Abstract
Importance
Uveal melanoma is characterized by mutations in GNAQ and GNA11, resulting in MAPK pathway activation.
Objective
To assess the efficacy of selumetinib, a selective, non-ATP competitive inhibitor of MEK1 and MEK2, in uveal melanoma.
Design
Randomized open-label phase II clinical trial comparing selumetinib versus chemotherapy. Those receiving chemotherapy could receive selumetinib at the time of radiographic progression.
Setting
Fifteen academic oncology centers.
Participants
120 patients with metastatic uveal melanoma.
Interventions
101 patients were randomized on a 1:1 ratio to selumetinib 75 mg orally twice daily on a continual basis (n=50) or chemotherapy (temozolomide 150 mg/m2 orally daily for 5 of every 28 days or DTIC 1000 mg/m2 intravenously every 21 days; investigator choice; n=51) until disease progression, death, intolerable toxicity, or withdrawal of consent. Following primary outcome analysis, enrollment continued in a non-randomized fashion to the superior therapy.
Main Outcomes
Final analysis of progression-free survival, the primary endpoint, was assessed as of April 22, 2013. Additional endpoints, including overall survival, response rate, and safety/toxicity, were assessed as of December 31, 2013.
Results
Median progression-free survival for those randomized to chemotherapy and selumetinib was 7 (95% CI, 4.3 – 8.4; median treatment duration of 8 weeks (IQR, 4.3–16)) and 15.9 weeks (95% CI, 8.4 – 21.1; median treatment duration of 16.1 weeks (IQR, 8.1–25.3)), respectively (hazard ratio 0.46; 95% CI, 0.30 – 0.71; p < 0.001). Median overall survival was 9.1 (95% CI, 6.1 – 11.1) and 11.8 months (95% CI, 9.8 – 15.7) for those randomized to chemotherapy and selumetinib, respectively (hazard ratio 0.66; 95% CI, 0.41–1.06; p=0.09). No objective responses were observed with chemotherapy. 49% of patients treated with selumetinib achieved tumor regression, with 14% achieving an objective radiographic response to therapy. Treatment-related adverse events were observed in 97% patients treated with selumetinib, with 37% requiring at least one dose reduction.
Conclusions and Relevance
In this hypothesis-generating study of patients with advanced uveal melanoma, selumetinib compared with chemotherapy resulted in a modestly improved progression-free survival and response rate; however, no improvement in overall survival was observed. Improvement in clinical outcomes was accompanied by a high adverse event rate.
Keywords: Uveal, melanoma, MEK, GNAQ, GNA11
INTRODUCTION
Uveal melanoma arises from melanocytes within the choroid of the eye, has an incidence of 1500 cases per year in the United States, and is biologically distinct from cutaneous melanoma.1, 2 Despite enucleation or definitive radiotherapy of the primary lesion, metastases develop in 50% of patients and outcomes are subsequently poor, with a median survival of under 12 months.3–5 While improved outcomes have been achieved in patients with advanced cutaneous melanoma, no effective therapy has been identified for those with metastatic uveal melanoma.
Oncogenic mutations in GNAQ or GNA11, genes encoding for widely expressed G-protein alpha subunits, are observed in more than 80% of primary uveal melanomas and activate signaling pathways including the MAPK pathway.6–8 We and others demonstrated the genotype-dependent anti-tumor effects of inhibition of the MAPK pathway at the level of the mitogen-activated protein kinase kinase enzymes MEK1 and MEK2 in preclinical models.9–11 Furthermore, subset analysis of 20 patients with advanced uveal melanoma treated on a previously completed trial of selumetinib (AZD6244; ARRY-142886), a selective, orally-available, non-ATP competitive small molecule inhibitor of MEK1/2,12, 13 versus temozolomide demonstrated a progression-free survival with selumetinib double that achieved with chemotherapy.14
We therefore developed and conducted this National Cancer Institute (NCI) Cancer Therapy Evaluation Program (CTEP)-sponsored, multicenter, randomized phase II trial of selumetinib versus chemotherapy to formally assess the efficacy of MEK inhibition in advanced uveal melanoma.
METHODS
Study Design
This trial assessed the efficacy of selumetinib in patients with metastatic uveal melanoma who had not received prior therapy with temozolomide or DTIC. Enrollment began on August 25, 2010, with 10 patients on therapy as of the data-lock date of December 31, 3013. Tumor samples from all patients were prospectively genotyped for mutations in exon 5 of GNAQ and GNA11.
