Summary
Iron is a critical and tightly regulated nutrient for both the malaria parasite and its human host. The importance of the relationship between host iron and the parasite has been underscored recently by studies showing that host iron supplementation may increase the risk of falciparum malaria. It is unclear what host iron sources the parasite is able to access. We developed a flow cytometry-based method for measuring the labile iron pool (LIP) of parasitized erythrocytes using the nucleic acid dye STYO 61 and the iron sensitive dye, calcein acetoxymethyl ester (CA-AM). This new approach enabled us to measure the LIP of P. falciparum through the course of its erythrocytic life cycle and in response to the addition of host serum iron sources. We found that the LIP increases as the malaria parasite develops from early ring to late schizont stage, and that the addition of either transferrin or ferric citrate to culture media increases the LIP of trophozoites. Our method for detecting the LIP within malaria parasitized RBCs provides evidence that the parasite is able to access serum iron sources as part of the host vs. parasite arms race for iron.
Keywords: Plasmodium falciparum, iron, labile iron pool, malaria, calcein acetoxymethyl ester
Each year up to 250 million clinical cases of malaria and nearly 1 million deaths from malaria are reported in official statistics (WHO, 2011). Plasmodium falciparum malaria is the most deadly of all the species of malaria that infect humans. The malaria parasite has a complex life cycle in the human host. Anopheles mosquitoes inject sporozoite stage P. falciparum parasites during a blood meal; sporozoites then migrate to the liver where they infect hepatocytes and multiply over a clinically silent 7–10 d period (Sinnis et al, 1996). During the asexual erythrocyte stage of the parasite, merozoites invade red blood cells (RBCs) and progress from the metabolically inactive ring stage to the metabolically active trophozoite stage to the schizont stage. DNA replication is initiated during the schizont stage and results in the production of new merozoites that burst from the host RBC into the blood stream and invade new RBCs. The RBC stage of the malaria parasite is responsible for the morbidity and mortality associated with P. falciparum infection and is exquisitely sensitive to iron chelators (Ferrer et al, 2012).
Despite the essential role of iron in parasite development, it is unknown what host iron sources P. falciparum utilizes during any stage of the human infection. The two principal sources of host iron available to the parasite during the RBC stage are extra-erythrocytic (serum) iron and intra-erythrocytic iron. The intra-erythrocytic iron pool amounts to 100 fg (20 mmol/l) iron, partitioned into haemoglobin, ferritin, and the cytoplasmic labile iron pool (LIP). The erythrocytic LIP consists of residual cytoplasmic bioavailable iron that was not incorporated into haemoglobin or stored within ferritin during the maturation of erythrocyte precursors (Prus & Fibach, 2008a). The majority of host serum iron is bound to host protein transferrin (0·6–1·5 g/l), with a residual amount of iron, non-transferrin-bound iron (NTBI), circulating in the serum chelated by low molecular weight molecules such as citrate (Cook & Skikne, 1989). To date there is no evidence that P. falciparum is able to release iron from haem or host ferritin (Sigala & Goldberg, 2012). The relationship between the host erythrocyte LIP and the malaria parasite infectivity and maturation is unknown (Scholl et al, 2005). It is unknown how the host LIP impacts the malaria parasite’s infectivity and maturation. The ability of the parasite to access serum iron is unclear, and data are conflicting (Pollack & Fleming, 1984; Haldar et al, 1986; Rodriguez & Jungery, 1986; Sanchez-Lopez & Haldar, 1992).
Calcein acetoxymethyl ester (CA-AM) has been widely used to examine the cytoplasmic LIP of mammalian cells (Breuer et al, 1995a,b, 1996; Epsztejn et al, 1997; Tenopoulou et al, 2007). CA-AM is non-fluorescent, non-iron binding, neutrally charged, and easily permeates cell membranes. Upon cellular entry, intracellular esterases cleave CA-AM into the green-fluorescent molecule calcein, which is then trapped within the cell. Calcein fluorescence is quenched by 1:1 stoichiometric binding of iron in pH range of 7–7·5 (Breuer et al, 1995a, 1996). The addition of non-fluorescent, high affinity iron chelators removes iron from calcein and consequently increases calcein fluorescence, providing an effective method for assessing the labile iron content of cells. Alternatively the addition of iron, capable of being incorporated by a cell, quenches calcein fluorescence (Tsien, 1989; Breuer et al, 1995b). Previous investigators have utilized a microscopy-based approach for the measurements of calcein fluorescence, to investigate the site of action of anti-malarial iron chelators and gain preliminary insight into the LIP of parasitized human erythrocytes (Loyevsky et al, 1999). More recently, CA-AM has been utilized to assess the LIP of the heterogeneous cell populations of peripheral blood and bone marrow by flow cytometry (Prus & Fibach, 2008b).
In the present study, we adapted the CA-AM flow cytometry method in order to assess the LIP of P. falciparum-infected erythrocytes (Prus & Fibach, 2008a,b). We combined the technique of identifying parasitized erythrocytes with the fluorescent DNA dye, SYTO 61 (Fu et al, 2010), with the CA-AM method for assessing cellular labile iron to determine the LIP of P. falciparum during asexual maturation by flow cytometry. This flow cytometry approach allows for the analysis of the LIP of a mixed population of uninfected and P. falciparum -infected erythrocytes. Furthermore, we utilized this approach to investigate the effect of extracellular iron sources, transferrin and ferric citrate, on the LIP of the erythrocytic stage of P. falciparum.
