Abstract
We explored molecules involved in in vitro exsheathment of Oesophagostomum dentatum L3s using a proteomic-transcriptomic-bioinformatic approach. Analysis of L3s before, during and after exsheathment identified 11 proteins that were over-expressed exclusively during exsheathment. These proteins (including key enzymes, heat shock, structural and nematode-specific proteins) were inferred to be involved in development, metabolism, structure, motility and/or host-parasite interactions. Some of these molecules represented homologues linked to entry into and exit from the dauer stage in the free-living nematode Caenorhabditis elegans. The approach established here provides a basis for investigations of ecdysis in other strongylid nematodes.
Keywords: Exsheathment, Parasitic nematode, Oesophagostomum dentatum, Proteomics
Ecdysis (εκδυο, ekduo, “to take or strip off”) is a key developmental process involving the moulting of the cuticle in many invertebrates, including arthropods, horse-hair worms, velvet worms, tardigrades and nematodes, which are collectively called ecdysozoans (Aguinaldo et al., 1997). Stimulated by recent studies of an economically important and model nematode, Oesophagostomum dentatum (see Joachim et al., 2005; Ondrovics et al., 2013), we became interested in understanding this fundamental process in parasitic nematodes. This and related nematodes (order Strongylida) undergo three moults from L1-L4 and another from L4 to the adult stage (Lee, 2002). At each moult, the nematode enters a period of decreased activity, called lethargus (Page and Johnstone, 2007). At this point, the old cuticle begins to disconnect from the underlying hypodermis, a process called apolysis (Page and Johnstone, 2007), and a new cuticle is formed in the space between these two layers. The sloughing of the old cuticle then concludes the moulting process (Lee, 2002).
Interestingly, the L3 of strongylid nematodes retains its cuticle from the L2 stage as a sheath, and is thus called an ensheathed L3. This sheath prevents the L3 stage from feeding, such that it is dependent solely on accumulated nutrient reserves to live and survive, but protects it from environmental stresses such as desiccation and low temperatures (Lee, 2002). In the case of O. dentatum, ensheathed L3s are ingested and pass through the stomach to the intestine; during this passage, the sheath provides protection from gastric juices, enabling L3s to reach the small intestine unscathed, after which the larvae emerge from within their sheath under the influence of pH change, and an increase in both temperature and carbon dioxide concentration (Lee, 2002). This exsheathment process marks the transition from the free-living to the parasitic L3 stage. The latter is able to feed on nutrients in the gut and now infects the host. During a histotropic phase in the intestinal wall, this larva undergoes development to the L4 stage, which returns to the gut lumen of the large intestine to establish and mature to the adult stage.
Clearly, all four moults are crucial for the nematode to develop, mature and reproduce. As the process of exsheathment relates to the transition from the free-living to the parasitic phase of the life cycle, we have become particularly interested in gaining insight into the molecular and/or biochemical changes that take place during this transition in O. dentatum. Therefore, in the present study, we use a combination of transcriptomic, electrophoretic, mass spectrometric and bioinformatic methods to explore proteomic profiles in O. dentatum L3s before (eL3), during (L3dx) and after (xL3) in vitro exsheathment. The goal was to identify key molecules involved in this crucial step of this nematode's development.
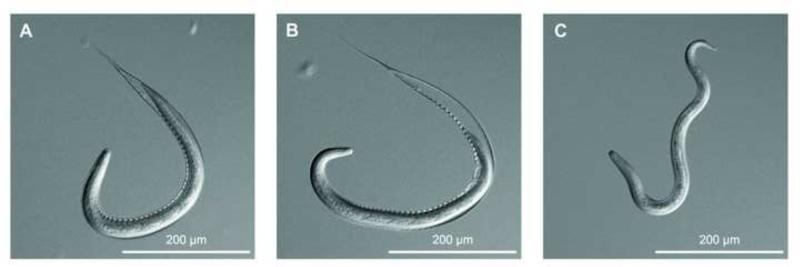
Oesophagostomum dentatum (OD-Hann strain) was maintained in pigs (Talvik et al., 1997) in facilities of the University of Veterinary Medicine Vienna, Austria, under animal ethics approval GZ 68.205/103-II/10b/2008. Faecal samples were collected from two mono-specifically infected pigs on four different days. Each day, faeces from the two pigs were pooled and set up for coproculture (Talvik et al., 1997). The four batches of L3s were designated replicates. For each of the four replicates of O. dentatum L3s (n = 1,000,000), we made three distinct preparations (cf. Fig. 1): (i) eL3s were purified by agar gel migration (Talvik et al., 1997) and snap-frozen in liquid nitrogen; (ii) L3dxs were exposed to sodium hypochlorite (12-15% v/v) at 22 °C on a 100 μm filter (CellTrics®, Partec GmbH, Germany); exsheathment was monitored using an inverted light microscope (Diaphot 300, Nikon, Japan) and stopped by washing larvae in distilled water when more than 90% of them started to emerge from their sheath. Then, larvae were washed in PBS (pH 7.4) and snap frozen in liquid nitrogen; (iii) xL3s were exsheathed in hypochlorite (the same concentration as for L3dx) and then incubated in 10 ml of Luria-Bertani (LB) medium and 10% sterile pig serum in 75 cm2 cell culture flasks (200,000 larvae/flask) at 38.5 °C and 10% CO2 for 24 h. Then, the larvae were washed three times with PBS and snap-frozen in liquid nitrogen. Proteins were extracted from each of the four replicates of eL3, L3dx and xL3 and quantified using standard methods (Ondrovics et al., 2013).