Eligible patients were randomized on a 1:1 ratio using the method of random permuted block to receive open-label treatment to chemotherapy with either temozolomide 150 mg/m2 orally daily for 5 of every 28 days or DTIC 1000 mg/m2 intravenously every 21 days (investigator choice), or selumetinib 75 mg orally twice daily on a continual basis.13 Randomization was stratified by mutation status (GNAQ mutant versus GNA11 mutant versus GNAQ and GNA11 wild-type), American Joint Committee on Cancer cutaneous melanoma staging criteria M stage (M1a/b versus M1c), and number of prior systemic therapies for metastatic disease (0 versus ≥1). Dose modification was permitted for toxicity (eTable 1). Patients received study treatment until the first occurrence of disease progression, death, intolerable toxicity, or withdrawal of consent. Clinical and laboratory assessments were conducted at baseline, every 2 weeks for 4 weeks, and every 4 weeks subsequently for up to 30 days following the off-study date or until resolution of treatment-associated toxicities. Patients treated with selumetinib at Memorial Sloan Kettering Cancer Center who had accessible tumor underwent tumor biopsies at baseline and after 14 (+/−3) days of therapy. Adverse events were graded using the NCI Common Terminology Criteria for Adverse Events, v4.0. Investigator-determined tumor response was measured radiographically every 4 weeks for 8 weeks and every 8 weeks subsequently using the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.15 Those randomized to chemotherapy who experienced disease progression could receive selumetinib subsequently if they remained eligible for study therapy.
Selumetinib was supplied by the Division of Cancer Treatment and Diagnosis (DCTD) of the NCI and was provided to the NCI under a collaborative agreement with AstraZeneca. Temozolomide and DTIC were obtained as commercially available agents.
Patients
Eligible patients had documented metastatic uveal melanoma; age >18 years; life expectancy >3 months; Eastern Cooperative Oncology Group performance status ≤1 (able to conduct normal activity or carry out work of a light nature); measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) v1.1;15 adequate organ function; and either untreated metastatic uveal melanoma or disease that progressed on prior anti-cancer therapy in the opinion of the investigator. Prior therapy with a MEK inhibitor, temozolomide or DTIC was not permitted.
Both men and women and members of all races and ethnic groups were eligible. The protocol and amendments were approved by relevant institutional review boards. All participants provided written informed consent before initiating study procedures.
Statistical Analysis
The primary endpoint was progression-free survival. Secondary endpoints included overall survival, response rate, and safety/tolerability. Patients who received at least one dose of therapy or who experienced objective disease progression during the first cycle of therapy were evaluable for the primary endpoint. Progression-free and overall survival were calculated as time from randomization to the earlier date of disease progression by RECIST 1.1 criteria or death due to any cause in the absence of progression, and death due to any cause, respectively, with distributions estimated via Kaplan-Meier methodology and compared across arms using the logrank test. Hazard ratios were estimated using a Cox proportional hazards model. Proportional hazards assumptions were assessed using a plot of the log(−log(survival) versus log(time). We tested for proportional hazard assumption by including in a Cox model a term for the interaction between treatment arm and progression-free survival time. We used SAS version 9.2 (SAS Institute Inc) and considered 2-tailed P values of less than .05 as significant. All data available on December 31, 2013 are reported.
A randomized phase II design was employed to evaluate the primary endpoint. Assuming a median progression-free survival of 1.5 months, a 24 month accrual period, and 12 a month followup period, the design had 80% power (10% significance level, one-sided) to detect a treatment difference if the true hazard ratio was 0.6. Final analysis was pre-specified to occur after ≥68 progression events were observed in patients with tumor harboring a GNAQ or GNA11 mutation. Randomization of ≥80 patients with tumor harboring GNAQ or GNA11 mutation was planned. As antitumor effects were observed in GNAQ and GNA11 wild-type uveal melanoma in preclinical models, ≤40 additional patients could be randomized regardless of mutational status.10 Following this analysis, randomization to the inferior arm was discontinued; however, accrual could continue to complete the planned 120 patient enrollment. An unplanned analysis of progression-free and overall survival that included 72 patients with a data cut-off of September 25, 2012 was performed. No correction for multiplicity across testing of the primary and secondary endpoints was performed, as the goal of this hypothesis generating study was to assess for a signal than proof of efficacy.
Correlative Analyses
Mutational analysis of exon 5 of GNAQ and GNA11 was conducted in a CLIA certified laboratory. Standard PCR amplification of a 250bp and 245bp fragment for GNAQ and GNA11, respectively, including the entire coding region of exon 5, was performed in duplicate using HotStar Taq DNA polymerase (Qiagen) and primers listed in eTable 2. PCR was also performed using standard primers with a 10–mer locked nucleic acid (LNA) oligonucleotide, designed to suppress amplification of wild-type DNA. Sequencing and analysis were performed using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) on an ABI3730 running ABI Prism DNA Sequence Analysis Software.
