Abstract
The goal of our study was to develop a simple and practical method for simulating diving in humans using facial cold exposure and apnea stimuli to measure neural and circulatory responses during the stimulated diving reflex. We hypothesized that responses to simultaneous facial cold exposure and apnea (simulated diving) would be synergistic, exceeding the sum of responses to individual stimuli. We studied 56 volunteers (24 female and 32 male), average age 39 years. All subjects were healthy, free of cardiovascular and other diseases, and on no medications. Although muscle sympathetic nerve activity (MSNA), blood pressure, and vascular resistance increased markedly during both early and late phases of simulated diving, significant reductions in heart rate were observed only during the late phase. Total MSNA during simulated diving was greater than combined MSNA responses to the individual stimuli. We found that simulated diving is a powerful stimulus to sympathetic nerve traffic with significant bradycardia evident in the late phase of diving and eliciting synergistic sympathetic and parasympathetic responses. Our data provide insight into autonomic triggers that could help explain catastrophic cardiovascular events that may occur during asphyxia or swimming, such as in patients with obstructive sleep apnea and congenital long QT syndrome.
Keywords: microneurography, sympathetic nervous system, diving, bradycardia, arrhythmias, long QT syndrome
INTRODUCTION
The diving reflex has been demonstrated in animal studies to be a powerful autonomic stimulus (Andersen, 1966; Blix et al., 1983; Hong, 1989). Unlike other stresses that accentuate either sympathetic or parasympathetic outflow, with reciprocal inhibition of one or the other, diving stimulates simultaneously both of these major components of the autonomic nervous system in birds and mammals (Butler et al., 1997; Davis et al., 2004; McPhail et al., 1999). Diving reflex responses include vagally-mediated bradycardia in humans (Asmussen et al., 1968), simultaneous with diffuse and marked increases in sympathetic-mediated peripheral vasoconstriction in several vascular beds, for the purpose of maintaining circulation to vital organs such as the brain and heart in humans (Elsner et al., 1971; Leuenberger et al., 2001). The primary objective of this reflex response is to conserve oxygen in order to enable underwater exploration by birds and mammals (Butler et al., 1997) while maintaining neural and cardiac integrity.
The role of the diving reflex in circulatory homeostasis in humans is poorly understood. However, it has been implicated in drowning and catastrophic cardiovascular events, particularly in patients with congenital long QT syndrome (LQTS) (Ackerman et al., 1999; Batra et al., 2002). Enhanced sympathetic activity is an established cause of ventricular tachyarrhythmia (Vassalle et al., 1970). Diving-induced bradycardia is associated with ventricular ectopic beats in healthy humans (Smith et al., 1997). Vagal bradycardia and simultaneous sympathetic activation during the diving reflex (Paton et al., 2006) may be a potential trigger for arrhythmias and possibly sudden death in patients with vulnerable substrates, such as LQTS in general and type 1 LQTS in particular (Ackerman et al., 1999). Activation of the diving reflex also may occur during apneic episodes in patients with obstructive sleep apnea (Rial et al., 2000), sometimes resulting in pacemaker placement, and has been implicated in increased cardiovascular mortality. Although the diving reflex has been studied extensively in animals for more than a century, human studies are few, in part due to the complexity of appropriate stimuli. Immersion of the face in water is an important component of the stimulus (Anderson, 1963; Dykes, 1974; Schuitema et al., 1988), and face immersion alone increases vagal activity (Hayashi et al., 1997). Therefore, in the laboratory setting, simulated “diving” and “facial cold” have been introduced to study the diving reflex in humans (Heath et al., 1990; Heistad et al., 1968; Whayne et al., 1967). Although less marked than in some animals, bradycardia due to increased vagal activity during simulated diving has been observed in humans (Andersson et al., 2004; Craig, 1963; Foster et al., 2005; Kawakami et al., 1967; Leuenberger et al., 2001). However, little is known about the sympathetic neural responses associated with simulated diving. Microneurography is a unique method for direct measurement of sympathetic nerve outflow in humans (Mano et al., 2006; Shamsuzzaman et al., 2003; Vallbo et al., 2004; Wallin et al., 2007). To our knowledge, the only study that systematically evaluated the diving reflex and its effect on muscle sympathetic nerve activity (MSNA) in humans noted marked increases in SNA accompanied by peripheral vasoconstriction and increased blood pressure (BP) (Fagius et al., 1986). How these changes compare to the effects of apnea alone or facial cold exposure alone was not studied. Furthermore, subjects were studied in the prone position with only 12 seconds of facial immersion, and bradycardia as a key component of the diving response was not statistically significant (Fagius et al., 1986). Short stimulus duration may have affected the measurements.
