Complications (yes/no) | .17 | .20* | .13 | .11 | .15 | -- | -- |
| Insulin (yes/no) | .26** | .27** | .26** | .18 | .15 | .33** | -- |
| Social Support (number) | −.08 | −.14 | −.06 | −.12 | −.11 | −.09 | −.08 |
| Satisfaction with Support (level) | −.20* | −.19* | −.11 | −.11 | −.33** | −.03 | .01 |
Note. DDS = Diabetes Distress Scale. Pearson correlation coefficients were used except for the dichotomous variables (i.e., presence of complication and insulin use), in which the phi-coefficient was used.
p < .05,
p < .01.
Buffering models: Perceived satisfaction with social support as a moderator
Linear regression models tested whether satisfaction with social support moderated the relationship between diabetes complications or insulin and diabetes distress (Table 3). Neither diabetes complications nor insulin use were independently associated with diabetes distress in Step 1 of these models. Support satisfaction was significantly associated with lower total diabetes distress, emotional burden and interpersonal distress, independent of insulin use, complications and covariates. The interaction between insulin use and support satisfaction (Step 2b) was significant in the prediction of total diabetes distress (R2Δ = .03, p = .036) and physician-related distress (R2Δ = .06, p = .007).
Table 3.
Summary of hierarchical regression analyses: the moderating role of level of satisfaction with social support in the relationship between diabetes burden and diabetes distress.
| DDS total | Emotional | Regimen | Interpersonal | Physician | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | p | R2 | b | p | R2 | b | p | R2 | b | p | R2 | b | p | R2 | |
| Step 1: Age | −.02 | .067 | .14 | −.02 | .138 | .14 | −.04 | .011 | .14 | −.01 | .453 | .16 | −.01 | .345 | .06 |
| Sex | .10 | .671 | .14 | .612 | .25 | .355 | −.38 | .143 | .20 | .438 | |||||
| Insulin | .46 | .060 | .57 | .052 | .52 | .074 | .29 | .290 | .38 | .169 | |||||
| Comp | .37 | .127 | .49 | .094 | .40 | .171 | .33 | .238 | .22 | .436 | |||||
| SAT | −.16 | .039 | −.19 | .045 | −.10 | .281 | −.29 | .001 | −.11 | .232 | |||||
|
| |||||||||||||||
| Step 2a: Age | −.02 | .066 | .14 | −.02 | .133 | .14 | −.04 | .011 | .14 | −.01 | .429 | .18 | −.01 | .350 | .06 |
| Sex | .09 | .689 | .13 | .647 | .26 | .344 | −.41 | .121 | .21 | .434 | |||||
| Insulin | .46 | .061 | .56 | .053 | .52 | .075 | .29 | .293 | .38 | .170 | |||||
| Comp | .61 | .430 | 1.1 | .221 | .03 | .970 | 1.4 | .101 | .06 | .950 | |||||
| SAT | −.14 | .215 | −.12 | .358 | −.14 | .291 | −.17 | .160 | −.12 | .327 | |||||
| Comp x SAT | −.05 | .746 | −.13 | .464 | .08 | .677 | −.23 | .181 | .03 | .847 | |||||
|
| |||||||||||||||
| Step 2b: Age | −.02 | .058 | .18 | −.02 | .127 | .16 | −.04 | .010 | .15 | −.01 | .438 | .18 | −.01 | .309 | .12 |
| Sex | .10 | .672 | .14 | .613 | .25 | .355 | −.38 | .140 | .20 | .431 | |||||
| Insulin | 2.0 | .010 | 2.1 | .027 | 1.8 | .057 | .1.4 | .109 | 2.7 | .003 | |||||
| Comp | .37 | .120 | .49 | .090 | .40 | .168 | .33 | .235 | .22 | .418 | |||||
| SAT | −.04 | .679 | −.07 | .546 | .00 | .996 | −.20 | .068 | .07 | .503 | |||||
| Insulin x SAT | −.33 | .036 | −.32 | .090 | −.27 | .153 | −.24 | .178 | −.47 | .007 | |||||
|
| |||||||||||||||
| Step 3: Age | −.02 | .059 | .18 | −.02 | .126 | .16 | −.04 | .010 | .15 | −.01 | .421 | .19 | −.01 | .319 | .12 |
| Sex | .10 | .670 | .13 | .643 | .26 | .334 | −.40 | .123 | .21 | .405 | |||||
| Insulin | 2.0 | .011 | 2.0 | .037 | 1.9 | .046 | 1.3 | .169 | 2.8 | .002 | |||||
| Comp | .31 | .685 | .86 | .357 | −.23 | .805 | 1.3 | .158 | −.40 | .647 | |||||
| SAT | −.05 | .700 | −.04 | .794 | −.06 | .684 | −.12 | .374 | .02 | .898 | |||||
| Comp x SAT | .01 | .934 | −.08 | .678 | .13 | .478 | −.19 | .272 | .13 | .456 | |||||
| Insulin x SAT | −.33 | .039 | −.30 | .114 | −.29 | .125 | −.20 | .266 | −.50 | .005 | |||||
|
| |||||||||||||||
| Conditional effects for Insulin | Effect | p | 95% CI | Effect | p | 95% CI | |||||||||
|
| |||||||||||||||
| −1SD | 1.1 | .001 | .45–1.7 | 1.1 | .001 | .46–1.8 | |||||||||
| M | .60 | .006 | .17-.10 | .46 | .057 | −.01–.94 | |||||||||
| +1SD | −.22 | .441 | −.34–.78 | −.10 | .761 | −.72–.53 | |||||||||
Note: COMP = Presence of complications, SAT = level of perceived satisfaction with social support. Significant p-values (p < .05) are in bold.