Materials and methods
Plasmodium falciparum culture
Plasmodium falciparum parasite lines FCR3-FMG (MR4, MRA-736) and Dd2 (MR4, MRA-156) were routinely cultured in iron-replete O-positive (O+) RBCs obtained from healthy individuals at the Clinical and Translational Research Center at the University of North Carolina, Chapel Hill, NC (approved by the University of North Carolina Institutional Review Board, reference 09–0559). Cultures were maintained with 2% haematocrit in complete media containing RPMI 1640 medium with 10% albumax II, 1 mM hypoxanthine, 20 mmol/l L-glutamine, .45% glucose, and 10 μg/l gentamicin (ACM). Cultures were incubated on a shaker at 37°C in 5% O2, 5% CO2 and 90% Nitrogen. Parasite density was maintained between 0·5% and 10% P. falciparum parasitized RBCs (pRBCs). pRBC cultures were synchronized to within 4–6 h of each other by first treating cultures with 5% D-sorbitol to select for ring stage parasites, followed by magnetic-activated cell sorting (MACS) (Miltenyi Biotec, Auburn, CA, USA) isolation of haemozoin-containing trophozoite and schizont stage pRBCs 24 h later. pRBC cultures were next incubated with 30 iu heparin to prevent invasion of merozoites for 18 h, at which point heparin was washed from cultures with subsequent incubated under normal culture conditions for an additional 24 h before a final MACS isolation of trophozoites and schizonts was performed (Boyle et al, 2010). To obtain a culture with all stages (ring, trophozoite, schizont, and merozoites) synchronized cultures were monitored until approximately 50% of schizonts had ruptured.
Plasmodium falciparum LIP assay
Plasmodium falciparum lines FCR3-FMG or Dd2 at a parasite density of 5–10% pRBCs were washed twice with phosphatebuffered saline (PBS) 0. 5% Albumax II (PBS+), inoculated into a 96-well plate at 2 × 106 cells per well and subsequently labelled with 0·125 umol/l calcein acetoxymethyl ester (CA-AM [Invitrogen, Grand Island, NY, USA] for 15 min in the dark. Following CA-AM labelling, cells were washed twice with PBS+ and allowed to rest for 15 min under standard culture conditions in the dark. Cells were then labeled with 0·5 μmol/l DNA dye SYTO 61 (Invitrogen) in the presence or absence of 100 μmol/l of iron chelators: deferiprone, dipyridyl, or deferoxamine for 1 h under standard culture conditions in the dark. Following incubation with SYTO 61 and iron chelators, unfixed cells were immediately analysed by flow cytometry using a Cytek-modified FACS-Calibur under BSL-2 containment. For experiments assessing the impact of extracellular transferrin or ferric citrate on the pRBC LIP, P. falciparum cultures were incubated with transferrin or ferric citrate prior to CA-AM and SYTO 61 labelling. Individual experiments were performed in triplicate. The statistical significance for each individual experiment was calculated using the Student’s t-test. For experiments where data is expressed as delta mean fluorescence intensity (ΔMFI), ΔMFI represents the absolute difference in MFI.
Flow cytometry analysis
Flow cytometry was performed at the UNC Flow Cytometry Core Facility, Chapel Hill, NC on a Cytek-modified FACS-Calibur with 2 lasers: a 30 mW 488 Diode Pumped Solid State laser and a 25 mW 637 red diode laser (FACS-Calibur; Becton Dickinson, Mount View CA, modified by Cytek Development, Freemont, CA). Channels and fluorescent probes used on the FACS-Calibur included: SYTO 61 (63 × nm excitation, 666·27 nm bandpass), and calcein (488 nm, 530/30 bandpass). Detector gain settings were varied between experiments to optimize signal but were kept constant within individual experiments and no compensation was applied to any of the channels. pRBC were gated based on the SYTO 61 signal and detector gains for calcein fluorescence were adjusted to achieve a calcein MFI of 10–20 for uninfected RBCs. A minimum 1000 pRBC (SYTO 61 +) events were acquired. FACS-Calibur data was collected using FlowJo CE (Treestar, Ashland, OR) and analysed with Summit v 5.1 (Beckman Coulter, Miami, Florida).
Results
Detection of LIP in P. falciparum-infected erythrocytes by flow cytometry
To assess the LIP of P. falciparum infected erythrocytes, P. falciparum (FCR3-FMG strain)-infected erythrocyte cultures were loaded with CA-AM, stained with DNA dye SYTO 61 to identify parasitized RBCs (pRBCs) and finally incubated with membrane permeable iron chelator deferiprone to enable the determination of LIP. We first confirmed that neither calcein nor SYTO 61 interfered with the others’ fluorescence profile (Figure S1A, B), and that SYTO 61 did not interfere with calcein’s sensitivity to either iron chelator or extracellular iron (Figure S1C). To characterize the basal calcein fluorescence of uninfected and pRBCs, we examined the SYTO 61 profile of stained cells to identify uninfected (SYTO 61 negative, R2- lower region), pRBCs infected with rings (SYTO 61 positive, R3-middle region) and pRBCs infected with trophozoites (SYTO 61 positive, R4-upper region) (Fig 1A). Each of these three populations was gated upon and the basal calcein fluorescence of each population was determined. We observed that the pRBCs had greater steady state calcein basal fluorescence than uninfected erythrocytes, and calcein fluorescence increased with increasing parasite maturation. Compared to uninfected RBCs, the calcein fluorescence of ring pRBCs and trophozoite pRBCs was 18% (P < 0·02) and 153% (P < 0·0002) greater than uninfected RBCs respectively (Fig 1B).
Fig 1.
