Abstract
Objective
To determine if an abnormal CA-125 level in a menopausal female without ovarian cancer is associated with an increase in mortality.
Methods
The Prostate, Lung, Colorectal, and Ovarian Cancer Screening Randomized Controlled (PLCO) Trial was a large multicenter prospective trial conducted by the National Cancer Institute (NCI). Over 78,000 healthy women aged 55–74 were randomized to a screening arm versus a usual medical care arm to evaluate the efficacy of screening in reducing mortality due to ovarian cancer. Women in the screening arm underwent annual screening for ovarian cancer with transvaginal ultrasound and CA-125 levels. There were 38,818 patients without ovarian cancer that had at least one CA-125 level drawn; 1201 (3.09%) had at least one abnormal level. The current study compares mortality in patients that had one or more abnormal CA-125 levels without ovarian cancer versus those with all normal levels.
Results
Patients with one or more abnormal CA-125 levels, without ovarian cancer, had a significantly higher mortality than patients with all normal CA-125 levels in the PLCO screening trial (p<0.0001). This increased risk extended throughout the follow-up period. Analysis of cause of death listed on the death certificate showed an excess mortality attributable to lung cancer, digestive disease, and endocrine, nutritional, and metabolic disease.
Conclusion
Menopausal females with an elevated CA-125 and without ovarian cancer are exposed to an increased risk of premature mortality.
INTRODUCTION
The Prostate, Lung, Colorectal, and Ovarian Cancer Screening Randomized Controlled (PLCO) Trial was a large multicenter prospective trial conducted by the National Cancer Institute (NCI) to assess the efficacy of screening for four index cancers. Over 76,000 healthy menopausal female volunteers were enrolled and randomized to a screening arm and a usual medical care arm. Women in the usual medical care arm did not undergo specific screening intervention. Women in the screening arm underwent annual screening for ovarian cancer with pelvic ultrasound and CA-125 levels. Approximately 8% of patients in the screening arm experienced false positive results for ovarian cancer screening. [1] The purpose of the current study is to examine the clinical significance of a false positive CA-125 level in menopausal women undergoing screening for ovarian cancer.
The serum CA-125 level is widely recognized and utilized as a tumor marker for epithelial ovarian cancer. The PLCO trial, and other studies, however, have found that CA-125 lacks sufficient positive predictive value to be utilized as a screening test. [1, 2] It is unknown, however, whether there is any clinical significance to a false positive CA-125. CA-125 levels are often elevated in pre-menopausal women with benign gynecologic conditions, i.e. endometriosis, myomata, and benign ovarian cysts. [3] In post-menopausal women, however, these conditions are generally quiescent. Elevated CA-125 levels are also associated with non-gynecologic malignancies, cirrhosis, and heart failure. [4,5,6] One expects these pathologic conditions to be more prevalent in a menopausal population. There are no published studies, however, examining the long-term consequences of false positive CA-125 levels in post-menopausal females. The current study utilizes data from the PLCO trial to determine if asymptomatic healthy post-menopausal female volunteers with elevated CA-125 levels are at risk for increased mortality compared to women with normal CA-125 levels.