Western blotting was performed for pERK and cyclinD1, and quantitated by densitometry using ImageJ software. Cells were lysed in radioimmunoprecipitation assay buffer supplemented with protease inhibitor cocktail tablets (Roche Diagnostics) and 1 mmol/L Na3VO4. Equal amounts of protein were loaded on 4% to 12% PAGE gels (Invitrogen). Polyvinylidene difluoride membranes were blocked with 5% nonfat dried milk and probed with pERK, ERK, cyclin D1, and α-tubulin (Cell Signaling). Wilcoxon rank sum test was used to evaluate associations between radiographic regression (RECIST response or stable disease of >16 weeks) and suppression of pERK and cyclin D1.
RESULTS
Patient Characteristics
Between August 25, 2010 and July 23, 2013, 101 patients from 15 centers (eTable 3) were randomized, with 51 assigned to chemotherapy and 50 to selumetinib. One patient in each arm was randomized but not treated due to rapid clinical decline. Patient characteristics (Table 1) were balanced between treatment arms. The median treatment duration for those randomized to chemotherapy and selumetinib was 8 (interquartile range (IQR), 4.3–16) and 16.1 weeks (IQR, 8.1–25.3), respectively. Nineteen patients were subsequently registered and 18 treated with selumetinib without randomization in order to complete the planned 120 patient enrollment.
Table 1.
Baseline patient characteristics for all patients treated as of December 31, 2013.
| Randomized | Non-Randomized | ||
|---|---|---|---|
| TMZ/DTIC (n=51) |
Selumetinib (n=50) |
Selumetinib (n=19) |
|
| Age, Median (Range) | 62 (34–86) | 62 (32–86) | 63 (42–81) |
| Gender | |||
| Male (%) | 31 (62%) | 26 (52%) | 9 (47%) |
| Female (%) | 20 (39%) | 24 (48%) | 10 (53%) |
| Performance Status, Median (Range) | 0 (0–1) | 0 (0–1) | 0 (0–1) |
| Stage | |||
| M1a/b (%) | 3 (6%) | 2 (4%) | 0 (0%) |
| M1c (%) | 48 (94%) | 48 (96%) | 19 (100%) |
| Elevated Lactate Dehydrogenase† (%) | 30 (59%) | 25 (50%) | 14 (74%) |
| Number of Prior Systemic Therapies, Median (Range) | 0 (0–2) | 0 (0–3) | 0 (0–2) |
| Ipilimumab (%) | 11 (22%) | 8 (16%) | 4 (21%) |
| Number of Prior Liver-Directed Therapies, Median (Range) | 0 (0–2) | 0 (0–2) | 0 (0–1) |
| Radiofrequency Ablation (%) | 3 (6%) | 5 (10%) | 0 (0%) |
| Chemoembolization (%) | 5 (10%) | 4 (8%) | 0 (0%) |
| Immunoembolization (%) | 1 (2%) | 1 (2%) | 0 (0%) |
| Other (%) | 2 (4%) | 4 (8%) | 1 (5.3%) |
| Tumor Mutation | |||
| GNAQ Mutant | 19 (37%) | 20 (40%) | 6 (32%) |
| GNA11 Mutant | 25 (49%) | 21 (42%) | 8 (42%) |
| Wild-type+ | 7 (14%) | 9 (18%) | 5 (26%) |
ECOG denotes Eastern Cooperative Oncology Group
Value exceeding institutional laboratory upper limit of normal range
Wild-type indicates wild-type for Q209 mutations in GNAQ/11
Mutational testing was performed on 117 metastatic and 3 primary specimens, with 37% harboring mutations in exon 5 of GNAQ (13-Q209L, 26-209P, 4-Q209H, and 1-Q209R) and 45% harboring mutations in exon 5 of GNA11 (53-Q209L and 1-Q209P).
Clinical Activity
The primary endpoint of progression-free survival was analyzed using data available as of April 22, 2013. At this time, 98 patients were randomized and 96 evaluable for progression-free survival (Figure 1). The median progression-free survival was 7 (95% confidence interval (CI), 4.3–8.4) and 15.9 weeks (95% CI, 8.4–21.1; Figure 2a) for those randomized to and treated with chemotherapy (n=49) and selumetinib (n=47), respectively. The hazard ratio for progression-free survival was 0.46 (95% CI, 0.30–0.71; p<0.001) in favor of selumetinib. Similar improvement was observed when limiting analysis to patients with tumor harboring a GNAQ or GNA11 mutation (n=80; Figure 2b). Two patients randomized to chemotherapy were progression-free, with a median follow-up of 12.8 weeks (range, 7.9–17.9). Eight patients randomized to selumetinib were progression-free, with a median follow-up of 15.2 weeks (range, 4–80). Four- and 6-month progression-free survival rates were 43.1% and 22.9% with selumetinib, and 8.5% and 5.7% with chemotherapy. As of April 22, 2013, the median overall survival was 9.4 (95% CI, 6.0–11.4) and 10.8 months (95% CI, 7.5–12.9) for those randomized to chemotherapy and selumetinib, respectively, with a hazard ratio of 0.79 (95% CI, 0.46–1.37; p=0.40; eFigure 1).