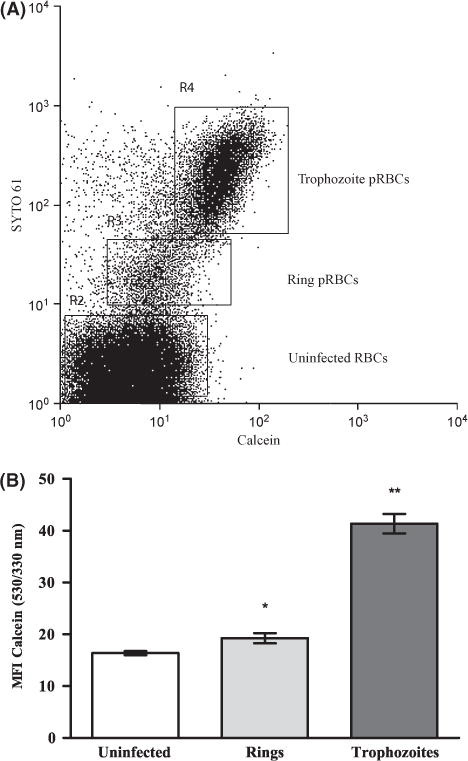
Measurement of the basal levels of CA-AM fluorescence. Calcein fluorescence of parasitized and uninfected RBCs was determined by assessing the calcein fluorescence of SYTO 61− (uninfected) and SYTO 61 + (parasitized) RBCs. Cells from P. falciparum (FCR3-FMG strain)-infected erythrocyte cultures were seeded in triplicate into a 96-well plate, loaded with CA-AM, and then stained with DNA dye SYTO 61 and analysed by flow cytometry. (A) Dot-plot of cell distribution of DNA stain SYTO 61 vs. calcein reveals the distribution of uninfected RBCs, rings and trophozoites. (B) Gates were set on uninfected RBCs, rings, and trophozoite populations and, mean calcein-fluorescence intensity (MFI) (530/330 nm) was assessed for each population. The bar graph (mean ± SD, n = 3) shows calcein fluorescence of uninfected and parasitized RBCs. Student’s t-test statistical analysis was performed comparing uninfected to parasitized cells, *P < 0·02 **P < 0·0002. Data is from a single representative experiment, and the experiment was performed five independent times with each parasite line, FCR-FMG and Dd2.
Before calcein fluorescence can be made susceptible to iron quenching, cellular esterases must cleave the acetoxymethyl ester group from CA-AM, converting the non-fluorescent CA-AM molecule to the fluorescent calcein. Parasitized RBCs have greater enzymatic activity than uninfected RBCs (Vander Jagt et al, 1982). To assess the impact of iron on calcein fluorescence, we employed the use of iron chelators, which, when added to calcein-loaded cells, chelate any iron bound to calcein. The resulting increase in calcein fluorescence achieved after adding an iron chelator to calcein-loaded cells represents the cell LIP. To assess the LIP of uninfected and pRBCs, we measured the calcein fluorescence of each of these populations in the absence (gray line/diagonal hatch) and presence (black line/vertical hatch) of the iron chelator deferiprone (Fig 2A, B). The addition of deferiprone resulted in significant increases in calcein fluorescence in uninfected as well as in the ring and trophozoite stage parasites, indicating the presence of a LIP. In addition to deferiprone, we employed a second membrane-permeable iron chelator (2, 2 biypridyl) and a membrane-impermeable iron chelator (desferioxamine) to assess the LIP within uninfected RBCs, ring pRBCs and trophozoite pRBCs (Fig 2C). Use of the membrane-permeable iron chelator deferiprone resulted in ΔMFI in both ring pRBCs (ΔMFI = 4·55 ± 0·426, P < 0·005) and trophozoite pRBCs (ΔMFI = 15·02 ± 1·11, P < 0·005) as well as uninfected RBCs (ΔMFI = 1·93 ± 0·12, P < 0·005). Use of a second membrane-permeable iron chelator, 2,2 biypridyl, resulted in an increase in ΔMFI in both ring pRBCs (ΔMFI = 3·88 ± 0·44, P < 0·005) and trophozoite pRBCs (ΔMFI = 16·48 ± 1–10, P < 0·005) compared to the increase in ΔMFI observed in uninfected RBCs (ΔMFI = 2·22 ± 0·043, P < 0·005). Utilizing membrane-permeable iron chelators, we consistently observed 15–20% greater ΔMFI (P < 0·005) in ring pRBCs and 40–50% greater ΔMFI (P < 0·005) in trophozoite pRBCs as compared to the ΔMFI observed in uninfected RBCs in independent experiments. However, with the less permeable chelator, deferoxamine, an increase in ΔMFI in both ring pRBCs (ΔMFI = 3·1 ± 0·0·21, P < 0·005) and trophozoite pRBCs (ΔMFI = 8·67 ± 0·089, P < 0·005) was seen, but no ΔMFI was observed in uninfected RBCs (ΔMFI = 0·19 ± 0·32, P > 1·0). This finding is consistent with evidence that pRBCs are more permeable than uninfected RBCs (Pouvelle et al, 1991; Nguitragool et al, 2011).
Fig 2.
