Abstract
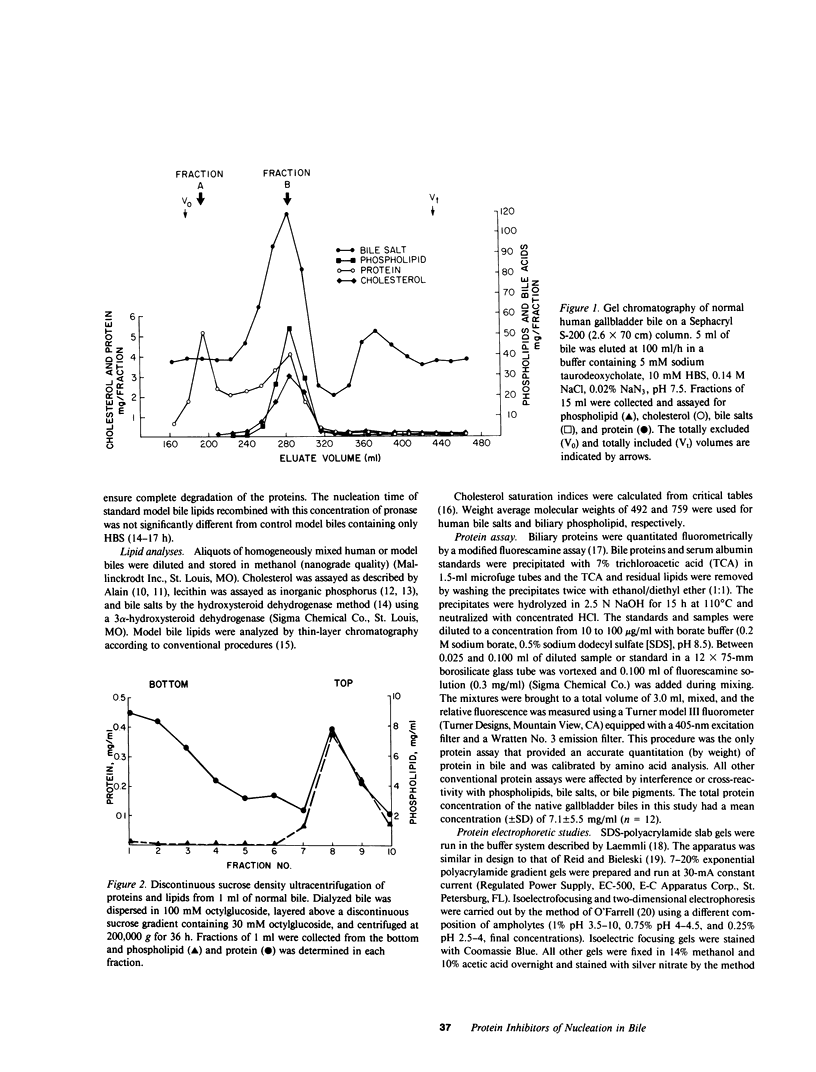
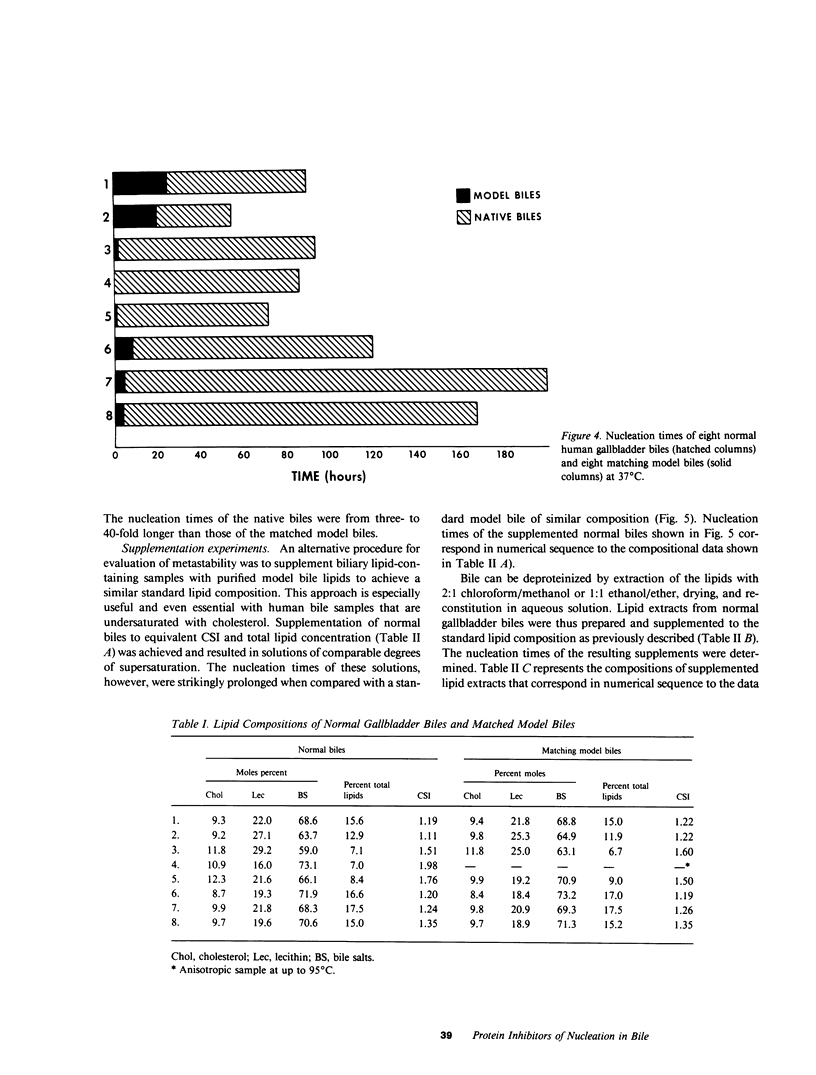
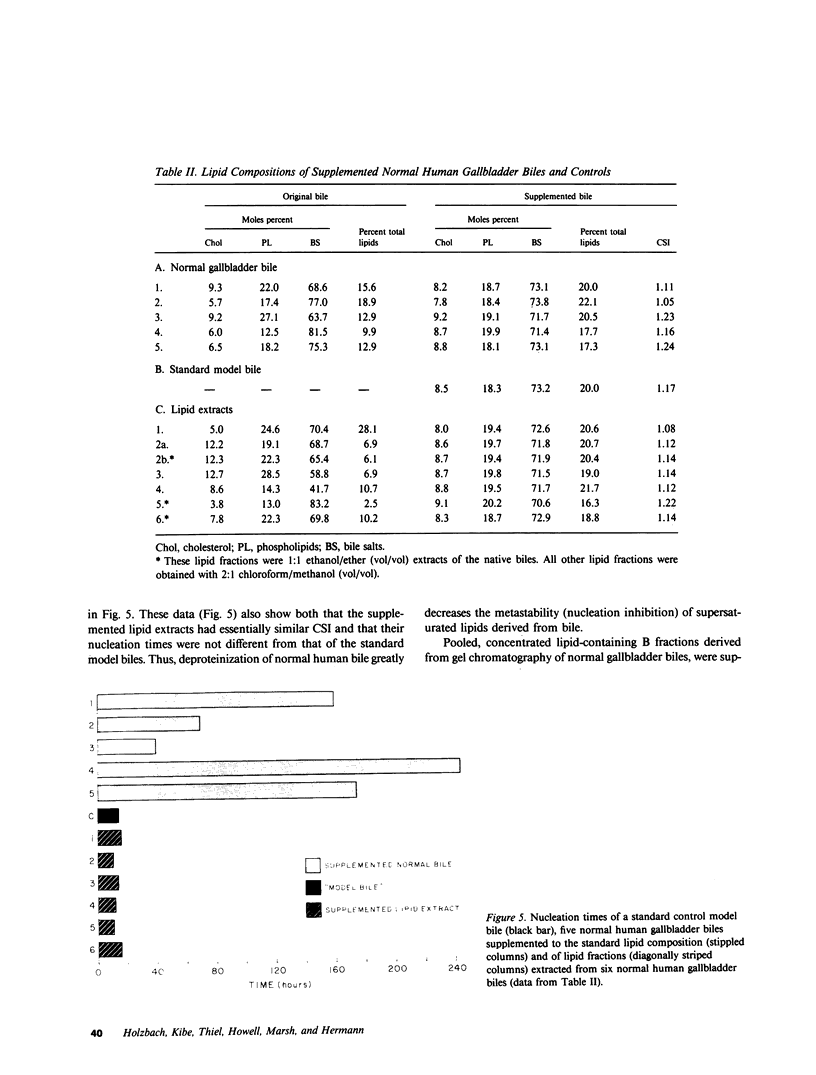
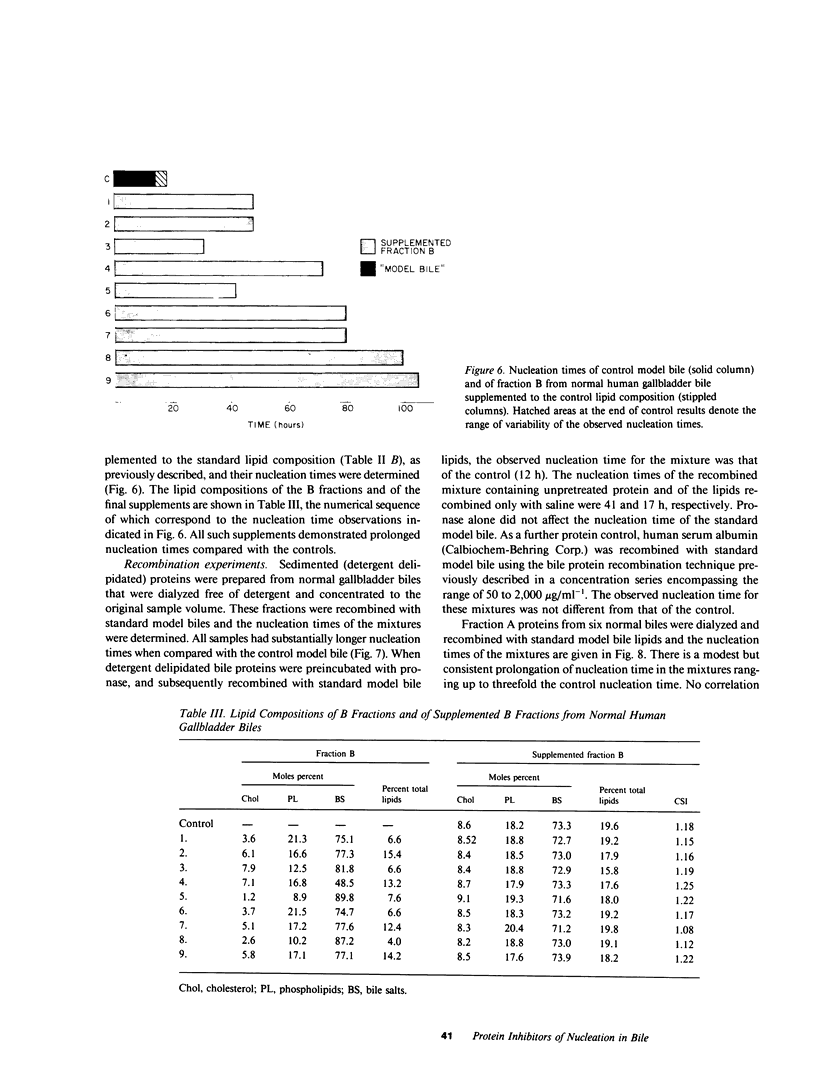
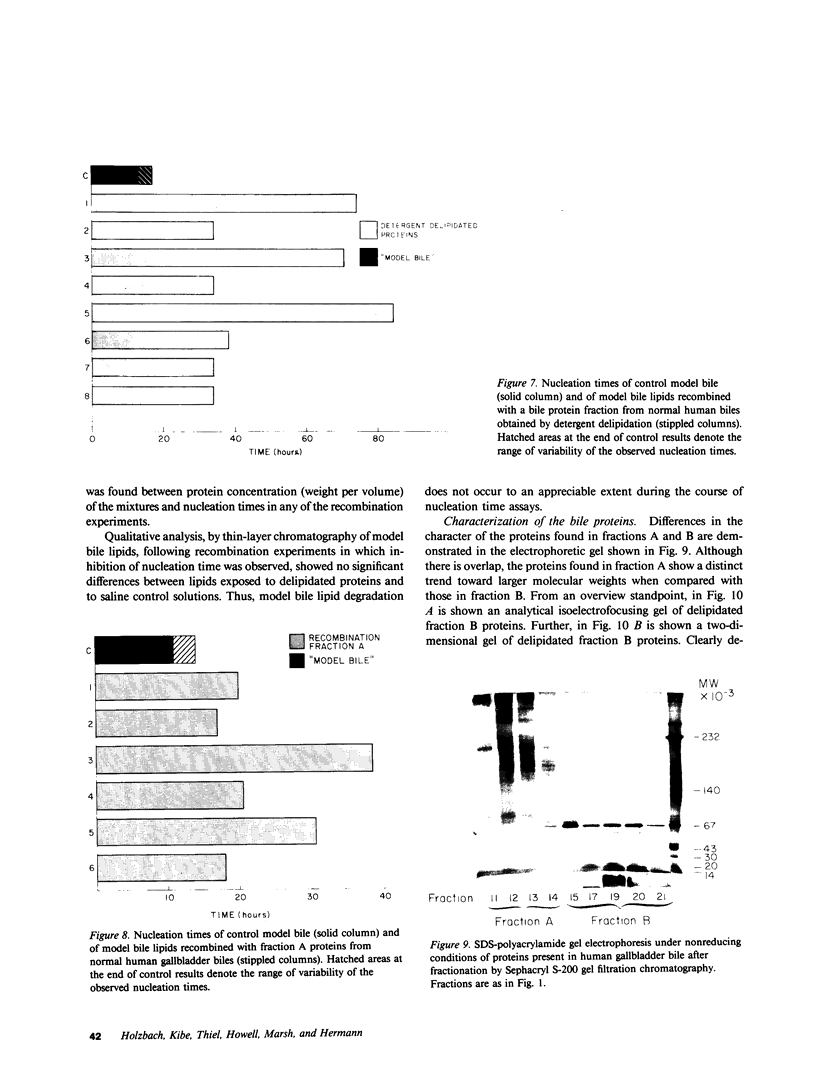
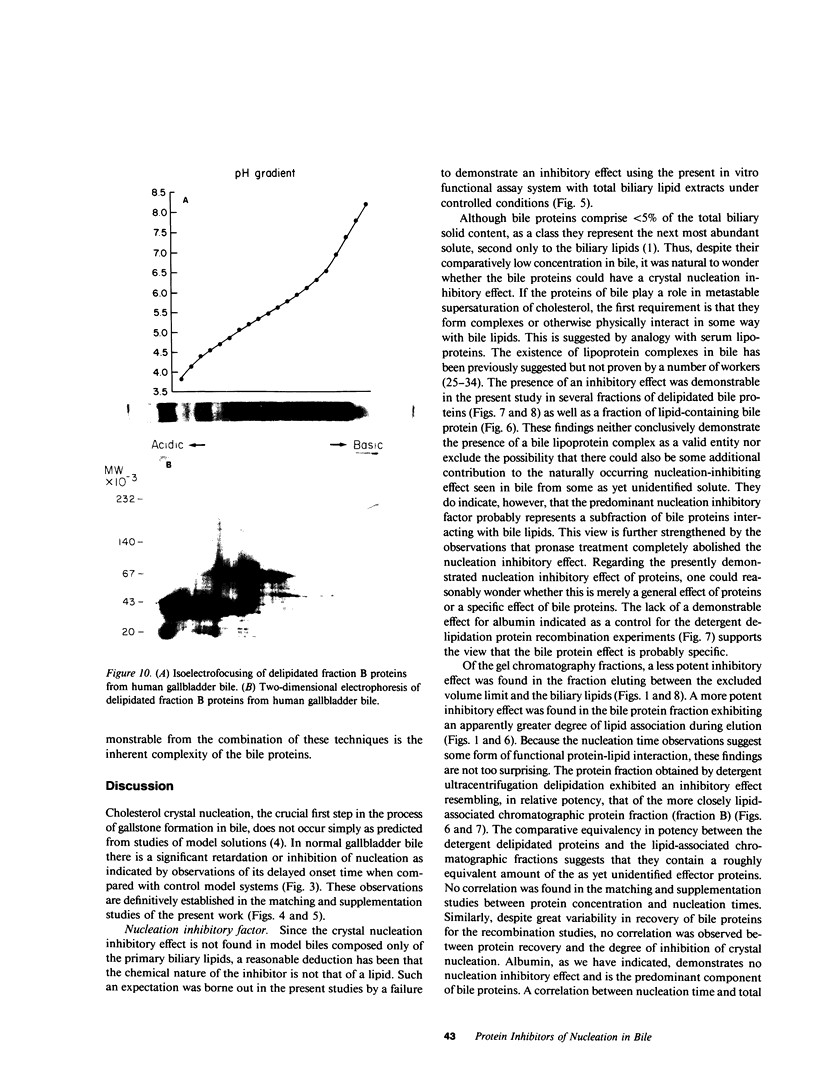
The onset time for cholesterol crystal nucleation of supersaturated normal human gallbladder biles is consistently prolonged when compared with biles from patients with cholesterol gallstone disease. Investigation of the factor(s) responsible for the suspended supersaturation (metastability) of normal human biles revealed that model bile solutions of cholesterol saturation index (CSI) and molar lipid composition identical to individual gallbladder bile specimens had much shorter crystal nucleation times, i.e., exhibited decreased metastability. Unsaturated normal biles, after supplementation with lecithin, cholesterol, and sodium taurocholate to a 'standard' supersaturated lipid composition, also demonstrated nucleation times three- to 15-fold longer than the comparable standard model bile. Total lipid extracts of normal biles, however, when similarly supplemented, did not differ in nucleation time from the control model solution. Gallbladder biles were fractionated by gel chromatography and the eluted fractions were