The goal of our study was to develop a simple and practical method for simulating diving in humans using facial cold exposure and apnea stimuli to measure neural and circulatory responses during the stimulated diving reflex. We hypothesized that neural circulatory responses to simulated diving induced by both facial cold and apnea are synergistic, exceeding the sum of the individual responses to either facial cold or apnea.
METHODS
Subjects
We studied 56 healthy volunteers (24 female and 32 male), average age 39±14 years with body mass index (BMI) 26±4. All female volunteers were pre-menopausal and not menstruating during the study. All subjects were healthy, free of cardiovascular and other diseases, on no medications, and were asked to avoid exercise and caffeinated beverages for at least 24 hours before the study. Informed written consent was obtained from all subjects. The study was approved by the Mayo Clinic’s Institutional Review Board.
Measurements and facial cold exposure material
A 12-lead electrocardiogram (ECG) was recorded continuously by ECG Bioamplifier (Gould Electronics) and respiration by a thoracic belt (Pneumotrace, Gould Electronics). Beat-to-beat changes in BP were recorded continuously by a Finapres (Ohmeda, Louisville, CO). BP also was measured every minute from the brachial artery with an automatic sphygmomanometer (Dinamap, Critikon Inc.). Blood flow was measured from the left calf by venous occlusion plethysmography (EC4, Hokanson) in 11 subjects. Multiunit efferent intraneural recordings of MSNA to the skeletal muscle blood vessels were obtained from the right peroneal nerve posterior to the head of the fibula, using microneurography (Shamsuzzaman et al., 2003).
For facial cold exposure, a specially constructed water-proof polyethylene bag with a central opening for the nose to maintain spontaneous nasal breathing was used. The bag, filled with ice and water, was applied to cover the entire face except the nose for facial cold. Before applying the bag, two pieces of shielding gauze were applied over closed eyelids to protect them from cold and pressure, thus minimizing the possibility of activating the oculocardiac reflex.
Protocol
All subjects were studied in the supine position. Measurements were made in a quiet, air-conditioned room with 22–25°C ambient temperature. After 15 minutes of recovery following instrumentation, technically high-quality recordings of MSNA (signal to noise ratio ≥ 3:1) together with measurements of BP, heart rate (HR), respiration, and calf blood flow were obtained while the subject rested in the supine horizontal position in an undisturbed environment. After an acceptable recording of MSNA was established, subjects rested supine for 15 minutes, during which MSNA and all cardiovascular measures were recorded continuously. At the end of baseline recordings, subjects were asked to hold their breath after full expiration (end-expiratory apnea) for maximum possible duration. Subjects then rested for a 10-minute period to permit hemodynamics and MSNA to return to baseline levels. A plastic bag was filled with approximately 50% ice and 50% water. The plastic bag was stored in a bucket containing a mixture of ice and water to keep it wet. A towel was placed around the subject’s head and neck to protect from dripping water, before application of the facial cold. The cold and wet plastic bag was applied to the face for 1 minute for facial cold alone, after which the subject was asked to hold his or her breath after full expiration for a maximum duration for apnea while facial cold continued (simulated diving). Blood flow was measured from the left calf for 2 minutes at the end of the baseline resting period and continuously during individual apnea and facial cold exposure, as well as during simulated diving.
Data storage and analysis
The data were digitized on a computer and simultaneously stored on digital audio tape. Baseline data were averaged for each minute. The duration of apnea and thus simulated diving was different between subjects. Therefore, apnea, facial cold, and simulated diving periods were divided into halves, an early phase and a late phase, and results were expressed as per minute. HR was calculated from the ECG by counting the number of R-waves per minute. Systolic, diastolic, and mean BP were calculated from the Finapres BP wave after careful identification of the peak and trough. Blood flow was measured by venous occlusion plethysmography as previously described (Kato et al., 2000). Vascular resistance (VR) was calculated by dividing mean arterial pressure (MAP) by calf blood flow and is expressed in arbitrary units.
Sympathetic bursts were identified and counted manually after careful inspection of the mean voltage neurogram and expressed as MSNA burst frequency (bursts/min) and burst incidence (bursts/100 heart beats). MSNA burst amplitude was determined, and total MSNA was calculated as burst rate multiplied by mean burst amplitude and expressed as arbitrary units per minute. Neural circulatory changes during apnea, facial cold, and simulated diving were calculated as both absolute changes and percentage changes from the baseline resting period, considering baseline activity as 100%.
Statistical Analysis
Data were expressed as means ± standard deviation. Neural and circulatory responses to apnea, face cold, and simulated diving during early and late phases were analyzed by ANOVA with post-hoc comparison between the stimuli using Student’s t-test. In all tests, a value of P<0.05 was considered statistically significant.