Figure 1.
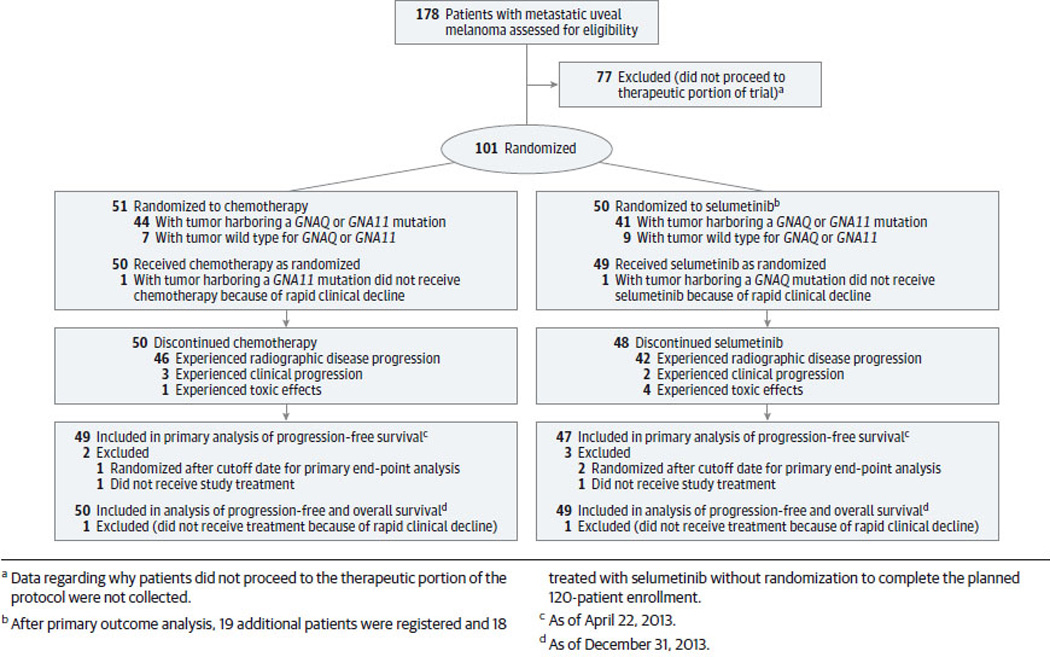
Consolidated Standard for the Reporting of Trials (CONSORT) diagram for the 98 patients randomized to either chemotherapy (n=50) or selumetinib (n=48) as of April 22, 2013. *Of the 148 patients who provided informed consent for mutational analysis of tumor, 50 did not proceed to the therapeutic portion of the protocol. Data regarding why patients did not proceed to the therapeutic portion of the protocol were not collected.
Figure 2. Progression-free Survival.
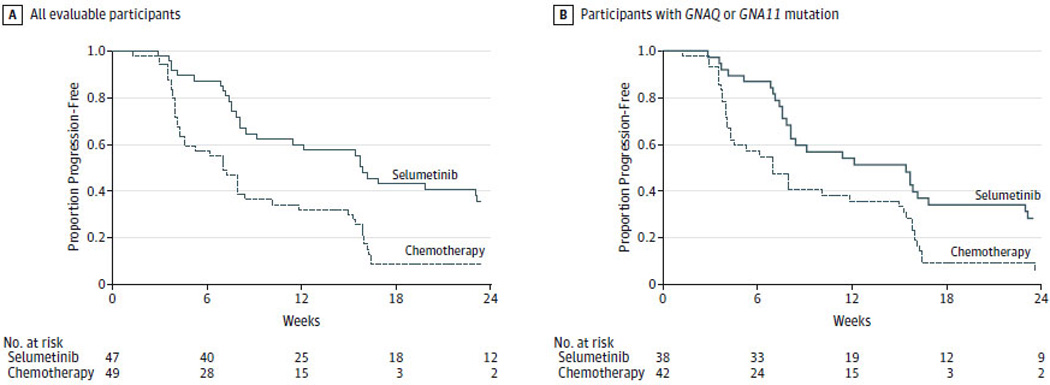
Kaplan-Meier estimates of progression-free survival in all evaluable patients (n=96; Panel A) and in patients with tumor harboring mutations in exon 5 of GNAQ or GNA11 (n=80; Panel B) treated as of as of April 22, 2013 are shown. The vertical lines indicate that patients’ data were censored. The median progression-free survival was 7 weeks (95% confidence interval (CI), 4.3–8.4) and 15.9 weeks (95% CI, 8.4–21.1) for all evaluable patients randomized to chemotherapy (n=49) and selumetinib (n=47), respectively. The hazard ratio for progression-free survival in all evaluable patients was 0.46 (95% CI, 0.30 – 0.71; p < 0.001) in favor of selumetinib (Panel A). When limiting analysis to the 80 patients with tumor harboring a mutation treated with chemotherapy (n=42) and selumetinib (n=38), the hazard ratio was 0.55 (95% CI, 0.34 – 0.87; p = 0.01) in favor of selumetinib (Panel B).
