Abstract
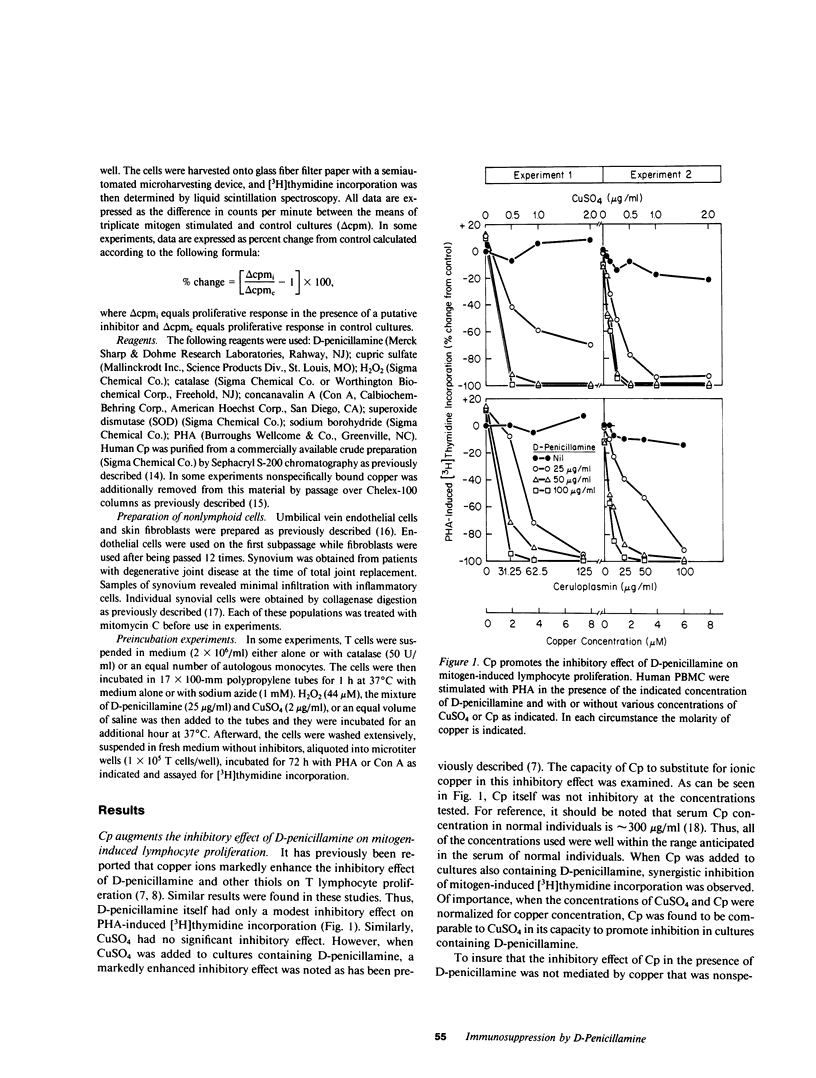
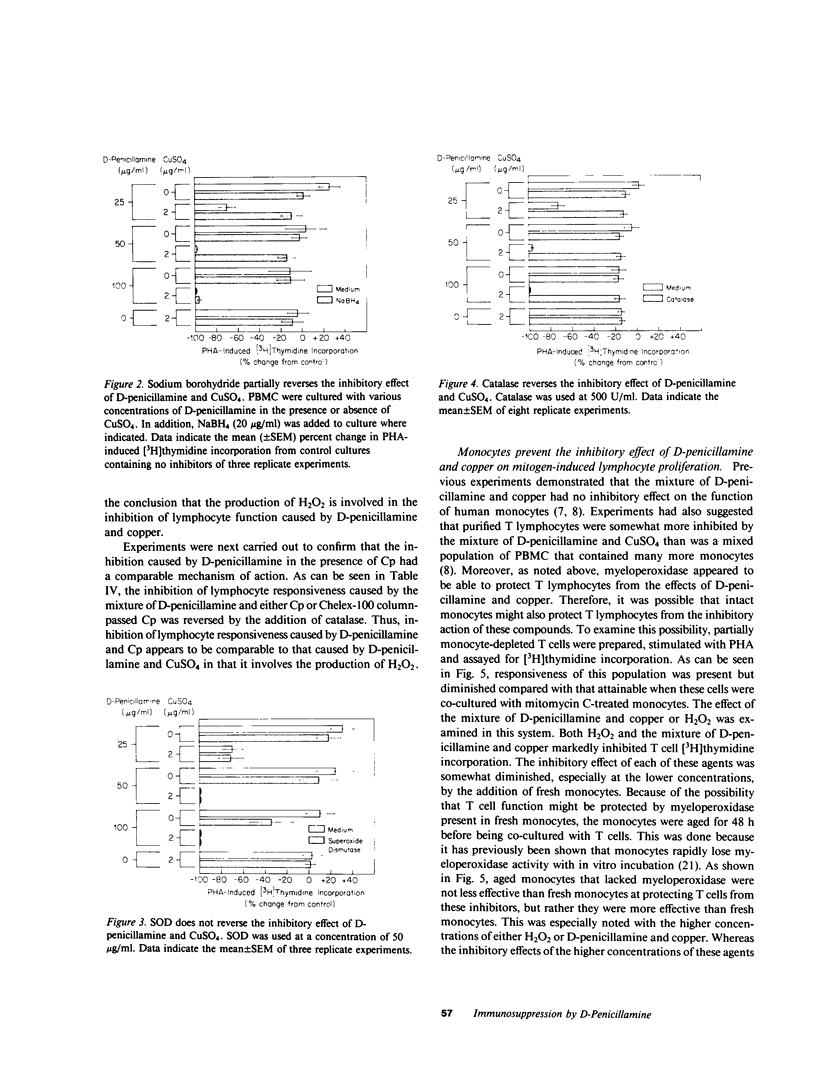
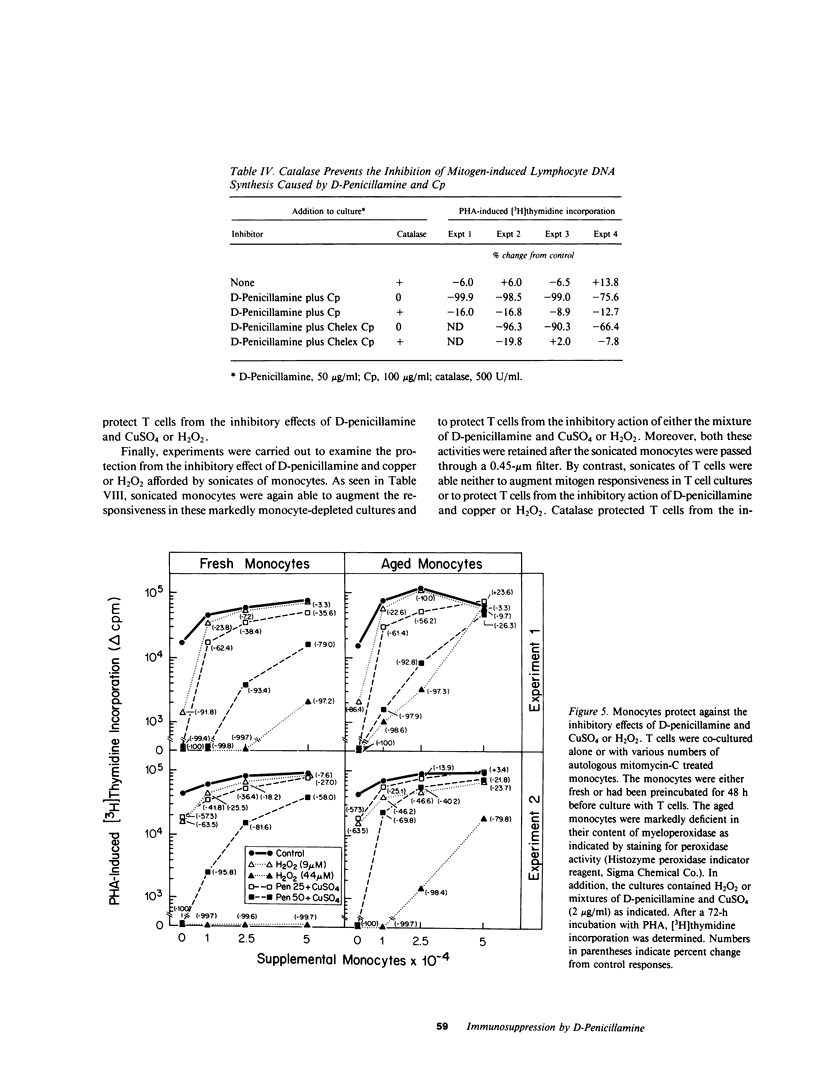
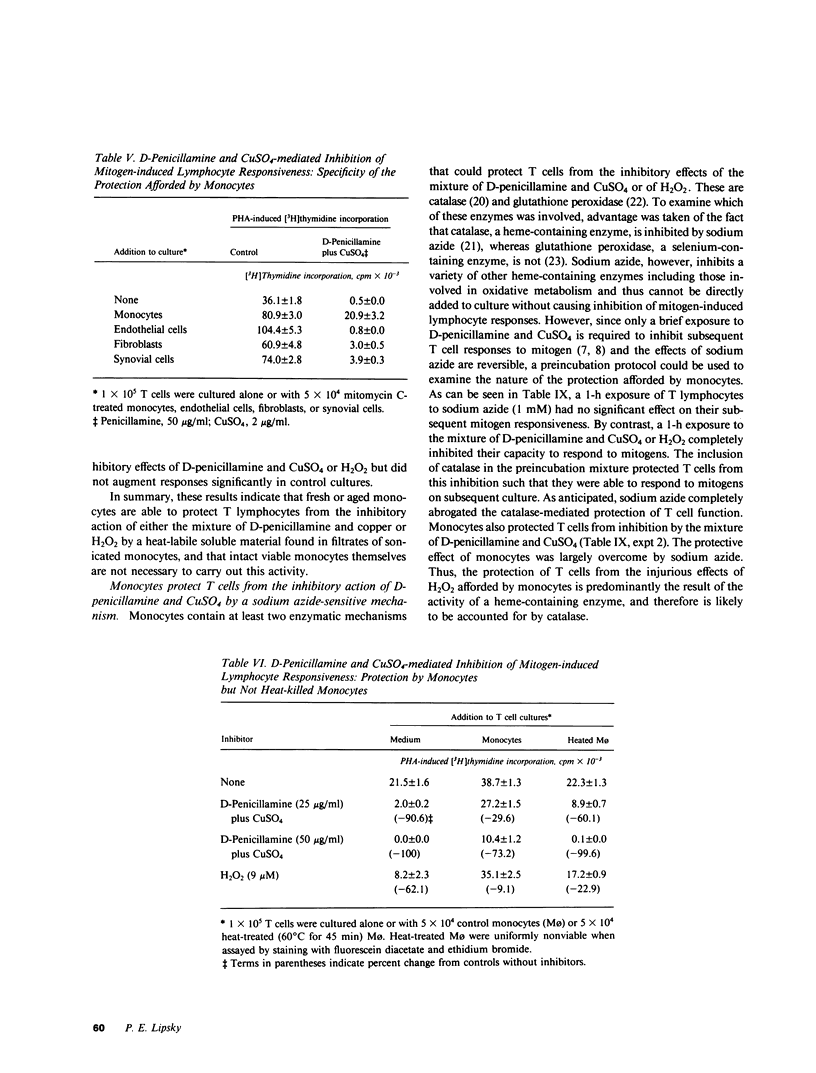
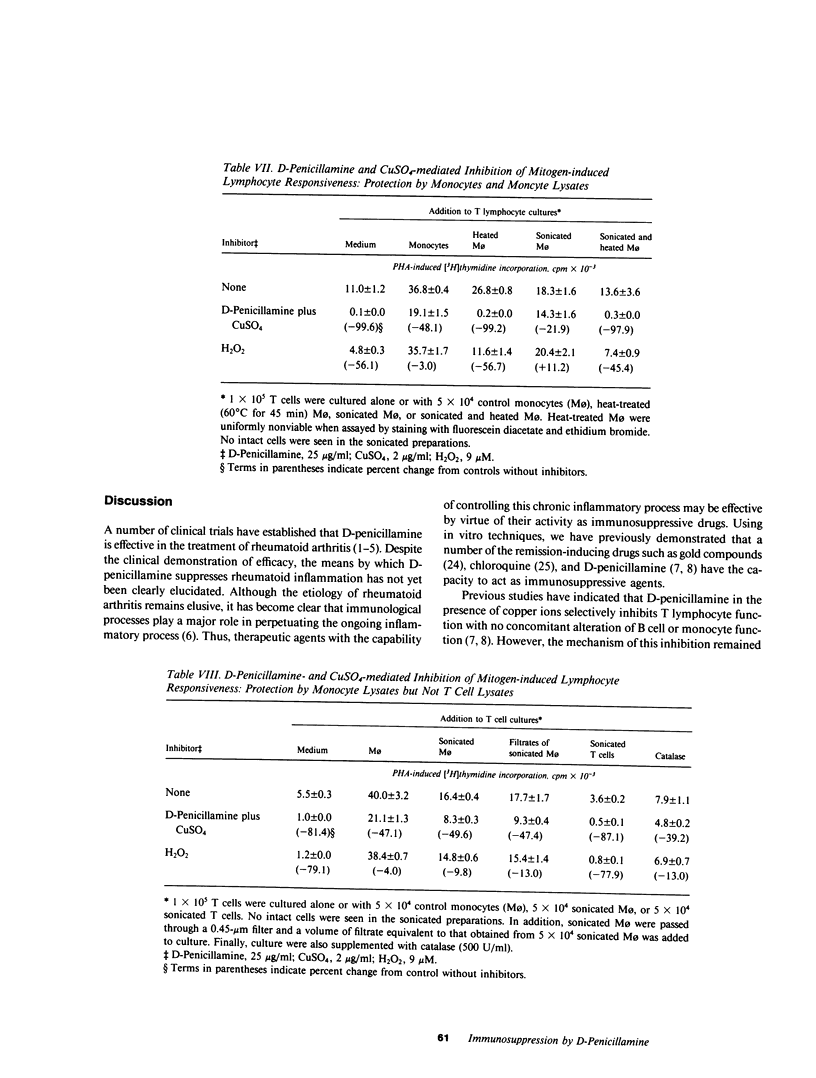
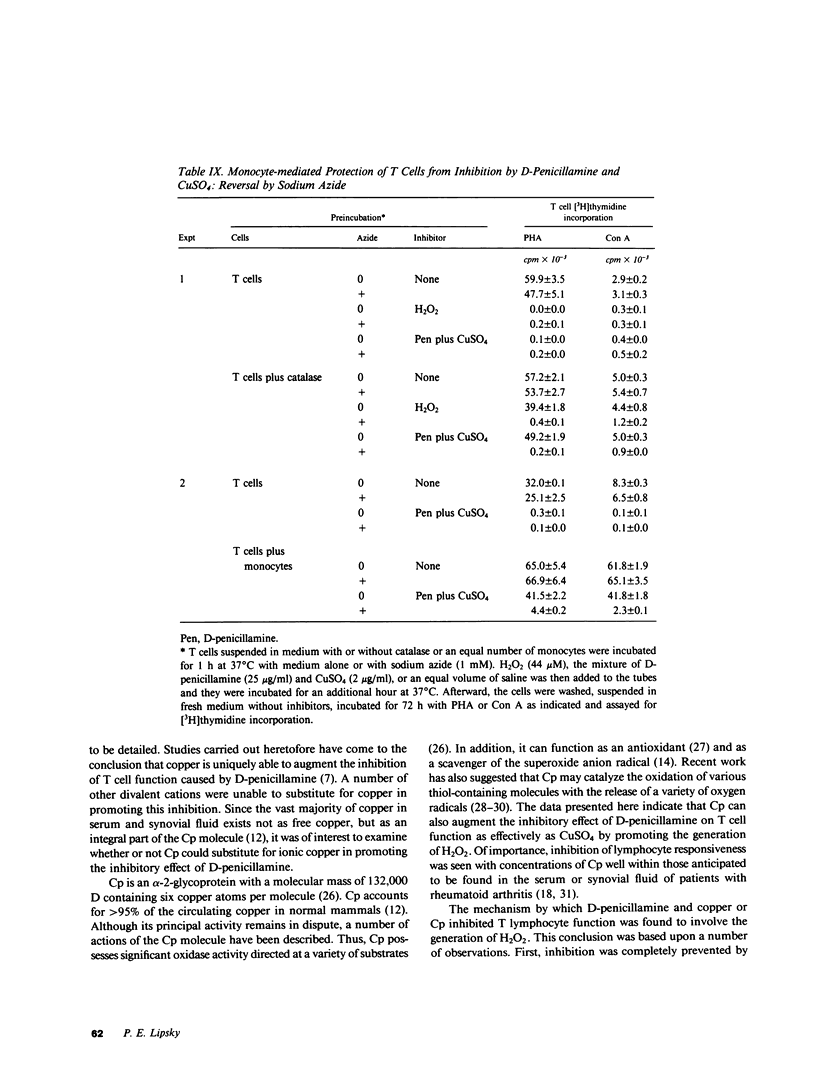
It has been suggested that D-penicillamine is active in rheumatoid arthritis because of its capacity to function as a selective inhibitor of T lymphocyte function. The basis for the immunosuppressive action of this drug as well as mechanisms whereby the effect of D-penicillamine could be modified by elements of rheumatoid synovial tissue were examined. As previously reported, D-penicillamine, in the presence of copper ions markedly inhibited mitogen-induced human T lymphocyte DNA synthesis. Since the vast majority of copper in the body exists as an integral part of the ceruloplasmin molecule, the capacity of this cuproprotein to augment D-penicillamine-mediated inhibition of T cell function was examined. The requirement for copper ions could be entirely replaced by purified ceruloplasmin, which had been depleted of nonspecifically bound copper by passage over Chelex-100 columns. The mechanism by which D-penicillamine in the presence of either copper ions or ceruloplasmin caused inhibition of T lymphocyte responsiveness was examined. Partial protection from this inhibitory effect was accomplished by sodium borohydride. While superoxide dismutase had no protective effect, catalase was found to protect lymphocyte responsiveness totally from the inhibitory action of D-penicillamine and either copper ions or ceruloplasmin. Similarly, horseradish peroxidase