Abstract
OBJECTIVES
To describe recent epidemiologic trends in stage IV prostate cancer. Although advances in screening and diagnostic techniques have led to earlier detection of prostate cancer, a portion of patients still present with late-stage disease.
METHODS
Population-based cancer registry data from the Surveillance, Epidemiology, and End Results Program (cases from 1988 to 2003, follow-up through 2005) were used to calculate annual age-adjusted incidence rates of stage IV prostate cancer (overall and for the subset presenting with distant metastases) and to assess time trends in patient, tumor, and treatment characteristics and survival.
RESULTS
From 1988 to 2003, the age-adjusted incidence of stage IV prostate cancer significantly declined by 6.4% each year. The proportion of men diagnosed at younger ages, with poorly differentiated tumors, or who underwent a radical prostatectomy significantly increased over time. Five-year relative survival improved across the study period (from 41.6% to 62.3%), particularly in those diagnosed at younger ages or with moderately to well-differentiated tumors. Later years of diagnosis were independently associated with a decreased risk of death (from all causes and from prostate cancer specifically) after controlling for important patient, tumor, and treatment characteristics. Tumor grade and receipt of radical prostatectomy appeared to be the strongest independent prognostic indicators. Temporal trends were similar in the subset presenting with distant metastases, except that no significant improvement in survival was observed.
CONCLUSIONS
As younger men may expect to live longer with advanced prostate cancer, there remains a need to widen the range of therapeutic and supportive care options.
Prostate cancer is the most common invasive cancer diagnosed among men in the United States and is the second leading cause of cancer death, with an expected 192 280 new patients and 27 360 deaths in 2009.1 Although the development and widespread use of the prostate-specific antigen screening test for early prostate cancer detection in the late 1980s led to an approximate 2-fold increase in the incidence of the disease, the American Cancer Society has reported a 2.7% average annual decline in prostate cancer death rates since 1993.2 A downward shift in clinical and pathologic stage has also been observed, with the greatest proportion of prostate cancer patients being diagnosed with organ-confined disease;3–5 but this trend appears to have diminished in recent years.6
Despite the apparent stage migration and improvement in long-term survival, however, an estimated 4% of prostate cancer patients currently present with metastatic disease at the time of diagnosis.7 For these individuals, successfully managing the disease still poses a significant challenge and the prognosis is discouraging. Although several studies have previously reported on trends in prostate cancer epidemiology,8 –13 limited data exist on recent temporal changes in those diagnosed specifically with advanced disease specifically. To better understand the changing characteristics and prognosis in this subset of patients, we examined recent time trends in the epidemiology of newly diagnosed stage IV prostate cancer in the United States using data from the Surveillance, Epidemiology and End Results (SEER) Program.
MATERIAL AND METHODS
Data Source
We used data from the National Cancer Institute’s SEER Program, an authoritative source of population-based information on cancer incidence and survival in the United States.14 SEER registries routinely collect information on patient demographics; primary tumor site, morphology, and stage at diagnosis; first course of treatment; and follow-up for vital status. To assess epidemiologic trends over time and maximize years of coverage, we limited our analyses to the first 9 registries supported by SEER (Connecticut, Iowa, New Mexico, Utah, Hawaii, Atlanta, Detroit, San Francisco-Oakland, and Seattle–Puget Sound), which cover approximately 10% of the US population.
Study Population
For incidence estimates, we selected male patients with a primary diagnosis of stage IV (American Joint Committee on Cancer, 3rd ed. staging criteria) prostate cancer (C61.9, International Classification of Diseases for Oncology, 3rd ed.), diagnosed between January 1, 1988 and December 31, 2003 (n = 29 447 [100%]). For all remaining analyses, we limited our sample to include patients with a first and only primary diagnosis of stage IV prostate cancer (n = 24 657 [84%]). We excluded patients aged less than 35 years (n = 18 [<1%]), those who with unknown treatment status (n = 327 [1%]), and those that were identified by death certificate or autopsy only (n = 61 [<1%]). Given that the natural history of primary distant stage prostate cancer may be unique compared with the larger stage IV population, we also performed analyses separately on this subset.
Statistical Analysis
We calculated incidence rates by year of diagnosis (age-adjusted to the 2000 US standard population) and estimated the average annual percentage change in incidence by fitting a least squares regression line to the natural logarithm of the incidence rates (with year of diagnosis as the regressor variable) using the National Cancer Institute’s SEER/Stat software version 6.4.4 (Available at: http://www.seer.cancer.gov/seerstat).
We examined the distribution of incident stage IV prostate cancer patients by 3 approximately equivalent time periods of diagnosis (1988–1992, 1993–1997, and 1998–2003) according to age, ethnicity/race, tumor grade, and receipt of surgery and radiation as part of first-line therapy (ie, treatment within 4 months after diagnosis). Surgery was categorized according to receipt of cancer-directed surgery; radical/total prostatectomy, other surgery (including local tumor destruction/excision and subtotal/simple prostatectomy), or none. Receipt of radiation was classified into external beam radiation, other (radioactive implants only, radioisotopes only, or radiation not otherwise specified), and none. Cochran–Armitage chi-square tests for trend15,16 were used to describe changes in the proportion of patients according to these characteristics by time period of diagnosis. The statistical software Stata/SE 9.1 for Windows (StataCorp LP, College Station, Texas) was used for these analyses.
