Abstract
Adolescence and early adulthood is a time when peer groups become increasingly influential in the lives of young people. Youth exposed to deviant peers risk susceptibility to externalizing behaviors and related psychopathology. In addition to environmental correlates of deviant peer affiliation, a growing body of evidence suggests that affiliation with deviant peers is heritable. This study examined the magnitude of genetic and environmental influences on affiliation with deviant peers, changes in the relative importance of these factors, and which of these factors contribute to the stability of affiliation across this critical developmental period using a longitudinal twin study design that assessed same-sex twins (485 monozygotic pairs, 271 dizygotic pairs) at three discrete ages-15-, 18-, and 21-years-old. Biometric models revealed that genetic influences increased with age. New genetic influences appeared during late adolescence, and no new genetic influences emerged by age 21. Environmental influences shared by sibling pairs decreased with age, while the proportion of nonshared environmental effects unique to each individual remained relatively stable over the course of development. Shared environmental influences were largely age-overlapping whereas nonshared environmental influences were largely age-specific. In summary, this study found variance in affiliation with deviant peers is explained by shared and nonshared environment effects as well as by genetic influences (46% by age 21), supporting the role of genetically influenced selection factors. The shared environment was almost exclusively responsible for the stability in late adolescence, while genetic influences were primarily responsible for stability in early adulthood.
Keywords: peers, deviance, genes, environmental factors
Developmental changes in the nature of peer relationships (e.g., Gavin & Furman, 1989), time spent with peers (e.g., Warr, 1993), and the influence of peer relationships on youth development (e.g., Gardner & Steinberg, 2005; O'Brien & Bierman, 1988) have been the focus of considerable research. Affiliation with deviant peers during adolescence is predictive of various adolescent and young adult externalizing behaviors—including sexual risk (Lansford et al., 2010), illicit drug use (Petraitis, Flay, Miller, Torpy, & Greiner, 1998), and violence (Henry, Tolan, & Gorman-Smith, 2001), as well as psychiatric disorders in adulthood (e.g., substance use disorder; Cornelius, Clark, Reynolds, Kirisci, & Tarter, 2007). Far less is known about the factors that influence youth's affiliation with peers and how those factors may change across adolescence and into early adulthood. Various "environmental" variables have been found to be genetically influenced (McGue, Elkins, Walden, & Iacono, 2005; Plomin & Bergeman, 1991), including affiliation with deviant peers (Button et al., 2007; Kendler et al., 2007).
Longitudinal behavior genetics studies present a unique method for studying changes in the influence of genetic factors relative to environmental circumstances over key developmental transitions. For example, during late adolescence, youths interact less with parents and families and exert greater autonomy as they transition to adulthood and out of their parent's home (Roisman, Masten, Coatsworth, & Tellegen, 2004). It is likely that shifts in the relative importance of genetic and environmental influences (especially family environmental influences) on peer deviance also occur during this period. Moreover, it is unclear if the same genetic and environmental factors influence deviant peer affiliation across adolescence and early adulthood, if new genetic and environment influences emerge and exert influences as the nature of peer relationships and normality of affiliation with deviant peers shifts, or if both factors shared across age and age-specific factors influence affiliation with deviant peers. Using a longitudinal design, the current study examines these questions as they relate to peer deviance from middle adolescence to early adulthood.
How genes shape our environments
Phenotypic research has primarily focused on environmental correlates of peer deviance. Family life is often found to be influential, with findings of peer deviance being positively associated with parent-child conflict (Brook, Brook, Zhang, & Cohen, 2009), poor parental monitoring and discipline (Dishion, Patterson, Stoolmiller, & Skinner, 1991), and parental deviance and substance abuse (Fergusson & Horwood, 1999). Furthermore, peer deviance has been linked to socioeconomic and community level factors (i.e., social and neighborhood disadvantage; Brody et al., 2001; Fergusson & Horwood, 1999). Youth may also be predisposed to select or be chosen by such peer groups; for example, longitudinal research shows that individual characteristics (e.g., fearlessness, hyperactivity) are predictive of peer deviance (Lacourse et al., 2006). Social constructs and other putatively environmental factors are influenced by genes; heritability of these environmental variables is thought to be a byproduct of genetic influences on the psychological or behavioral traits that influence exposure to certain environments (Rutter, Moffitt, & Caspi, 2006), with researchers noting the potential mediatory role of personality (Kendler & Baker, 2007). Social behaviors, such as deviant peer affiliation, are complex and influenced by many individual characteristics (e.g., fearlessness; need for approval), making investigation of genetic etiology challenging. Studying genetic contributions to peer affiliation itself provides insight about the sum of all potential genetic factors influencing peer group selection.
Developmental changes in the salience of genetic and environmental influences may be related to shifts in genotype-environment correlations (rGE; i.e., the tendency of individuals to experience environments consistent with their genotype). There are several types of rGE (Jaffee & Price, 2007). Youth are primarily influenced by environments shaped by their parents (passive rGE) in early childhood. As they grow older and more autonomous, they may be more likely to actively seek environments that reinforce their genetically influenced dispositions (active rGE). Simultaneously, their genetically influenced behavior may shape their social environment by evoking reactions in others (i.e., evocative or reactive rGE; Plomin, DeFries, & Loehlin, 1977; Scarr & McCartney, 1983). From this perspective, with active and evocative/reactive rGE increasing with age and passive rGE decreasing with age, peer deviance should be influenced more by environmental influences during adolescence and more by genetic influences as young people move into adulthood. It should be noted that while age-related increases in genetic influences on various behavioral and psychiatric phenotypes have been well-documented (see Bergen, Gardner, & Kendler, 2007 for a meta-analysis), empirical support for increases in rGE is less common.
Twin study methodology provides a means to distinguish genetic from environmental sources of influence on behaviors, using comparisons of co-twin similarity in phenotypes, such as peer deviance. Biometric modeling with twin pairs takes advantage of the fact that twins provide a natural experiment; monozygotic (MZ; identical) twins are the result of one fertilized egg that splits in-utero and thus they share 100% of their genetic material, while dizygotic (DZ; fraternal) twins are the result of two eggs fertilized at the same time and thus they share on average 50% of their genetic material (as with any two non-twin full siblings). Comparisons are quantified using biometric statistical models that provide estimates of additive genetic effects, shared environmental effects (i.e., environmental effects shared by reared-together relatives that are sources of behavioral similarity), and nonshared environmental effects (i.e., environmental effects that differ for reared-together relatives and are sources of behavioral dissimilarity) on peer deviance.
Research on genetic and environmental contributions to peer deviance
Several studies have examined genetic and environmental contributions to deviant peer affiliation and deviant peer characteristics. Some studies find moderate to large genetic influences on peer deviance or delinquency (21%, Button et al., 2007; 49 to 71%, Manke, McGuire, Reiss, Hetherington, & Plomin, 1995) and peer substance use (27%, Dick et al., 2007; 31%, Fowler et al., 2007; 41%, Harden, Hill, Turkheimer, & Emery, 2008) and others find little or no genetic contributions to peer deviance, delinquency, or substance use (Bullock, Deater-Deckard, & Leve, 2006; Iervolino et al., 2002; Walden, McGue, lacono, Burt, & Elkins, 2004). Research supports both nonshared (Bullock et al., 2006; Iervolino et al., 2002) and shared environmental influences on peer deviance and substance use (Button et al., 2007). The discrepancies in heritability estimates are difficult to explain because of the diverse samples, measures and informants, and statistical approaches. Nevertheless, it appears that genetic influences on peer delinquency, deviance, and substance use are higher when measured through self-report (see Manke et al., 1995 for an exception) and among older samples.