and myeloperoxidase also protected responsiveness from these inhibitors while boiled catalase was without effect. These results indicate that inhibition of T lymphocyte responsiveness resulted from the generation of hydrogen peroxide. Since a number of cells likely to be present at chronic inflammatory sites, such as mononuclear phagocytes, contain enzymatic mechanisms to degrade hydrogen peroxide, the modulatory influence of these cells on the inhibition of T cell function caused by D-penicillamine and copper was examined. Monocytes, whose function was not suppressed by D-penicillamine and copper, were found to protect T lymphocyte responsiveness from the inhibitory effects of either the mixture of D-penicillamine and CuSO4 or of hydrogen peroxide. By contrast, endothelial cells, fibroblasts, or cells obtained from enzyme-digested noninflamed synovium could not protect T cells from the inhibitory effects of D-penicillamine and copper. Protection of T cells was afforded by means of a heat labile, azide-sensitive soluble factor present in lysates of human monocytes. These results indicate that the mechanism whereby D-penicillamine in the presence of copper or ceruloplasmin inhibits T lymphocyte responsiveness involves the generation of hydrogen peroxide and that other neighboring cells likely to be found w
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Timimi D. J., Dormandy T. L. The inhibition of lipid autoxidation by human caeruloplasmin. Biochem J. 1977 Nov 15;168(2):283–288. doi: 10.1042/bj1680283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albergoni V., Cassini A. The oxidation of cysteine by ceruloplasmin. FEBS Lett. 1975 Jul 15;55(1):261–264. doi: 10.1016/0014-5793(75)81006-4. [DOI] [PubMed] [Google Scholar]