Data on vital status were available through December 31, 2005. To describe 5-year survival, we limited our analyses to patients diagnosed up through December 31, 2001 to ensure at least 5 years of follow-up for each patient. Using SEER/Stat, we estimated 5-year relative and observed survival rates through the actuarial method first by the year of diagnosis and then by select demographic, tumor, and treatment variables according to time period of diagnosis. To further evaluate the association between period of diagnosis and risk of death after adjusting for age, ethnicity/race, tumor grade, and cancer-directed treatment, we used Stata/SE 9.1 statistical software to build Cox proportional hazard models.17 Patients were followed from the date of diagnosis (1988–2003) until the date of death and censored at the date last known to be alive (if lost to follow-up) or December 31, 2005, whichever came first. We separately examined overall and prostate cancer-specific survival. For the latter analysis, patients who died of nonprostate cancer causes were considered censored at their date of death. Because SEER reports survival time in months, patients who survived for less than 31 days were excluded from these analyses.
RESULTS
Incidence
The annual age-adjusted incidence of stage IV prostate cancer decreased from 28.1 per 100 000 (95% confidence interval [CI], 26.9–29.3) in 1988 to 12.3 per 100 000 (95% CI, 11.7–13.0) in 2003, representing an average 6.4% annual decline (95% CI, −7.8 to −4.9). For the subset with distant metastases at diagnosis, this downward trend was even steeper; age-adjusted incidence fell from 18.4 per 100 000 (95% CI, 17.4–19.4) in 1988 to 6.7 per 100 000 (95% CI, 6.3–7.3) in 2003, representing an average annual drop of 8.0% (95% CI, −9.3 to −6.7).
Patient, Tumor, and Treatment Characteristics
Patient, tumor, and treatment characteristics for stage IV prostate cancer patients diagnosed in the SEER 9 registries by time period of diagnosis as well as an assessment of trends in these characteristics over time are described in Table 1 for all stage IV patients (n = 24 251) and for the stage IV patients with distant metastases (n = 14 774). For all stage IV prostate cancer patients combined, the proportion of men diagnosed at younger ages increased significantly across the study period. Specifically, among men diagnosed during 1988–1992, just 1.0% were aged 35–50, 2.6% were aged 51–55, and 6.8% were aged 56–60 years. For those diagnosed in more recent years (1998–2003), these proportions increased to 4.4% (P <.0001), 8.5% (P <.0001), and 12.5% (P <.0001), respectively. For the distant stage subset, similar but less pronounced increases in proportions of younger men were evident, but there was also a rise in the proportion of those aged 81+ years.
Table 1.
Distribution of stage IV prostate cancer patients according to select variables by time period of diagnosis in the SEER 9 registries
| Characteristics* | Period of Diagnosis
|
Overall Trend†
|
|||
|---|---|---|---|---|---|
| 1988–1992 | 1993–1997 | 1998–2003 | |||
| All Stage IV | (n = 10 026) | (n = 6454) | (n = 7771) | +/− | P |
| Age | |||||
| 35–50 y | 1.0% | 2.9% | 4.4% | + | <.0001 |
| 51–55 y | 2.6% | 4.4% | 8.5% | + | <.0001 |
| 56–60 y | 6.8% | 8.1% | 12.5% | + | <.0001 |
| 61–65 y | 13.7% | 14.5% | 15.3% | + | .0020 |
| 66–70 y | 20.3% | 18.3% | 16.0% | − | <.0001 |
| 71–75 y | 21.2% | 18.5% | 14.6% | − | <.0001 |
| 76–80 y | 16.3% | 14.4% | 12.1% | − | <.0001 |
| 81+ y | 18.2% | 19.1% | 16.5% | − | .0095 |
| Mean (SD), years | 71.9 (9.1) | 70.9 (10.3) | 68.7 (11.1) | ||
| Ethnicity/race | |||||
| White | 80.2% | 77.4% | 77.8% | − | .0001 |
| Black | 14.2% | 15.2% | 14.4% | + | .6822 |
| Other/unknown | 5.6% | 7.4% | 7.8% | + | <.0001 |
| Tumor grade | |||||
| Surgically treated patients | |||||
| Well-differentiated | 5.2% | 2.8% | 0.5% | − | <.0001 |
| Moderately differentiated | 36.2% | 41.1% | 45.4% | + | <.0001 |
| Poorly differentiated | 48.8% | 49.3% | 51.4% | + | .0274 |
| Undifferentiated | 2.9% | 1.9% | 1.0% | − | <.0001 |
| Unknown | 6.9% | 4.9% | 1.8% | − | <.0001 |
| Nonsurgically treated patients | |||||
| Well-differentiated | 4.6% | 2.4% | 0.6% | − | <.0001 |
| Moderately differentiated | 31.2% | 28.7% | 22.4% | − | <.0001 |
| Poorly differentiated | 37.5% | 40.8% | 46.9% | + | <.0001 |
| Undifferentiated | 2.1% | 1.5% | 1.3% | − | .0012 |
| Unknown | 24.6% | 26.7% | 28.7% | + | <.0001 |
| Overall | |||||
| Well-differentiated | 4.9% | 2.5% | 0.6% | − | <.0001 |
| Moderately differentiated | 33.5% | 33.3% | 31.9% | − | .0297 |