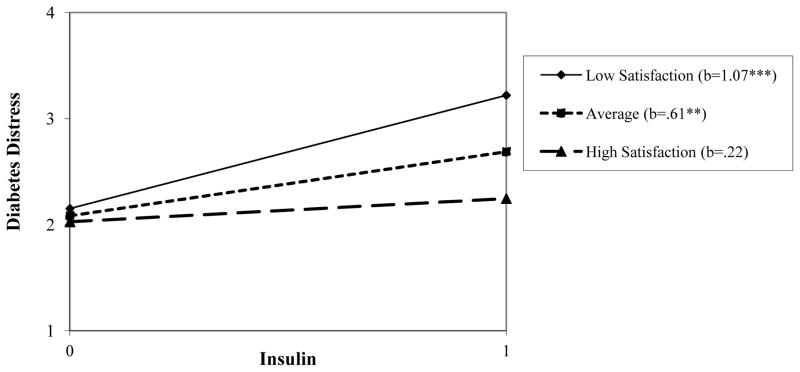
Post-hoc probing of these significant moderation effects showed that at 1SD below the mean of support satisfaction, the conditional effect of insulin use on total diabetes distress was significant, increasing predicted distress scores by 1.07 units. The conditional effect of insulin was smaller at average levels of satisfaction, but remained significant. However, at high levels of support satisfaction (1SD above the mean), the effect of insulin on total diabetes distress was reduced to non-significance. See bottom row of Table 3 and Figure 1. A similar pattern of conditional effects was found for insulin predicting physician-related distress: The effect was significant at low levels of support satisfaction, more modest (and short of significance) at average levels, and near zero at high levels of support. No significant interaction effects were found for complications and satisfaction support. See Table 3, Step 2a and Step 3.
Figure 1.
Regression lines for the relationship between insulin prescription (0 = not prescribed, 1 = prescribed) and diabetes distress, as moderated by level of social support satisfaction. b = unstandardized regression coefficient (i.e., simple slope) **p < .01, ***p < .001
Buffering Models: Number of social supports as a moderator
Another multiple linear regression model was used to test the moderating effect of number of social supports on the relationship between diabetes complications and diabetes distress (Table 4). The log of reported number of individuals available to provide social support was not significantly associated with total diabetes distress but was significantly associated with lower emotional burden and interpersonal distress in Step 1 of these models. Step 2a results demonstrated significant interaction effects between diabetes complications and the number of social supports for total diabetes distress and interpersonal distress. However, these interaction effects were reduced to non-significance in the presence of significant insulin-by-support interactions, indicating a lack of independence (Step 3). In contrast, support number significantly moderated the effect of insulin use in relation to total diabetes distress (R2Δ = .09, p = .001) and each DDS subscale (See Step 2b). These effects were independent of complication by support interactions (Step 3). Therefore, only Step 2b insulin interactions were examined via probing.
Table 4.