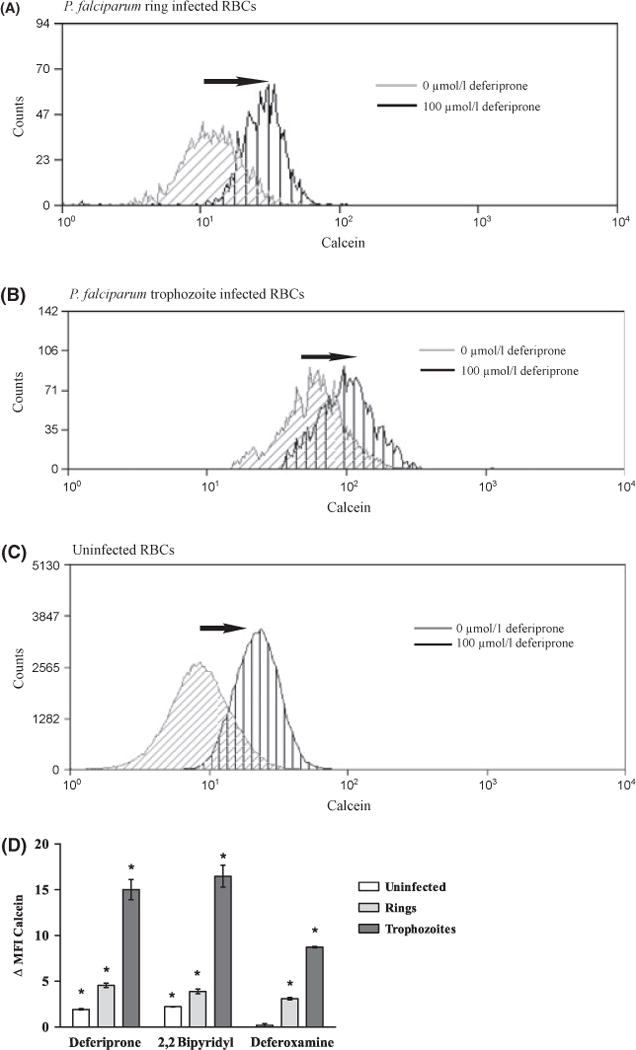
Measurement of LIP in P. falciparum infected RBCs using flow cytometry. The LIP of uninfected (SYTO 61−) and parasitized RBCs (SYTO 61 +) was determined by evaluating the change in MFI of calcein-loaded cells achieved in the presence of different iron chelators. Cells from P. falciparum (FCR3-FMG strain) infected erythrocyte cultures were loaded with CA-AM and then incubated with DNA dye SYTO 61 in either the presence or absence of 100 μmol/l of the indicated iron chelator for 1 h. Cells were immediately analysed by flow cytometry. (A) Ring infected RBCs (B) trophozoite infected RBCs or (C) uninfected RBCs following 1 h incubation in the absence (gray line/diagonal hatch) or presence (black line/vertical hatch) of the iron chelator, deferiprone. Black arrows denote the deferiprone-induced shift in calcein fluorescence. (C) Change in calcein MFI (530/330 nm) with the addition of either deferiprone, 2,2 bipyridyl or deferoxamine in uninfected and ring and trophzoite parasitzed RBCs. The bar graph (mean ± SD, n = 3) shows calcein ΔMFI of uninfected and parasitized RBCs. Student’s t-test statistical analysis was performed comparing MFI calcein before and after the addition of iron chelator to cells, *P < 0·005. Data is from a single representative experiment using strain FCR3-FMG, and the experiment was performed three independent times with each parasite line, FCR3-FMG and Dd2.
Characterization of LIP during maturation of P. falciparum within host RBCs
Based upon our observation that pRBCs contain a greater LIP than uninfected RBCs, and that labile iron appeared to increase with the maturation of the parasite from the ring stage to the trophozoite stage, we sought to characterize the dynamics of the LIP during maturation of erythrocyte stage P. falciparum. To this end, we tightly synchronized parasites to within 4–6 h of each other by a combination of (i) ring stage selection by sorbitol treatment, (ii) haemozoin-containing trophozoite and schizont stage isolation by MACS and (iii) merozoite invasion inhibition with heparin. Following synchronization, the parasite culture was allowed to progress to late schizony, to the point at which at least 50% of schizonts had ruptured. We were able to observe uninfected, newly invaded ring stage, late stage trophozoites, schizonts, and free merozoites by microscopic analysis of Geimsa stained thin blood smears (data not shown) and flow cytometry analysis of STYO 61 stained pRBCs (Fig 3A). We observed that the addition of the iron chelator, deferiprone, to these calcein-loaded parasite cultures resulted in a calcein ΔMFI that increased with parasite maturation. As shown in Fig 3B, compared to uninfected RBCs (ΔMFI = 80·61 ± 0·99, P < 0·0002), the ΔMFI was increased in ring pRBCs (ΔMFI = 95·19 ± 2·59, P < 0·0002), trophozoite pRBCs (ΔMFI = 162·13 ± 6·4, P < 0·0002), and schizont pRBCs (ΔMFI = 173·91 ± 16·5, P < 0·0002). Despite differences in cytometer settings between independent experiments, the increase in calcein fluorescence of rings, trophozoites and schizonts was consistently 15–20%, 40–50% and 55–60% greater than the increase observed in uninfected RBCs. There was little to no detectable change in calcein fluorescence in merozoites with the addition of deferiprone (ΔMFI = 0·963 ± 0·076) (Fig 3B). These results suggest that as erythrocyte stage P. falciparum parasites mature, the level of the LIP increases, in response to increased iron demands as the parasite becomes more metabolically active and begins to replicate DNA.
Fig 3.