Longitudinal research of genetic and environmental effects to peer deviance shows how these influences change over time. Variance estimates of peer deviance indicate a steady increase in genetic effects during late childhood to early adulthood (from 39% to 50%), a decrease in shared environmental effects (from 27% to 13%), and a relatively stable amount of nonshared environmental effects (from 33% to 35%; Kendler et al., 2007). Despite Kendler et al.’s (2007) main finding that distinct developmental trajectories are produced via genetic versus environmentally-influenced peer deviance, it was not without limitations, including use of an all-male sample, retrospective reporting of adults, and occasion-specific reporting of peer deviance that spanned three years. Most importantly, this paper did not provide insight about age-specific genetic or environmental effects (also known as innovation) relative to age-overlapping effects.
Assessing the stability of peer deviance across development
While Kendler, Jacobson, Myer, and Eaves (2008)’s study was primarily designed to test causal hypotheses about the relationship between conduct disorder and peer deviance, they also commented less specifically on genetic and environmental factors that may account for the stability of peer deviance. They reported that genetic effects on peer deviance had a pervasive effect across occasions while environmental (both shared and nonshared) influences were occasion-specific. Of note, they did not report the proportions of the shared variance in peer deviance across ages as a function of occasion-overlapping relative to occasion-specific effects. That is, they compared models of shared (common) genetic and environmental effects versus occasion-specific effects, without introducing models in which both could be correct. This last limitation is worth noting. Genetic and environmental effects may be novel to discrete points during development. Etiological influences may also accumulate throughout development. It is likely that both novel and accumulated influences contribute to peer deviance at each age, and thus models that account for changes in both age-overlapping and ages-specific influences are important.
Indeed, a recent twin study (using the same sample as the current study) that assessed a related phenotype (i.e., nicotine dependence; Tully et al., 2010) at discrete ages found some overlap in genetic effects across ages 15, 18, and 21 but also the emergence of new genetic influences at age 18. Similarly, Malone, Taylor, Marmorstein, McGue, and Iacono (2004) used an older (assessments at ages 18, 21, and 25), all-male sample and showed significant genetic innovation at age 21 in both antisocial behavior and alcohol dependence (59 and 60% genetic variance was genetic innovation, respectively). Baker, Maes, Larsson, Lichtenstein, and Kendler (2011) found genetic innovation around the same age for a latent substance use factor (25% innovation). In contrast, one study found that a single set of genetic risk factors present in middle adolescence was responsibility for continuity in alcohol abuse and dependence in early adulthood (van Beek et al., 2012). These studies support the potential importance of both age-overlapping and age-specific effects.
Modeling peer deviance stability in this way can inform interventions to prevent peer deviance. For example, strong genetic influences on peer deviance at each age and developmentally stable genetic influences across adolescence (i.e., the same genetic influences contributing to peer deviance across development) would support peer deviance as a genetically-influenced trait-like characteristic that would likely contribute to persistence in risk behaviors across adolescence, leaving unclear what candidate interventions would be worth trying or how effective they could be expected to be. Alternatively, knowledge that genetic influences do not contribute to the stability of peer deviance across adolescence or do so only during certain periods of adolescence would inform timing and selection of interventions, for example employing school-based interventions during periods when environmental influences are relatively greater than genetic influences.
Current Study
We tested several hypotheses about developmental changes in peer deviance (PD) at three ages during adolescence (15, 18, and 21 years). First, based on the assumption of developmental shifts toward increased independence, less time spent with parents and family, and thus likely greater niche-fitting during adolescence and early adulthood as well as prior research supporting age-related increases in the heritability of many behavioral traits (Bergen et al., 2007), we expected age-related increases in genetic influences on PD and age-related decreases in shared environmental influences on PD. Second, given evidence of common latent genetic factors contributing to PD across this developmental period (Kendler et al., 2008), we predicted common genetic factors would influence PD across all three ages (i.e., age-overlapping effects). At the same time, we also predicted that not all of the genetic influences on PD would be shared across the ages but rather new genetic influences would emerge (i.e., age-specific effects), which would be consistent with findings of genetic innovations on related phenotypes during this developmental period (e.g., Baker et al., 2011; Malone et al., 2004; Tully et al., 2010). Third, we expect that some of the shared influences would be shared across ages and some new, age-specific shared environmental influences would emerge during this period based on prior research supporting both occasion-specific phenotypes (Kendler et al., 2008) and common shared environmental influences of related phenotypes (e.g., Baker et al., 2011; van Beek et al., 2012). Fourth, consistent with findings of age-specific nonshared environmental influences on several phenotypes (e.g., Baker et al., 2011; Kendler et al., 2008; Tully et al., 2010) and the idea that many unique environmental influences change across development (e.g., from extracurricular activities in adolescence to college/work environment in young adulthood), we hypothesized that nonshared environmental influences would be largely age-specific and thus would not contribute to the covariance in peer deviance across ages (i.e., its stability).
Method
Sample
The sample of 756 same-sex twin pairs (50.3% female, 485 monozygotic, 271 dizygotic) was drawn from the Minnesota Twin and Family Study (MTFS). The MTFS is a large, population-based, longitudinal study of twins and their families who were recruited from Minnesota birth records of twins born between 1971 and 1985. The participation rate was 83% for the twins who met inclusion criteria (i.e., twins were free from major cognitive and physical handicaps, lived within a day's drive from the laboratory, and were not adopted by non-relatives). The participating families were representative of the population of Minnesota at the time the twins were born. The sample was 98% Caucasian; mean occupational status (Hollingshead) for fathers and mothers were 3.9 and 3.7, respectively (which corresponds to clerical, sales, and technician). All participants provided written, informed consent. Additional information about recruitment and sample characteristics can be found elsewhere (Iacono, Carlson, Taylor, Elkins, & McGue, 1999; Iacono, McGue, & Krueger, 2006). Participants were recruited at age 11 (M=11.72, SD=0.43) and completed three follow-up assessments approximately every three years. Means and standard deviations for ages at the three follow-up assessments are 14.80 (0.51), 18.16, (0.65), and 21.46 (0.77). The peer group survey was administered to all participants only at the three follow-up assessments. These assessments will be referred to by the rounded age of participants (age 15, 18, 21).
Attrition analyses showed that mean peer deviance scores were slightly lower for participants who completed the peer deviance measures at follow-up compared to participants who did not. At age 15, PD scores were .23 SDs lower for participants who completed the peer deviance measure at the age 18 follow-up (85.5% of the sample) versus those who did not and were .34 SDs lower for participants who completed the peer deviance measure at the age 21 follow-up (85.6%) versus those who did not. Symptoms of nicotine dependence (NicD), a related phenotype, at age 15 were .43 SDs lower in completers compared to non-completers of the age 18 follow-up and were .15 SDs lower for completers compared to non-completers of the age 21 follow-up. Female participants were also more likely than male participants to complete the age 18 follow-up (89% of females vs. 81% of males) and the age 21 follow-up (91% vs. 80%).