| Poorly differentiated | 42.7% | 44.0% | 48.8% | + | <.0001 |
| Undifferentiated | 2.5% | 1.7% | 1.2% | − | <.0001 |
| Unknown | 16.4% | 18.6% | 17.6% | + | .0276 |
| Cancer-directed surgery | |||||
| None | 54.1% | 62.6% | 58.7% | + | <.0001 |
| Radical/total prostatectomy | 10.0% | 13.9% | 32.7% | + | <.0001 |
| Other cancer-directed surgery | 36.0% | 23.5% | 8.6% | − | <.0001 |
| Radiation | |||||
| None | 78.4% | 80.3% | 76.5% | − | .0040 |
| Beam radiation | 21.2% | 19.3% | 22.7% | + | .0422 |
| Other radiation | 0.4% | 0.5% | 0.9% | + | <.0001 |
| Stage IV with distant metastases | (n = 6 542) | (n = 4 193) | (n = 4 039) | ||
| Age | |||||
| 35–50 y | 0.9% | 2.2% | 3.0% | + | <.0001 |
| 51–55 y | 2.0% | 2.8% | 4.9% | + | <.0001 |
| 56–60 y | 5.6% | 5.8% | 7.5% | + | .0002 |
| 61–65 y | 11.3% | 10.9% | 9.7% | − | .0085 |
| 66–70 y | 17.6% | 15.9% | 13.3% | − | <.0001 |
| 71–75 y | 20.5% | 19.7% | 17.0% | − | <.0001 |
| 76–80 y | 19.1% | 17.9% | 17.6% | − | .0417 |
| 81+ y | 23.0% | 24.9% | 27.2% | + | <.0001 |
| Mean (SD), years | 73.4 (9.2) | 73.2 (10.1) | 72.9 (11.0) | ||
| Ethnicity/race | |||||
| White | 78.3% | 76.0% | 75.0% | − | .0001 |
| Black | 16.9% | 16.2% | 16.2% | − | .3132 |
| Other/unknown | 4.8% | 7.8% | 8.8% | + | <0.0001 |
| Tumor grade | |||||
| Surgically treated patients | |||||
| Well-differentiated | 5.5% | 2.1% | 0.5% | − | <.0001 |
| Moderately differentiated | 28.6% | 27.5% | 18.0% | − | <.0001 |
| Poorly differentiated | 53.9% | 60.2% | 72.6% | + | <.0001 |
| Undifferentiated | 3.6% | 2.5% | 2.5% | − | .1053 |
| Unknown | 8.4% | 7.7% | 6.5% | − | .1493 |
| Nonsurgically treated patients | |||||
| Well-differentiated | 4.1% | 2.2% | 0.5% | − | <.0001 |
| Moderately differentiated | 30.3% | 26.9% | 19.3% | − | <.0001 |
| Poorly differentiated | 36.4% | 40.0% | 45.5% | + | <.0001 |
| Undifferentiated | 2.2% | 1.3% | 1.2% | − | .0002 |
| Unknown | 27.0% | 29.6% | 33.6% | + | <.0001 |
| Overall | |||||
| Well-differentiated | 4.6% | 2.2% | 0.5% | − | <.0001 |
| Moderately differentiated | 29.7% | 27.1% | 19.1% | − | <.0001 |
| Poorly differentiated | 42.1% | 43.9% | 48.5% | + | <.0001 |
| Undifferentiated | 2.6% | 1.6% | 1.3% | − | <.0001 |
| Unknown | 21.0% | 25.4% | 30.6% | + | <.0001 |
| Cancer-directed surgery | |||||
| None | 67.4% | 80.8% | 88.9% | + | <.0001 |
| Radical/total prostatectomy | 0.9% | 1.2% | 0.8% | − | .6554 |
| Other cancer-directed surgery | 31.7% | 18.0% | 10.3% | − | <.0001 |
| Radiation | |||||
| None | 83.6% | 82.7% | 78.3% | − | <.0001 |
| Beam radiation | 16.2% | 16.7% | 20.7% | + | <.0001 |
| Other radiation | 0.2% | 0.6% | 1.0% | + | <.0001 |
SD indicates standard deviation; SEER, Surveillance, Epidemiology, and End Results.
Percentages may not add up to 100 due to rounding.
Based on Cochran–Armitage chi-squares tests for trends in proportions by time period of diagnosis.
The percentage of stage IV tumors that were poorly differentiated at diagnosis significantly increased across the study period, from 42.7% (1988–1992) to 48.8% (1998–2003) (P <.0001), whereas the proportion of those of lower grades decreased. Trends were similar in the distant stage subset, although the decline in moderately differentiated tumors in this group was much greater (29.7% in 1988–1992 to 19.1% in 1998–2003, P <.0001). When temporal changes in tumor grade were examined by receipt of cancer-directed surgery, patterns were similar except that in surgically treated patients, the percentage of moderately differentiated tumors significantly increased in the overall stage IV group (with a less pronounced increase in the proportion of poorly differentiated tumors), whereas there was a steeper increase in the proportion of poorly differentiated tumors in the subset with distant metastases.
Over time, there was a significant increase in the proportion of stage IV prostate cancer patients electing a radical prostatectomy as first-line treatment, from 10.0% in 1988–1992 to 32.7% in 1998–2003 (P <.0001), and a concurrent sharp decline in those receiving other types of cancer-directed surgeries. However, for those with distant metastases at diagnosis, there was an upward shift in the percentage of men not receiving any type of cancer-directed surgery (67.4% [1988–1992] to 88.9% [1998–2003], P <.0001), and relatively few underwent radical prostatectomy during any period of diagnosis. Although there was a detectable increase in the proportion of stage IV patients who received radiation as part of first-line therapy, primarily in the distant stage subset, over 75% did not receive any form of radiation across the study period.