Fig. 1.
Exsheathment of Oesophagostomum dentatum L3s. (A) Ensheathed L3s (eL3s); (B) L3 during exsheathment (L3dxs); (C) exsheathed L3s (xL3s).
Proteins (50 μg) of each of the four replicates of eL3, L3dx and xL3 were labelled with 300 pmol of the dyes Cy3 and Cy5 (CyDye Fluor minimal dyes; GE Healthcare, USA). As an internal standard, aliquots from each replicate and preparation were mixed in equal amounts and labelled with Cy2. To exclude dye-specific effects, Cy3 and Cy5 were used interchangeably and combined in a randomized design (Supplementary Table S1). The labelling mixes were incubated on ice in the dark for 30 min; reactions were quenched with 1 μl of 10 mM lysine (Sigma Aldrich, USA) and an equal volume of sample buffer (8 M urea, 2% (w/v) 3-[(3-cholamidopropyl)dimethylammonio]-1- propanesulfonate (CHAPS), 12.7 mM DTT, 2% immobilized pH gradient (IPG) buffer 3-10 non-linear; GE Healthcare) added and incubated on ice for 10 min.
Labelled proteins of all four replicates of eL3, L3dx and xL3 were pooled and 120 μl of each sample subjected to two-dimensional difference gel electrophoresis (2D-DIGE; Ondrovics et al., 2013), using the Cy2-labelled internal control to ensure optimum separation and reproducibility of results. Gels were scanned at 10 μm resolution on a 9400 Typhoon imager (GE Healthcare) and then silver-stained (Ondrovics et al., 2013). Image analysis, spot detection, matching and normalization were performed using DeCyder® 2D software v.6.0 (GE Healthcare). Gel-to-gel comparisons and statistical analysis were performed with a DeCyder® 2D Differential In-gel Analysis (DIA) and the DeCyder® 2D Biological Variation Analysis (BVA) software module. Protein spots significantly (P ≤ 0.01) over-expressed (1.5 to 5.37-fold) in L3dx were selected for further analyses.
Protein spots were digested within the gel with trypsin, and peptides recovered and annotated using a Matrix Assisted Laser Desorption Ionisation Tandem Time-of-Flight (MALDI-TOF/TOF) mass spectrometer (Ultraflex II; Ondrovics et al., 2013). MS spectral data were acquired and analysed by MS/MS based on the most intense ions present (trypsin and major keratin ions being excluded) using FlexAnalysis and Biotools (Bruker Daltonics, USA). Protein sequence matches with a significant MS/MS score (P < 0.05) were used for analysis. The Basic Local Alignment Search Tool (BLAST) was used to find sequence similarities between MS/MS data and amino acid sequences characterized from processed spectra and sequences present within public eukaryotic databases, including an inferred protein database of O. dentatum (www.nematode.net), the UniProtKB/Swiss-Prot database (http://www.uniprot.org) and the National Center for Biotechnology Information (NCBI) non-redundant (nr) database (www.ncbi.nlm.nih.gov) employing the database-based programs Proteinscape (v.1.3; Bruker Daltonics) and MASCOT (www.matrixscience.com). The search parameters were: global modifications carbamido-methylation on cysteine; variable modifications oxidation on methionine; peptide mass tolerance = 100 ppm; MS/MS tolerance = 1 Da; and one missed cleavage permitted. Proteins identified by MS were annotated (Ondrovics et al., 2013) based on their best match (BLASTp, E-value cut-off ≤ 1×10−05) in the UniProtKB/Swiss-Prot database and gene ontology (GO). To confirm correct protein annotation, sequences were searched against the InterPro member databases (www.ebi.ac.uk/interpro). GO annotations were performed using the AmiGO BLAST tool. Biological pathways were inferred (KOBAS algorithm) by mapping sequences to those in the Kyoto Encyclopedia of Genes and Genomes (KEGG) database using BLASTp (E-value cut-off ≤ 10−20). The identity of selected O. dentatum proteins assigned by MS was verified by Western blot analysis (Joachim et al., 2001) employing commercial antibodies raised against mammalian proteins with at least 70% sequence similarity to these proteins. Genetic interaction partners of homologous proteins in C. elegans were predicted using Gene Orienteer (www.geneorienteer.org).