Summary of hierarchical regression analyses: the moderating role of number of social supports in the relationship between diabetes burden and diabetes distress
| DDS total | Emotional | Regimen | Interpersonal | Physician | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | p | R2 | b | p | R2 | b | p | R2 | b | p | R2 | b | p | R2 | |
| Step 1: Age | −.02 | .052 | .13 | −.03 | .086 | .15 | −.04 | .013 | .12 | −.01 | .316 | .14 | −.02 | .298 | .07 |
| Sex | .10 | .687 | .20 | .486 | .16 | .580 | −.38 | .168 | .25 | .351 | |||||
| Insulin | .44 | .081 | .53 | .081 | .54 | .072 | .25 | .393 | .37 | .198 | |||||
| Comp | .31 | .234 | .43 | .158 | .32 | .289 | .25 | .391 | .17 | .562 | |||||
| SS# | −.64 | .087 | −.97 | .031 | .00 | .994 | −1.2 | .008 | −.66 | .119 | |||||
|
| |||||||||||||||
| Step 2a: Age | −.03 | .046 | .16 | −.03 | .079 | .17 | −.04 | .012 | .15 | −.02 | .292 | .18 | −.02 | .292 | .08 |
| Sex | .06 | .813 | .16 | .581 | .11 | .690 | .43 | .110 | .23 | .399 | |||||
| Insulin | .46 | .068 | .54 | .070 | .56 | .062 | .27 | .348 | .37 | .189 | |||||
| Comp | 1.3 | .021 | 1.4 | .028 | 1.4 | .035 | 1.5 | .015 | .71 | .247 | |||||
| SS# | .24 | .682 | −.05 | .947 | .97 | .160 | .01 | .990 | −.15 | .815 | |||||
| Comp x SS# | −1.5 | .048 | −1.5 | .082 | −1.6 | .067 | −1.9 | .022 | −.84 | .315 | |||||
|
| |||||||||||||||
| Step 2b: Age | −.03 | .026 | .22 | −.03 | .058 | .20 | −.04 | .007 | .18 | −.02 | .212 | .23 | −.02 | .197 | .17 |
| Sex | .15 | .503 | .25 | .375 | .21 | .445 | −.32 | .228 | .31 | .222 | |||||
| Insulin | 2.0 | .000 | 1.8 | .002 | 2.0 | .001 | 2.0 | .001 | 2.1 | .000 | |||||
| Comp | .37 | .133 | .49 | .104 | .38 | .196 | .32 | .249 | .24 | .387 | |||||
| SS# | .44 | .346 | −.02 | .967 | 1.0 | .069 | .07 | .896 | .55 | .293 | |||||
| Insulin x SS# | −2.5 | .001 | −2.1 | .012 | −2.4 | .006 | −2.8 | .001 | −2.8 | .001 | |||||
|
| |||||||||||||||
| Step 3: Age | −.03 | .026 | .23 | −.03 | .059 | .20 | −.04 | .007 | .19 | −.02 | .215 | .24 | −.02 | .198 | .17 |
| Sex | .13 | .571 | .22 | .439 | .18 | .515 | .35 | .181 | .32 | .215 | |||||
| Insulin | 1.8 | .001 | 1.7 | .009 | 1.8 | .004 | 1.7 | .004 | 2.1 | .000 | |||||
| Comp | .76 | .162 | 1.0 | .125 | .91 | .166 | .98 | .117 | .09 | .880 | |||||
| SS# | .72 | .271 | .35 | .620 | 1.4 | .046 | .53 | .427 | .45 | .486 | |||||
| Comp x SS# | −.62 | .417 | −.84 | .366 | −.83 | .368 | −1.0 | .239 | .23 | .790 | |||||
| Insulin x SS# | −2.2 | .003 | −1.8 | .045 | −2.1 | .024 | −2.4 | .005 | −2.8 | .001 | |||||
|
| |||||||||||||||
| Conditional effects for Insulin | Effect | p | 95% CI | Effect | p | 95 % CI | Effect | p | 95% CI | Effect | p | 95% CI | Effect | p | 95% CI |
|
| |||||||||||||||
| −1SD | 1.3 | .000 | .68–1.9 | 1.3 | .001 | .58–2.0 | 1.4 | .000 | .63–2.7 | 1.2 | .001 | .48–1.8 | 1.2 | .000 | .57–1.9 |
| M | .54 | .016 | .10–.97 | .68 | .013 | .14–1.2 | .65 | .017 | .12–1.2 | .32 | .210 | −.18–.81 | .40 | .105 | −.08–.89 |
| +1SD | −.20 | .516 | −.82–.42 | .04 | .914 | −.72–.80 | −.07 | .862 | −.82–.68 | −.53 | .142 | −1.2–.18 | −.44 | .211 | −1.1–.25 |
Note. Comp = Presence of complications, SS#= number of social supports. Significant p-values (p < .05) are in bold.
Examination of conditional effects of insulin on diabetes distress revealed a consistent pattern: For total distress and all subscales, at low levels (−1SD) of log number of available social supports, insulin use was associated with significant elevations in diabetes distress. Though reduced in magnitude, these effects remained significant at average number of social supports for total diabetes distress, emotional burden and regimen distress. Across all distress variables, when the number of available social supports was high (+1SD), there was no significant effect of insulin use on distress. These conditional effects are reported in the bottom row of Table 4. As an example of the pattern of these consistent relationships, Figure 2 depicts the conditional relationship between insulin use and predicted total diabetes distress scores across levels of number of sources of social support.
Figure 2.