Survival
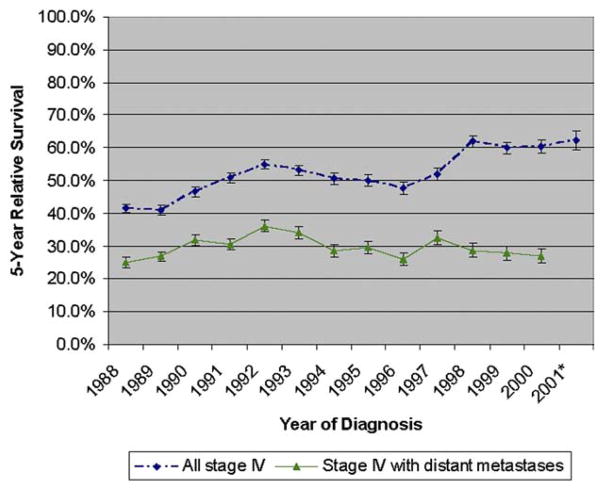
Five-year relative survival for stage IV prostate cancer patients increased from 41.6% for individuals diagnosed in 1988 to 62.3% for those diagnosed in 2001 (Fig. 1). However, for the distant stage subset, survival was 25.0% for patients diagnosed in 1988, peaked to 36.2% in 1992, and declined to 27.0% in 2001, resulting in very little net improvement in prognosis across the study period (Fig. 1). When survival was examined according to patient, tumor, and treatment characteristics by time period of diagnosis, estimates of 5-year relative survival increased among all stage IV patients except in those who were older than 70 years or had undergone cancer-directed surgery other than radical prostatectomy (Table 2). Improvements in survival were particularly dramatic for patients diagnosed with moderately to well-differentiated tumors and for those diagnosed at younger ages, especially for those aged 35–60 years. Trends in survival over time were very different for the distant stage subset, remaining stable or decreasing in all groups except for those who were younger than 71 years or diagnosed with moderately to well-differentiated tumors (Table 2).
Figure 1.
Time trend in 5-year relative survival (%) for all stage IV prostate cancer patients and for those with distant metastases at diagnosis by year of diagnosis with follow-up through 2005. *indicates unable to calculate for those diagnosed with distant metastases in the year 2001 based on too few patients and insufficient follow-up time.
Table 2.
Five-year observed and relative survival for stage IV prostate cancer patients according to select variables by time period of diagnosis based on data from the SEER 9 registries*
| Characteristics | Period of Diagnosis
|
|||||
|---|---|---|---|---|---|---|
| 1988–1992
|
1993–1997
|
1998–2001
|
||||
| 5-Year Survival (SE)
| ||||||
| Observed | Relative | Observed | Relative | Observed | Relative | |
| All stage IV | (n = 10 026) | (n = 6 450) | (n = 5 192) | |||
| Age | ||||||
| 35–50 y | 32.0% (4.7) | 33.0% (4.8)† | 49.6% (3.7) | 51.0% (3.8)† | 62.9% (3.3) | 64.8% (3.4)† |
| 51–60 y | 46.0% (1.6) | 49.8% (1.8) | 55.8% (1.8) | 59.6% (1.9) | 72.2% (1.4) | 76.7% (1.5) |
| 61–70 y | 42.9% (0.8) | 50.5% (1.0) | 50.0% (1.1) | 57.6% (1.3) | 63.1% (1.2) | 72.0% (1.4) |
| 71+ y | 27.6% (0.6) | 44.1% (1.0) | 26.7% (0.8) | 42.0% (1.2) | 26.4% (0.9) | 41.1% (1.5) |
| Ethnicity/race | ||||||
| White | 35.9% (0.5) | 49.0% (0.7) | 38.9% (0.7) | 51.2% (0.9) | 49.5% (0.8) | 62.8% (1.0) |
| Black | 26.3% (1.2) | 36.8% (1.6) | 32.7% (1.5) | 44.2% (2.0) | 41.7% (1.8) | 53.5% (2.4)† |
| Other/unknown | 36.2% (2.0) | 46.4% (2.6) | 48.2% (2.3) | 59.7% (2.9) | 50.8% (2.5) | 61.8% (3.0) |
| Tumor grade | ||||||
| Well-/moderately differentiated | 46.8% (0.8) | 62.2% (1.1) | 56.1% (1.0) | 70.5% (1.3) | 74.1% (1.0) | 88.2% (1.2) |
| Poorly/undifferentiated | 29.7% (0.7) | 40.1% (0.9) | 34.0% (0.9) | 43.9% (1.1) | 43.0% (1.0) | 53.7% (1.3) |
| Unknown | 19.5% (1.0) | 29.1% (1.5) | 16.2% (1.1) | 24.5% (1.6) | 11.2% (1.1) | 17.1% (1.6) |
| Cancer-directed surgery | ||||||
| None | 24.7% (0.6) | 34.7% (0.8) | 26.9% (0.7) | 36.9% (1.0) | 29.5% (0.8) | 39.7% (1.1) |
| Radical/total prostatectomy | 83.0% (1.2) | 98.7% (1.4) | 88.6% (1.1) | 99.8% (1.2)†‡ | 91.3% (0.7) | 100.0% (0.2)†‡ |