Total RNA was isolated from two of four replicates of eL3, L3dx and xL3 (equivalent to 150,000 larvae for each) using a ReliaPrep® RNA Tissue Miniprep System (Promega, USA), treated with RNase-free DNase I (Thermo Fisher Scientific, USA) and stored at -80 °C; cDNA was synthesized (25 °C for 10 min, 48 °C for 30 min and 95 °C for 5 min) using TaqMan® Reverse Transcription Reagents (Life Technologies, USA). Quantitative PCR (qPCR) was conducted in 20 μl, using Power SYBR® Green PCR Master Mix (Applied Biosystems, USA) and specific intron-spanning primers (0.25 pmol; Supplementary Table S2) in a thermal cycler (Mx3000p, Stratagene, USA). For test samples (run in triplicate), 20 ng of cDNA were used; no-template (negative) and no-reverse transcription controls were included in each qPCR run. Cycling conditions were: 93 °C for 2.5 min for one cycle, followed by 35 cycles of 93 °C for 30 s, 59 °C for 30 s, 72 °C for 30 s. One additional cycle of 93 °C for 30 s, 59 °C for 30 s, 93 °C for 30 s was used for melting-curve analysis. Data were normalised against established reference genes (GTP-cyclohydrolase, triosephosphate isomerase and ubiquitin-conjugating enzyme-2; Ondrovics et al., 2012) that exhibited stable transcription across all larval preparations with M-values ranging from 0.609 to 0.971. Statistical analyses were performed using the SPSS (v.20.0) package for Windows. The relative quantification cycle (Cq) values were used as input data to automatically calculate the M-value and then measure the average pairwise variation of the reference against all other genes (http://medgen.ugent.be/~jvdesomp/genorm/). The Kruskal-Wallis and the Mann-Whitney U tests were used to assess significant differences in gene transcription among eL3, L3dx and L3x.
Protein and RNA were extracted from each eL3, L3dx and xL3 (Fig. 1); the yields obtained were 0.44 - 1.0 mg of protein and 14.6 - 35.6 μg of total RNA per 150,000 larvae from each of the three preparations, respectively. The 2D-DIGE analysis resolved ~3,000 protein spots per gel (Supplementary Fig. S1). The pairwise comparison of the profiles for eL3, L3dx and xL3 showed that the distribution and intensity of protein spots were consistent across all replicates and preparations; thus, background subtraction, quantification, normalization and gel-matching could be automated. Protein spots that were significantly (P ≤ 0.01) over-expressed in L3dx, based on the amount of protein and matches across all gels, were selected for MS analysis. Seventeen proteins spots represented 11 different proteins that shared sequence homology to individual conceptually translated proteins of O. dentatum (Table 1, Fig. 2).
Table 1.
Annotation of the different protein spots inferred for Oesophagostomum dentatum, MALDI-TOF-MS/MS results and closest homologues.
| Spot no. |
Contig IDa | MASCOT Score (MS/MS) |
%Cov | #Pep | Theoretical pI/MW |
Protein identity | Protein homologue | Species (UniProt ID) | Score | Identity (%) |
E-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Oden_isotig01423 | 166.8 | 9.3 | 2 | 5.5/61.2 | Heat shock 70 kDa protein (HSP-70) | HSP-70 | Dracunculus medinensis (D7RTV6) | 2689 | 94 | 0 |
| 2 | Oden_isotig19560 | 84.9 | 12.9 | 2 | 9.1/37.8 | Troponin T (TNT) | CBN-TNT-2 protein | Caenorhabditis brenneri (G0NFB1) | 1392 | 83 | |
| 3 | Oden_isotig17816 | 174.0 | 13.7 | 4 | 5.7/41.1 | Phosphoenolpyruvate carboxykinase (PEPCK) (GTP) | Putative uncharacterized protein | C. brenneri (G0MYR1) | 937 | 83 | 3*10−99 |