Ninety-nine patients were ultimately randomized and treated, with 98 patients off active therapy by December 31, 2013 (eFigure 2). At this time, the median progression-free survival was 7.3 (95% confidence interval (CI), 4.3–10.1) and 16 weeks (95% CI, 8.4–23) for those randomized to and treated with chemotherapy (n=50) and selumetinib (n=49), respectively (eFigure 3). The median overall survival was 9.1 (95% CI, 6.1–11.1) and 11.8 months (95% CI, 9.8–15.7) for those randomized to chemotherapy and selumetinib, respectively, with a hazard ratio of 0.66 (95% CI, 0.41–1.06; p=0.09; eFigure 4a). Ten patients randomized to chemotherapy were alive, with a median follow-up of 14.2 months (range, 8.8–23.8). Sixteen patients randomized to selumetinib were alive, with a median follow-up of 12.8 months (range, 6–35.2). No difference in overall survival was observed when limiting analysis to patients with tumor harboring a GNAQ or GNA11 mutation (n=83; eFigure 4b).
Tumor regression was uncommon with chemotherapy, with no RECIST responses observed (Figure 3a). In contrast, 49% of patients randomized to selumetinib achieved tumor regression (Figure 3b), with 7 of 49 (14%) patients evaluable for response achieving a 30% or greater tumor regression, consistent with a RECIST partial response. Five partial responses were confirmed on subsequent imaging studies, with durations of response of 23, 23.4, 25.3, 31.7 and 40.3 weeks. Two were unconfirmed, with durations of response of 7.9 and 15.7 weeks. Representative images from one patient are presented in eFigure 5.
Figure 3. Best Tumor Response for Each Patient.
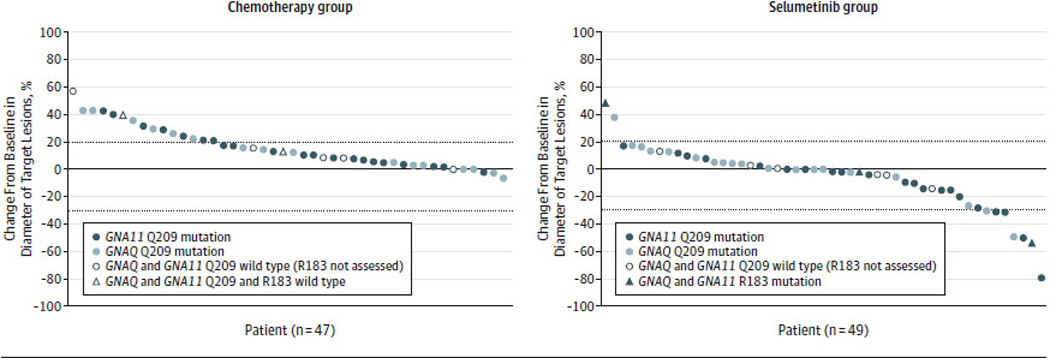
Data regarding the best tumor response observed as of December 31, 2013 are shown for the 47 patients evaluable for response in the chemotherapy group (Panel A) and the 49 patients evaluable for response in the selumetinib group (Panel B) who had undergone at least one tumor assessment after treatment before the clinical cutoff date on December 31, 2013. Each marker represents data for an individual patient. The specific markers indicate the mutational status for GNAQ and GNA11. The percentage change from baseline in the sum of the diameters of the target lesions is shown on the y-axis. Negative values indicate tumor shrinkage. The dotted horizontal lines indicate 20% tumor enlargement consistent with progression of disease by RECIST criteria and 30% tumor shrinkage consistent with a partial response by RECIST criteria. Five patients with tumors wild-type for exon 5 of GNAQ and GNA11 were tested for exon 4 mutations in GNAQ and GNA111 and are indicated by asterisks and triangles.
Forty-two (86%) patients randomized to chemotherapy experienced disease progression and subsequently received selumetinib. The median progression-free survival was 8 weeks (95% CI, 8–12; eFigure 6). Although tumor regression was observed in 11 of 40 (28%) patients evaluable for response, no objective RECIST response was observed (eFigure 7).