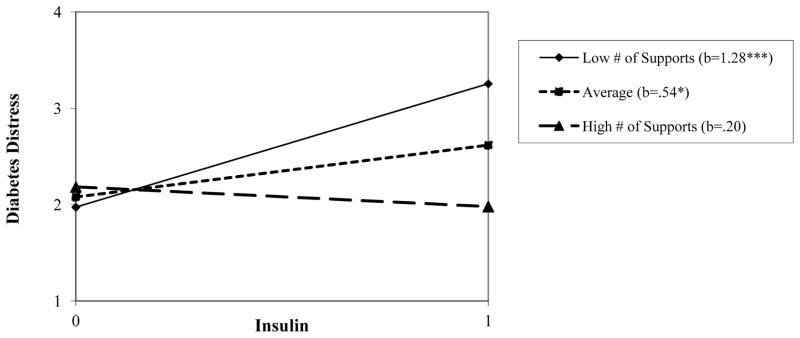
Regression lines for the relationship between insulin prescription (0 = not prescribed, 1 = prescribed) and diabetes distress, as moderated by number of social supports. b = unstandardized regression coefficient (i.e., simple slope) *p < .05, **p < .01, ***p < .001
Discussion
The present study documents evidence for increased diabetes distress among adults with type 2 diabetes who experience greater health- and treatment-related burden of diabetes. Prescription of insulin was significantly associated with increased overall diabetes distress, greater emotional burden of diabetes, and feelings of failure, inadequacy and lack of confidence in diabetes self-management (i.e., regimen distress). These relationships were independent of diabetes complications, which were reported by half our sample. Although complications were associated with greater diabetes emotional burden, this relationship was not independent of the effect of insulin. The independent relationship between insulin prescription and greater diabetes distress is consistent with prior work in type 2 diabetes and suggests that patients on insulin are at significant risk for illness-specific emotional distress [13,48].
Our primary aim was to evaluate social support as a potential protective factor against the distressing effects of diabetes burden. As social support is a multidimensional concept, we investigated two aspects: the level of satisfaction with available social support and the number of supportive individuals reported to be available in the participant’s network. We found support for the hypothesis that social support would be associated with reduced diabetes distress overall. Greater satisfaction with support was significantly associated with reduced total diabetes distress, reduced emotional burden of diabetes, and less diabetes-related interpersonal distress. Greater social network size was significantly associated with less diabetes-related emotional burden and interpersonal distress. These relationships were independent of diabetes burden. These findings are in line with earlier studies, which found that social support played an important role in diabetes-specific quality of life [54], and that enhanced social support was significantly associated with lower diabetes distress in women with type 2 diabetes [55]. Furthermore, another study reported that social support variables including supportive behaviors from healthcare providers and family were significantly associated with lower diabetes distress [29]. However, these studies did not examine the role of disease burden.
Findings also supported our expectations for a moderating role of social support in the relationship between diabetes burden and diabetes distress, as proposed by the buffering hypothesis [32, 35, 42]. Although moderation analyses demonstrated significant interaction effects between social support and both complications and insulin, only insulin interaction effects were independent. This suggests that to the extent that illness-related and treatment-related burden can be separated by the indicators used in the current study (discussed below), evidence is stronger for buffering effects for treatment-related burden. Post-hoc probing revealed a significant relationship between insulin use and total diabetes distress and physician-related distress at lower levels of support satisfaction, but at high levels of support satisfaction the effect of insulin was substantially attenuated and was non-significanct. Buffering effects for social network size were even more robust and consistent across every measured aspect of diabetes distress. When participants reported few individuals in their support network, insulin use was associated with increased emotional burden, interpersonal distress, regimen distress, and negative evaluations of physicians. When the number of available supports was high, none of these effects was significant. This demonstration of the buffering effect of social support is consistent and compelling.
Although we attempted to examine both illness-related burden and treatment-related burden associated with diabetes, it is important to acknowledge their overlap in this study and in the wider population of patients. Previous studies have consistently indicated worse health status and heath-related quality of life in individuals with type 2 diabetes treated with insulin [56–58]. For example, a recent cohort study showed that adults with type 2 diabetes who are treated with insulin have worse glycemic control at baseline and have increased risk for diabetes complications, cancer and all-cause mortality over time as compared to those on other glucose-lowering regimens [59]. A similar relationship between insulin and worse health was found in a primary care sample of adults with type 2 diabetes [13]. Thus, despite our control for complications, insulin prescription likely captured other health-related aspects of diabetes burden. Therefore, we cannot conclude that the increased distress associated with insulin treatment was due to the prescription of insulin per se. In fact, others have shown that, when accompanied by structured, group-based education and support, intensive treatment with insulin in type 2 diabetes can actually reduce diabetes distress [60].
Our results demonstrating the buffering effect of social support are consistent with prior work in other chronic illnesses, including cancer and cardiovascular disease. One study examined whether social support moderates the relationship between physical functioning and psychological outcomes in gynecologic cancer survivors [61]. Results indicated that social support moderated the relationship between physical symptoms and cancer-specific traumatic stress. Another study reported that high social support buffered the relationship between stress and C-reactive protein, an inflammatory marker associated with cardiovascular risk among middle-aged women [62]. There have been few studies on the buffering hypothesis in diabetes; to the authors’ knowledge, this is the first study to focus specifically on diabetes distress rather than depressive symptoms. Depressive symptoms and diabetes distress differ conceptually. Instead of the symptom-based and context-neutral approach to Major Depressive Disorder, for example, diabetes distress takes into consideration the role of chronic illness as a context for emotional distress [63]. Therefore, diabetes distress should be more sensitive to the emotional distress that is specifically related to the experience of disease burden associated with diabetes complications than measures of depression.