| Other cancer-directed surgery | 36.0% (0.8) | 49.1% (1.1) | 40.4% (1.3) | 52.4% (1.6) | 28.1% (2.1) | 39.4% (3.0) |
| Beam radiation | ||||||
| No | 32.1% (0.5) | 44.6% (0.7) | 37.4% (0.7) | 49.9% (0.9) | 47.1% (0.8) | 60.6% (1.0) |
| Yes | 43.8% (1.1) | 55.9% (1.4) | 43.9% (1.4) | 54.6% (1.8) | 53.4% (1.5) | 64.1% (1.8) |
| Overall | 34.6% (0.5) | 47.2% (0.6) | 38.6% (0.6) | 50.8% (0.8) | 48.5% (0.7) | 61.5% (0.9) |
| Stage IV with distant metastases at diagnosis | (n = 6 542) | (n = 4 191) | (n = 2 719) | |||
| Age | ||||||
| 35–50 y | 13.6% (4.5) | 14.0% (4.6)† | 23.3% (4.5) | 24.0% (4.6)† | 21.5% (4.7) | 22.2% (4.8)† |
| 51–60 y | 23.4% (1.9) | 25.4% (2.1) | 24.7% (2.3) | 26.4% (2.4) | 27.9% (2.7) | 29.7% (2.9) |
| 61–70 y | 24.2% (1.0) | 28.7% (1.2) | 26.6% (1.3) | 30.7% (1.5) | 30.7% (1.9) | 35.3% (2.1) |
| 71+ y | 19.5% (0.6) | 32.0% (1.0) | 19.5% (0.8) | 31.2% (1.2) | 15.5% (0.9) | 24.9% (1.5) |
| Ethnicity/race | ||||||
| White | 21.3% (0.6) | 30.4% (0.8) | 21.4% (0.7) | 29.8% (1.0) | 20.0% (0.9) | 28.0% (1.3) |
| Black | 18.2% (1.2) | 26.0% (1.7) | 17.9% (1.5) | 25.0% (2.1) | 18.8% (1.9) | 25.5% (2.6) |
| Other/unknown | 28.4% (2.6) | 37.6% (3.4) | 35.3% (2.7) | 44.9% (3.4) | 29.1% (3.0) | 36.9% (3.8) |
| Tumor grade | ||||||
| Well-/moderately differentiated | 29.9% (1.0) | 41.6% (1.3) | 34.5% (1.4) | 46.1% (1.8) | 42.8% (2.1) | 56.2% (2.7) |
| Poorly/undifferentiated | 17.2% (0.7) | 24.1% (1.0) | 19.4% (0.9) | 25.9% (1.2) | 18.0% (1.1) | 23.7% (1.5) |
| Unknown | 14.9% (1.0) | 22.8% (1.5) | 11.9% (1.0) | 18.4% (1.5) | 8.4% (1.0) | 12.9% (1.5) |
| Cancer-directed surgery | ||||||
| None | 20.5% (0.6) | 29.1% (0.9) | 21.8% (0.7) | 30.3% (1.0) | 20.6% (0.8) | 28.4% (1.2) |
| Radical/total prostatectomy | 72.1% (5.7) | 86.4% (6.9)† | 76.9% (5.8) | 86.6% (6.2)† | 68.9% (10.9) | 80.9% (12.8) |
| Other cancer-directed surgery | 21.0% (0.9) | 30.0% (1.3) | 18.8% (1.4) | 25.6% (1.9) | 17.3% (2.3) | 24.1% (3.2) |
| Beam radiation | ||||||
| No | 21.1% (0.6) | 30.3% (0.8) | 22.2% (0.7) | 31.0% (1.0) | 20.7% (0.9) | 29.0% (1.3) |
| Yes | 21.2% (1.3) | 28.3% (1.7) | 20.6% (1.5) | 26.9% (2.0) | 20.4% (1.8) | 26.3% (2.3) |
| Overall | 21.1% (0.5) | 30.0% (0.7) | 21.9% (0.6) | 30.3% (0.9) | 20.6% (0.8) | 28.4% (1.1) |
SE indicates standard error.
Survival calculated based on actuarial method.
The relative cumulative rate increased from a prior interval and has been adjusted.
The relative cumulative rate is over 100% and has been adjusted.
Mirroring these temporal trends in 5-year survival, results of Cox proportional hazard regression modeling indicated that after controlling for age, ethnicity/race, tumor grade, and receipt of cancer-directed treatments, there was a decreased risk of death (both from all causes and from prostate cancer specifically) over time for men diagnosed with stage IV prostate cancer (Table 3). Those diagnosed in either 1993–1997 or 1998–2003 had a 9% decreased risk of death from prostate cancer compared with those diagnosed in 1988–1992. For the subset presenting with distant metastases, however, there was no significant change in the risk of death across the study period. For all patients, tumor grade and receipt of radical prostatectomy appeared to be the strongest prognostic indicators.
Table 3.