- Ashida E. R., Johnson A. R., Lipsky P. E. Human endothelial cell-lymphocyte interaction. Endothelial cells function as accessory cells necessary for mitogen-induced human T lymphocyte activation in vitro. J Clin Invest. 1981 May;67(5):1490–1499. doi: 10.1172/JCI110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry H., Liyanage S. P., Durance R. A., Barnes C. G., Berger L. A., Evans S. Azathioprine and penicillamine in treatment of rheumatoid arthritis: a controlled trial. Br Med J. 1976 May 1;1(6017):1052–1054. doi: 10.1136/bmj.1.6017.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchill B. R., Oliver J. M., Pearson C. B., Leinbach E. D., Berlin R. D. Microtubule dynamics and glutathione metabolism in phagocytizing human polymorphonuclear leukocytes. J Cell Biol. 1978 Feb;76(2):439–447. doi: 10.1083/jcb.76.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester G. R., Yu D. T., Irani A. M., Kunkel H. G., Winchester R. J. Ia+ T cells in synovial fluid and tissues of patients with rheumatoid arthritis. Arthritis Rheum. 1981 Nov;24(11):1370–1376. doi: 10.1002/art.1780241106. [DOI] [PubMed] [Google Scholar]
- Dixon A. J., Davies J., Dormandy T. L., Hamilton E. B., Holt P. J., Mason R. M., Thompson M., Weber J. C., Zutshi D. W. Synthetic D(-)penicillamine in rheumatoid arthritis. Double-blind controlled study of a high and low dosage regimen. Ann Rheum Dis. 1975 Oct;34(5):416–421. doi: 10.1136/ard.34.