| 4 | Oden_isotig17816 | 116.7 | 10.9 | 4 | 5.7/41.1 | PEPCK (GTP) | Putative uncharacterized protein | C. brenneri (G0MYR1) | 937 | 83 | 3*10−99 |
| 5 | Oden_isotig17816 | 227.4 | 12.8 | 4 | 5.7/41.1 | PEPCK (GTP) | Putative uncharacterized protein | C. brenneri (G0MYR1) | 937 | 83 | 3*10−99 |
| 6 | Oden_isotig05043 | 121.3 | 4.9 | 2 | 5.8/57.1 | Glucose-6-phosphate 1-dehydrogenase (GPD) | GPD | Caenorhabditis elegans (Q27464) | 2115 | 78 | 0 |
| 7 | Oden_isotig05042 | 119.9 | 8.1 | 3 | 5.9/57.4 | GPD | GPD | C. elegans (Q27464) | 2115 | 78 | 0 |
| 8 | Oden_isotig12221 | 101.3 | 10.5 | 3 | 5.3/49.3 | Propionyl-CoA carboxylase beta chain (PCCB) | Uncharacterized protein | Caenorhabditis japonicum (H2WMP1) | 2098 | 86 | 0 |
| 9 | Oden_isotig18624 | 94.4 | 8.5 | 2 | 6.0/55.4 | UTP-glucose-1-phosphate uridylyltransferase (GPU) | Putative uncharacterized protein | Caenorhabditis remanei (E3LYH8) | 2151 | 84 | 0 |
| 10 | Oden_isotig18624 | 107.9 | 7.5 | 3 | 6.0/55.4 | GPU | Putative uncharacterized protein | C. remanei (E3LYH8) | 2151 | 84 | 0 |
| 11 | Oden_isotig20090 | 203.4 | 18.4 | 3 | 5.3/36.1 | Actin (ACT) | ACT, putative | Brugia malayi (A8P5A0) | 1629 | 99 | 1*10−179 |
| 12 | Oden_isotig12727 | 141.6 | 10.5 | 2 | 5.5/45.0 | GDP dissociation inhibitor (GDI) | Protein CBR-GDI-1 | Caenorhabditis briggsae (A8Y0A6) | 1822 | 86 | 0 |
| 13 | Oden_isotig20090 | 415.1 | 27.1 | 5 | 5.3/36.1 | ACT | ACT, putative | B. malayi (A8P5A0) | 1629 | 99 | 1*10−179 |
| 14 | Oden_isotig20228 | 111.1 | 8.0 | 2 | 5.0/33.0 | Cuticlin-1 (CUT-1) | CUT-1 | Haemonchus contortus (Q5BLX0) | 1232 | 77 | 1*10−168 |
| 15 | Oden_isotig13276 | 106.2 | 25.8 | 3 | 5.2/20.4 | ACT | ACT variant 1 | Dictyocaulus viviparus (B0LJD1) | 937 | 99 | 1*10−125 |
| 16 | Oden_isotig15772 | 91.2 | 23.8 | 3 | 5.5/15.6 | Transthyretin-like protein 5 (TTL-5) | Uncharacterized protein | Pristionchus pacificus (H3EKJ9) | 559 | 75 | 3*10−72 |
| 17 | Oden_isotig12590 | 122.0 | 23.1 | 3 | 6.1/12.4 | Heat shock 12.6 kDa protein (HSP-12.6) | HSP-12.6 | Nippostrongylus brasiliensis (E5RX10) | 361 | 67 | 4*10−43 |
Contig ID based on www.nematode.net
Cov, percentage sequence coverage
Pep, number of peptides found within the sequence for the resulting inferred protein.
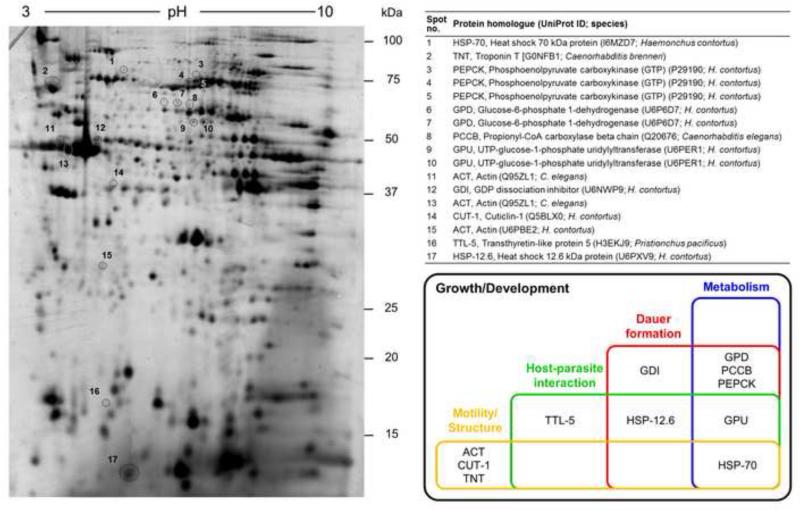
Fig. 2.
Two-dimensional difference gel electrophoretic (2D-DIGE) protein map and Venn diagram summarizing the main biological processes in which proteins over-expressed in Oesophagostomum dentatum L3s during exsheathment (L3dxs) are inferred to be involved. Silver-stained 2D-DIGE gel of total protein extracts of O. dentatum L3s displays the protein spots and the protein homologues identified in the UniProt database (Uniprot ID; species).
The expressed O. dentatum proteins were annotated based on their closest nematode protein homologues in the UniProt database; for each O. dentatum sequence, at least one homologue could be identified in each nematode species studied (Table 1). The UniProt BLAST scores of sequence similarity ranged from 361 to 2,689 for individual sequences, with homologous proteins identified in other strongylid species (i.e. Haemonchus contortus, Dictyocaulus viviparus and/or Nippostrongylus brasiliensis) and in related orders, such as the Rhabditida (i.e. Caenorhabditis spp. and/or Pristionchus pacificus) and Spirurida (i.e. Brugia malayi and/or Dracunculus medinensis). Protein annotations were confirmed by additional InterPro classification (Supplementary Table S3).