Multivariate regression analysis of factors associated with time to death (overall and prostate cancer-specific) based on the Cox proportional hazards model for males diagnosed with stage IV prostate cancer who survived at least 31 days based on data from the SEER 9 registries
| Characteristics | All Causes of Death
|
Prostate Cancer-Specific Death
|
||||
|---|---|---|---|---|---|---|
| aHR* | 95% CI | P | aHR* | 95% CI | P | |
| All stage IV (n = 23 845) | ||||||
| Time of diagnosis | ||||||
| 1988–1992 | 1.00 | Reference | 1.00 | Reference | ||
| 1993–1997 | 0.92 | 0.89–0.95 | <.001 | 0.91 | 0.87–0.95 | <.001 |
| 1998–2003 | 0.90 | 0.86–0.93 | <.001 | 0.91 | 0.87–0.95 | <.001 |
| Age | ||||||
| 35–50 y | 0.68 | 0.61–0.76 | <.001 | 0.98 | 0.87–1.10 | .744 |
| 51–60 y | 0.62 | 0.59–0.65 | <.001 | 0.82 | 0.78–0.87 | <.001 |
| 61–70 y | 0.70 | 0.68–0.73 | <.001 | 0.85 | 0.81–0.88 | <.001 |
| 71+ y | 1.00 | Reference | 1.00 | Reference | ||
| Ethnicity/race | ||||||
| White | 1.00 | Reference | 1.00 | Reference | ||
| Black | 1.16 | 1.12–1.21 | <.001 | 1.11 | 1.06–1.17 | <.001 |
| Other/unknown | 0.74 | 0.69–0.78 | <.001 | 0.68 | 0.63–0.74 | <.001 |
| Tumor grade | ||||||
| Well-/moderately differentiated | 1.00 | Reference | 1.00 | Reference | ||
| Poorly/undifferentiated | 1.62 | 1.56–1.68 | <.001 | 1.87 | 1.80–1.96 | <.001 |
| Unknown | 2.07 | 1.98–2.16 | <.001 | 2.32 | 2.20–2.44 | <.001 |
| Cancer-directed surgery | ||||||
| None | 1.00 | Reference | 1.00 | Reference | ||
| Radical/total prostatectomy | 0.18 | 0.17–0.19 | <.001 | 0.13 | 0.11–0.14 | <.001 |
| Other cancer-directed surgery | 0.79 | 0.76–0.82 | <.001 | 0.78 | 0.75–0.81 | <.001 |
| Beam radiation | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 0.82 | 0.79–0.86 | <.001 | 0.87 | 0.83–0.91 | <.001 |
| Stage IV with distant metastases at diagnosis (n = 14 417) | ||||||
| Time of diagnosis | ||||||
| 1988–1992 | 1.00 | Reference | 1.00 | Reference | ||
| 1993–1997 | 0.98 | 0.94–1.02 | .356 | 0.98 | 0.93–1.03 | .355 |
| 1998–2003 | 1.00 | 0.95–1.05 | .999 | 1.02 | 0.96–1.07 | .537 |
| Age | ||||||
| 35–50 y | 0.92 | 0.80–1.05 | .199 | 1.20 | 1.05–1.38 | .010 |
| 51–60 y | 0.78 | 0.73–0.83 | <.001 | 0.97 | 0.90–1.04 | .336 |
| 61–70 y | 0.78 | 0.75–0.82 | <.001 | 0.92 | 0.88–0.96 | <.001 |
| 71+ y | 1.00 | Reference | 1.00 | Reference | ||
| Ethnicity/race | ||||||
| White | 1.00 | Reference | 1.00 | Reference | ||
| Black | 1.12 | 1.07–1.17 | <.001 | 1.06 | 1.00–1.12 | .034 |
| Other/unknown | 0.74 | 0.69–0.80 | <.001 | 0.69 | 0.63–0.75 | <.001 |
| Tumor grade | ||||||
| Well-/moderately differentiated | 1.00 | Reference | 1.00 | Reference | ||
| Poorly/undifferentiated | 1.48 | 1.42–1.55 | <.001 | 1.70 | 1.61–1.78 | <.001 |
| Unknown | 1.89 | 1.80–1.98 | <.001 | 2.06 | 1.94–2.18 | <.001 |
| Cancer-directed surgery | ||||||
| None | 1.00 | Reference | 1.00 | Reference | ||
| Radical/total prostatectomy | 0.28 | 0.22–0.36 | <.001 | 0.20 | 0.14–0.27 | <.001 |
| Other cancer-directed surgery | 1.00 | 0.96–1.04 | .976 | 1.00 | 0.96–1.06 | .851 |
| Beam radiation | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 1.06 | 1.01–1.11 | .011 | 1.15 | 1.10–1.21 | <.001 |
CI indicates confidence interval.
Adjusted hazard ratio (aHR) based on Cox proportional hazards model, adjusted for time period of diagnosis, age, ethnicity/race, tumor grade, and cancer-directed surgery and radiation.
COMMENT
Consistent with studies reporting downward time trends in rates of metastatic prostate cancer,18,19 we observed a significant decline in the annual incidence of stage IV prostate cancer from 1988 to 2003. To our knowledge, the current study represents the only recent report focused on examining temporal trends in the distribution of patient, tumor, and treatment characteristics and survival for newly diagnosed stage IV prostate cancer. We observed significant changes in patient demographics across the study period, most notably with respect to age at diagnosis. Previous research has noted a shift toward younger ages at diagnosis for all stages of prostate cancer combined,10,13 and we found that this same trend holds when stage IV tumors are examined separately. However, Barnholtz-Sloan et al20 examined time trends in meta-static prostate cancer using data from the Detroit SEER registry (1973–1997), and they indicated a decrease in the percentage of men diagnosed at ages <60 years. Conversely, among our distant stage subset, the proportion of those aged 60 years or less rose from 8.5% (1988–1992) to over 15% (1998–2003). Discordance in these findings may be attributed to differences in time period and geographic coverage, as well as nuances in tumor inclusion criteria. Regardless, prostate cancer is still rare in younger men, particularly under the age of 50, and this group may therefore be less likely to suspect it, be screened for it, or have their doctor propose a diagnostic work-up when symptoms surface. Hence, it has been suggested that differential screening behavior may be causing younger men to account for a greater proportion of more advanced tumors in recent years.21
We also discovered an upward migration in stage IV prostate tumor grade at diagnosis, which is consistent with earlier reports on prostate cancer overall22,23 and metastatic disease specifically.10,20 Hankey et al10 proposed that, in addition to a screening effect, shifts in the grade distribution among all prostate tumors may reflect changes in the use of available therapies. In the early 1980s, radical prostatectomy was modified to preserve regional innervation and potency, which led to an increase in the uptake of this procedure through the 1980s and into the 1990s. It is widely recognized that tumors are upgraded following surgery (because of the amount of tissue given to the pathologist for review), so an increase in radical prostatectomy procedures may have at least partly contributed to the shift in tumor grade distribution toward higher grades. Indeed, we found a steep rise in the percentage of stage IV prostate cancer patients undergoing radical prostatectomy across the study period. However, in our subset of stage IV patients with distant metastases, although we observed relatively fewer moderately to well-differentiated tumors over time, only a small percentage underwent radical prostatectomy, and receipt of other types of cancer-directed surgeries declined considerably across the study period. Further, when temporal changes in tumor grade migration were examined separately for surgically versus nonsurgically treated patients, a shift toward higher tumor grades was consistently demonstrated across time for both groups.