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S. L., Hunter J. S., Zgirski A., Chidambaram M. V., Frieden E. Comparison of the catalytic oxidation of cysteine and o-dianisidine by cupric ion and ceruloplasmin. J Inorg Biochem. 1982 Aug;17(1):51–60. doi: 10.1016/s0162-0134(00)80229-9. [DOI] [PubMed] [Google Scholar]
- Fisher R. I., Bostick-Bruton F. Depressed T cell proliferative responses in Hodgkin's disease: role of monocyte-mediated suppression via prostaglandins and hydrogen peroxide. J Immunol. 1982 Oct;129(4):1770–1774. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Frieden E. Caeruloplasmin: a multi-functional metalloprotein of vertebrate plasma. Ciba Found Symp. 1980;79:93–124. doi: 10.1002/9780470720622.ch6. [DOI] [PubMed] [Google Scholar]
- GUBLER C. J., LAHEY M. E., CARTWRIGHT G. E., WINTROBE M. M. Studies on copper metabolism. IX. The transportation of copper in blood. J Clin Invest. 1953 May;32(5):405–414. doi: 10.1172/JCI102752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Edelson H. S., Weissmann G. Ceruloplasmin. A scavenger of superoxide anion radicals. J Biol Chem. 1979 May 25;254(10):4040–4045. [PubMed] [Google Scholar]
- Huber C. T., Frieden E. Substrate activation and the kinetics of ferroxidase. J Biol Chem. 1970 Aug 10;245(15):3973–3978. [PubMed] [Google Scholar]
- Klebanoff S. J. The iron-H2O2-iodide cytotoxic system. J Exp Med. 1982 Oct 1;156(4):1262–1267. doi: 10.1084/jem.156.4.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E. Modulation of T lymphocyte function by copper and thiols. Agents Actions Suppl. 1981;8:85–102. [PubMed] [Google Scholar]