The present study suggests that interventions to increase social support may have the potential to reduce distress in patients with type 2 diabetes, particularly among those on insulin therapy. A systematic review of social support interventions in type 2 diabetes in general indicated that these types of approaches are promising methods of improving not only psychological well being but also glycemic control and self-care [64]. Additionally, a social support intervention for Spanish-speaking Mexican Americans with type 2 diabetes found that the intervention not only improved behavioral outcomes related to diabetes self-care and diabetes knowledge, but also reduced diabetes distress [65]. These studies contribute to growing evidence of a relationship between increased social support and decreased psychological distress in patients with diabetes [66, 67, 68].
Peer support interventions may represent effective models for reducing the burden of psychosocial problems related to diabetes. In a randomized controlled trial, Heisler, et al. found that reciprocal peer support among male veterans with diabetes resulted in improved HbA1C when compared against nurse care management [69]. In another randomized trial, Long et al. found that African American veterans with diabetes experienced improved glycemic control following participation in a peer-mentoring program [70]. Prior work also suggests that family-based interventions may be effective in reducing diabetes-specific emotional burden [29, 71, 72]. These studies show the promise of enhancing social support to improve the psychological and physical well being of adults living with diabetes. Our findings suggest such approaches may be particularly helpful for patients experiencing distress related to disease and treatment burden.
Our results should be considered within the context of our design and methods. This study’s cross-sectional design limits our ability to infer causal relationships between diabetes burden and diabetes distress. We avoided ratings of severity of diabetes burden in an attempt to rule out the plausibility of reverse causality between diabetes distress and disease burden. Instead, we selected objective indicators of burden based on their salience for patients. Diabetes complications were assessed based on prior diagnosis or reported surgical procedures (e.g., laser eye surgery). Prescription of insulin was similarly salient to patients and represents an added component to the treatment regimen that often makes self-management more complex. We did not consider glycemic control as an adequate indicator of burden because patients are often unaware of their own HbA1c results or recommended HbA1c targets [73]. Furthermore, glycemic control is often conceptualized as an outcome of diabetes distress and problems with self-management. Although we did not include HbA1c in our models because of these reasons and plans to more fully evaluate relationships between distress, self-management and glycemic control in a separate paper, analyses that added baseline HbA1c as a control variable did not alter our findings: all interaction effects remained significant and were not appreciably attenuated (data not shown). However, one limitation of our approach is that we did not capture variation in our indicators of burden (e.g., some complications may be more distressing than others, insulin regimens can vary in their complexity). Further work is warranted that examines health-related and treatment-related aspects of diabetes burden in more detail. Another limitation is the relatively small sample size, which may have limited the power of the study. However, the socioeconomic and ethnic diversity of our sample may enhance generalizability. Measurement error is a common concern with self-report instruments and should also be recognized as a limitation.
In sum, the results of the current study demonstrate a significant protective role for social support in the relationship between increased illness and treatment burden and various aspects of diabetes emotional distress and perceived problems with self-management in adults with treated type 2 diabetes. Our findings suggest that interventions aimed at increasing social support availability and quality may hold promise in reducing the emotional toll of diabetes, particularly among those experiencing increased illness and treatment burden.
Acknowledgments
This work was partially supported by a pilot and feasibility grant from the Einstein Diabetes Research Center. Dr. Gonzalez’s efforts were additionally supported by grants P60 DK020541 and R18 DK098742 from the National Institutes of Health.
Footnotes
Conflict of Interest Statement
All authors declare they have no competing interests to report.