MATERIALS AND METHODS
The PLCO trial was conducted from 1993–2001. This was a randomized controlled trial of 78,216 women assigned to either a usual medical care arm versus a screening arm. Women in the screening arm (n=39,105) were screened annually for 6 years with CA-125 levels and for 4 years with transvaginal ultrasound. Women in the usual medical care arm (n=39,111) did not undergo screening for ovarian cancer. Patients with an abnormal screen were referred to their primary physician for additional evaluation and/or surgery. Patients without a specific diagnosis continued screening as per trial protocol. Follow-up continued until death or until 2010. Cause of death was ascertained and accessioned from the death certificates and/or medical records. All death records were reviewed by at least two independent and blinded reviewers. Previous publications from the PLCO study group have outlined the trial methods in detail. [1, 7, 8, 9, 10, 11]
Results of the trial were published in The Journal of the American Medical Association in 2011. [1] In 2012 the NCI allowed outside investigators with approved research projects access to the collected data through the Cancer Data Access System (CDAS). The current study was approved by the NCI in 2012; the data was received and analyzed by the Biostatistics Core at John A. Burns School of Medicine in 2013. To describe the CA-125 level by mortality status, we counted the total number of all abnormal CA-125 levels for the study period. We also created an indicator of patients who had at least one abnormal CA-125 level. A CA-125 level over 35 U/ml. was considered abnormal. Chi-square tests were used to compare the mortality rate by an indicator of abnormal CA-125 level. Relative risk (RR) of abnormal CA-125 levels and their 95% confidence intervals (95% CI) were estimated by comparing the mortality rate for the abnormal CA-125 group with that of the normal CA-125 group. Survivals between normal and abnormal CA-125 level group were compared with non-parametric tests. All data analyses were performed in SAS 9.3 (Cary, N.C., 2011). A two-tailed p-value of less than 0.05 was regarded as statistically significant.
RESULTS
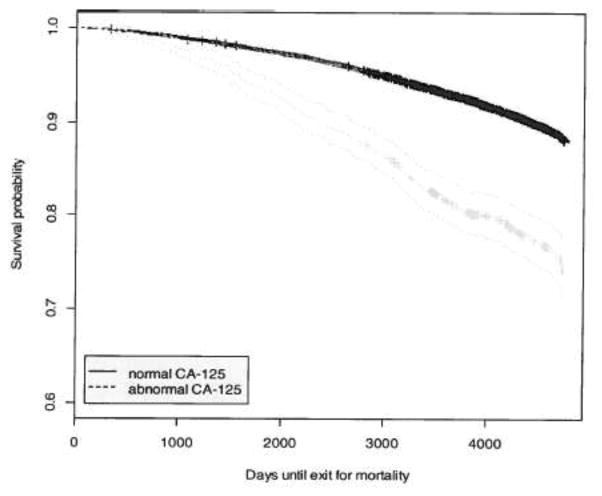
There were 38,818 patients abstracted that had at least one CA-125 level drawn and did not have a diagnosis of ovarian cancer. There were 1201 patients (3.09%) that had at least one abnormal level. Patients with an abnormal CA-125 level were slightly older than patients with all normal CA-125 levels (mean 63.5 years vs. 62.5 years, p<0.001). The relative risk of death for patients with at least one abnormal CA-125 was 2.29 (95% CI, 2.05–2.55). After adjusting for age, the relative risk remained significant at 2.02 (95% CI, 1.82–2.24). Table 1 presents the analysis of mortality rate and number of abnormal CA-125 levels. Patients that had one or more abnormal levels had a significantly higher mortality than patients with all normal levels (p<0.0001). Figure 1 demonstrates the survival analysis. Patients with at least one abnormal CA-125 experienced a significantly worse survival over time (p<0.0001). This trend persisted over the entire follow-up period of ten years. A subgroup analysis was performed on patients that had at least one CA-125 level over 100 U/ml. This subgroup appeared to have a particularly poor outcome, with a relative risk of death of 5.96 (95% CI, 4.97–7.15). Table 2 lists the cause of death from the Death Certificate. Both the relative risk and the confidence intervals are included. Patients with an abnormal CA-125 experienced a higher mortality due to lung cancer (p<0.001). This increase, however, was only statistically significant for the subgroup with one abnormal CA-125 (p=0.001). Women with two or more abnormal CA-125 levels did not experience an excess mortality due to lung cancer (p=0.37). Smoking status was also examined in patients with an abnormal CA-125; both current smokers and former smokers with an abnormal CA-125 experienced a higher mortality than never smokers with an abnormal CA-125 (p<0.05). Mortality due to ischemic heart disease (p=0.035) and cerebrovascular accident (p=0.015) was significantly lower. There was no difference in mortality associated with non-PLCO malignancies or infectious diseases (p=0.89). Both digestive diseases and the group of endocrine, nutritional, metabolic diseases and immunity disorders were associated with a higher mortality.
Table 1.