RESULTS
Effects of Apnea
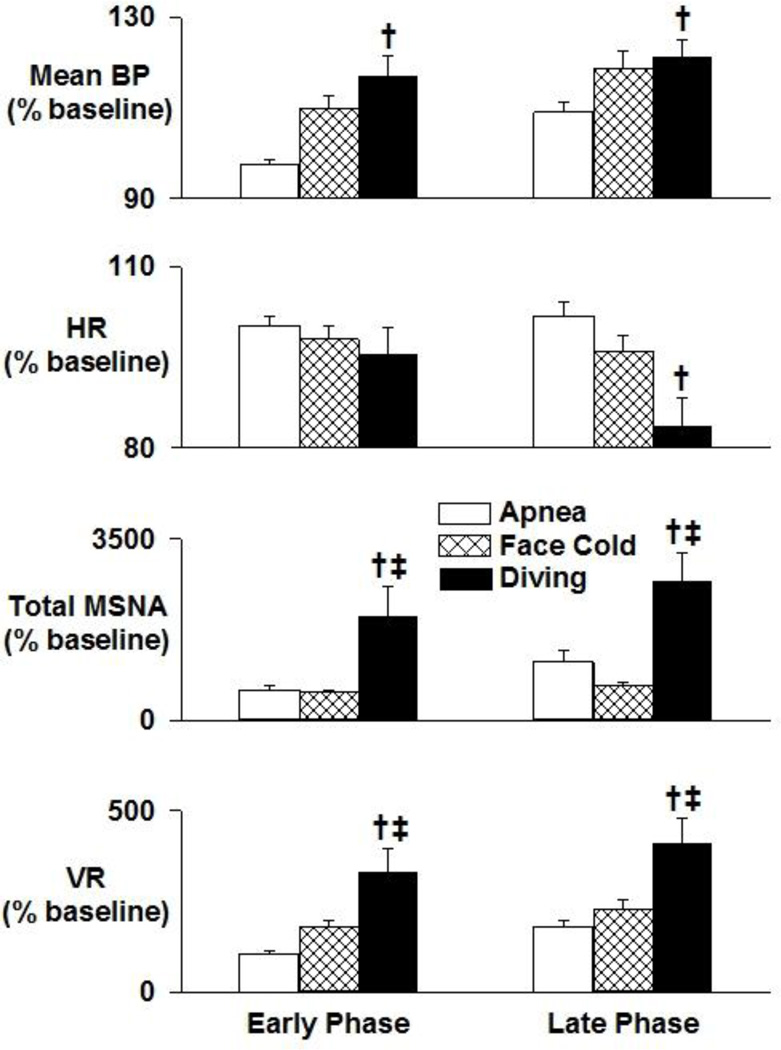
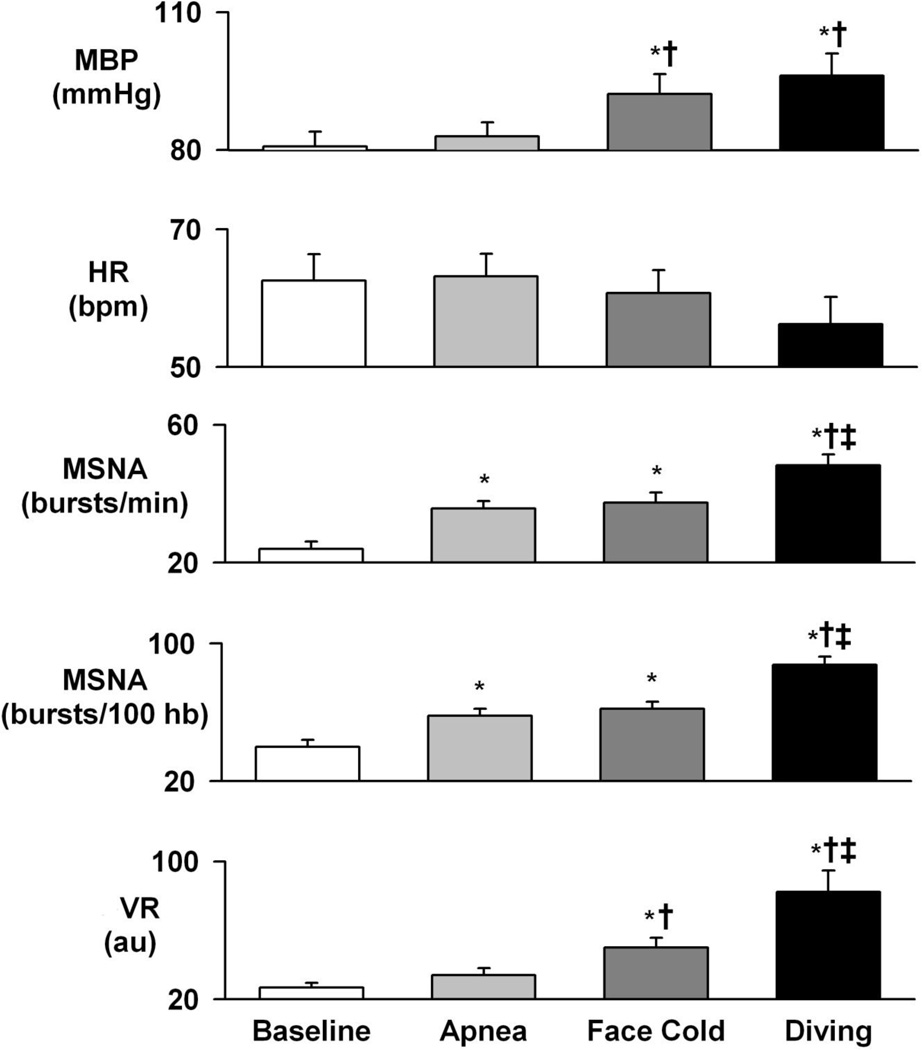
Prolonged apnea was associated with late potentiation of MSNA. Original recordings of ECG, BP, respiration, and integrated MSNA during baseline resting conditions and during apnea in a single subject are shown in Figure 1. Significant increases