References
- 1.Centers of Disease Control and Prevention. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States. [Accessibility verified April 29, 2013]. Available at. [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Schetman JM, Nadkarni MM, Voss JD. The association between diabetes metabolic control and drug adherence in an indigent population. Diabetes Care. 2002;25:1015–1021. doi: 10.2337/diacare.25.6.1015. [DOI] [PubMed] [Google Scholar]
- 4.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77–82. [Google Scholar]
- 5.Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. PHYS THER. 2008;88:1254–1264. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care. 2001;24(6):1069–78. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 7.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with type 2 diabetes: A systematic review and meta-analysis. Diabetic Medicine. 2006;23(11):1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 8.Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner PJ, Diez Roux AV, Lee HB, Lyketsos C. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751–2759. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan A, Lucas M, Sun Q, van Dam RM, Franco OH, Manson JE, Willett WC, Ascherio A, Hu FB. Arch Intern Med. 2010;22;170(21):1884–91. doi: 10.1001/archinternmed.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nouwen A, Nefs G, Caramlau I, Connock M, Winkley K, Lloyd CE, Pouwer F. Prevalence of depression in individuals with impaired glucose metabolism or undiagnosed diabetes. Diabetes Care. 2011;34:752–762. doi: 10.2337/dc10-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mezuk B, Johnson-Lawrence V, Lee H, Rafferty JA, Abdou CM, Uzogara EE, Jackson JS. Is ignorance bliss? Depression, antidepressants, and the diagnosis of prediabets and type 2 diabetes. Health Psychology. 2013;32:254–263. doi: 10.1037/a0029014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371(9626):1783–1789. doi: 10.1016/S0140-6736(08)60766-7. [DOI] [PubMed] [Google Scholar]
- 13.Delahanty LM, Grant RW, Wittenberg E, et al. Association of diabetes-related emotional distress with diabetes treatment in primary care patients with Type 2 diabetes. Diabet Med J Br Diabet Assoc. 2007;24(1):48–54. doi: 10.1111/j.1464-5491.2007.02028.x. [DOI] [PubMed] [Google Scholar]
- 14.Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30(3):542–548. doi: 10.2337/dc06-1614. [DOI] [PubMed] [Google Scholar]
- 15.Peyrot M, Rubin R. Levels and risks of depression and anxiety symptomatology among diabetic adults. Diabetes Care. 1997;20:585–590. doi: 10.2337/diacare.20.4.585. [DOI] [PubMed] [Google Scholar]
- 16.Welch GW, Jacobson AM, Polonsky WH. The Problem Areas in Diabetes Scale: an evaluation of its clinical utility. Diabetes Care. 1997;20(5):760–66. doi: 10.2337/diacare.20.5.760. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd C, Smith J, Weinger K. Stress and diabetes: a review of the links. Diabetes Spectrum. 2005;18(2):121–127. [Google Scholar]
- 18.Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33(5):1034–36. doi: 10.2337/dc09-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snoek FJ, Pouwer F, Welch GW, Polonsky WH. Diabetes-related emotional distress in Dutch and U.S. diabetic patients: cross-cultural validity of the Problem Areas in Diabetes Scale. Diabetes Care. 2000;23:1305–9. doi: 10.2337/diacare.23.9.1305. [DOI] [PubMed] [Google Scholar]
- 20.Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33(1):23–28. doi: 10.2337/dc09-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aikens JE, Perkins DW, Lipton B, Piette JD. Longitudinal analysis of depressive symptoms and glycemic control in type 2 diabetes. Diabetes Care. 2009;32(7):1177–81. doi: 10.2337/dc09-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georgiades A, Zucker N, Friedman KE, et al. Changes in depressive symptoms and glycemic control in diabetes mellitus. Psychosom Med. 2007;69(3):235–41. doi: 10.1097/PSY.0b013e318042588d. [DOI] [PubMed] [Google Scholar]
- 23.Fisher L, Mullan T, Skaff M, Glasgow R, Arean P, Hessler D. Predicting diabetes distress in patients with type 2 diabetes: a longitudinal study. Diabetic Medicine. 2009;26:622–27. doi: 10.1111/j.1464-5491.2009.02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zagarins SE, Allen NA, Garb JL, Welch G. Improvement in glycemic control following a diabetes education intervention is associated with change in diabetes distress but not change in depressive symptoms. J Behav Med. 2012;35(3):299–304. doi: 10.1007/s10865-011-9359-z. [DOI] [PubMed] [Google Scholar]
- 25.Leyva B, Zagarins SE, Allen NA, Welch G. The relative impact of diabetes distress vs depression on glycemic control in hispanic patients following a diabetes self-management education intervention. Ethn Dis. 2011;21(3):322–327. [PubMed] [Google Scholar]
- 26.Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev. 1999;15(3):205–218. doi: 10.1002/(sici)1520-7560(199905/06)15:3<205::aid-dmrr29>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 27.Vileikyte L, Leventhal H, Gonzalez JS, Peyrot M, Rubin RR, Ulbrecht JS, Garrow A, Waterman C, Cavanagh PR, Boulton AJ. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care. 2005;28(10):2378–83. doi: 10.2337/diacare.28.10.2378. [DOI] [PubMed] [Google Scholar]