It is well-documented that in recent years, survival has improved for men with prostate cancer in the United States.8,10,12,20 Our study demonstrates that this is also true for patients diagnosed with stage IV disease. We observed an absolute increase of nearly 21% in 5-year relative survival across the study period, and later years of diagnosis were independently associated with improvements in survival after controlling for important patient, tumor, and treatment characteristics. Based on advances in screening and diagnostic techniques, it is likely that at least a portion of individuals are being diagnosed earlier in the disease process and are therefore surviving longer; but improvements may also be credited to increasingly successful treatment options for patients with advanced disease.24 For the stage IV patients with distant metastases at diagnosis, however, rates of 5-year survival slightly increased only for younger men or for those with moderately to well-differentiated tumors, suggesting that only a subset of patients with distant stage prostate cancer has benefited from more aggressive or better systemic therapies. Further, no real improvement in prognosis for this group is yet evident, as multivariate analyses of survival revealed no significant change in the risk of all-cause or prostate cancer-specific death across the 3 time periods examined.
We also found that older age (71+ years), black ethnicity/race, higher tumor grade, and lack of radical prostatectomy generally conferred a poorer prognosis for stage IV patients, most likely reflecting differences between subgroups of patients with respect to biological factors related to survival, access to care, nature and extent of comorbidity, and response to available treatments. The prognostic role of age in prostate cancer, specifically, has been a subject of debate, and we detected a distinct pattern of disparate outcomes by age group for stage IV patients. Regardless of time period of diagnosis, 5-year relative survival increased, peaked, and then decreased with increasing age, and this trend was echoed in our adjusted age-specific death hazard ratios. Some research has demonstrated superior survival in younger patients, while other investigations have noted that younger men present with prostate cancer that is more aggressive, too advanced for radical surgery, and rapidly fatal.21 Although we observed improvements in survival over time for men diagnosed at younger ages, our results also provide evidence that, at least for advanced prostate cancer, men diagnosed at either especially young (≤50 years) or old (71+ years) ages tend to have a poorer prognosis compared to the middle-aged group.
The SEER Program provides high-quality data on cancer incidence and survival in the United States, but any time trend investigation can be complicated by modifications in disease classification, advancements in diagnostic techniques, fluctuations in the popularity and availability of screening, and stage and grade migration. Our study was focused exclusively on newly diagnosed prostate cancer patients classified as having stage IV disease consistent with the American Joint Committee on Cancer 3rd ed. staging criteria, and we performed separate analyses on the subset presenting with distant metastases in recognition of the heterogeneity of the stage IV group. We also attempted to limit the effect of tumors being upgraded because of surgery by describing time trends in tumor grade distribution separately for surgically versus nonsurgically treated patients and by combining moderately differentiated and well-differentiated tumors in our survival analyses. More generally, inherent to any population-based disease registry is the potential for inaccurate cause of death information (obtained from death certificates). Although the level of this type of attribution bias across SEER data are unknown, Penson et al25 reported that for prostate cancer patients from the Seattle-Puget Sound SEER Registry, there was excellent agreement (97%) between clinician-assigned cause of death (based on medical chart review) and the cause of death on the death certificate (kappa = 0.91). Further, trained nosologists code cause of death information on death certificates, which are based on standardized procedures developed by the National Center for Health Statistics and are routinely reviewed for quality. It seems reasonable to assume that any potential discrepancies in the coding of cause of death would be consistent across time, geographic regions, and population groups in the United States. We chose to examine relative survival, prostate cancer-specific mortality, and overall mortality, and all 3 of these approaches yielded consistent findings. Unfortunately, we were unable to evaluate additional factors of interest in prostate cancer, such as prostate-specific antigen levels, Karnofsky performance status, site of metastases, and differences in other important therapies (eg, androgen deprivation therapy) over the study period, because these data are either incomplete or unavailable in SEER.
CONCLUSIONS
This descriptive study on recent time trends in the epidemiology of stage IV prostate cancer in the United States has important clinical implications. Perhaps one of the most striking findings is that younger men are representing an increasingly higher proportion of stage IV prostate tumors, and survival in this group is improving. As younger men may expect to live longer with advanced disease, long-term management of their condition must be carefully planned, particularly with respect to quality of life. For stage IV patients who present with distant metastases, our findings suggest no significant change in overall or prostate cancer-specific survival and only modest improvements in 5-year relative survival for those who are younger or have moderately to well-differentiated tumors, further highlighting the need for a wider range of supportive care and therapeutic options available to treat late-stage disease.
Acknowledgments
Funded by Amgen Inc., Thousand Oaks, CA.