Mortality rate by number of abnormal CA-125 level during study period
| number of abnormal CA-125 level | Exit Status for Mortality | Total | |
|---|---|---|---|
| Dead | Alive | ||
| n (%) | n (%) | ||
| 0 | 3673 (9.76%) | 33944 (90.24%) | 37617 |
| 1 | 173 (24.64%) | 529 (75.36%) | 702 |
| 2 | 44 (20.85%) | 167 (79.15%) | 211 |
| 3 | 16 (17.58%) | 75 (82.42%) | 91 |
| 4 | 16 (20%) | 64 (80%) | 80 |
| 5 | 10 (17.24%) | 48 (82.76%) | 58 |
| 6 | 9 (15.25%) | 50 (84.75%) | 59 |
| Total | 3941 | 34877 | 38818 |
Figure 1.
Survival curves with 95% CI between normal and abnormal CA-125 level (p<0.0001).
Table 2.
Cause of death from death certificate by CA-125 level (normal vs. abnormal) during study period
| Cause of death from death certificate | CA-125 level | Relative risk [95% CI]: | |
|---|---|---|---|
| Normal | Abnormal | Abnormal vs. Normal | |
| Lung | 376 (10.26%) | 38 (14.18%) | 1.39 [1.02, 1.89] |
| Colorectal | 106 (2.89%) | 5 (1.87%) | 0.65 [0.27, 1.57] |
| Ovarian | 3 (0.08%) | 0 (0%) | NA |
| Non-PLCO Neoplasms | 863 (23.55%) | 64 (23.88%) | 1.02 [0.81, 1.27] |
| Ischemic Heart Disease | 403 (11%) | 18 (6.72%) | 0.61 [0.39, 0.97] |
| Cerebrovascular Accident | 259 (7.07%) | 8 (2.99%) | 0.42 [0.21, 0.85] |
| Other Circulatory Disease | 439 (11.98%) | 31 (11.57%) | 0.97 [0.69, 1.36] |
| Respiratory Illness | 339 (9.25%) | 31 (11.57%) | 1.25 [0.89, 1.77] |
| Digestive Disease | 113 (3.08%) | 16 (5.97%) | 1.94 [1.17, 3.23] |
| Infectious Disease | 88 (2.4%) | 7 (2.61%) | 1.09 [0.51, 2.33] |
| Endocrine, Nutritional and Metabolic Diseases, and Immunity Disorders | 131 (3.58%) | 18 (6.72%) | 1.88 [1.17, 3.03] |
| Diseases of the Nervous System | 141 (3.85%) | 6 (2.24%) | 0.58 [0.26, 1.31] |
| Accident | 140 (3.82%) | 9 (3.36%) | 0.88 [0.45, 1.71] |
| Other | 263 (7.18%) | 17 (6.34%) | 0.89 [0.55, 1.42] |
DISCUSSION
Occasionally, clinicians are faced with the dilemma of a menopausal patient with an abnormal CA-125 but without ovarian cancer. The CA-125 level is relatively non-specific and may be associated with a variety of diagnostic conditions. In pre-menopausal females, abnormal CA-125 levels are commonly associated with endometriosis, myomata, salpingitis, and benign ovarian cysts. In menopausal females, however, the risk of ovarian cancer increases, and an abnormal level requires additional evaluation. Nonetheless, in the initial PLCO report, less than 4% of patients with an abnormal CA-125 were diagnosed with ovarian cancer. [7] The current study was undertaken to assess the long term consequences of an abnormal CA-125 level in healthy menopausal volunteers without ovarian cancer.