pooled into two fractions. The fractions eluting in about the first 25% of the included volume when mixed with the supersaturated standard model bile induced a modest increase in nucleation time of approximately 1.5 times the control value. The fractions eluting in the second 25% of the included volume and which contained all of the bile lipids, were concentrated and supplemented with lipids to the standard composition. The nucleation times of these supplements were 3-10 times longer than the control nucleation times. Delipidated bile protein mixtures, purified by discontinuous sucrose gradient centrifugation, were recombined with purified lipids at the standard composition used previously. The nucleation times of these mixtures were significantly prolonged to the same extent as those associated with the second chromatographic fraction. These observations demonstrate that the delayed onset (inhibition) of cholesterol crystal nucleation observed in normal human gallbladder bile is produced by a factor(s) present in the biliary protein fraction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain C. C., Poon L. S., Chan C. S., Richmond W., Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Burnstein M. J., Ilson R. G., Petrunka C. N., Taylor R. D., Strasberg S. M. Evidence for a potent nucleating factor in the gallbladder bile of patients with cholesterol gallstones. Gastroenterology. 1983 Oct;85(4):801–807. [PubMed] [Google Scholar]
- Carey M. C. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res. 1978 Nov;19(8):945–955. [PubMed] [Google Scholar]
- Carey M. C., Small D. M. The physical chemistry of cholesterol solubility in bile. Relationship to gallstone formation and dissolution in man. J Clin Invest. 1978 Apr;61(4):998–1026. doi: 10.1172/JCI109025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castell J. V., Cervera M., Marco R. A convenient micromethod for the assay of primary amines and proteins with fluorescamine. A reexamination of the conditions of reaction. Anal Biochem. 1979 Nov 1;99(2):379–391. doi: 10.1016/s0003-2697(79)80022-6. [DOI] [PubMed] [Google Scholar]
- Gollish S. H., Burnstein M. J., Ilson R. G., Petrunka C. N., Strasberg S. M. Nucleation of cholesterol monohydrate crystals from hepatic and gall-bladder bile of patients with cholesterol gall stones. Gut. 1983 Sep;24(9):836–844. doi: 10.1136/gut.24.9.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Fries E., Kartenbeck J. Reconstitution of Semliki forest virus membrane. J Cell Biol. 1977 Dec;75(3):866–880. doi: 10.1083/jcb.75.