- Lipsky P. E., Ziff M. Inhibition of antigen- and mitogen-induced human lymphocyte proliferation by gold compounds. J Clin Invest. 1977 Mar;59(3):455–466. doi: 10.1172/JCI108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., Ziff M. Inhibition of human helper T cell function in vitro by D-penicillamine and CuSO4. J Clin Invest. 1980 May;65(5):1069–1076. doi: 10.1172/JCI109759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., Ziff M. The effect of D-penicillamine on mitogen-induced human lymphocyte proliferation: synergistic inhibition by D-penicillamine and copper salts. J Immunol. 1978 Mar;120(3):1006–1013. [PubMed] [Google Scholar]
- MARKOWITZ H., GUBLER C. J., MAHONEY J. P., CARTWRIGHT G. E., WINTROBE M. M. Studies on copper metabolism. XIV. Copper, ceruloplasmin and oxidase activity in sera of normal human subjects, pregnant women, and patients with infection, hepatolenticular degeneration and the nephrotic syndrome. J Clin Invest. 1955 Oct;34(10):1498–1508. doi: 10.1172/JCI103201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Mery C., Delrieu F., Ghozlan R., Saporta L., Simon F., Amor B., Menkes C. J., Delbarre F. Controlled trial of D-penicillamine in rheumatoid arthritis. Dose effect and the role of zinc. Scand J Rheumatol. 1976;5(4):241–247. doi: 10.3109/03009747609099913. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novogrodsky A., Ravid A., Rubin A. L., Stenzel K. H. Hydroxyl radical scavengers inhibit lymphocyte mitogenesis. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1171–1174. doi: 10.1073/pnas.79.