The PLCO trial was a multicenter, randomized trial conducted to determine if screening could effectively reduce mortality due to ovarian cancer. Healthy volunteers were enrolled and randomized to a screening arm versus a usual medical care/non-screening arm. Screening for ovarian cancer consisted of annual CA-125 levels and transvaginal ultrasound. An analysis of pathologic findings associated with false-positive screens was published 2011. [11] There were 540 patients that underwent a diagnostic procedure for either an abnormal CA-125 or an abnormal ultrasound. The majority of patients were found to have benign neoplastic or non-neoplastic conditions; however, 4% were found to have a non-ovarian cancer. The majority of these cancers were primary endometrial cancers. Although the outcome for this malignancy is generally favorable, it suggests that an abnormal CA-125 may reflect a potentially life threatening condition in a small proportion of patients. Patients with an abnormal screen in the trial were referred to their physician for further evaluation and treatment. Therefore a number of patients in the trial did not undergo a diagnostic procedure. Presumably these patients had no localizing symptoms or diagnostic findings; these patients remained in the trial and continued annual screening. In our study 41% of patients with an abnormal CA-125 had 2 or more abnormal levels.
The current study, therefore, was undertaken to determine if an abnormal CA-125 level in an apparently healthy woman is clinically relevant. If mortality remains unaffected by an abnormal CA-125, then an abnormal level in a patient without ovarian cancer can be safely disregarded. Potentially, however, an elevated CA-125 might be reflective of subclinical illness or non-ovarian malignancy. The abstracted PLCO data identified 1201 patients (3.09%) of the screened population with at least one abnormal CA-125 level in the absence of ovarian cancer. Follow-up data indicates an increased risk of mortality in these patients. This increased risk occurred for patients with one abnormal level and extended to patients with multiple, up to six (the duration of screening), abnormal levels. Survival curves comparing the population with at least one abnormal level versus those with all normal levels also shows a significantly worse survival over time; and the survival curves continued to diverge for the entire follow-up period.
The cause of death listed on the death certificate was assessed to determine possible contributions to the excess in mortality. There was a statistically significant excess mortality attributable to lung cancer in the abnormal CA-125 population. The excess mortality due to lung cancer occurred only in the group with one abnormal CA-125. This excess mortality did not extend to those with two or more abnormal levels. This suggests that an abnormal CA-125 occurring with the diagnosis of lung cancer is most likely associated with the presence of metastases and a poor prognosis. When smoking was examined as a possible contributing factor, both current and former smokers with an abnormal CA-125 were noted to have a significantly higher mortality than non-smokers with an abnormal CA-125. The PLCO trial, utilizing annual chest radiograph to screen for lung cancer, did not find a reduction in lung cancer mortality in the screening group. [12] It seems prudent, however, to screen patients with a smoking history and an abnormal CA-125 level with a routine chest radiograph.
There was no apparent increase in mortality due to non-PLCO neoplasms in the abnormal CA-125 group. This indicates that searching for other malignancies in patients without ovarian cancer is probably not an effective use of resources and is unlikely to be productive. Deaths due to ischemic heart disease and cerebrovascular accident were significantly lower in the abnormal CA-125 group compared to the normal group. Again, this confirms that this group is exposed to premature mortality rather than the anticipated normal process of aging. In both the ‘digestive disease’ category and the ‘endocrine, nutritional, and metabolic disease’ category, the abnormal CA-125 group exhibited a significantly higher mortality. This seems to indicate that abnormal CA-125 levels reflect chronic processes exposing these individuals to excess mortality. Abnormal CA-125 levels, therefore, might indicate ongoing non-neoplastic processes associated with increased mortality risk. In summary, the abnormal CA-125 group appears to be at risk for premature mortality primarily due to lung cancer and non-neoplastic, non-cardiovascular illness.
Variations in CA-125 levels may be associated with certain baseline conditions, such as ethnic group, obesity, etc. [8, 13]. This may introduce known or unknown confounding variables. The current study did not examine or adjust for all of these variables. Therefore it is possible that abnormal CA-125 levels are merely reflective of individual characteristics or variables, which may have influenced mortality. Patients with an abnormal CA-125 had a statistically significant higher mean age (63.5 vs. 62.5 years); age adjusted mortality, however, remained higher in the abnormal group.
Clinicians should avoid obtaining a CA-125 level in asymptomatic females. Nonetheless, an elevated CA-125 in a menopausal female without ovarian cancer should be regarded with concern. These individuals appear to be at risk for premature mortality. It is unclear whether further investigation is warranted. Continued health surveillance would appear prudent.