Measures
Deviant peer affiliation was assessed using the Friends Inventory, a computer-administered, self-report measure of peer characteristics that was developed by the MTFS researchers (Walden et al., 2004). This instrument contains 19 items for age 15 and 18 assessments and 27 items for age 21; for the current study, we used the 9 items that tap deviant peer affiliation (e.g., my friends steal things from others, my friends break the rules). The 9 items were identical at age 15 and 18 assessments. At age 21, two items (“my friends drink alcohol or beer” and "my friends get into trouble at school") were replaced with items more appropriate for 21-year-olds who are out of school and of legal drinking age (“my friends drink alcohol or beer a lot” and “my friends enjoy getting drunk”). Items are rated on a 4-point scale (1=none of my friends are like that, 2=just a few of my friends are like that, 3=most of my friends are like that, 4=all of my friends are like that), and these 9 items were summed to create a total deviant peer scale. Alphas at ages 15, 18, and 21 are .89, .88, and .82, respectively.
Zygosity of the twins was determined using three measures: (1) parental report on a standard measure of zygosity, (2) MTFS research staff member's subjective evaluations of twin's physical similarity, such as hair color and face and ear shape, and (3) anthropometric measures of ponderal index, cephalic index, and finger print ridge count. When these measures did not agree, zygosity was determined through serological analysis. Validation of this zygosity method was supported through analysis of a subsample (n = 50), which confirmed agreement among the three measures with serological analysis.
Data Analyses
Biometric model-fitting
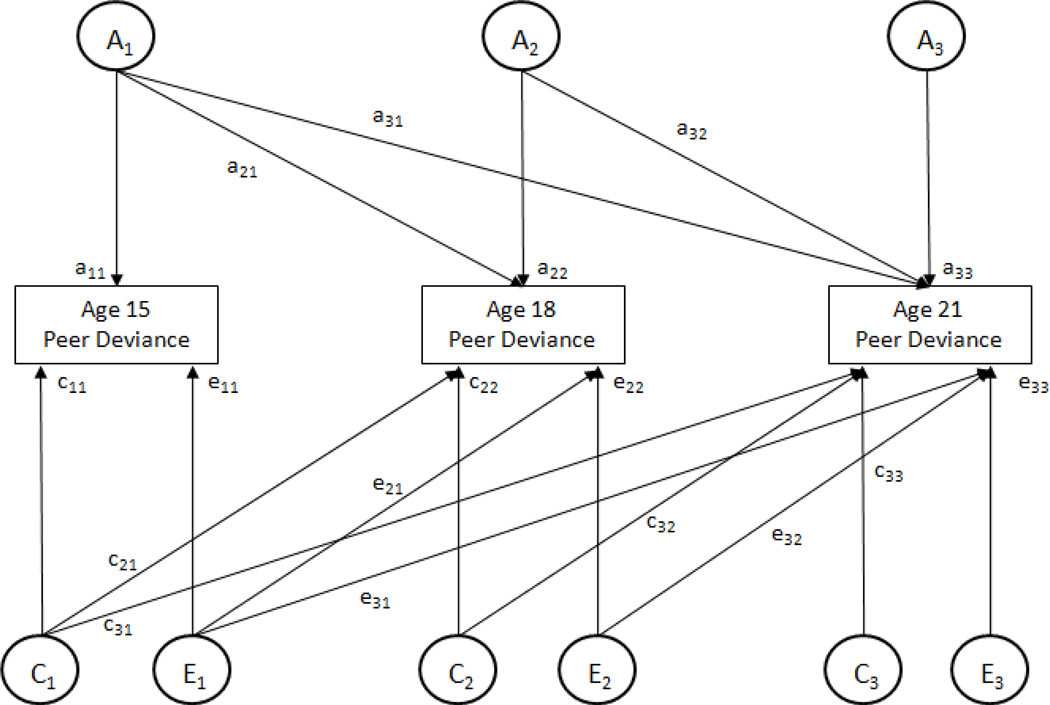
Genetic and environmental contributions to variance in affiliation with deviant peers at each age and covariance in affiliation with deviant peers across ages 15, 18, and 21 were estimated using a 3-factor Cholesky decomposition model (Figure 1). This model provides estimates of additive genetic effects (A), shared environmental effects (C, environmental effects that are shared by reared-together relatives and are sources of behavioral similarity), and nonshared environmental effects (E, environmental effects that differ for reared-together relatives and are a source of behavioral dissimilarity) on each phenotype in the model (here, deviant peer affiliation at three different ages). Further, it separates the A, C, and E effects into effects common across ages and effects specific to a particular age. Genetic and environmental contributions to variance in age 15 phenotypes are obtained by squaring the respective path-coefficients (a11, c11, e11). Variance in the phenotypes at age 18 is divided into components attributable to genetic and environmental influences present at age 15 (a21, c21, e21) and residual (new) components that are independent of the genetic and environmental variance at age 15 (a22, c22, e22). Variance in the phenotypes at age 21 is divided into components attributable to genetic and environmental influences present at age 15 (a31, c31, e31) and present at age 18 but not 15 (a32, c32, e32) and residual (new) components that are independent of the genetic and environmental influences present at age 15 and 18 (a33, c33, e33). Correlations between the latent variables that represent the total A influences (i.e., not divided into previously present and new influences) at each of the three ages, correlations for the C influences at each age, and correlations for the E influences at each age are also reported.
Figure 1. Three-Factor Cholesky Decomposition Model for Investigating Developmental Changes in Genetic and Environmental Influences on Deviant Peers.
This figure depicts the 3-factor Cholesky decomposition model used in this study. The model decomposes the variance in deviant peers into components attributable to additive genetic effects (Ai), shared environmental effects (Ci), and nonshared environmental effects (Ei) at each of the three assessments (i = 1, 2, 3). The individual paths, which are represented by lowercase letters followed by numerals, when squared estimate the proportion of variance accounted for by the genetic and environmental influences. This figure represents only one twin, and identical model is also calculated for the co-twin.
To account for the changes in wording of items on the Friends Inventory version used at ages 15 and 18 and the version used at age 21, models were fit to correlation matrices rather than raw data. Separate correlation matrices for males and females with parameters constrained to be equal across sexes were used to account for sex differences in mean levels, variance, and covariance of deviant peer affiliation. To determine which parameter estimates were meaningful, fit statistics for an unconstrained model in which all parameter estimates were free to vary across the ages was compared to a series of models in which the A, C, and/or E estimates were constrained to be equal across ages. These models were calculated using the the maximum likelihood option in Mx statistical software system (Neale, Boker, Xie, & Maes, 1999). Parameters were estimated to minimize minus two times the natural logarithm of the multivariate normal likelihood (−2lnL), and the minimized values of −2lnL for the baseline model (in which A, C, and E effects were free to vary across ages) were compared with more restrictive models (with effects constrained across ages) using the likelihood χ2 difference tests. These tests evaluate the comparative fit of models with age constraints, with a nonsignificant χ2 difference test indicating that the more restrictive model provides an appropriate fit to the data. The Akaike Information Criteria (AIC = χ2 − 2Δdf) is an alternative to the χ2 goodness-of-fit test statistic that is less likely to result in rejection of the more restrictive model when deviations between the baseline and restricted model are relatively small but the sample size is large. Models that minimize AIC are preferred.