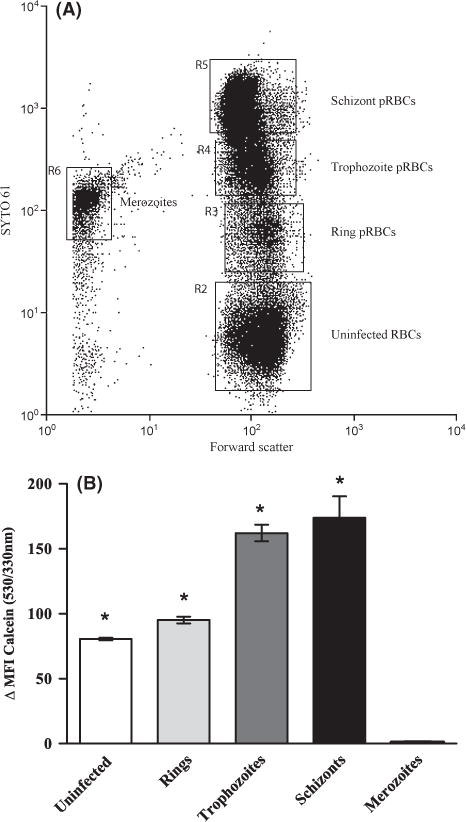
The LIP increases as the parasite matures within the host red blood cell. The LIP of uninfected and different stage parasitized RBCs (pRBCs) was determined by assessing the change in MFI of calcein-loaded cells with the addition of the iron chelator, deferiprone. Cells from P. falciparum (FCR3-FMG strain)-infected erythrocyte cultures were loaded with CA-AM and then incubated with DNA dye SYTO 61 either in the presence or absence of 100 μmol/l deferiprone for 1 h and analysed by flow cytometry. (A) Dot-plot of cell distribution of DNA stain (SYTO 61) vs. forward scatter showing the distribution of uninfected RBC, ring, trophozoite, schizont pRBCs and merozoites. (B) Change in calcein MFI (530/330 nm) with the addition of deferiprone in uninfected RBCs and RBCS infected with ring, trophozoite and schizont stage P. falciparum parasites and the extracellular merozoite stage. The change in the MFI of calcein before and after addition of deferiprone represents the LIP of each cell population. The bar graph (mean ± SD, n = 3) shows calcein ΔMFI of uninfected and parasitized RBCs. Student’s t-test statistical analysis was performed comparing the MFI of calcein before and after the addition of iron chelator to cells, *P < .0002. Data is from a single representative experiment, and the experiment was performed three independent times with parasite line FCR-FMG.
Investigation of the impact of host serum iron sources on the LIP in P. falciparum-infected erythrocytes
To determine whether serum iron sources (transferrin bound iron and ferric citrate) can be accessed by erythrocyte stage P. falciparum and incorporated into the LIP of pRBCs, we incubated P. falciparum (mixed ring and trophozoite stage)-infected erythrocyte cultures with increasing physiological concentrations of either holo-transferrin (0·20–1·2 g/l) or ferric citrate (7·16–26·85 μmol/l) for 6 h. Cells were then loaded with CA-AM and subsequently stained with SYTO 61. The presence of both transferrin and ferric citrate in culture media corresponded with significant decreases in calcein fluorescence in trophozoite pRBC (—ΔMFI calcein), which indicates that additional iron entered the LIP and quenched the calcein fluorescence (Fig 4). Compared to untreated trophozoite pRBCs a decrease of 14% (P < 0·005), 15% (P < 0·005) and 26% (P < 0·0009) was observed in the MFI calcein after the addition of .2 g/L, .4 g/L and 1·2 g/L human transferring, respectively. Compared to untreated trophozoite pRBCs a decrease of 10% (P < 0·005), 15% (P < 0·005) and 16% (P < 0·0009) was observed in the calcein fluorescence after the addition of 7·16, 11·19 and 26·85 μmol/l ferric citrate. No significant changes in calcein fluorescence were observed for uninfected RBCs or ring stage pRBC (data not shown). These results demonstrate that the LIP of trophozoite stage P. falciparum increases with increasing concentrations of transferrin and ferric citrate, this suggests that late stage parasites are capable of accessing serum iron in both the transferrin and non-transferrin bound form (ferric citrate).
Fig 4.
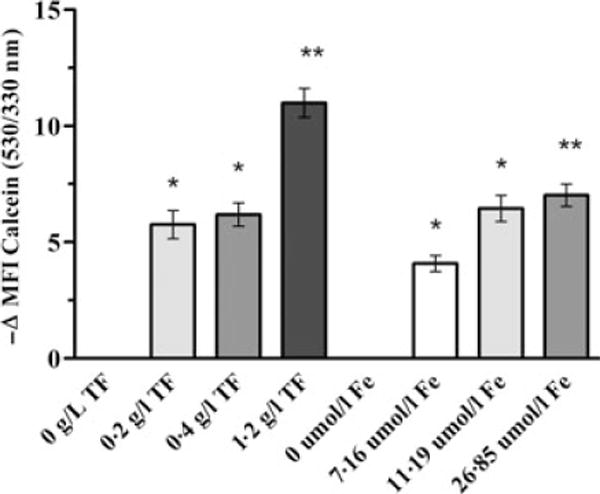
Addition of human transferrin or ferric citrate increases the LIP in parasitized RBCs. Impact of human transferrin or ferric citrate on the LIP of RBCs infected with trophozoite stage parasites was determined by assessing the MFI of calcein in cells cultured in the absence and presence of increasing concentrations of transferrin or ferric citrate. Cells from P. falciparum (FCR3-FMG strain)-infected erythrocyte cultures were incubated in the presence or absence of increasing physiological concentrations of human transferrin (TF, 0·2-1·2 g/l) or ferric citrate (Fe, 7·16-26·85 μmol/l) for 6 h. Cells were then loaded with CA-AM, subsequently stained with DNA dye SYTO 61 and analysed by flow cytometry. The LIP was measured by the change in the mean fluorescence intensity of calcein following the addition of the exogenous iron source. The decrease in calcein fluorescence (−ΔMFI calcein (530/330 nm)) indicates the addition of iron to the cellular LIP that results in increased quenching of calcein fluorescence. The bar graph (mean ± SD, n = 3) shows calcein ΔMFI of trophozoite pRBCs with increasing concentrations of either transferrin or ferric citrate. Student’s t-test statistical analysis was performed comparing cellular calcein fluorescence of increasing concentrations of transferrin and ferric citrate to the addition of no iron, *P < 0·005 **P < 0·0009. Data is from a single representative experiment, and the experiment was performed three independent times with parasite line FCR-FMG.