Nineteen patients were registered to receive selumetinib without randomization. Eighteen were treated and 17 evaluable for progression-free survival. With 9 progression events and a median follow-up for those without progression of 12 weeks, the median progression-free survival was 16 weeks (95% CI, 6–not reached; eFigure 8). Of 17 patients evaluable for response, 1 (6%) achieved a partial response.
Tolerability
Select adverse events observed in >5% of patients attributed to temozolomide, DTIC and selumetinib are presented in Table 2. All observed adverse events observed attributed to therapy and regardless of attribution are presented in eTables 4–7.
Table 2.
Select adverse events observed in >5% of patients adjudicated as possibly, probably or definitely related to therapy. Data is presented on all patients treated as of December 31, 2013.
| Randomized | Non-Randomized | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Temozolomide/DTIC* (n = 50) |
Selumetinib*^ (n = 49) |
Selumetinib* (n = 18) |
|||||||
| Gr 1/2 | Gr 3 | Gr 4 | Gr 1/2 | Gr 3 | Gr 4 | Gr 1/2 | Gr 3 | Gr 4 | |
| Hematologic Adverse Events | |||||||||
| Anemia | 8 (16%) | - | - | 15 (31%) | - | - | 5 (28%) | 1 (6%) | - |
| Leukopenia | 9 (18%) | - | - | 6 (12%) | - | - | 6 (33%) | - | - |
| Lymphopenia | 4 (8%) | 1 (2%) | - | 1 (2%) | 3 (6%) | - | 2 (11%) | 2 (11%) | - |
| Neutropenia | 4 (8%) | 1 (2%) | - | 3 (6%) | - | - | 3 (17%) | - | - |
| Thrombocytopenia | 8 (16%) | - | - | 5 (10%) | - | - | 5 (28%) | - | - |
| Non-hematologic Adverse Events | |||||||||
| Alanine Amino Transferase Elevation | 4 (8%) | - | - | 17 (35%) | 4 (8%) | - | 7 (39%) | - | - |
| Alopecia | - | - | - | 6 (12%) | - | - | 1 (6%) | - | - |
| Anorexia | 7 (14%) | - | - | 4 (8%) | - | - | 1 (6%) | - | - |
| Arthralgias | - | - | - | 5 (10%) | - | - | - | - | - |
| Aspartate Amino Transferase Elevation | 6 (12%) | - | - | 20 (41%) | 5 (10%) | - | 7 (39%) | - | - |
| Blurred Vision | - | - | - | 3 (6%) | - | - | 1 (6%) | - | - |
| Constipation | 15 (30%) | - | - | 3 (6%) | - | - | 1 (6%) | - | - |
| CPK Elevation | - | - | - | 18 (37%) | 8 (16%) | - | 13 (72%) | 1 (6%) | - |
| Diarrhea | 4 (8%) | - | - | 20 (41%) | - | - | 8 (44%) | - | - |
| Dyspnea | - | - | - | 7 (14%) | 1 (2%) | - | 2 (11%) | - | - |
| Edema Face | - | - | - | 6 (12%) | - | - | 3 (17%) | - | - |
| Edema Limbs | 1 (2%) | - | - | 14 (29%) | 1 (2%) | - | 10 (56%) | - | - |
| Eye Disorder | - | - | - | 4 (8%) | - | - | 1 (6%) | - | - |
| Fatigue | 22 (44%) | - | - | 30 (61%) | - | - | 8 (44%) | - | - |
| Mucositis | 1 (2%) | - | - | 6 (12%) | - | - | - | - | - |
| Nausea | 20 (40%) | - | - | 18 (37%) | - | - | 7 (39%) | - | - |
| Pruritis | - | - | - | 8 (16%) | - | - | 1 (6%) | - | - |
| Rash Acneiform | 3 (6%) | - | - | 37 (76%) | 1 (2%) | - | 12 (67%) | - | - |
| Vomiting | 12 (24%) | - | - | 11 (22%) | - | - | 3 (17%) | - | - |
Adverse events observed in patients initially randomized to selumetinib only; does not include events observed in those receiving selumetinib after experiencing disease progression with chemotherapy.
One patient in each of the randomized treatment arms and one patient in the non-randomized portion of this trial did not receive study therapy and are not included.
Treatment-related adverse events (any grade) were observed in 65 of 67 (97%) patients treated with selumetinib, with the most common being acneiform rash (75%), CPK elevation (60%), fatigue (57%), AST elevation (48%), and ALT elevation (42%). Blurred vision (6%) and other visual changes (7%) were observed. Twenty-five (37%) experienced grade 3–4 treatment-related adverse events, including CPK elevation (13%), AST elevation (7%), and ALT elevation (6%). Most cases of CPK elevation were asymptomatic and clinically insignificant; however, neck myopathy or myositis, was observed in 3 patients (4%).16 Twenty-five (37%) patients required at least one dose reduction of selumetinib due to adverse events (eTable 1). Four (6%) patients discontinued therapy due to treatment-related adverse events.