Results
Descriptive statistics, phenotypic correlations, and twin correlations
Table 1 presents means, standard deviations, and cross-time phenotypic correlations for peer deviance (PD) at each assessment, separately for males and females. A repeated measures ANOVA revealed significant quadratic change in levels of PD from age 15 to age 21 [F(1, 514) = 15.348, p < .001] with a steeper increase in PD at younger ages. The quadratic rise in PD was significantly stronger for males than females [F(1, 514) = 5.872, p = .016]. The repeated measures ANOVA was run on a subsample of one twin per family and then verified on the subsample of the other twins. Results were the same across the two subsamples. Phenotypic correlations were estimated in Mplus using the weighted least squares with robust standard errors (to use all available data) and twins were clustered within twin pairs to account for nonindependence. These correlations were significant and positive and were moderate to high in magnitude, indicating stability of PD across adolescence to early adulthood, particularly across successive time points.
Table 1.
Means, Standard Deviations, and Phenotypic Correlations for Peer Deviance at ages 15, 18, and 21 for Males and Females
| Full Sample | Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 15 years | 18 years | 21 years | 15 years | 18 years | 21 years | 15 years | 18 years | 21 years | |
| Means (SD) | 13.86 (4.26) | 15.94 (4.46) | 17.15 (3.64) | 14.33 (4.09) | 16.99 (4.83) | 18.19 (3.87) | 13.30 (4.09) | 15.02 (3.85) | 16.28 (3.43) |
| Phenotypic Correlations | |||||||||
| Age 18 | .58 | .61 | .54 | ||||||
| Age 21 | .37 | .59 | .44 | .60 | .30 | .53 | |||
Note. All correlations are significant at p < .001.
Prior to biometric model fitting, twin intraclass correlations for PD within each age group by zygosity and gender were calculated to assess genetic and environmental influences on PD (Table 2). These correlations give a general indication of the magnitude of genetic and environmental influences; MZ correlations less than 1.0 indicate nonshared environmental effects, and MZ correlations that are greater than DZ correlations suggest significant genetic influences. Intraclass correlations were positive, moderate to strong in magnitude, and significant for MZ and DZ twins at all ages. As expected, correlations were larger for MZ twins than for DZ twins, with the exception of the correlations for females at age 15 when the magnitudes of the correlations were higher for DZ twins but were fairly similar across zygosity. The zygosity differences in the correlations increased with age. These correlations suggest low genetic influences on PD at age 15 and increases in genetic influences with age. The widening gap in the magnitude of the MZ and DZ correlations also indicates that shared environmental influences generally decreased during this time period.
Table 2.
Twin Intraclass Correlations for Trait Deviant Peers by Age and Gender
| Full Sample | Male | Female | |||||
|---|---|---|---|---|---|---|---|
| Mean Age |
MZ | DZ | MZ | DZ | MZ | DZ | |
| 15 | n | 418 | 228 | 207 | 96 | 211 | 132 |
| r | .64 | .60 | .65 | .54 | .60 | .65 | |
| 95% CI | (.58, .74) | (.51, .67) | (.56, .72) | (.38, .67) | (.51, .68) | (.54, .74) | |
| 18 | n | 372 | 204 | 181 | 91 | 191 | 113 |
| r | .73 | .57 | .73 | .57 | .68 | .51 | |
| 95% CI | (.68, .77) | (.46, .65) | (.65, .79) | (.41, .69) | (.59, .75) | (.36, .64) | |
| 21 | n | 381 | 214 | 185 | 87 | 196 | 127 |
| r | .62 | .43 | .59 | .33 | .60 | .420 | |
| 95% CI | (.56, .68) | (.31, .53) | (.49, .68) | (.12, .50) | (.50, .68) | (.27, .55) | |
Note. All correlations are significant at p < .001.
Biometric Model Fitting
Table 3 presents the standardized variance estimates for genetic, shared environmental, and nonshared environmental influences on PD at each age. These estimates allow for comparisons in the proportions of variance in PD that can be attributed to genetic and environmental influences across the three ages. The standardized estimates are consistent with initial genetic and environmental estimates from twin correlations, as there were increases in heritability and decreases in shared environmental influences on PD from middle adolescence to young adulthood. These estimates show that variance accounted for by genetic influences has a large increase (nearly tripling in magnitude) from age 15 to 18 and a smaller increase from age 18 to 21. Conversely, variance explained by shared environmental effects decreases from age 15 to 18 and then again from age 18 to 21. The estimate of nonshared environmental effects varied a bit across the three ages, including a small dip between ages 15 and 18 before rising at age 21.
Table 3.
Standardized Variance Estimates at Three Ages
| Standardized Variance Estimates with 95% Confidence Intervals | |||
|---|---|---|---|
| Mean Age | a2 | c2 | e2 |
| Age 15 | .12 (.01, .28) | .52 (.38, .61) | .37 (.32, .42) |
| Age 18 | .34 (.20, .50) | .37 (.23, .50) | .29 (.25, .33) |
| Age 21 | .47 (.27, .62) | .13 (.00, .30) | .40 (.35, .47) |
Notes. a2, c2, e2 = proportion of variance accounted for by genetic, shared environmental, and nonshared environmental influences, respectively.
Before comparing fits of the proposed age-constrained models, models constraining the parameters across sexes were fit to the data. Constraining all parameters in the model to be equal across sexes did not significantly reduce the fit of the model (ΔX2 = 27.89, p = .06), indicating negligible differences in parameter estimates for male and female participants. Similarly, constraining only the genetic parameters to be equal across sexes (while allowing the others to vary across the sexes) also did not significantly reduce the fit of the model (ΔX2 = 3.16, p = .76), further supporting no meaningful sex differences in estimates of genetic influences on PD across adolescence and young adulthood. Therefore, in all subsequent models, parameters were constrained to be equal across sexes.