Discussion
During the course of microbial infections, there is an arms race between the pathogen and host for iron. In the course of this arms race pathogens have evolved sophisticated methods of scavenging host iron while the host acute activation of the nutritional immune response effectively limits the availability of iron to invading pathogens (Skaar, 2010). Iron chelating agents suppress the growth of P. falciparum in vitro and in vivo (Hershko & Peto, 1988; Gordeuk et al, 1992). In addition, iron chelators also bolster the host innate immune response by synergistically acting with cytokines to increase stimulation of NO production, which is protective against severe malaria infection (Weiss et al, 1997; Fritsche et al, 2001). The importance of iron to malaria is additionally demonstrated by clinical studies that have documented an increased susceptibility to malaria infection in individuals given high doses of iron supplementation (Murray et al, 1975; Smith et al, 1989; Oppenheimer, 2001; Sazawal et al, 2006). The sources of host iron used by P. falciparum and the strategies used by the parasite to evade host nutritional immunity have remained elusive.
The labile iron pool represents the transition zone for iron between import, cellular utilization and storage and it is thought to change in response to the metabolic needs of the cell. As a cells’ metabolic demand for iron increases, it will increase the amount of iron in the labile iron pool. The CA-AM LIP assay measures the LIP present in uninfected RBCs and pRBCs. Calcein fluorescence is sensitive to iron at physiological pH 7·2–7·4 (Tenopoulou et al, 2007). As RBC precursors mature, all their organelles are lost, producing an anucleate mature erythrocyte with a cytoplasm of pH 7·2–7·4 (Tenopoulou et al, 2007). At this pH, calcein is sensitive to iron and is able to detect the entire LIP. Upon infection of the RBC, P. falciparum introduces new organelles and structures including: a nucleus, mitochondria, apicoplast, endoplasmic reticulum (ER) and golgi apparatus as well as a parasitophorous vacuole and a food vacuole with pH 3·7–6·5 (Hayward et al, 2006).
The LIP, which is detected within pRBCs using the CA-AM method, is the bioavailable/labile iron present in the neutral pH regions of the residual RBC cytoplasm, parasitophorous vacuole, and parasite cytoplasm. We defined LIP as the ΔMFI of calcein that occurred with the addition of an iron chelator or iron source. We observed that the basal calcein fluorescence was greater within pRBCs than within uninfected RBCs and that fluorescence increased with parasite maturation. The addition of iron chelators to calceinloaded uninfected and pRBCs resulted in greater ΔMFI within pRBCs than uninfected RBCs, and the ΔMFI further increases in iron-replete increased with increasing parasite maturation. Interestingly, the extra-erythrocytic merozoite stage of P. falciparum had no detectable LIP. Our data indicate that the parasite may be able to access both intra-erythrocytic and serum iron. Given that total iron does not differ between uninfected and pRBCs (Marvin et al, 2012), our observation that LIP increases with parasite maturation when it is grown in very low (1·79–2·685 μmol/l) iron media, suggests that the parasite may be able to release iron from either RBC haemoglobin or ferritin, redistributing but not altering the total cellular iron. Increasing LIP with parasite maturation is consistent with the increasing iron demands of the parasite during the trophozoite and schizont stage as the parasite’s metabolic activity dramatically increases and commences DNA replication. Alternatively, changes in intracellular iron levels may not only reflect iron consumption by the parasite but may be due to regulation of iron import/export in infected cells, as has been shown in macrophages targeted by intracellular bacteria (Nairz et al, 2007; Paradkar et al, 2008).
To provide new insight into the potential ability of P. falciparum to access serum iron, either transferrin or non-transferrin bound iron (ferric citrate), we measured the impact of holo-transferrin and ferric citrate on the LIP of uninfected and pRBCs. We observed that the addition of increasing physiological concentrations of either ferric citrate or holo-transferrin increased the LIP of trophozoite pRBCs to a significantly greater degree than of ring pRBCs and uninfected RBC. This provides evidence that trophozoite stage pRBC can access serum iron sources. These results do not address whether pRBCs specifically bind or internalize transferrin. Rodriguez and Jungery (1986) and Haldar et al (1986) independently postulated the existence of a P. falciparum transferrin receptor, however such a receptor has yet to be isolated and cloned. Alternatively, it is well established that pRBCs are able to non-specifically incorporate both micro- and macro-molecules from the serum (Pouvelle et al, 1991; Nguitragool et al, 2011). Human transferrin, like other abundant serum proteins, such as albumin, may be non-specifically internalized into pRBCs (El Tahir et al, 2003).
Our results are relevant to the clinical question of whether host iron status and host iron supplementation affects risk of malarial infection. The relationship between host iron and the malaria parasite is complex and is tightly regulated by both host and parasite. A study published in 2006 that was conducted in Pemba, Zanzibar, involving more than 24,000 children in a setting where anti-malarial treatment was not readily available, showed that routine supplementation with iron and folic acid increased the rates of severe illness and death from malaria in iron-replete children who took iron supplements (Sazawal et al, 2006). Because non-transferrin bound serum iron transiently increases in iron-replete individuals who are given oral iron supplementation (Schümann et al, 2012), we speculated that P. falciparum may scavenge serum iron in order to augment intraerythrocytic growth and thereby potentiate the risk of malaria.
Our application of the flow cytometry based CA-AM LIP assay has revealed that the LIP content of infected RBCs steadily increases with increasing maturation of the intra-erythrocytic stage of the parasite. Additionally, we demonstrated that the LIP content of late stage trophozoite pRBCs is increased in the presence of extracellular transferrin and ferric citrate. Further studies are needed to elucidate the mechanisms by which the malaria parasite senses, acquires, utilizes, regulates, and stores iron during the erythrocytic stage of its life cycle and the impact of host serum iron on these processes. Elucidation of parasite iron biology will provide therapeutic insights into how to augment the host innate immune response and may reveal targets for anti-malarial drug development.