The 11 proteins shared highest sequence homology to four distinct enzymes (phosphoenolpyruvate carboxykinase (PEPCK (GTP), EC 4.1.1.32), glucose-6-phosphate 1-dehydrogenase (GPD, EC 1.1.1.49), propionyl-CoA carboxylase beta chain (PCCB, EC 6.4.1.3), UTP-glucose-1-phosphate uridylyltransferase (GPU, EC 2.7.7.9)), two nematode- specific proteins (cuticlin-1 (CUT-1) and transthyretin-like protein 5 (TTL-5)), two heat shock proteins (heat shock 70 kDa protein (HSP-70) and heat shock 12.6 kDa protein (HSP-12.6)), two structural proteins (troponin T (TNT) and actin (ACT)) and one GDP dissociation inhibitor (GDI) (Fig. 2). We confirmed the identity of selected O. dentatum proteins, including HSP-70 and phosphoenolpyruvate carboxykinase (GTP), by Western blotting employing specific antibodies (Supplementary Table S4). Interestingly, although expressed at the protein level, there was no significant difference in transcription of genes encoding CUT-1, GDI, GPD, GPU, HSP-12.6, HSP-70, PCCB, PEPCK (GTP) and TTL-5 among eL3, L3dx and xL3 (Supplementary Fig. S2).
An integrated functional annotation of proteins by KEGG and/or GO revealed that the 11 proteins over-expressed in L3dx were associated with various biological processes, including growth and development, metabolism, structure and motility, host-parasite interactions and/or dauer formation (Fig. 2 and Supplementary Tables S3, S5 and S6). All 11 proteins were predicted to contribute to parasite growth and development. Four proteins (GPD, PEPCK (GTP), PCCB and GPU) were linked to amino acid, carbohydrate or energy metabolism. Three proteins (ACT, CUT-1 and TNT) were inferred to be involved in body structure and/or motility. In addition, the proteins GPU, HSP-12.6 and protein TTL-5 were predicted to be associated with host-parasite interactions (Fig. 2, Supplementary Table S3). Finally, HSP-70 was predicted to be involved in such interactions as well as metabolism and/or motility. Genetic networking linked six of 11 annotated proteins to dauer stage entry and/or exit. This networking inferred direct interactions of GDI, HSP-12.6 and HSP-70 with C. elegans DAF-2 (a key regulator of dauer entry and exit; Hu, 2007), and indirect interactions of GPD and PCCB with this regulator. Associated with these interactions was an enrichment of PEPCK (GTP), in accordance with findings for C. elegans dauer stages (Wang and Kim, 2003; Fig. 2; Supplementary Table S3).
Essentiality predictions revealed nine of 17 protein spots (52.9%) encoded by at least five genes with C. elegans orthologs with non-wildtype RNA interference (RNAi) phenotypes upon gene knockdown, including lethality, defects in postembryonic development, organism homeostasis metabolism, physiology of the reproductive system, movement and organism segment morphology (Supplementary Tables S3 and S7; cf. WormBase, www.wormbase.org).
In the present study, we employed a newly established filter-based technique to explore L3 (specifically L3dx) during the actual exsheathment process in vitro. In this stage, we recorded an over-expression of HSP-70, TNT, PEPCK (GTP) and PCCB, which are the same four proteins that displayed reduced expression following specific chemical inhibition of the moulting process in O. dentatum L3 by comparison with uninhibited controls (Ondrovics et al., 2013), confirming their crucial role during exsheathment/moulting and subsequent larval development. Importantly, we showed a considerable increase in the expression of proteins inferred to be associated with development and growth, dauer formation, metabolism, structure and motility as well as host-parasite interactions during the transition from the free-living to the parasitic L3 stage of O. dentatum.
Regarding development, eL3 of O. dentatum and related nematodes show features that are similar to those of the dauer (arrested) form of C. elegans larvae. We showed here that six of 11 proteins over-expressed in L3dx compared with eL3 and xL3 were homologues of proteins linked to dauer in C. elegans. While homologues of GDI, HSP-12.6 and HSP-70 are known to interact with DAF-2 in C. elegans, GPD and PCCB interact via gamma-glutamine cysteine synthetase (GCS-1) and COP9/Signalosome and eIF3 complex shared subunit (CIF-1), respectively, and then, via DAF-16, with DAF-2 (cf. WormBase, www.wormbase.org). In C. elegans, dauer entry and exit are regulated by various signalling pathways, including the insulin-like pathway which contains the receptor tyrosine kinase DAF-2 (Hu, 2007). Following its activation by various agonists, DAF-2 stimulates the AGE-1 phosphoinositide 3-kinase (PI3K) and the downstream protein kinases PDK-1, AKT-1 and AKT-2 (Hu, 2007). Subsequently, AKT-1 phosphorylates the FOXO orthologue DAF-16, resulting in a cytoplasmic localisation of DAF-16. Under particular conditions, such as high population density, poor food supply and/or high ambient temperature, a lack of DAF-2 activation results in the nuclear localisation of DAF-16 and entry into dauer (Hu, 2007). In addition, the expression of PEPCK is upregulated during dauer (Wang and Kim, 2003). In accordance with the „dauer hypothesis' (Crook, 2014), our findings indicate that homologous pathways are operational in O. dentatum in the transition from free-living to parasitic L3s in vitro.