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M., Albertini D. F., Berlin R. D. Effects of glutathione-oxidizing agents on microtubule assembly and microtubule-dependent surface properties of human neutrophils. J Cell Biol. 1976 Dec;71(3):921–932. doi: 10.1083/jcb.71.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967 Jul;70(1):158–169. [PubMed] [Google Scholar]
- Paulus H. E., Machleder H. I., Levine S., Yu D. T., MacDonald N. S. Lymphocyte involvement in rheumatoid arthritis. Studies during thoracic duct drainage. Arthritis Rheum. 1977 Jul-Aug;20(6):1249–1262. doi: 10.1002/art.1780200614. [DOI] [PubMed] [Google Scholar]
- Rogoff T. M., Lipsky P. E. Characterization of isolated guinea pig Kupffer cells: accessory cell function in mitogen-induced T lymphocyte activation. J Immunol. 1979 Nov;123(5):1920–1927. [PubMed] [Google Scholar]
- Roos D., Weening R. S., Voetman A. A., van Schaik M. L., Bot A. A., Meerhof L. J., Loos J. A. Protection of phagocytic leukocytes by endogenous glutathione: studies in a family with glutathione reductase deficiency. Blood. 1979 May;53(5):851–866. [PubMed] [Google Scholar]
- Rosenberg S. A., Lipsky P. E. Monocyte dependence of pokeweed mitogen-induced differentiation of immunoglobulin-secreting cells from human peripheral blood mononuclear cells. J Immunol. 1979 Mar;122(3):926–931. [PubMed] [Google Scholar]
- Scudder P. R., McMurray W., White A. G., Dormandy T. L. Synovial fluid copper and related variables in rheumatoid and degenerative arthritis. Ann Rheum Dis. 1978 Feb;37(1):71–72. doi: 10.1136/ard.37.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman W. E., Gindhart T. D., Blackman M. A., Dalal B., Talal N., Werb Z. Suppression of natural killing in vitro by monocytes and polymorphonuclear leukocytes: requirement for reactive metabolites of oxygen. J Clin Invest. 