One (2%) patient treated with chemotherapy required dose reduction due to toxicity. Eight (16%) patients initially randomized to chemotherapy were unable to receive selumetinib after disease progression due to death or declining performance status.
Pharmacodynamic Analysis
We observed sustained MAPK pathway inhibition in available tumor specimens, with median decreases in pERK and cyclinD1 of 48% (p=0.03) and 76% (p=0.03; sign test), respectively (eTable 8). Of the 18 patients for whom specimens were available, 5 achieved radiographic regression, with 2 achieving RECIST partial responses. Six achieved clinical benefit as defined as a RECIST response or stable disease of >16 weeks. Radiographic regression correlated with suppression of pERK (p=0.03), but not cyclinD1 (p=0.97; Wilcoxon rank sum test). No statistically significant association between pERK suppression and clinical benefit was observed (p=.07).
DISCUSSION
In this hypothesis-generating phase II clinical trial, selumetinib compared with chemotherapy resulted in improved progression-free survival in patients with uveal melanoma not previously treated with temozolomide or DTIC. The median progression-free survival was improved from 7 to 15.9 weeks, in favor of selumetinib. The 4-month progression-free survival rate was improved from 8.5% with chemotherapy to 43.1% with selumetinib. Tumor regression was more common with selumetinib, occurring in 50% of patients treated, with only 2 patients experiencing greater than 20% tumor growth (consistent with RECIST disease progression) at the time of the first scan. No effect upon overall survival was observed.
Our results are similar to those of an unplanned subset analysis of patients with advanced uveal melanoma treated on a prior trial of selumetinib.14 This analysis demonstrated a median progression-free survival of 16.3 weeks (80% CI, 10–28.8 weeks) in 7 patients randomized to selumetinib versus 7.1 weeks (80% CI, 6.1–11.8 weeks) in 13 patients randomized to temozolomide. The limited activity observed with temozolomide is similar to that achieved in a phase II study of temozolomide by Bedikian et. al. which reported a median time-to-progression of 1.84 months (range, 0.7–3.8)17 and that observed in our study which demonstrated a progression-free survival of 7 weeks with chemotherapy.
The efficacy of MEK inhibition in uveal melanoma is predicted by the frequent activation of the MAPK pathway by functionally activating mutations in GNAQ or GNA11. This strategy may be applicable to other tumors characterized by MAPK pathway activation via additional mechanisms including receptor tyrosine kinase activation,18 RAS mutation,19 NF1 loss,20 and others. Single agent activity has been observed with MEK162, another small molecule inhibitor of MEK, in NRAS-mutant melanoma.21 Selumetinib has been demonstrated to enhance iodine update and retention in radioiodine refractory thyroid cancer, with the most prominent activity observed in NRAS-mutant tumors.22 Additionally, phase II trials comparing the efficacy of chemotherapy alone or in combination with selumetinib have demonstrated improved outcomes with combination therapy in KRAS-mutant non-small cell lung cancer and BRAF-mutant melanoma, with hazard ratios for progression of 0.58 (p<0.001) and 0.63 (p=0.021), respectively.23, 24
Our data suggest that progression-free survival is greater in the GNAQ and GNA11 wild-type population (25.9 [range, 3.7–40.4] vs 15.4 weeks [range, 8.1–16.9]). This observation may be explained by the presence of other mechanisms of MAPK pathway activation in exon 5 GNAQ or GNA11 wild-type tumors. After study initiation, activating mutations affecting exon 4 of GNAQ and GNA11 were reported to occur in 12% of metastatic uveal melanomas in a pattern mutually exclusive with exon 5 mutations.8 Thus, it is likely that the majority of the patients classified as wild-type in this study had tumors harboring exon 4 GNAQ or GNA11 mutations. Of the five exon 5 wild-type cases with sufficient remaining tumor material, we identified exon 4 mutations in three, one of whom achieved a major response to selumetinib.
As of December 31, 2013, we observed a median overall survival of 9.1 and 11.8 months for those randomized to chemotherapy and selumetinib, respectively, which did not reach statistical significance. As 86% of patients received selumetinib after experiencing disease progression to chemotherapy, which was permitted to maximize accrual from this rare cancer population, analysis of survival data is confounded. We observed that the efficacy of selumetinib may be affected by prior therapy with temozolomide or DTIC. The median progression-free survival and response rate was 15.9 weeks and 14%, respectively, for those initially randomized to selumetinib, and 8 weeks and 0%, respectively, for those receiving selumetinib after experiencing progression to chemotherapy. While our study was not designed to assess the effects of prior therapy upon clinical outcome with selumetinib, these observations may reflect the induction of survival pathways or enhancement of angiogenesis with chemotherapy that confer resistance to MEK inhibition, or the more advanced disease in patients who have received prior therapies.25
Treatment-related adverse events were observed in 97% patients treated with selumetinib and were consistent with those observed with other inhibitors of MEK, including rash, CPK elevation, edema, and visual changes.21, 26 While most events were manageable with supportive measures, 37% required at least one dose reduction and 6% discontinued therapy due to toxicity.