Coinciding with a substantial up-regulation of key proteins involved in developmental pathways is a rapid and massive increase in metabolism linked to increased energy requirements of the L3 stage as it develops through to its parasitic phase. The high expression of GPD, GPU, PCCB and PEPCK (GTP), all enzymes involved in carbohydrate metabolism was conspicuous. In mammals, PEPCK functions primarily in gluconeogenesis, converting oxaloacetate to phosphoenolpyruvate, whereas in parasitic nematodes its function is reversed. It preferentially carboxylates phosphoenolpyruvate to oxaloacetate, introducing the products of glycolysis into mitochondrial metabolism and, thus, plays a pivotal role in metabolic pathways delivering energy (Saz and Lescure, 1969). Moreover, the energy obtained by fixing carbon dioxide is crucial for the anaerobic metabolism of gastrointestinal nematodes (Iglesias et al., 2005). In agreement with our findings, phosphoenolpyruvate carboxykinases have also been reported to exhibit their highest activity during the moulting process in anisakid nematodes (Iglesias et al., 2005), indicating their essential role in this process more generally.
The two structural proteins ACT and TNT were over-expressed in L3dx. These molecules are essential for muscle contraction and movement (Myers et al., 1996) and are likely to be involved in active movement of larvae necessary to shed their cuticular sheath during ecdysis, and to migrate to the appropriate section of the large intestine of the host. Moreover, CUT-1, a component of nematode cuticle, was over-expressed during exsheathment. This insoluble, immunogenic protein has been identified in a number of nematodes, including C. elegans, Heterorhabditis sp., Ascaris lumbricoides and Brugia spp., and appears to be critical to body structure (Favre et al., 1998; Lewis et al., 1999; Page and Johnstone, 2007). Thus, O. dentatum CUT-1 seems to be necessary for the maintenance of body shape and structure, and for protection against and contact with the external environment and the host interface.
TTL-5 was significantly up-regulated (4.3-fold) in L3dx compared with eL3 and xL3. TTLs represent one of the largest groups of nematode-specific proteins (Parkinson et al., 2004). A subset of TTL proteins has also been identified in other strongylid nematodes, including Ancylostoma caninum (hookworm), H. contortus (barber's pole worm), and Ostertagia ostertagi (brown stomach worm) (Yatsuda et al., 2003; Saverwyns et al., 2008; Mulvenna et al., 2009). For example, in O. ostertagi, at least 18 ttl genes have been identified, most of which are constitutively transcribed in the free-living L3 through to adult females and males (Saverwyns et al., 2008). In H. contortus, a spectrum of ttl genes is transcribed in different developmental stages (Schwarz et al., 2013). Some secreted TTLs of adult stages have been characterized previously to be immunogenic (Yatsuda et al., 2003) and some homologues are abundant in excretory/secretory products (ESPs) of A. caninum (see Mulvenna et al., 2009). Taken together, this information suggests that TTLs play key roles in host-parasite interactions during and following exsheathment, a hypothesis that will require rigorous experimental testing. Based on knowledge of immune responses against helminths in animals (Hewitson et al., 2009), HSP-12.6, HSP-70 and GPU might play immunomodulatory or immunogenic roles. In B. malayi, for instance, HSPs appear to have key immunoregulatory capacity in the host (Dakshinamoorthy et al., 2012). In addition, proteins glycosylated by GPU might mask parasite antigens by mimicking host molecules (such as C-type lectins, galectins or concanavalin A; Hewitson et al., 2009).
In the present study, protein abundance did not always relate to mRNA level, which is a common finding in comparative proteomic-transcriptomic work and likely relates to (i) the storage of precursor RNAs in the larvae required for immediate translation of proteins essential for development and survival and/or (ii) post-transcriptional or post-translational regulation mechanisms (e.g. Foss et al., 2011). However, it is also possible that this discrepancy between expression and transcription might relate to a rapid (minutes) and synchronous exsheathment in vitro compared with the natural and more gradual process in the host's gut in vivo (Joachim et al., 2005). Although in vitro exsheathment appears to reflect what happens in vivo (Hertzberg et al., 2002), further study is warranted to show that the discrepancy observed is not an artefact.
In conclusion, the integrated approach combining an efficient filter-based technique for exsheathment with the application of proteomic, transcriptomic and bioinformatic methods was highly effective in elucidating key molecules of the transition of the free-living eL3 of O. dentatum to the parasitic xL3 stage in vitro. Our approach should be directly applicable to a wide range of related strongylid nematodes and could have implications for the exploration of the ecdysis process in various nematodes.