in MSNA, VR, and BP were observed only during the late phase of apnea (Figure 4).
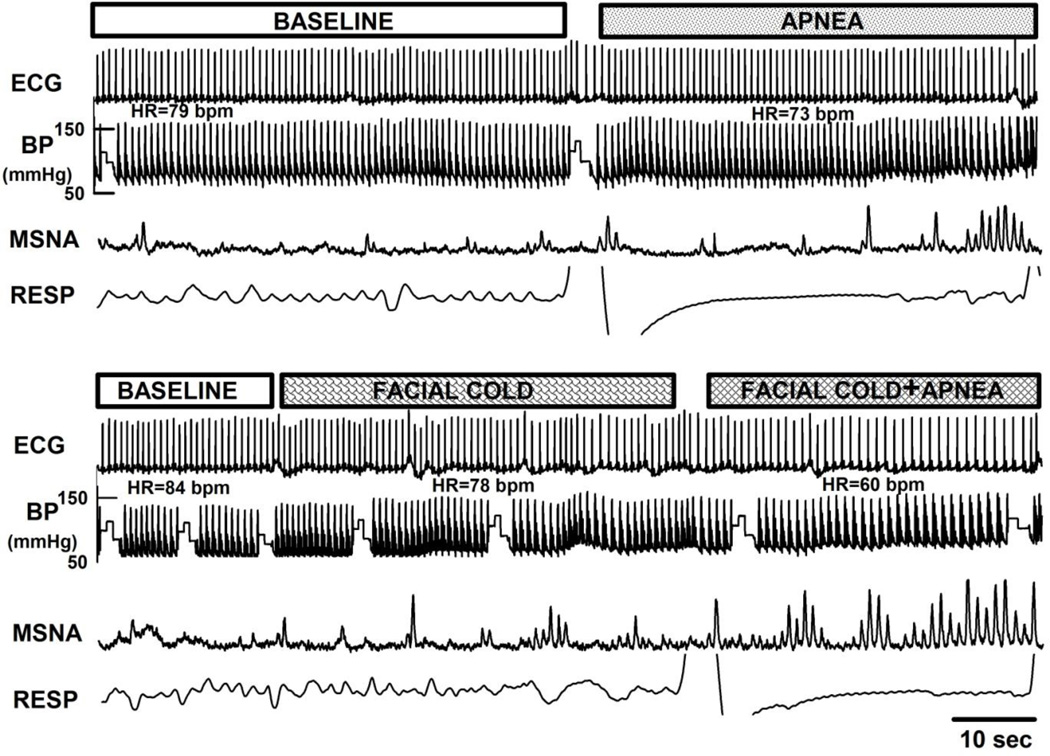
Figure 1.
Original recordings of ECG, BP, MSNA, and respiration during baseline, apnea, facial cold, and simulated diving (facial cold and apnea). Note increase in MSNA with bradycardia during simulated diving.
Figure 4.