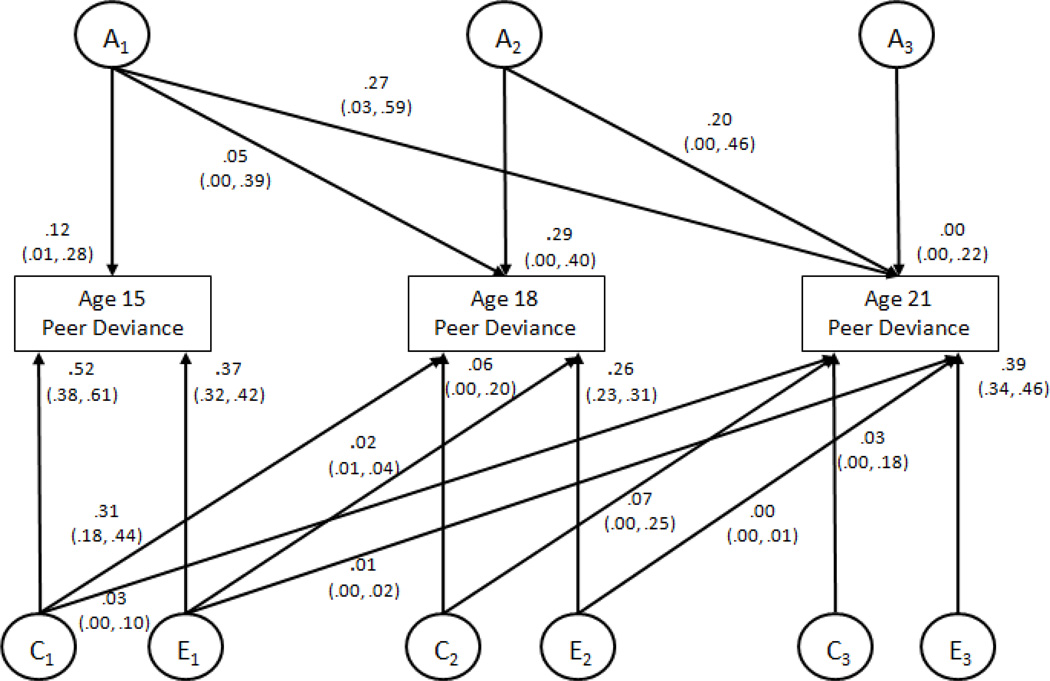
Figure 2 presents the standardized variance estimates for the fully age-constrained (baseline) model for all estimates of the unique and overlapping (or common) genetic, shared, and nonshared environmental influences on PD at each assessment. The estimates suggest that most of the genetic influences (.29 of .34; 85%)1 on PD at age 18 were new or not shared with genetic influences at age 15, whereas all (.27 common with age 15 and .20 common with age 18 of .47; 100%) the genetic influences at age 21 were present at earlier ages. The genetic correlations, that is correlations between the latent variables representing all genetic influence on PD at a given age, were large and significant between ages 15 and 21 (rA1A3 = .76, 95% CI =.27, 1.00) and ages 18 and 21 (rA2A3 = .89, 95% CI = .70, 1.00) but not between ages 15 and 18 (rA1A2 = .38, 95% CI= −.14, 1.00). Conversely, most (.31 of .37; 83%) of the shared environmental influences on PD at age 18 were present at age 15. The shared environmental influences on PD at age 21 were small and nonsignificant but nearly half (.03 and .07; 43%) were present at earlier ages. Shared environmental correlations were moderate to large and significant across all ages: rC1C3 = .91, 96% CI = .73, .1.00; rC2C3 = .73, 95% CI = .32, 1.00; rC1C3 = .46, 95% CI = .07, 1.00. Finally, nonshared environmental influences on PD were almost entirely age-specific, with 92% (.26 of .29) of the nonshared environmental influences new at age 18 and 97% (.39 of .40) new at age 21. As follows, the nonshared environmental correlations were small, rE1E2 = .28, 95% CI = .18, .37; rE1E3 = .13, 95% CI = .03, .23); rE2E3 = .11, 95% CI = (.01, .21). The parameters in this model can also be summed to inform the cause of stability in PD (noted in the phenotypic correlations) across this age period. The common variance in PD between age 15 and age 18 (.38) was primarily accounted for by shared environmental influences (.31; 81%), whereas the common variance in PD between ages 18 and 21 (.28) was primarily accounted for by genes (.20; 73%).
Figure 2. Developmental Changes in Genetic and Environmental Influences on Deviant Peers at Ages 15, 18, and 21.
This figure is a standardized path diagram of the full, sex-constrained Cholesky decomposition model for deviant peers with 95% confidence intervals for the parameters shown in the model. Path coefficient estimates have been squared and represent the proportion of variance in deviant peers accounted for by the components. Ai = variance attributable to additive genetic effects; Ci = variance attributable to shared environmental effects; Ei = variance attributable to nonshared environmental effects.
The effect of constraining parameters to be equal across ages on the fit of the models was examined using the χ2 difference test and the AIC (see Table 4). Compared to the fully age unconstrained "baseline" model (Model 1), a model constraining A to be equal across all ages, C to be equal across all ages, and E to be equal across all ages (Model 2) provided a significantly worse fit to the data as indicated by the significant χ2 difference test. This indicates that estimates of genetic, shared environmental, and nonshared environmental influences should not be constrained to be equal across all ages, and we should move forward with testing models that constrain the A, C, and E parameters at the different ages. Constraining A to be equal across the three ages (Model 3) also worsened the fit of the model compared to Model 1 as did constraining A to be equal at ages 15 and 18 (Model 4), but constraining A to be equal at ages 18 and 21 (Model 5) did not significantly reduce the fit of the model. These findings indicate that the proportion of variance that can be attributed to genetic influences is significantly smaller at age 15 than at ages 18 and 21, but this proportion is not significantly different at ages 18 and 21.
Table 4.
Test Statistics for Cholesky Decomposition Model for Deviant Peers with Constraints across Age
| Model | −2lnL | df | Δ−2lnL (df) | p value | AIC |
|---|---|---|---|---|---|
| 1. Fully age unconstrained (baseline) | 133.06 | 66 | |||
| 2. Fully age constrained | 164.68 | 75 | 31.61 (9) | .0002 | 14.68 |
| 3. Constrain A at all ages | 141.23 | 69 | 8.17 (3) | .04 | 3.23 |
| 4. Constrain A at ages 15 and 18 years | 137.28 | 67 | 4.22 (1) | .04 | 3.28 |
| 5. Constrain A at ages 18 and 21 years | 134.15 | 67 | 1.09 (1) | .30 | 0.15 |
| 6. Constrain C at all ages | 146.71 | 69 | 13.65 (3) | .003 | 8.71 |
| 7. Constrain C at ages 15 and 18 | 135.46 | 67 | 2.40 (1) | .12 | 1.46 |
| 8. Constrain C at ages 18 and 21 | 138.68 | 67 | 5.62 (1) | .02 | 4.68 |
| 9. Constrain E at all ages | 143.84 | 69 | 10.78 (3) | .01 | 5.84 |
| 10. Constrain E at ages 15 and 21 | 138.55 | 67 | 5.49 (1) | .02 | 4.55 |
| 11. Constrain E at ages 18 and 21 | 143.12 | 67 | 10.06 (1) | .002 | 9.12 |
Notes.
Abbreviations: A=additive genetic effects. C=shared environmental effects. E=nonshared environmental effects. −2lnL= −2 times the log likelihood. AIC=Akaike Information Criteria.
Δ−2lnL=differences in 2lnL values between the sex-constrained but age unconstrained model (model 1). All χ2 tests compare models to model 1 (fully age unconstrained model). All models constrain parameters across sexes.
Model 6 constrained C across the three ages and provided a significantly worse fit compared to the baseline model (Model 1). Constraining C to be equal at age 15 and 18 but allowing C to vary between age 21 and the two younger ages (Model 7) did not significantly reduce the fit of the model. Conversely, constraining C to be equal at age 18 and 21 significantly worsened model fit (Model 8). The nonshared environmental influences, which fluctuated a bit with age, could not be constrained to be equal across the three ages (Model 9), between ages 15 and 18 (Model 10) or between ages 18 and 21 (Model 11).