Supplementary Material
Highlights.
In vitro exsheathment in L3s of the parasitic nematode Oesophagostomum dentatum.
Identification (2D-DIGE and MS) of 11 proteins over-expressed during exsheathment.
These proteins represented key molecules involved in several biological processes.
Processes included growth and development, metabolism, structure and motility.
Proteins were also involved in host-parasite interaction, immunobiology and dauer.
Acknowledgements
MO is recipient of a DOC-fFORTE-fellowship of the Austrian Academy of Sciences. The O. dentatum genome and transcriptome data are generated as part of the Strongylida genome sequencing project funded by National Institutes of Health (NIH), USA (grant number AI081803). RBG's research is funded mainly through the Australian Research Council (ARC), the National Health and Medical Research Council of Australia (NHMRC), Melbourne Water Corporation, Australia and Yourgene Bioscience, Taiwan, and supported by a Victoria Life Sciences Computation Initiative (VLSCI), Australia (grant number VR0007) on its Peak Computing Facility at The University of Melbourne, Australia, an initiative of the Victorian Government. NDY is an NHMRC Early Career Research Fellow (ECRF). We thank Ebrahim Razzazi-Fazeli, Katharina Nöbauer and Karin Hummel (VetCore, University of Veterinary Medicine Vienna, Austria) for their technical support in mass spectrometric analysis. Ingrid Miller (Department for Biomedical Sciences, University of Veterinary Medicine Vienna, Austria) is gratefully acknowledged for assistance with the scanning of 2DDIGE gels. We also thank Hanna Lucia Worliczek (Institute of Parasitology, University of Veterinary Medicine Vienna) for her technical support with microscopic imaging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguinaldo AM, Turbeville JM, Linford LS, Rivera MC, Garey JR, Raff RA, Lake JA. Evidence for a clade of nematodes, arthropods and other moulting animals. Nature. 1997;387:489–493. doi: 10.1038/387489a0. [DOI] [PubMed] [Google Scholar]
- Crook M. The dauer hypothesis and the evolution of parasitism: 20 years on and still going strong. Int. J. Parasitol. 2014;44:1–8. doi: 10.1016/j.ijpara.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakshinamoorthy G, Samykutty AK, Munirathinam G, Shinde GB, Nutman T, Reddy MV, Kalyanasundaram R. Biochemical characterization and evaluation of a Brugia malayi small heat shock protein as a vaccine against lymphatic filariasis. PLoS One. 2012;7:e34077. doi: 10.1371/journal.pone.0034077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre R, Cermola M, Nunes CP, Hermann R, Muller M, Bazzicalupo P. Immuno-cross-reactivity of CUT-1 and cuticlin epitopes between Ascaris lumbricoides, Caenorhabditis elegans, and Heterorhabditis. J. Struct. Biol. 1998;123:1–7. doi: 10.1006/jsbi.1998.4012. [DOI] [PubMed] [Google Scholar]
- Foss EJ, Radulovic D, Shaffer SA, Goodlett DR, Kruglyak L, Bedalov A. Genetic variation shapes protein networks mainly through non-transcriptional mechanisms. PLoS Biol. 2011;9:e1001144. doi: 10.1371/journal.pbio.1001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg H, Huwyler U, Kohler L, Rehbein S, Wanner M. Kinetics of exsheathment of infective ovine and bovine strongylid larvae in vivo and in vitro. Parasitology. 2002;125:65–70. doi: 10.1017/s0031182002001816. [DOI] [PubMed] [Google Scholar]
- Hewitson JP, Grainger JR, Maizels RM. Helminth immunoregulation: The role of parasite secreted proteins in modulating host immunity. Mol. Biochem. Parasitol. 2009;167:1–11. doi: 10.1016/j.molbiopara.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu PJ. Dauer. WormBook 1 - 19. 2007 doi: 10.1895/wormbook.1.144.1. http://www.wormbook.org/chapters/www_dauer/dauer.html. [DOI] [PMC free article] [PubMed]
- Iglesias L, Malagon D, Valero A, Benitez R, Adroher FJ. CO2-fixing enzymes during moulting from third larval to fourth larval stage of Anisakis simplex and Hysterothylacium aduncum (Nematoda: Anisakidae). Parasitol. Res. 2005;96:212–215. doi: 10.1007/s00436-005-1342-6. [DOI] [PubMed] [Google Scholar]
- Joachim A, Ruttkowski B, Daugschies A. Characterisation of stage-specific proteins of Oesophagostomum dentatum by preparative isoelectric focusing and lectin blotting. Parasitol. Int. 2001;50:41–45. doi: 10.1016/s1383-5769(00)00070-2. [DOI] [PubMed] [Google Scholar]