1982 Apr;69(4):876–888. doi: 10.1172/JCI110527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiokawa Y., Horiuchi Y., Honma M., Kageyama T., Okada T., Azuma T. Clinical evaluation of D-penicillamine by multicentric double-blind comparative study in chronic rheumatoid arthritis. Arthritis Rheum. 1977 Nov-Dec;20(8):1464–1472. doi: 10.1002/art.1780200804. [DOI] [PubMed] [Google Scholar]
- Silver R. M., Redelman D., Zvaifler N. J., Naides S. Studies of rheumatoid synovial fluid lymphocytes. I. Evidence for activated natural killer- (NK) like cells. J Immunol. 1982 Apr;128(4):1758–1763. [PubMed] [Google Scholar]
- Sorenson J. R. Copper chelates as possible active forms of the antiarthritic agents. J Med Chem. 1976 Jan;19(1):135–148. doi: 10.1021/jm00223a024. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Selenium-dependent enzymes. Annu Rev Biochem. 1980;49:93–110. doi: 10.1146/annurev.bi.49.070180.000521. [DOI] [PubMed] [Google Scholar]
- Van Boxel J. A., Paget S. A. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975 Sep 11;293(11):517–520. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- Younes M., Weser U. Superoxide dismutase activity of copper-penicillamine: possible involvement of Cu(I) stabilized sulphur radical. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1247–1253. doi: 10.1016/0006-291x(77)91427-9. [DOI] [PubMed] [Google Scholar]
- Zvaifler N. J. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16(0):265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]