In summary, the proportion of variance in PD scores explained by genetic influences increased from ages 15 to 21, particularly between ages 15 and 18. Almost all of the genetic influences on PD at age 18 were new (i.e., not shared with previous ages), while none of the genetic influence on PD were new at age 21. The proportion of variance explained by shared environmental influences decreased with age, and almost all the shared environmental influences on PD at age 18 were present at age 15. Nonshared environmental influences on PD were unique to each age. Thus, the covariance (stability) of PD scores across ages is primarily due to common shared environmental influences between ages 15 and 18 and largely due to genetic influences between ages 18 and 21. Nonshared environmental effects contributed little to the covariance of PD scores across ages.
Discussion
The current study sought to examine developmental changes in genetic and environmental contributions to the variance and covariance in PD during a critical period of development—middle adolescence to early adulthood—when the relative importance of peer groups is high, the nature of peer relationships tends to change, and peer influence on the development of individual problem behavior, such as antisocial behavior and alcohol and drug use, is at its peak. Of primary significance is our finding that genes influence PD throughout this period. Consistent with behavioral genetic studies of other putative environmental measures (family environment, social support, and marital quality; see Kendler & Baker, 2007 for review), this finding is in line with research that environmental risk factors are in part heritable, possibly through genetic transmission of associated psychological and behavioral traits that predispose individuals to particular environments through complex underlying gene-environment interplay (Plomin & Bergeman, 1991). In addition, environmental influences (both shared and nonshared) to PD were also found. Moreover, although PD is a stable characteristic, it was found to be maintained by different genetic and environmental factors during middle adolescence to early adulthood.
Genetic influences to PD: Stability versus innovation
Support was found for our hypothesis that like many other phenotypes, the magnitude of genetic influences on PD increased with age. This increase was most pronounced from age 15 to 18 and remained relatively stable from 18 to 21. One explanation for this shift in genetic contributions is the growing independence from families and active selection of friends during late adolescence which might contribute to increases in active gene-environment correlations (rGEs) through the process of niche fitting. An evocative rGE could also exist whereby individuals are selected by others similar to themselves. These mechanisms remain largely speculative; however, evidence is growing for their existence (e.g., Burt, 2008). Moreover, age-related increases in rGEs responsible for the rising heritability of PD may be due to within-pair differences in PD that lead to reallocation of environmental resources to one sibling over another, which through a feedback loop, fosters PD growth in the more genetically-prone sibling and PD decline in the less genetically-prone sibling (Beam & Turkheimer, 2013).
No new genetic influences emerged at age 21 that were not already present by age 18. This finding is consistent with research on developmental trajectories of deviant behavior and PD. Deviant behavior is more normative for youth during adolescence than during adulthood, as evidenced by findings of life-course-persistent versus adolescent-limited antisocial behavior (Moffitt, 1993; Moffitt & Caspi, 2005). Mean differences in PD by age in our sample indicate a sharper rise in PD during middle to late adolescence than from late adolescence to young adulthood, a quadratic pattern that if extended further into adulthood may result in declining levels later in adulthood. Kendler et al. (2007) found that greater genetic than environmental influences on PD during childhood was associated with sharper increases in PD through adolescence and adulthood. Our finding that the stability of PD into adulthood (from 18 to 21) is driven by genetic influences lends further support to this idea, while also extending these findings by showing that while the stability in PD between late adolescence to young adulthood was almost entirely driven by genetic influences, the stability in PD from age 15 to 18 was very minimally driven by genetic influences. Unlike prior research (Kendler et al., 2008), we were able to pinpoint an age when genetic influences began to drive this stability. Furthermore, we found no differences in genetic and environmental influences across sexes, thus extending a literature that has focused primarily on males to show these findings apply equally well to males and females.
The transitional nature of late adolescence has been the subject of considerable research (e.g., Way & Greene, 2006). This often turbulent period of late adolescence may trigger the expression of novel genetic pathways via gene-environment interactions, consistent with a stress-diathesis model (Shanahan & Hofer, 2005). Furthermore, this age period may mark an important transition for the nature of PD, as it goes from being more environmentally malleable to more genetically driven and trait-like; the heritability of PD increased dramatically between age 15 and 18 and stability of PD was driven by genetic factors only after age 18. Efforts to prevent PD in youth at-risk for maladjustment should thus focus on transitions in middle adolescence, when PD may be more susceptible to intervention.
The environment and PD
As hypothesized, the relative importance of the shared environment decreased with age, with shared environmental influences accounting for about half of the variance in PD at age 15, one third of the variance by age 18, and a nonsignificant proportion by age 21. Common family life, school environment, and neighborhood quality are likely to underlie the greater shared environmental influences in middle and late adolescence when cohabitation between twins is expected. The shared environmental effects in our study were largely age-overlapping, wherein most of the variance explained by shared environmental influences during late adolescence and adulthood was explained by influences present at earlier ages. Given the small amount of variance in PD explained by the shared environment by age 21, this finding is most relevant to the period between middle to late adolescence. Thus, our finding is consistent with the idea that some environmental factors common to both twins exert influence when children are likely to live at home and underscores the promise of interventions that target changes to shared environmental experiences—for instance, greater parental monitoring—during this high risk period.
Unlike genetic and shared environmental influences, the nonshared environment influences on PD were, as hypothesized, largely age-specific. This means that the environmental influences that were uniquely experienced by each twin were specific to each age. During middle and late adolescence, parents may contribute to these nonshared experiences by adjusting their parenting to match each child's unique behavior. Later on, individuals are less influenced by parental monitoring, and other nonshared developmentally-relevant environmental factors (e.g., a university setting) are likely to influence friendship patterns. Our finding that nonshared environmental influences were largely age-specific is consistent with studies of PD (e.g., Kendler et al., 2008) and related behavioral phenotypes (Baker et al., 2011; Tully et al., 2010), and suggests that certain environmental characteristics are moving targets, changing overtime to correspond with discrete developmental periods. Interventions aimed at minimizing PD should adjust to salient risk and protective factors corresponding to age categories.
Limitations
A few methodological limitations of this study should be noted. First, we assessed predominantly Caucasian participants, a reflection of the community from which the twins were selected. Our findings may not generalize to other ethnic and racial groups. Second, biometric models partition variance into genetic and environmental effects but do not separate gene-environment or epistatic genetic effects and do not provide information about the specific genes or environmental factors involved. Given our findings, future research in the growing field of molecular genetics (e.g., Lee, 2011) should examine underlying genes that influence PD, particularly during the period from middle to late adolescence. Third, the assessment of PD occurred every three years and more frequent assessments may have uncovered even more subtle developmental shifts in the influences of these effects. Fourth, there were slightly lower levels of PD for individual who completed the follow-up assessments than individuals who did not, and thus the results are potentially biased towards less maladjusted adolescents and young adults.