- Joachim A, Ruttkowski B, Daugschies A. Ecdysis of Oesophagostomum: Possible involvement of eicosanoids and development of a bioassay. Parasitol. Res. 2005;95:391–397. doi: 10.1007/s00436-005-1302-1. [DOI] [PubMed] [Google Scholar]
- Lee DL. Cuticle, moulting and exsheathment. In: Lee DL, editor. The Biology of Nematodes. Taylor & Francis; London: 2002. pp. 171–209. [Google Scholar]
- Lewis E, Hunter SJ, Tetley L, Nunes CP, Bazzicalupo P, Devaney E. Cut-1-like genes are present in the filarial nematodes, Brugia pahangi and Brugia malayi, and, as in other nematodes, code for components of the cuticle. Mol. Biochem. Parasitol. 1999;101:173–183. doi: 10.1016/s0166-6851(99)00070-5. [DOI] [PubMed] [Google Scholar]
- Mulvenna J, Hamilton B, Nagaraj SH, Smyth D, Loukas A, Gorman JJ. Proteomics analysis of the excretory/secretory component of the blood-feeding stage of the hookworm, Ancylostoma caninum. Mol. Cell. Proteomics. 2009;8:109–121. doi: 10.1074/mcp.M800206-MCP200. [DOI] [PubMed] [Google Scholar]
- Myers CD, Goh PY, Allen TS, Bucher EA, Bogaert T. Developmental genetic analysis of troponin T mutations in striated and nonstriated muscle cells of Caenorhabditis elegans. J. Cell Biol. 1996;132:1061–1077. doi: 10.1083/jcb.132.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondrovics M, Gasser RB, Ruttkowski B, Nisbet AJ, Joachim A. Transcription profiles for two key gender-specific gene families in Oesophagostomum dentatum during development in vivo and in vitro. Infect. Genet. Evol. 2012;12:137–141. doi: 10.1016/j.meegid.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Ondrovics M, Silbermayr K, Mitreva M, Young ND, Razzazi-Fazeli E, Gasser RB, Joachim A. Proteomic analysis of Oesophagostomum dentatum (Nematoda) during larval transition, and the effects of hydrolase inhibitors on development. PLoS One. 2013;8:e63955. doi: 10.1371/journal.pone.0063955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AP, Johnstone IL. The cuticle. WormBook 1 - 15. 2007 doi: 10.1895/wormbook.1.138.1. http://www.wormbook.org/chapters/www_cuticle/cuticle.html. [DOI] [PMC free article] [PubMed]
- Parkinson J, Mitreva M, Whitton C, Thomson M, Daub J, Martin J, Schmid R, Hall N, Barrell B, Waterston RH, McCarter JP, Blaxter ML. A transcriptomic analysis of the phylum Nematoda. Nat. Genet. 2004;36:1259–1267. doi: 10.1038/ng1472. [DOI] [PubMed] [Google Scholar]
- Saverwyns H, Visser A, Van Durme J, Power D, Morgado I, Kennedy MW, Knox DP, Schymkowitz J, Rousseau F, Gevaert K, Vercruysse J, Claerebout E, Geldhof P. Analysis of the transthyretin-like (TTL) gene family in Ostertagia ostertagi--comparison with other strongylid nematodes and Caenorhabditis elegans. Int. J. Parasitol. 2008;38:1545–1556. doi: 10.1016/j.ijpara.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Saz HJ, Lescure OL. The functions of phosphoenolpyruvate carboxykinase and malic enzyme in the anaerobic formation of succinate by Ascaris lumbricoides. Comp. Biochem. Physiol. 1969;30:49–60. doi: 10.1016/0010-406x(69)91296-1. [DOI] [PubMed] [Google Scholar]
- Schwarz EM, Korhonen PK, Campbell BE, Young ND, Jex AR, Jabbar A, Hall RS, Mondal A, Howe AC, Pell J, Hofmann A, Boag PR, Zhu XQ, Gregory TR, Loukas A, Williams BA, Antoshechkin I, Brown CT, Sternberg PW, Gasser RB. The genome and developmental transcriptome of the strongylid nematode Haemonchus contortus. Genome Biol. 2013;14:R89. doi: 10.1186/gb-2013-14-8-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talvik H, Christensen CM, Joachim A, Roepstorff A, Bjørn H, Nansen P. Prepatent periods of different Oesophagostomum spp. isolates in experimentally infected pigs. Parasitol. Res. 1997;83:563–568. doi: 10.1007/s004360050298. [DOI] [PubMed] [Google Scholar]
- Wang J, Kim SK. Global analysis of dauer gene expression in Caenorhabditis elegans. Development. 2003;130:1621–1634. doi: 10.1242/dev.00363. [DOI] [PubMed] [Google Scholar]
- Yatsuda AP, Krijgsveld J, Cornelissen AW, Heck AJ, de Vries E. Comprehensive analysis of the secreted proteins of the parasite Haemonchus contortus reveals extensive sequence variation and differential immune recognition. J. Biol. Chem. 2003;278:16941–16951. doi: 10.1074/jbc.M212453200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.