Footnotes
Presented at the 44th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30, 2008–June 3, 2008.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Byers T, Barrera E, Fontham ET, et al. A midpoint assessment of the American Cancer Society challenge goal to halve the US cancer mortality rates between the years 1990 and 2015. Cancer. 2006;107:396–405. doi: 10.1002/cncr.21990. [DOI] [PubMed] [Google Scholar]
- 3.Desireddi NV, Roehl KA, Loeb S, et al. Improved stage and grade-specific progression-free survival rates after radical prostatectomy in the PSA era. Urology. 2007;70:950–955. doi: 10.1016/j.urology.2007.06.1119. [DOI] [PubMed] [Google Scholar]
- 4.Jhaveri FM, Klein EA, Kupelian PA, et al. Declining rates of extracapsular extension after radical prostatectomy: evidence for continued stage migration. J Clin Oncol. 1999;17:3167–3172. doi: 10.1200/JCO.1999.17.10.3167. [DOI] [PubMed] [Google Scholar]
- 5.Roehl KA, Han M, Ramos CG, et al. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 6.Dong F, Reuther AM, Magi-Galluzzi C, et al. Pathologic stage migration has slowed in the late PSA era. Urology. 2007;70:839–842. doi: 10.1016/j.urology.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2005 (Based on November 2007 SEER Data Submission) Bethesda, MD: National Cancer Institute; 2008. [Google Scholar]
- 8.Brenner H, Arndt V. Long-term survival rates of patients with prostate cancer in the prostate-specific antigen screening era: population-based estimates for the year 2000 by period analysis. J Clin Oncol. 2005;23:441–447. doi: 10.1200/JCO.2005.11.148. [DOI] [PubMed] [Google Scholar]
- 9.Dennis LK, Resnick MI. Analysis of recent trends in prostate cancer incidence and mortality. Prostate. 2000;42:247–252. doi: 10.1002/(sici)1097-0045(20000301)42:4<247::aid-pros1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 10.Hankey BF, Feuer EJ, Clegg LX, et al. Cancer surveillance series: interpreting trends in prostate cancer—part I: evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999;91:1017–1024. doi: 10.1093/jnci/91.12.1017. [DOI] [PubMed] [Google Scholar]
- 11.Potosky AL, Feuer EJ, Levin DL. Impact of screening on incidence and mortality of prostate cancer in the United States. Epidemiol Rev. 2001;23:181–186. doi: 10.1093/oxfordjournals.epirev.a000787. [DOI] [PubMed] [Google Scholar]
- 12.Sarma AV, Schottenfeld D. Prostate cancer incidence, mortality, and survival trends in the United States: 1981–2001. Semin Urol Oncol. 2002;20:3–9. doi: 10.1053/suro.2002.30390. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson RA, Stanford JL. Population-based prostate cancer trends in the United States: patterns of change in the era of prostate-specific antigen. World J Urol. 1997;15:331–335. doi: 10.1007/BF01300179. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute, DCCPS, Surveillance Research Program Cancer Statistics Branch. [Accessed 2008.];Surveillance, Epidemiology, and End Results (SEER) Program. Limited-Use Data (1973–2005) released April 2008, based on the November 2007 submission. Available at: http://www.seer.cancer.gov.
- 15.Armitage P. Tests for linear trends in proportions and frequencies. Biometrics. 1955;11:375–386. [Google Scholar]
- 16.Cochran WG. Some methods of strengthening the common chi-square tests. Biometrics. 1954;10:417–451. [Google Scholar]
- 17.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 18.Feuer EJ, Mariotto A, Merrill R. Modeling the impact of the decline in distant stage disease on prostate carcinoma mortality rates. Cancer. 2002;95:870–880. doi: 10.1002/cncr.10726. [DOI] [PubMed] [Google Scholar]
- 19.Newcomer LM, Stanford JL, Blumenstein BA, et al. Temporal trends in rates of prostate cancer: declining incidence of advanced stage disease, 1974–94. J Urol. 1997;158:1427–1430. doi: 10.1016/s0022-5347(01)64231-9. [DOI] [PubMed] [Google Scholar]
- 20.Barnholtz-Sloan JS, Severson RK, Vaishampayan U, et al. Survival analysis of distant prostate cancer by decade (1973–97) in the Detroit Metropolitan Surveillance, Epidemiology and End Results (SEER) Program registry: has outcome improved? (United States) Cancer Causes Control. 2003;14:681–685. doi: 10.1023/a:1025675303370. [DOI] [PubMed] [Google Scholar]
- 21.Merrill RM, Bird JS. Effect of young age on prostate cancer survival: a population-based assessment (United States) Cancer Causes Control. 2002;13:435–443. doi: 10.1023/a:1015764507609. [DOI] [PubMed] [Google Scholar]
- 22.Gallina A, Chun FK, Suardi N, et al. Comparison of stage migration patterns between Europe and the USA: an analysis of 11 350 men treated with radical prostatectomy for prostate cancer. BJU Int. 2008;101:1513–1518. doi: 10.1111/j.1464-410X.2008.07519.x. [DOI] [PubMed] [Google Scholar]
- 23.Jani AB, Master VA, Rossi PJ, et al. Grade migration in prostate cancer: an analysis using the Surveillance, Epidemiology, and End Results registry. Prostate Cancer Prostatic Dis. 2007;10:347–351. doi: 10.1038/sj.pcan.4500977. [DOI] [PubMed] [Google Scholar]
- 24.Pienta KJ, Smith DC. Advances in prostate cancer chemotherapy: a new era begins. CA Cancer J Clin. 2005;55:300–318. doi: 10.3322/canjclin.55.5.300. [DOI] [PubMed] [Google Scholar]
- 25.Penson DF, Albertsen PC, Nelson PS, et al. Determining cause of death in prostate cancer: are death certificates valid? J Natl Cancer Inst. 2001;93:1822–1823. doi: 10.1093/jnci/93.23.1822. [DOI] [PubMed] [Google Scholar]