Future directions in research on peer group affiliation
To build on the findings from the current study, future research should consider the role individual differences which may help explain genetic vulnerability to PD. The covariance between PD and behavioral maladjustment—for example, conduct problems (Burt, McGue, & Iacono, 2009; Button et al., 2007; Kendler et al., 2008) and substance use (Button et al., 2009; Dick et al., 2007; Gillespie, Neale, Jacobson, & Kendler, 2009; Walden et al., 2004)—indicates that socialization and peer selection processes during these discrete, high-risk developmental periods should be isolated to more accurately inform etiological models of psychiatric disorders (e.g., antisocial personality disorder and substance use disorders).
Individuals, however, will ultimately experience their environments in unique ways, shaping interpersonal relationships. For example, researchers considered the role of externalizing behaviors in affecting PD and found the nonshared environment to mediate the association between externalizing behaviors and PD in middle and late adolescence (Burt et al., 2009). This suggests a genetic vulnerability for PD and conduct problems will be expressed differently depending on the unique environmental experiences of each twin. This finding coupled with our finding that the nonshared environment exerts entirely age-specific influences but consistently influences PD into early adulthood suggests the need for research into the exact nature of these nonshared environmental influences at discrete ages.
Uncovering precise shared environmental influences on PD is also an avenue for inquiry. For example, early childhood behavior problems have been found to be influenced in part by the shared environment via the effects of neighborhood disadvantage (Caspi, Taylor, Moffitt, & Plomin, 2000). The pervasiveness of shared environmental effects that contributed to the stability of PD during adolescence in our sample may be related to enduring neighborhood conditions. Genetically-informed studies of shared environmental influences on peer group affiliation would benefit from a consideration of ecological theories of child development (Bronfenbrenner, 1979) in order to understand how neighborhoods and community settings affect family life and parenting, which in turn, shape interpersonal relations among adolescents in a lasting way.
In summary, this study makes important contributions to our understanding of PD. The findings support the influences of the social environment, both influences shared among family members and influences unique to each individual, as well as genetically influenced factors on youth's affiliation with deviant peers. The relative importance of the genetic and environmental influences shift across development with late adolescence emerging as a critical period for changes in these influencing factors.
Acknowledgments
This study was supported in part by National Institutes of Health Grants DA-05147, AA-09367, and MH-017069. We thank the participating families who generously gave their time to the study and the many staff members and research assistants who contributed to the overall conduct of the study.
Footnotes
For accuracy, percentages were calculated prior to rounding estimates to two decimals.
Contributor Information
Nicholas Tarantino, Department of Psychology, Georgia State University.
Erin C. Tully, Department of Psychology, Georgia State University
Sarah E. Garcia, Department of Psychology, Georgia State University
Susan South, Department of Psychology, Purdue University.
William G. Iacono, Department of Psychology, University of Minnesota
Matt McGue, Department of Psychology, University of Minnesota.
References
- Baker JH, Maes HH, Larsson H, Lichtenstein P, Kendler KS. Sex differences and developmental stability in genetic and environmental influences on psychoactive substance consumption from early adolescence to young adulthood. Psychological Medicine. 2011;41(9):1907–1916. doi: 10.1017/S003329171000259X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LA, Daniels D. Nonshared environmental influences and personality differences in adult twins. Journal of Personality and Social Psychology. 1990;58(1):103–110. doi: 10.1037//0022-3514.58.1.103. [DOI] [PubMed] [Google Scholar]
- Beam CR, Turkheimer E. Phenotype–environment correlations in longitudinal twin models. Development and Psychopathology. 2013;25(1):7–16. doi: 10.1017/S0954579412000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: A meta-analysis. Twin Research and Human Genetics. 2007;10(3):423–433. doi: 10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- Brody GH, Conger R, Gibbons FX, Ge X, McBride MV, Gerrard M, Simons RL. The influence of neighborhood disadvantage, collective socialization, and parenting on african american children's affiliation with deviant peers. Child Development. 2001;72(4):1231. doi: 10.1111/1467-8624.00344. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U. The Ecology of Human Ddevelopment: Experiments by Nature and Design. Cambridge, MA: Harvard University Press; 1979. [Google Scholar]
- Brook JS, Brook DW, Zhang C, Cohen P. Pathways from adolescent parent-child conflict to substance use disorders in the fourth decade of life. American Journal on Addictions. 2009;18(3):235–242. doi: 10.1080/10550490902786793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock B, Deater-Deckard K, Leve LD. Deviant peer affiliation and problem behavior: A test of genetic and environmental influences. Journal of Abnormal Child Psychology. 2006;34(1):27–39. doi: 10.1007/s10802-005-9004-9. [DOI] [PubMed] [Google Scholar]
- Burt SA. Genes and popularity: Evidence of an evocative gene-environment correlation. Psychological Science. 2008;19(2):112–113. doi: 10.1111/j.1467-9280.2008.02055.x. [DOI] [PubMed] [Google Scholar]
- Burt SA, McGue M, Iacono WG. Nonshared environmental mediation of the association between deviant peer affiliation and adolescent externalizing behaviors over time: Results from a cross-lagged monozygotic twin differences design. Developmental Psychology. 2009;45(6):1752–1760. doi: 10.1037/a0016687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button TMM, Corley RP, Rhee SH, Hewitt JK, Young SE, Stallings MC. Delinquent peer affiliation and conduct problems: A twin study. Journal of Abnormal Psychology. 2007;116(3):554–564. doi: 10.1037/0021-843X.116.3.554. [DOI] [PubMed] [Google Scholar]
- Button TMM, Stallings MC, Rhee SH, Corley RP, Boardman JD, Hewitt JK. Perceived peer delinquency and the genetic predisposition for substance dependence vulnerability. Drug and Alcohol Dependence. 2009;100(1–2):1–8. doi: 10.1016/j.drugalcdep.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Taylor A, Moffitt TE, Plomin R. Neighborhood deprivation affects children's mental health: Environmental risks identified in a genetic design. Psychological Science. 2000;11(4):338–342. doi: 10.1111/1467-9280.00267. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Clark DB, Reynolds M, Kirisci L, Tarter R. Early age of first sexual intercourse and affiliation with deviant peers predict development of SUD: A prospective longitudinal study. Addictive Behaviors. 2007;32(4):850–854. doi: 10.1016/j.addbeh.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Daniels D, Plomin R. Origins of individual differences in infant shyness. Developmental Psychology. 1985;21(1):118–121. [Google Scholar]
- Dick DM, Pagan JL, Holliday C, Viken R, Pulkkinen L, Kaprio J, Rose RJ. Gender differences in friends’ influences on adolescent drinking: A genetic epidemiological study. Alcoholism: Clinical & Experimental Research. 2007;31(12):2012–2019. doi: 10.1111/j.1530-0277.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- Dishion TJ, Patterson GR, Stoolmiller M, Skinner ML. Family, school, and behavioral antecedents to early adolescent involvement with antisocial peers. Developmental Psychology. 1991;27(1):172–180. [Google Scholar]