Supplementary Material
Fig S1. (A) Calcein fluorescence is unaffected by the presence of SYTO 61. (B) SYTO 61 fluorescence is unaffected by the presence of Calcein. (C) SYTO 61 does not interfere with binding of Calcein to iron nor the uptake of iron into the cell.
Acknowledgments
The project described was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant 1U01HD061235–01 to CCH) and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Award Number UL1TR000083. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The UNC Flow Cytometry Core Facility is supported in part by an NCI Center Core Support Grant (P30CA06086) to the UNC Lineberger Comprehensive Cancer Center. We also thank Dr. S. Meshnick for helpful discussions and critically reading the manuscript.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
References
- Boyle MJ, Wilson DW, Richards JS, Riglar DT, Tetteh KKA, Conway DJ, Ralph SA, Baum J, Beeson JG. Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14378–14383. doi: 10.1073/pnas.1009198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer W, Epsztejn S, Cabantchik ZI. Iron acquired from transferrin by K562 cells is delivered into a cytoplasmic pool of chelatable iron(II) The Journal of Biological Chemistry. 1995a;270:24209–24215. doi: 10.1074/jbc.270.41.24209. [DOI] [PubMed] [Google Scholar]
- Breuer W, Epsztejn S, Millgram P, Cabantchik IZ. Transport of iron and other transition metals into cells as revealed by a fluorescent probe. The American Journal of Physiology. 1995b;268:C1354–1361. doi: 10.1152/ajpcell.1995.268.6.C1354. [DOI] [PubMed] [Google Scholar]
- Breuer W, Epsztejn S, Cabantchik ZI. Dynamics of the cytosolic chelatable iron pool of K562 cells. FEBS letters. 1996;382:304–308. doi: 10.1016/0014-5793(96)00190-1. [DOI] [PubMed] [Google Scholar]
- Cook JD, Skikne BS. Iron deficiency: definition and diagnosis. Journal of Internal Medicine. 1989;226:349–355. doi: 10.1111/j.1365-2796.1989.tb01408.x. [DOI] [PubMed] [Google Scholar]
- El Tahir A, Malhotra P, Chauhan VS. Uptake of proteins and degradation of human serum albumin by Plasmodium falciparum-infected human erythrocytes. Malaria Journal. 2003;2:11. doi: 10.1186/1475-2875-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epsztejn S, Kakhlon O, Glickstein H, Breuer W, Cabantchik I. Fluorescence analysis of the labile iron pool of mammalian cells. Analytical Biochemistry. 1997;248:31–40. doi: 10.1006/abio.1997.2126. [DOI] [PubMed] [Google Scholar]
- Ferrer P, Tripathi AK, Clark MA, Hand CC, Rienhoff HY, Jr, Sullivan DJ., Jr Anti-malarial iron chelator, FBS0701, shows asexual and gametocyte Plasmodium falciparum activity and single oral dose cure in a murine malaria model. PLoS ONE. 2012;7:e37171. doi: 10.1371/journal.pone.0037171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche G, Larcher C, Schennach H, Weiss G. Regulatory Interactions between Iron and Nitric Oxide Metabolism for Immune Defense against Plasmodium falciparum Infection. Journal of Infectious Diseases. 2001;183:1388–1394. doi: 10.1086/319860. [DOI] [PubMed] [Google Scholar]
- Fu Y, Tilley L, Kenny S, Klonis N. Dual labeling with a far red probe permits analysis of growth and oxidative stress in P. falciparum-infected erythrocytes. Cytometry. Part A: The Journal of the International Society for Analytical Cytology. 2010;77:253–263. doi: 10.1002/cyto.a.20856. [DOI] [PubMed] [Google Scholar]
- Gordeuk V, Thuma P, Brittenham G, McLaren C, Parry D, Backenstose A, Biemba G, Msiska R, Holmes L, McKinley E. Effect of iron chelation therapy on recovery from deep coma in children with cerebral malaria. The New England Journal of Medicine. 1992;327:1473–1477. doi: 10.1056/NEJM199211193272101. [DOI] [PubMed] [Google Scholar]
- Haldar K, Henderson CL, Cross GA. Identification of the parasite transferrin receptor of Plasmodium falciparum-infected erythrocytes and its acylation via 1,2-diacyl-sn-glycerol. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:8565–8569. doi: 10.1073/pnas.83.22.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward R, Saliba KJ, Kirk K. The pH of the digestive vacuole of Plasmodium falciparum is not associated with chloroquine resistance. Journal of Cell Science. 2006;119:1016–1025. doi: 10.1242/jcs.02795. [DOI] [PubMed] [Google Scholar]
- Hershko C, Peto TE. Deferoxamine inhibition of malaria is independent of host iron status. The Journal of Experimental Medicine. 1988;168:375–387. doi: 10.1084/jem.168.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyevsky M, John C, Dickens B, Hu V, Miller JH, Gordeuk VR. Chelation of iron within the erythrocytic Plasmodium falciparum parasite by iron chelators. Molecular and Biochemical Parasitology. 1999;101:43–59. doi: 10.1016/s0166-6851(99)00053-5. [DOI] [PubMed] [Google Scholar]
- Marvin RG, Wolford JL, Kidd MJ, Murphy S, Ward J, Que EL, Mayer ML, Penner-Hahn JE, Haldar K, O’Halloran TV. Fluxes in ‘Free’ and Total Zinc Are Essential for Progression of Intraerythrocytic Stages of Plasmodium falciparum. Chemistry & Biology. 2012;19:731–741. doi: 10.1016/j.chembiol.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MJ, Murray NJ, Murray AB, Murray MB. Refeeding-malaria and hyperferraemia. Lancet. 1975;1:653–654. doi: 10.1016/s0140-6736(75)91758-4. [DOI] [PubMed] [Google Scholar]