- 28.Vileikyte L, Peyrot M, Gonzalez JS, Rubin RR, Garrow AP, Stickings D, Waterman C, Ulbrecht JS, Cavanagh PR, Boulton AJ. Predictors of depressive symptoms in persons with diabetic peripheral neuropathy: a longitudinal study. Diabetologia. 2009;52(7):1265–73. doi: 10.1007/s00125-009-1363-2. [DOI] [PubMed] [Google Scholar]
- 29.Karlsen B, Oftedal B, Bru E. The relationship between clinical indicators, coping styles, perceived support and diabetes-related distress among adults with type 2 diabetes. J Adv Nurs. 2011;68(2):391–401. doi: 10.1111/j.1365-2648.2011.05751.x. [DOI] [PubMed] [Google Scholar]
- 30.Lloyd CE, Pambianco G, Orchard TJ. Does diabetes-related distress explain the presence of depressive symptoms and/or poor self-care in individuals with type 1 diabetes? Diabetic Medicine. 2010;27:234–7. doi: 10.1111/j.1464-5491.2009.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polonsky W, Anderson B, Lohrer P, Welch G, Jacobson A, Aponte K, Schwartz C. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754–60. doi: 10.2337/diacare.18.6.754. [DOI] [PubMed] [Google Scholar]
- 32.Cohen S. Psychosocial models of the role of social support in the etiology of physical disease. Health Psychol. 1988;7:269–297. doi: 10.1037//0278-6133.7.3.269. [DOI] [PubMed] [Google Scholar]
- 33.Schradle SB, Dougher MJ. Social support as a mediator of stress: theoretical and empirical issues. Clin Psychol Rev. 1985;5:641–61. [Google Scholar]
- 34.Feldman P, Cohen S. Social support. In: Kazdin AE, editor. Encyclopedia of Psychology. NY: Oxford University Press; 2000. [Google Scholar]
- 35.Cobb S. Social support as a moderator of life stress. Psychosomatic Medicine. 1976;38:300–314. doi: 10.1097/00006842-197609000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Shumaker SA, Brownell A. Towards a theory of social support: closing conceptual gaps. J Soc Issues. 1984;40:11–36. [Google Scholar]
- 37.Sarason BR, Sarason IG, Pierce GR. Traditional Views of social Support and Their Impact on Assessment. In: Sarason BR, Sarason IG, Pierce GR, editors. Social Support: An Interactional View. New York: Wiley; 1990. pp. 9–25. [Google Scholar]
- 38.Bukberg J, Penman D, Holland JC. Depression in hospitalized cancer patients. Psychosom Med. 1984;46:199–121. doi: 10.1097/00006842-198405000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Hann DM, Oxman HE, Ahles TA, Furstenberg CT, Stuke TA. Social support adequacy and depression in older patients with metastatic cancer. Psycho-Oncology. 1995;4:213–21. [Google Scholar]
- 40.Trunzo JJ, Pinto BM. Social support as a mediator of optimism and distress in breast cancer survivors. J Consult Clin Psychol. 2003;71(4):805–11. doi: 10.1037/0022-006x.71.4.805. [DOI] [PubMed] [Google Scholar]
- 41.Serovich JM, Kimberly JA, Mosack KE, Lewis TL. The role of family and friend support in reducing emotional distress among HIV-positive women. AIDS Care. 2001;13(3):335–41. doi: 10.1080/09540120120043982. [DOI] [PubMed] [Google Scholar]
- 42.Cohen S, McKay G. Social support, stress and the buffering hypothesis: A theoretical analysis. Handbook of Psychology and Health. 1984:253–267. [Google Scholar]
- 43.Beekman A, Penninx B, Deeg D, Ormel J, Braam A, van Tilburg W. Depression and physical health in later life: results from the Longitudinal Aging Study Amsterdam (LASA) J Affect Disord. 1997;46:219–31. doi: 10.1016/s0165-0327(97)00145-6. [DOI] [PubMed] [Google Scholar]
- 44.Thomas J, Jones G, Scarinci I, Brantley P. Social support and the association of type 2 diabetes and depressive and anxiety disorders among low-income adults seen in primary care clinics. J Clin Psychol Med Settings. 2007;14:351–9. [Google Scholar]
- 45.Littlefield C, Rodin G, Murray M, Craven J. Influence of functional impairment and social support on depressive symptoms in persons with diabetes. Health Psychology. 1990;9:737–49. doi: 10.1037//0278-6133.9.6.737. [DOI] [PubMed] [Google Scholar]
- 46.Vileikyte L, Leventhal H, Gonzalez JS, et al. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care. 2005;28:2378–2383. doi: 10.2337/diacare.28.10.2378. [DOI] [PubMed] [Google Scholar]
- 47.Sarason IG, Sarason BR, Shearin EN, Pierce GR. A brief measure of social support: Practical and theoretical implications. J Soc Pers Relat. 1987;(4):497–510. [Google Scholar]
- 48.Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626–631. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- 49.Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful? Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259–64. doi: 10.2337/dc11-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.IBM Corp. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp; Released 2011. [Google Scholar]
- 51.Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- 52.Hayes AF. Statistical methods for communication science. Mahwah, NJ: Erlbaum; 2005. [Google Scholar]