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenders H. J., Schoenmakers J. G., Gerding J. J., Tesser G. I., Bloemendal H. N-terminus of alpha-crystallin. Exp Eye Res. 1968 Apr;7(2):291–300. doi: 10.1016/s0014-4835(68)80080-6. [DOI] [PubMed] [Google Scholar]
- Holan K. R., Holzbach R. T., Hermann R. E., Cooperman A. M., Claffey W. J. Nucleation time: a key factor in the pathogenesis of cholesterol gallstone disease. Gastroenterology. 1979 Oct;77(4 Pt 1):611–617. [PubMed] [Google Scholar]
- Holzbach R. T., Corbusier C. Liquid crystals and cholesterol nucleation during equilibration in supersaturated bile analogs. Biochim Biophys Acta. 1978 Mar 30;528(3):436–444. doi: 10.1016/0005-2760(78)90033-4. [DOI] [PubMed] [Google Scholar]
- Holzbach R. T., Marsh M., Olszewski M., Holan K. Cholesterol solubility in bile. Evidence that supersaturated bile is frequent in healthy man. J Clin Invest. 1973 Jun;52(6):1467–1479. doi: 10.1172/JCI107321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Coe F. L. Acidic peptide and polyribonucleotide crystal growth inhibitors in human urine. Am J Physiol. 1977 Nov;233(5):F455–F463. doi: 10.1152/ajprenal.1977.233.5.F455. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafont H., Lairon D., Domingo N., Nalbone G., Hauton J. C. Does a lecithin-polypeptide association in bile originate from membrane structural subunits? Biochimie. 1974;56(3):465–468. doi: 10.1016/s0300-9084(74)80156-2. [DOI] [PubMed] [Google Scholar]