- Nairz M, Theurl I, Ludwiczek S, Theurl M, Mair SM, Fritsche G, Weiss G. The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella typhimurium. Cellular Microbiology. 2007;9:2126–2140. doi: 10.1111/j.1462-5822.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- Nguitragool W, Bokhari AAB, Pillai AD, Rayavara K, Sharma P, Turpin B, Aravind L, Desai SA. Malaria parasite clag3 genes determine channel-mediated nutrient uptake by infected red blood cells. Cell. 2011;145:665–677. doi: 10.1016/j.cell.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer SJ. Iron and its relation to immunity and infectious disease. The Journal of Nutrition. 2001;131:616S–633S. doi: 10.1093/jn/131.2.616S. discussion 633S–635S. [DOI] [PubMed] [Google Scholar]
- Paradkar PN, De Domenico I, Durchfort N, Zohn I, Kaplan J, Ward DM. Iron depletion limits intracellular bacterial growth in macrophages. Blood. 2008;112:866–874. doi: 10.1182/blood-2007-12-126854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack S, Fleming J. Plasmodium falciparum takes up iron from transferrin. British Journal of Haematology. 1984;58:289–293. doi: 10.1111/j.1365-2141.1984.tb06087.x. [DOI] [PubMed] [Google Scholar]
- Pouvelle B, Spiegel R, Hsiao L, Howard RJ, Morris RL, Thomas AP, Taraschi TF. Direct access to serum macromolecules by intraerythrocytic malaria parasites. Nature. 1991;353:73–75. doi: 10.1038/353073a0. [DOI] [PubMed] [Google Scholar]
- Prus E, Fibach E. The labile iron pool in human erythroid cells. British Journal of Haematology. 2008a;142:301–307. doi: 10.1111/j.1365-2141.2008.07192.x. [DOI] [PubMed] [Google Scholar]
- Prus E, Fibach E. Flow cytometry measurement of the labile iron pool in human hematopoietic cells. Cytometry. Part A: The Journal of the International Society for Analytical Cytology. 2008b;73:22–27. doi: 10.1002/cyto.a.20491. [DOI] [PubMed] [Google Scholar]
- Rodriguez MH, Jungery M. A protein on Plasmodium falciparum-infected erythrocytes functions as a transferrin receptor. Nature. 1986;324:388–391. doi: 10.1038/324388a0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Lopez R, Haldar K. A transferrin-independent iron uptake activity in Plasmodium falciparum-infected and uninfected erythrocytes. Molecular and Biochemical Parasitology. 1992;55:9–20. doi: 10.1016/0166-6851(92)90122-z. [DOI] [PubMed] [Google Scholar]
- Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, Kabole FM. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- Scholl PF, Tripathi AK, Sullivan DJ. Bioavailable iron and heme metabolism in Plasmodium falciparum. Current Topics in Microbiology and Immunology. 2005;295:293–324. doi: 10.1007/3-540-29088-5_12. [DOI] [PubMed] [Google Scholar]
- Schümann K, Kroll S, Romero-Abal ME, Georgiou NA, Marx JJM, Weiss G, Solomons NW. Impact of oral iron challenges on circulating non-transferrin-bound iron in healthy Guatemalan males. Annals of Nutrition & Metabolism. 2012;60:98–107. doi: 10.1159/000336177. [DOI] [PubMed] [Google Scholar]
- Sigala PA, Crowley JR, Hsieh S, Henderson JP, Goidberg DE. Direct tests of enzymatic heme degradation by the malaria parasite Plasmodium falciparum. Journal of Biological Chemistry. 2012;287:37793–37807. doi: 10.1074/jbc.M112.414078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnis P, Willnow TE, Briones MR, Herz J, Nussenzweig V. Remnant lipoproteins inhibit malaria sporozoite invasion of hepatocytes. The Journal of Experimental Medicine. 1996;184:945–954. doi: 10.1084/jem.184.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaar EP. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathogens. 2010;6:e1000949. doi: 10.1371/journal.ppat.1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AW, Hendrickse RG, Harrison C, Hayes RJ, Greenwood BM. The effects on malaria of treatment of iron-deficiency anaemia with oral iron in Gambian children. Annals of Tropical Paediatrics. 1989;9:17–23. doi: 10.1080/02724936.1989.11748589. [DOI] [PubMed] [Google Scholar]
- Tenopoulou M, Kurz T, Doulias PT, Galaris D, Brunk UT. Does the calcein-AM method assay the total cellular ‘labile iron pool’ or only a fraction of it? The Biochemical Journal. 2007;403:261–266. doi: 10.1042/BJ20061840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RY. Fluorescent probes of cell signaling. Annual Review of Neuroscience. 1989;12:227–253. doi: 10.1146/annurev.ne.12.030189.001303. [DOI] [PubMed] [Google Scholar]
- Vander Jagt DL, Intress C, Heidrich JE, Mrema JE, Rieckmann KH, Heidrich HG. Marker enzymes of Plasmodium falciparum and human erythrocytes as indicators of parasite purity. Journal of Parasitology. 1982;68:1068–71. [PubMed] [Google Scholar]
- Weiss G, Thuma PE, Mabeza G, Werner ER, Herold M, Gordeuk VR. Modulatory potential of iron chelation therapy on nitric oxide formation in cerebral malaria. The Journal of Infectious Diseases. 1997;175:226–230. doi: 10.1093/infdis/175.1.226. [DOI] [PubMed] [Google Scholar]
- WHO. World Malaria Report 2011. 2011 Available at: http://www.who.int/malaria/world_malaria_report_2011/en/ [Accessed August 13, 2012]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. (A) Calcein fluorescence is unaffected by the presence of SYTO 61. (B) SYTO 61 fluorescence is unaffected by the presence of Calcein. (C) SYTO 61 does not interfere with binding of Calcein to iron nor the uptake of iron into the cell.