- 53.American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(Suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 54.Tang T, Brown M, Funnell M, Anderson R. Social support, quality of life, and self-care behaviors among African Americans with type 2 diabetes. The Diabetes Educator. 2008;34:266–76. doi: 10.1177/0145721708315680. [DOI] [PubMed] [Google Scholar]
- 55.Whittemore R, D’Eramo Melkus G, Grey M. Metabolic control, self-management and psychosocial adjustment in women with type 2 diabetes. J Clin Nurs. 2005;14:195–203. doi: 10.1111/j.1365-2702.2004.00937.x. [DOI] [PubMed] [Google Scholar]
- 56.Gamble JM, Simpson SH, Eurich DT, Majumdar SR, Johnson JA. Insulin use and increased risk of mortality in type 2 diabetes: a cohort study. Diabetes Obes Metab. 2010;12:47–53. doi: 10.1111/j.1463-1326.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- 57.Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in persons with advanced heart failure. Am Heart J. 2005;149:168–174. doi: 10.1016/j.ahj.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 58.Bowker SL, Yasui Y, Veugelers P, Johnson JA. Glucose-lowering agents and cancer mortality rates in type 2 diabetes: assessing effects of time-varying exposure. Diabetologia. 2010;53:1631–1637. doi: 10.1007/s00125-010-1750-8. [DOI] [PubMed] [Google Scholar]
- 59.Currie CJ, Poole CD, Evans M, Peters JR, Morgan CL. Mortality and Other Important Diabetes-Related Outcomes With Insulin vs Other Antihyperglycemic Therapies in Type 2 Diabetes. J Clin Endocrinol Metab. 2013;98:668–677. doi: 10.1210/jc.2012-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hermanns N, Kulzer B, Maier B, Mahr M, Haak T. The effect of an education programme (MEDIAS 2 ICT) involving intensive insulin treatment for people with type 2 diabetes. Patient Education and Counseling. 2012;86:226–232. doi: 10.1016/j.pec.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 61.Carpenter K, Fowler J, Maxwell G, Anderson B. Direct and buffering effects of social support among gynecologic cancer survivors. Ann Behav Med. 2010;39(1):79–90. doi: 10.1007/s12160-010-9160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mezuk B, Diez Roux AV, Seeman T. Evaluating the buffering vs. direct effects hypotheses of emotional social support on inflammatory markers: The Multi-Ethnic Study of Atherosclerosis. Brain, Behavior, and Immunity. 2010;24:1294–1300. doi: 10.1016/j.bbi.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gonzalez JS, Fisher L, Polonsky WH. Depression in diabetes: Have we been missing something important? Diabetes Care. 2011;34:236–239. doi: 10.2337/dc10-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Dam H, van der Horst F, Ryckman R, Crebolder H, van den Borne B. Social support in diabetes: a systematic review of controlled intervention studies. Patient Education and Counseling. 2005;59:1–12. doi: 10.1016/j.pec.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 65.McEwen M, Pasvogel A, Gallegos G, Barrera L. Type 2 diabetes self-management social support intervention in the U.S.-Mexico Border. Public Health Nurs. 2010;27(4):310–19. doi: 10.1111/j.1525-1446.2010.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McEwen M, Baird M, Pasvogel A, Gallegos G. Health–illness transition experiences among Mexican immigrant women with diabetes. Family and Community Health. 2007;30(3):201–212. doi: 10.1097/01.FCH.0000277763.70031.0d. [DOI] [PubMed] [Google Scholar]
- 67.Mauldon M, Melkus G, Cagganello M. Tomando Control: A culturally appropriate diabetes education program for Spanish-speaking individuals with Type 2 diabetes mellitus-Evaluation of a pilot project. Diabetes Educator. 2006;32(5):751–760. doi: 10.1177/0145721706291999. [DOI] [PubMed] [Google Scholar]
- 68.Due-Christensen M, Zoffmann V, Hommel E, Lau M. Can sharing experiences in groups reduce the burden of living with diabetes, regardless of glycaemic control? Diabetic Medicine. 2012;29(2):251–6. doi: 10.1111/j.1464-5491.2011.03521.x. [DOI] [PubMed] [Google Scholar]
- 69.Heisler M, Vijan S, Makki F, Piette JD. Diabetes control with reciprocal peer support versus nurse care management. Annals of Internal Medicine. 2010:507–516. doi: 10.7326/0003-4819-153-8-201010190-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Long JA, Jahnle EC, Richardson DM, et al. Peer mentoring and financial incentives to improve glucose control in African American veterans: a randomized trial. Ann Intern Med. 2012;156:416–24. doi: 10.1059/0003-4819-156-6-201203200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keogh KM, Smith SM, White P, McGilloway S, Kelly A, Gibney J, O’Dowd T. Psyhosocial family intervention for poorly controlled type 2 diabetes. Am J Manag Care. 2011;17(2):105–13. [PubMed] [Google Scholar]
- 72.Franks MM, Sahin ZS, Seidel AJ, Shields CG, Oates SK, Boushey CJ. Table for two: diabetes distress and diet-related interactions of married patients with diabetes and their spouses. Fam Syst Health. 2012;30(2):154–65. doi: 10.1037/a0028614. [DOI] [PubMed] [Google Scholar]
- 73.Stark Casagrande S, Ríos Burrows N, Geiss LS, Bainbridge KE, Fradkin JE, Cowie CC. Diabetes knowledge and its relationship with achieving treatment recommendations in a national sample of people with type 2 diabetes. Diabetes Care. 2012;35(7):1556–1565. doi: 10.2337/dc11-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]