Neural circulatory changes from baseline during early and late phases of apnea, facial cold, and simulated diving. MSNA increases in the early phase of facial cold and increases further in the late phase of simulated diving. BP and VR also increase during the late phase of facial cold and into the late phase of simulated diving. A significant fall in HR was observed only during the late phase of simulated diving. The effect of simulated diving was synergistic, greater than the sum of the individual effects of either apnea or facial cold. Data are means ±SD. *, P<0.05 vs baseline; †, P<0.05 vs apnea; ‡, P<0.05 vs facial cold
Effects of Facial Cold Exposure
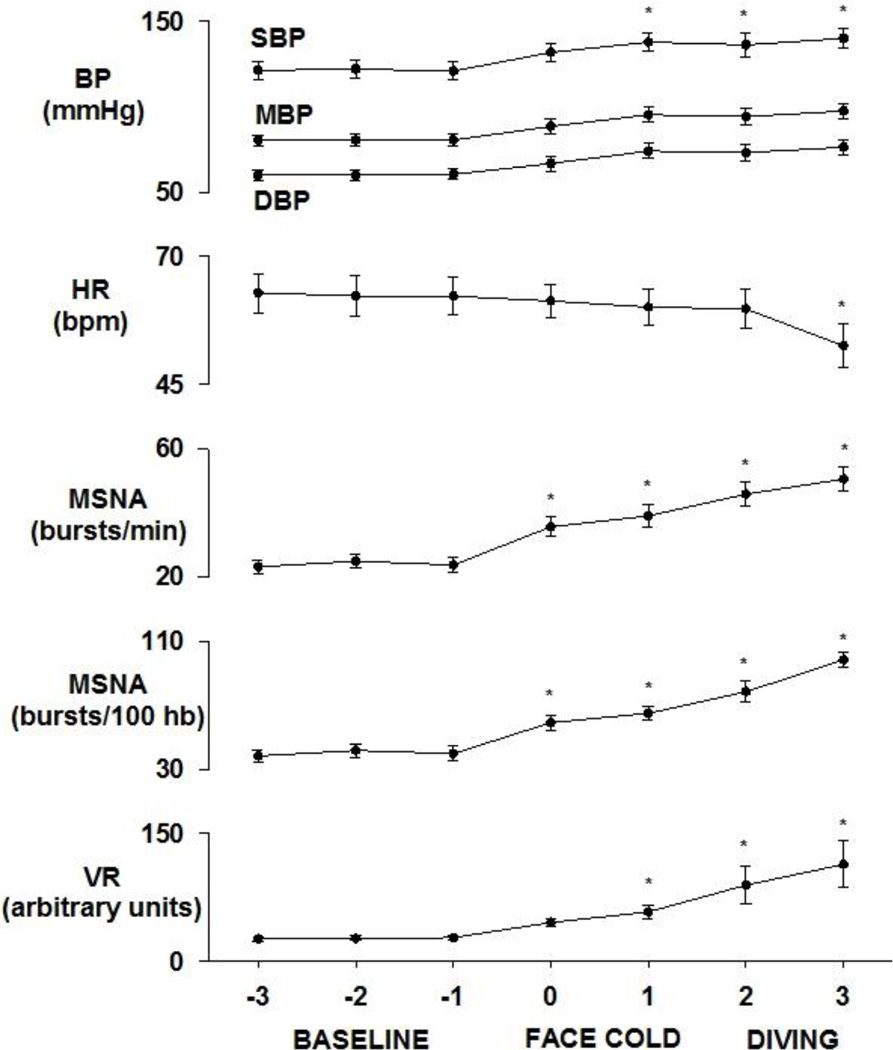
Original recordings of ECG, BP, respiration, and integrated MSNA during baseline resting, facial cold, and simulated diving in the same subject are shown in Figure 1. Facial cold for one minute was associated with increased MSNA, VR, and BP during both early and late phases (Figures 2 – 4). However, facial cold had no effect on breathing rate.
Figure 2.
Neural circulatory responses to facial cold and simulated diving. BP, MSNA, and VR increase significantly during facial cold and simulated diving with significant bradycardia during the late phase of simulated diving. Data are means ±SD. * P<0.05 vs baseline
Effects of Simulated Diving (simultaneous facial cold exposure and apnea)
Bradycardia and increased MSNA in one subject during simulated diving are shown in Figure 1. Average MSNA, BP, and VR increased markedly during both early and late phases of simulated diving (Figures 2 – 4). Significant reductions in HR were observed only during the late phase of simulated diving (Figures 2 and 4). Note that the early suppression of MSNA during apnea alone was absent during simulated diving, with simulated diving inducing the highest increases in MSNA (Figure 4). The effect of simulated diving on MSNA was synergistic, in that the total MSNA response during the late phase of simulated diving (2661±576 arbitrary units/min) was greater than the combined MSNA responses to the individual stimuli of either apnea (1117±217 units/min) or facial cold (658±58 units/min) (Figure 4). In addition, duration of breath hold during apnea with and without facial cold was not significantly different (31.5±3.7 vs 36.7±3.8 s, P>0.05).