- Lafont H., Nalbone G., Lairon D., Dagorn J. C., Domingo N., Amic J., Hauton J. C. Zone electrophoresis study of the bile lipoprotein complex. Biochimie. 1977;59(5-6):445–452. doi: 10.1016/s0300-9084(77)80049-7. [DOI] [PubMed] [Google Scholar]
- Lairon D., Lafont H., Hauton J. C. Lack of mixed micelles bile salt--lecithin--cholesterol in bile and presence of a lipoproteic complex. Biochimie. 1972;54(4):529–530. doi: 10.1016/s0300-9084(72)80237-2. [DOI] [PubMed] [Google Scholar]
- Lee S. P., Carey M. C., LaMont J. T. Aspirin prevention of cholesterol gallstone formation in prairie dogs. Science. 1981 Mar 27;211(4489):1429–1431. doi: 10.1126/science.7466399. [DOI] [PubMed] [Google Scholar]
- Lee S. P., LaMont J. T., Carey M. C. Role of gallbladder mucus hypersecretion in the evolution of cholesterol gallstones. J Clin Invest. 1981 Jun;67(6):1712–1723. doi: 10.1172/JCI110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzato E., Fellin R., Baggio G., Walch S., Neubeck W., Seidel D. Formation of lipoprotein-X. Its relationship to bile compounds. J Clin Invest. 