- Fergusson DM, Horwood LJ. Prospective childhood predictors of deviant peer affiliations in adolescence. Journal of Child Psychology & Psychiatry & Allied Disciplines. 1999;40(4):581. [PubMed] [Google Scholar]
- Fowler T, Shelton K, Lifford K, Rice F, McBride A, Nikolov I, van den Bree MBM. Genetic and environmental influences on the relationship between peer alcohol use and own alcohol use in adolescents. Addiction. 2007;102(6):894–903. doi: 10.1111/j.1360-0443.2007.01824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Developmental psychology. 2005;41(4):625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Gavin LA, Furman W. Age differences in adolescents' perceptions of their peer groups. Developmental Psychology. 1989;25(5):827–834. [Google Scholar]
- Gillespie NA, Neale MC, Jacobson K, Kendler KS. Modeling the genetic and environmental association between peer group deviance and cannabis use in male twins. Addiction. 2009;104(3):420–429. doi: 10.1111/j.1360-0443.2008.02457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Hill JE, Turkheimer E, Emery RE. Gene-environment correlation and interaction in peer effects on adolescent alcohol and tobacco use. Behavior Genetics. 2008;38(4):339–347. doi: 10.1007/s10519-008-9202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry DB, Tolan PH, Gorman-Smith D. Longitudinal family and peer group effects on violence and nonviolent delinquency. Journal of Clinical Child Psychology. 2001;30(2):172–186. doi: 10.1207/S15374424JCCP3002_5. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: Findings from the Minnesota Twin and Family Study. Development and Psychopathology. 1999;11(4):869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, McGue M, Krueger RF. Minnesota Center for Twin and Family Research. Twin Research and Human Genetics. 2006;9(6):978–984. doi: 10.1375/183242706779462642. [DOI] [PubMed] [Google Scholar]
- Iervolino AC, Pike A, Manke B, Reiss D, Hetherington EM, Plomin R. Genetic and environmental influences in adolescent peer socialization: Evidence from two genetically sensitive designs. Child Development. 2002;73(1):162. doi: 10.1111/1467-8624.00398. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. Gene-environment correlations: A review of the evidence and implications for prevention of mental illness. Molecular Psychiatry. 2007;12(5):432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Baker JH. Genetic influences on measures of the environment: A systematic review. Psychological Medicine. 2007;37(5):615–626. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson K, Myer JM, Eaves LJ. A genetically informative developmental study of the relationship between conduct disorder and peer deviance in males. Psychological Medicine. 2008;38(7):1001–1011. doi: 10.1017/S0033291707001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Gardner CO, Gillespie N, Aggen SA, Prescott CA. Creating a social world: A developmental twin study of peer-group deviance. Archives of General Psychiatry. 2007;64(8):958–965. doi: 10.1001/archpsyc.64.8.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacourse E, Nagin DS, Vitaro F, Côté S, Arseneault L, Tremblay RE. Prediction of early-onset deviant peer group affiliation: A 12-year longitudinal study. Archives of General Psychiatry. 2006;63(5):562–568. doi: 10.1001/archpsyc.63.5.562. [DOI] [PubMed] [Google Scholar]
- Lansford JE, Yu T, Erath SA, Pettit GS, Bates JE, Dodge KA. Developmental precursors of number of sexual partners from ages 16 to 22. Journal of Research on Adolescence. 2010;20(3):651–677. doi: 10.1111/j.1532-7795.2010.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS. Deviant peer affiliation and antisocial behavior: Interaction with monoamine oxidase A (MAOA) genotype. Journal of Abnormal Child Psychology. 2011;39(3):321–332. doi: 10.1007/s10802-010-9474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone SM, Taylor J, Marmorstein NR, McGue M, Iacono WG. Genetic and environmental influences on antisocial behavior and alcohol dependence from adolescence to early adulthood. Development and Psychopathology. 2004;16(4):943–966. doi: 10.1017/s0954579404040088. [DOI] [PubMed] [Google Scholar]
- Manke B, McGuire S, Reiss D, Hetherington EM, Plomin R. Genetic contributions to adolescents' extrafamilial social interactions: Teachers, best friends, and peers. Social Development. 1995;4(3):238–256. [Google Scholar]
- McGue M, Elkins I, Walden B, Iacono WG. Perceptions of the parent-adolescent relationship: A longitudinal investigation. Developmental Psychology. 2005;41(6):971–984. doi: 10.1037/0012-1649.41.6.971. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100(4):674–701. [PubMed] [Google Scholar]
- Moffitt TE, Caspi A. Life-course persistent and adolescence-limited antisocial males: Longitudinal followup to adulthood. In: Stoff DM, Susman EJ, editors. Developmental psychobiology of aggression. New York, NY: Cambridge University Press; 2005. pp. 161–186. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. 5th ed. Richmond, VA: Virginia Commonwealth University, Department of Psychiatry; 1999. [Google Scholar]
- O'Brien SF, Bierman KL. Conceptions and perceived influence of peer groups: Interviews with preadolescents and adolescents. Child Development. 1988;59(5):1360. doi: 10.1111/j.1467-8624.1988.tb01504.x. [DOI] [PubMed] [Google Scholar]
- Petraitis J, Flay BR, Miller TQ, Torpy EJ, Greiner B. Illicit substance use among adolescents: A matrix of prospective predictors. Substance Use & Misuse. 1998;33(13):2561–2604. doi: 10.3109/10826089809059341. [DOI] [PubMed] [Google Scholar]
- Pike A, Manke B, Reiss D, Plomin R. A genetic analysis of differential experiences of adolescent siblings across three years. Social Development. 2000;9(1):96–114. [Google Scholar]
- Plomin R, Bergeman CS. The nature of nurture: Genetic influence on 'environmental' measures. Behavioral and Brain Sciences. 1991;14(3):373–427. [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84(2):309–322. [PubMed] [Google Scholar]
- Roisman GI, Masten AS, Coatsworth JD, Tellegen A. Salient and emerging developmental tasks in the transition to adulthood. Child Development. 2004;75(1):123–133. doi: 10.1111/j.1467-8624.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene–environment interplay and psychopathology: multiple varieties but real effects. Journal of Child Psychology & Psychiatry. 2006;47(3/4):226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype→environment effects. Child Development. 1983;54(2):424. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Shanahan MJ, Hofer SM. Social context in gene-environment interactions: retrospect and prospect. Journals of Gerontology Series B: Psychological Sciences & Social Sciences. 2005;60B(1):65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- Tully EC, Iacono WG, McGue M. Changes in genetic and environmental influences on the development of nicotine dependence and major depressive disorder from middle adolescence to early adulthood. Development and Psychopathology. 2010;22(4):831–848. doi: 10.1017/S0954579410000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek JHDA, Kendler KS, de Moor MHM, Geels LM, Bartels M, Vink JM, Boomsma DI. Stable genetic effects on symptoms of alcohol abuse and dependence from adolescence into early adulthood. Behavior Genetics. 2012;42(1):40–56. doi: 10.1007/s10519-011-9488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden B, McGue M, lacono WG, Burt SA, Elkins I. Identifying shared environmental contributions to early substance use: The respective roles of peers and parents. Journal of Abnormal Psychology. 2004;113(3):440–450. doi: 10.1037/0021-843X.113.3.440. [DOI] [PubMed] [Google Scholar]
- Warr M. Age, peers, and delinquency. Criminology. 1993;31(1):17–40. [Google Scholar]
- Way N, Greene ML. Trajectories of perceived friendship quality during adolescence: The patterns and contextual predictors. Journal of Research on Adolescence. 2006;16(2):293–320. [Google Scholar]