DISCUSSION
We found significant increases in MSNA, VR, and BP during apnea, facial cold, and simulated diving. However, significant bradycardia was observed only during the late phase of simulated diving. The effect of simulated diving on MSNA was synergistic, greater than the combined effects of apnea and facial cold individually.
MSNA responses to voluntary apnea have been studied extensively in humans in physiologic and pathologic conditions (Eckberg et al., 1985; Somers et al., 1995; Somers et al., 1989; Van De Borne et al., 2001), and apnea is routinely used as a stimulus to MSNA during microneurographic recordings. In this study, we noted lower MSNA during the early phase of apnea, while the late phase of apnea was associated with marked increases in MSNA likely due to activation of chemoreceptors with a consequent rise in VR and BP. Facial cold exposure was well tolerated by all subjects. MSNA, VR, and BP increased progressively during both the early and late phases of facial cold. However, bradycardia was not evident during facial cold. During simulated diving (simultaneous facial cold exposure and apnea), dramatic increases in MSNA were observed. The total MSNA response was about ten-fold greater than that reported in previous studies (2800% vs 250%) (Fagius et al., 1986). The lesser activation of MSNA in the previous report may have been due to the short duration of facial immersion, possibly inadequate to potentiate chemoreceptors. In our study, the lower MSNA evident during the early phase of apnea was replaced by marked sympathetic activation during simulated diving, suggesting a powerful stimulus of apnea simultaneous with facial cold exposure on central sympathetic outflow. Furthermore, high MSNA in association with the marked increase in BP during simulated diving suggests that the diving reflex overrides activation of the arterial baroreflex. We also noted clear and significant bradycardia during simulated diving that was not evident during either apnea or facial cold alone. This finding speaks directly to the importance of the combined stimulus of both apnea and facial cold in eliciting combined cardiac vagal outflow and central sympathetic outflow.
In this study, several practical limitations in generating a simulated diving reflex in humans were circumvented. First, the necessity of the prone position for diving in humans was avoided. The supine position is more comfortable, and the preferred position for microneurographic recording of the sympathetic nerve traffic. Second, we observed significant bradycardia during the later phase of simulated diving, which is considered a hallmark of the diving reflex response, confirming successful simulation of diving. Third, the longer duration of simulated diving in our study (1 minute for facial cold and over 30 seconds for simulated diving) compares well to a previous study (12 seconds) (Fagius et al., 1986) and more closely mimics physiologic conditions. Furthermore, MSNA is synchronous with cardiac cycles and is quantified as bursts per minute or bursts per 100 heart beats. Duration is therefore an important factor for measuring MSNA, particularly during conditions that induce bradycardia. In our study, neural circulatory changes were calculated over longer durations, thus minimizing the potential errors in MSNA quantification. For example, low frequency oscillations in MSNA simultaneous with BP oscillations (Montano et al., 1998) require longer durations of diving to reduce the possibility of counting MSNA only during peaks or troughs of oscillatory changes. A limitation of our study was the calculation of VR by dividing MAP measured with Finapres by blood flow measured in the left calf with venous occlusion plethysmography, which did not provide an accurate estimation of systemic VR.
We describe here neural and circulatory responses to simulated diving in humans. Simulated diving is a powerful stimulus to sympathetic nerve traffic with significant bradycardia also evident in the late phase of diving. Diving elicits synergistic sympathetic and parasympathetic responses, greater than the sum of the individual responses to either apnea or facial cold exposure alone. Our findings contribute toward a better understanding of the neural and circulatory responses activated by cold immersion and by swimming. Furthermore, these data provide insight into autonomic triggers that could help explain catastrophic cardiovascular events that may occur during asphyxia or swimming, such as in patients with LQTS.
Figure 3.
Average neural and circulatory changes during baseline, apnea, facial cold, and simulated diving. Mean BP, MSNA, and VR increase significantly during facial cold and simulated diving without significant changes in HR. Data are means ±SD. *, P<0.05 vs baseline; †, P<0.05 vs apnea; ‡, P<0.05 vs facial cold
ACKNOWLEDGEMENTS
The authors are grateful to J. Denise Wetzel, CCHMC Medical Writer, for critical review of the manuscript.
Funding support: These studies were supported by a Perkins Memorial Award, AHA Scientist Development Grant (0730129N, AS), and AHA Fellowship Grant (09-20069G, FSK), and by NIH grants HL-70302, HL-65176, TW05463, TW05469, and 1 UL1 RR024150 (VS).
Dr. Somers has served as a consultant for Cardiac Concepts, Sepracor, Boston Scientific, and ResMed and is an investigator on studies funded with grants from the Respironics Sleep and Breathing Foundation, Sorin, Inc., and Select Research. None of these companies were involved in this study.