1976 May;57(5):1248–1260. doi: 10.1172/JCI108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- NAKAYAMA F., MIYAKE H. CHOLESTEROL COMPLEXING BY MACROMOLECULAR FRACTIONS IN HUMAN GALL BLADDER BILE. J Lab Clin Med. 1965 Apr;65:638–648. [PubMed] [Google Scholar]
- Nakagawa Y., Margolis H. C., Yokoyama S., Kézdy F. J., Kaiser E. T., Coe F. L. Purification and characterization of a calcium oxalate monohydrate crystal growth inhibitor from human kidney tissue culture medium. J Biol Chem. 1981 Apr 25;256(8):3936–3944. [PubMed] [Google Scholar]
- Nalbone G., Lafont H., Domingo N., Lairon D., Pautrat G., Hauton J. Ultramicroscopic study of the bile lipoprotein complex. Biochimie. 1973;55(11):1503–1506. doi: 10.1016/s0300-9084(74)80560-2. [DOI] [PubMed] [Google Scholar]
- Nalbone G., Lafont H., Lairon D., Vigne J. L., Domingo N., Leonardi J., Hauton J. C. Immunogenicity of the apoprotein of the bile lipoprotein complex. Biochimie. 1978 Sep 29;60(6-7):691–694. doi: 10.1016/s0300-9084(78)80790-1. [DOI] [PubMed] [Google Scholar]
- Nalbone G., Lafont H., Vigne J. L., Domingo N., Lairon D., Chabert C., Lechene P., Hauton J. C. The apoprotein fraction of the bile lipoprotein complex: isolation, partial characterization and phospholipid binding properties. Biochimie. 1979;61(9):1029–1041. doi: 10.1016/s0300-9084(80)80257-4. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pope J. L. Crystallization of sodium taurocholate. J Lipid Res. 1967 Mar;8(2):146–147. [PubMed] [Google Scholar]