Highlights.
An abnormal CA-125 was noted in 3% of menopausal females without ovarian cancer.
These patients appeared to have an excess risk of premature mortality.
Acknowledgments
Authors thank the National Cancer Institute for access to the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial database. The interpretation and reporting of these data are the sole responsibility of the authors.
FUNDING ACKNOWLEDGEMENT: Biostatistical support was partially funded by grants from the National Institute on Minority Health and Health Disparities U54MDOO7584 and G12MDOO7601 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest Statement
All authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, et al. Effect of screening on ovarian cancer mortality. JAMA. 2011;305:2295–2303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi H, Yamada Y, Sado T, Sakata M, Yoshida S, Kawaguchi R, et al. A randomized study of screening for ovarian cancer: a multicenter study in Japan. Int J Gynecol Cancer. 2008;18:414–420. doi: 10.1111/j.1525-1438.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 3.Meden H, Fattahi-Meibodi A. CA 125 in benign gynecological conditions. Int J Biol Markers. 1998;13:231–237. doi: 10.1177/172460089801300411. [DOI] [PubMed] [Google Scholar]
- 4.Miralles C, Orea M, Espana P, Provencio M, Sanchez A, Cantos B, et al. Cancer antigen 125 associated with multiple benign and malignant pathologies. 2003;10:150–154. doi: 10.1245/aso.2003.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Moss EL, Hollingworth J, Reynolds TM. The role of CA125 in clinical practice. 2005;58:308–312. doi: 10.1136/jcp.2004.018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silberstein LB, Rosenthal AN, Coppack SW, Noonan K, Jacobs IJ. Ascites and a raised serum CA-125 – a confusing combination. J R Soc Med. 2001;94:581–582. doi: 10.1177/014107680109401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buys SS, Partridge E, Greene MH, Prorok PC, Reding D, Riley TL, et al. Ovarian cancer screening in the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial: findings from the initial screen of a randomized trial. Am J Obstet Gynecol. 2005;193:2183–2184. doi: 10.1016/j.ajog.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Johnson CC, Kessel B, Riley TL, Ragard LR, Williams CR, Xu JL, et al. The epidemiology of CA-125 in women without evidence of ovarian cancer in the prostate, lung, colorectal and ovarian cancer (PLCO) screening trial. Gynecol Oncol. 2008;10:383–389. doi: 10.1016/j.ygyno.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Partridge EE, Greenlee RT, Riley TL, Commins J, Ragard L, Xu JL, et al. Assessing the risk of ovarian malignancy in asymptomatic women with abnormal CA 125 and transvaginal ultrasound scans in the prostate, lung, colorectal, and ovarian screening trial. Obstet Gynecol. 2012;121:25–31. doi: 10.1097/aog.0b013e3182755e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenlee RT, Kessel B, Williams CR, Riley TL, Ragard LR, Hartge P, et al. Prevalence, incidence, and natural history of simple ovarian cysts among women >55 years old in a large cancer screening trial. Am J Obstet Gynecol. 2010;202:373, e1–9. doi: 10.1016/j.ajog.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyante SJ, Black AB, Kreimer AR, Duggan MA, Carreon JD, Kessel B, et al. Pathologic findings following false-positive screening tests for ovarian cancer in the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Gynecol Oncol. 2011;120:474–479. doi: 10.1016/j.ygyno.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oken MM, Hocking WG, Kvale PA, Andriole GL, Buys SS, Church TR, et al. Screening by chest radiograph and lung cancer mortality, the prostate, lung, colorectal, and ovarian (PLCO) randomized trial. JAMA. 2011;306:1865–1873. doi: 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]
- 13.Pauler DK, Menon U, McIntosh M, Symecko, Skates SJ, Jacobs IJ. Factors influencing serum CA- 125II levels in healthy postmenopausal women. Cancer Epidemiol Biomarkers and Prevention. 2001;10:489. [PubMed] [Google Scholar]