Dr. Ackerman is a consultant for Boston Scientific, Gilead Sciences, Medtronic, and St. Jude Medical and receives royalties from Transgenomic for FAMILION-LQTS and FAMILION-CPVT genetic tests. None of these companies were involved in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Other authors have no disclosures.
REFERENCES
- Ackerman MJ, Tester DJ, Porter CJ. Swimming, a gene-specific arrhythmogenic trigger for inherited long QT syndrome. Mayo Clinic Proceedings. 1999;74:1088–1094. doi: 10.4065/74.11.1088. [DOI] [PubMed] [Google Scholar]
- Andersen HT. Physiological adaptations in diving vertebrates. Physiol Rev. 1966;46:212–243. doi: 10.1152/physrev.1966.46.2.212. [DOI] [PubMed] [Google Scholar]
- Anderson HT. Factors determining the circulatory adjustments to diving: I. Water immersion. Acta Physiologica Scandinavica. 1963;58:173–185. doi: 10.1111/j.1748-1716.1963.tb02639.x. [DOI] [PubMed] [Google Scholar]
- Andersson JP, Liner MH, Fredsted A, Schagatay EK. Cardiovascular and respiratory responses to apneas with and without face immersion in exercising humans. J Appl Physiol. 2004;96:1005–1010. doi: 10.1152/japplphysiol.01057.2002. [DOI] [PubMed] [Google Scholar]
- Asmussen E, Kristiansson NG. The "diving bradycardia" in exercising man. Acta Physiol Scand. 1968;73:527–535. doi: 10.1111/j.1365-201x.1968.tb10892.x. [DOI] [PubMed] [Google Scholar]
- Batra AS, Silka MJ. Mechanism of sudden cardiac arrest while swimming in a child with the prolonged QT syndrome. J Pediatr. 2002;141:283–284. doi: 10.1067/mpd.2002.126924. [DOI] [PubMed] [Google Scholar]
- Blix AS, Folkow B. Cardiovascular adjustments to diving in mammals birds. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology: The Cardiovascular System. Vol. 3. Bethesda, MD, USA: American Physiological Society; 1983. pp. 917–945. [Google Scholar]
- Butler PJ, Jones DR. Physiology of diving of birds and mammals. Physiol Rev. 1997;77:837–899. doi: 10.1152/physrev.1997.77.3.837. [DOI] [PubMed] [Google Scholar]
- Craig AJ. Heart rate responses to apneic underwater diving and to breath holding in man. J Appl Physiol. 1963;18:854–862. doi: 10.1152/jappl.1963.18.5.854. [DOI] [PubMed] [Google Scholar]
- Davis RW, Polasek L, Watson R, Fuson A, Williams TM, Kanatous SB. The diving paradox: new insights into the role of the dive response in air-breathing vertebrates. Comp Biochem Physiol A Mol Integr Physiol. 2004;138:263–268. doi: 10.1016/j.cbpb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Dykes RW. Factors related to the dive reflex in harbor seals: sensory contributions from the trigeminal region. Can J Physiol Pharmacol. 1974;52:259–265. doi: 10.1139/y74-035. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Nerhed C, Wallin BG. Respiratory modulation of muscle sympathetic and vagal cardiac outflow in man. J Physiol. 1985;365:181–196. doi: 10.1113/jphysiol.1985.sp015766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner R, Gooden BA, Robinson SM. Arterial blood gas changes and the diving response in man. Aust J Exp Biol Med Sci. 1971;49:435–444. doi: 10.1038/icb.1971.47. [DOI] [PubMed] [Google Scholar]
- Fagius J, Sundlof G. The diving response in man: effects on sympathetic activity in muscle and skin nerve fascicles. J Physiol. 1986;377:429–443. doi: 10.1113/jphysiol.1986.sp016196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GE, Sheel AW. The human diving response, its function, and its control. Scand J Med Sci Sports. 2005;15:3–12. doi: 10.1111/j.1600-0838.2005.00440.x. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Ishihara M, Tanaka A, Osumi T, Yoshida T. Face immersion increases vagal activity as assessed by heart rate variability. Eur J Appl Physiol Occup Physiol. 1997;76:394–399. doi: 10.1007/s004210050267. [DOI] [PubMed] [Google Scholar]
- Heath ME, Downey JA. The cold face test (diving reflex) in clinical autonomic assessment: methodological considerations and repeatability of responses. Clin Sci (Lond) 1990;78:139–147. doi: 10.1042/cs0780139. [DOI] [PubMed] [Google Scholar]