- Reid M. S., Bieleski R. L. A simple apparatus for vertical flat-sheet polyacrylamide gel electrophoresis. Anal Biochem. 1968 Mar;22(3):374–381. doi: 10.1016/0003-2697(68)90278-9. [DOI] [PubMed] [Google Scholar]
- Sedaghat A., Grundy S. M. Cholesterol crystals and the formation of cholesterol gallstones. N Engl J Med. 1980 Jun 5;302(23):1274–1277. doi: 10.1056/NEJM198006053022302. [DOI] [PubMed] [Google Scholar]
- Sutor D. J., Percival J. M. Presence or absence of inhibitors of crystal growth in bile. 1. Effect of bile on the formation of calcium phosphate, a constituent of gallstones. Gut. 1976 Jul;17(7):506–510. doi: 10.1136/gut.17.7.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley S. D., Dietschy J. M. Re-evaluation of the 3 alpha-hydroxysteroid dehydrogenase assay for total bile acids in bile. J Lipid Res. 1978 Sep;19(7):924–928. [PubMed] [Google Scholar]
- VERSCHURE J. C., DE WAEL J., MIJNLIEFF P. F. Further investigations on the macromolecular complex in human bile. Clin Chim Acta. 1956 Nov-Dec;1(6):511–518. doi: 10.1016/0009-8981(56)90038-9. [DOI] [PubMed] [Google Scholar]