- Heistad DD, Abbound FM, Eckstein JW. Vasoconstrictor response to simulated diving in man. J Appl Physiol. 1968;25:542–549. doi: 10.1152/jappl.1968.25.5.542. [DOI] [PubMed] [Google Scholar]
- Hong S. Diving Physiology. In: Wood S, editor. Comparative pulmonary physiology. Vol. 39. New York: Marcel Dekker Inc.; 1989. pp. 787–802. [Google Scholar]
- Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, Somers VK. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Natelson BH, DuBois AR. Cardiovascular effects of face immersion and factors affecting diving reflex in man. J Appl Physiol. 1967;23:964–970. doi: 10.1152/jappl.1967.23.6.964. [DOI] [PubMed] [Google Scholar]
- Leuenberger UA, Hardy JC, Herr MD, Gray KS, Sinoway LI. Hypoxia augments apnea-induced peripheral vasoconstriction in humans. J Appl Physiol. 2001;90:1516–1522. doi: 10.1152/jappl.2001.90.4.1516. [DOI] [PubMed] [Google Scholar]
- Mano T, Iwase S, Toma S. Microneurography as a tool in clinical neurophysiology to investigate peripheral neural traffic in humans. Clin Neurophysiol. 2006;117:2357–2384. doi: 10.1016/j.clinph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- McPhail LT, Jones DR. The autonomic nervous control of heart rate in ducks during voluntary diving. Physiol Biochem Zool. 1999;72:164–169. doi: 10.1086/316662. [DOI] [PubMed] [Google Scholar]
- Montano N, Cogliati C, Porta A, Pagani M, Malliani A, Narkiewicz K, Abboud FM, Birkett C, Somers VK. Central vagotonic effects of atropine modulate spectral oscillations of sympathetic nerve activity. Circulation. 1998;98:1394–1399. doi: 10.1161/01.cir.98.14.1394. [DOI] [PubMed] [Google Scholar]
- Paton JF, Nalivaiko E, Boscan P, Pickering AE. Reflexly evoked coactivation of cardiac vagal and sympathetic motor outflows: observations and functional implications. Clin Exp Pharmacol Physiol. 2006;33:1245–1250. doi: 10.1111/j.1440-1681.2006.04518.x. [DOI] [PubMed] [Google Scholar]
- Rial RV, Barbal F, Canellas F, Gamundi A, Akaarir M, Nicolau MC. Human Sleep Apneas and Animal Diving Reflexes: The Comparative Link. Sleep Breath. 2000;4:31–42. doi: 10.1007/s11325-000-0033-x. [DOI] [PubMed] [Google Scholar]
- Schuitema K, Holm B. The role of different facial areas in eliciting human diving bradycardia. Acta Physiol Scand. 1988;132:119–120. doi: 10.1111/j.1748-1716.1988.tb08306.x. [DOI] [PubMed] [Google Scholar]
- Shamsuzzaman A, Somers V. Microneurographic recordings of sympathetic nerve traffic in humans. In: Michael Aminoff M, Daroff R, editors. Encyclopedia of the Neurological Sciences. Vol. 3. San Diego: Academic Press; 2003. pp. 140–145. [Google Scholar]
- Smith JC, Stephens DP, Winchester PK, Williamson JW. Facial cooling-induced bradycardia: attenuating effect of central command at exercise onset. Med Sci Sports Exerc. 1997;29:320–325. doi: 10.1097/00005768-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol. 1989;67:2101–2106. doi: 10.1152/jappl.1989.67.5.2101. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Wallin BG. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol. 2004;96:1262–1269. doi: 10.1152/japplphysiol.00470.2003. [DOI] [PubMed] [Google Scholar]
- Van De Borne P, Montano N, Narkiewicz K, Degaute JP, Malliani A, Pagani M, Somers VK. Importance of ventilation in modulating interaction between sympathetic drive and cardiovascular variability. Am J Physiol Heart Circ Physiol. 2001;280:H722–H729. doi: 10.1152/ajpheart.2001.280.2.H722. [DOI] [PubMed] [Google Scholar]
- Vassalle M, Mandel WJ, Holder MS. Catecholamine stores under vagal control. Am J Physiol. 1970;218:115–123. doi: 10.1152/ajplegacy.1970.218.1.115. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Charkoudian N. Sympathetic neural control of integrated cardiovascular function: Insights from measurement of human sympathetic nerve activity. Muscle Nerve. 2007 doi: 10.1002/mus.20831. [DOI] [PubMed] [Google Scholar]
- Whayne TF, Jr, Killip T., 3rd Simulated diving in man: comparison of facial stimuli and response in arrhythmia. J Appl Physiol. 1967;22:800–807. doi: 10.1152/jappl.1967.22.4.800. [DOI] [PubMed] [Google Scholar]