We previously observed effective MAPK pathway inhibition as demonstrated by pERK suppression with selumetinib in uveal melanoma cell lines.10 Using human tumor specimens, we similarly demonstrated effective and sustained pathway blockade after 14 days of therapy, with inhibition of both pERK and cyclinD1. Furthermore, radiographic regression correlated with the degree of pERK suppression, suggesting that optimal antitumor effects require more complete pathway inhibition.
Limitations of this study include the unblinded trial design and lack of central review of imaging studies. Additionally, this trial was designed before activating mutations in exon 4 of GNAQ and GNA11 were reported, thus prospective assessment for these alterations was not performed.
CONCLUSIONS
In this hypothesis-generating study of patients with advanced uveal melanoma, selumetinib compared with chemotherapy resulted in a modestly improved progression-free survival and rate of response; however, no improvement in overall survival was observed. Improvement in clinical outcomes was accompanied by a high adverse event rate.
Acknowlegements
This study was sponsored by the Cancer Therapy Evaluation Program, which worked jointly with the academic co-authors under a confidentiality agreement to participate in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the review and approval of the manuscript; and the decision to submit the manuscript for publication. All drafts of the manuscript were written and prepared by the authors. The authors vouch for the accuracy and completeness of the reported data and for the fidelity of this report to the protocol. We gratefully acknowledge the support of the Division of Cancer Treatment and Diagnosis at the National Cancer Institute which provided selumetinib for this clinical trial. We acknowledge the assistance of Daniel J. Paucar, BS, Sloane Smith, MPH and Anne Fusco, MPA, all from Memorial Sloan Kettering Cancer Center for their contributions to this study, which included data acquisition, data analysis, and administrative support. No compensation was provided to Mr. Paucar, Ms. Smith or Ms. Fusco for this work. Richard D. Carvajal, MD of Memorial Sloan Kettering Cancer Center had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Katherine S. Panageas, DrPH of Memorial Sloan Kettering Cancer Center conducted independent statistical analysis of the study data. No compensation was provided to Drs. Carvajal or Panageas for this work. Financial support for this trial was provided by the National Cancer Institute, the Conquer Cancer Foundation, Cycle for Survival, and the Fund for Ophthalmic Knowledge.
Footnotes
Presented in part at the Fall 2011 CTEP Early Drug Development Meeting, October 3–4, 2011, Bethesda, MD; the 15th Biennial Meeting of the International Society of Ocular Oncology, November 14–17, 2011, Buenos Aires, Argentina; Macula 2012, January 20–21, 2012, New York, NY; the 4th Annual Scientific Retreat of the Melanoma Research Alliance, March 1–2, Washington, DC; the 48th Annual Meeting of the American Society of Clinical Oncology, June 1–5, 2012 Chicago, IL; the 2012 Beijing International Melanoma Congress, November 2–4, 2012, Beijing, China; and, the 49th Annual Meeting of the American Society of Clinical Oncology, May 31–June 4, 2013 Chicago, IL.
Trial Registration: ClinicalTrials.gov number NCT01143402
REFERENCES
- 1.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998 Oct 15;83(8):1664–1678. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Singh AD, Topham A. Incidence of uveal melanoma in the United States: 1973–1997. Ophthalmology. 2003 May;110(5):956–961. doi: 10.1016/S0161-6420(03)00078-2. [DOI] [PubMed] [Google Scholar]
- 3.Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch Ophthalmol. 2001 May;119(5):670–676. doi: 10.1001/archopht.119.5.670. [DOI] [PubMed] [Google Scholar]
- 4.Rietschel P, Panageas KS, Hanlon C, Patel A, Abramson DH, Chapman PB. Variates of survival in metastatic uveal melanoma. J Clin Oncol. 2005 Nov 1;23(31):8076–8080. doi: 10.1200/JCO.2005.02.6534. [DOI] [PubMed] [Google Scholar]
- 5.Diener-West M, Reynolds SM, Agugliaro DJ, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005 Dec;123(12):1639–1643. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- 6.Onken MD, Worley LA, Long MD, et al. Oncogenic mutations in GNAQ occur early in uveal melanoma. Invest Ophthalmol Vis Sci. 2008 Dec;49(12):5230–5234. doi: 10.1167/iovs.08-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009 Jan 29;457(7229):599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010 Dec 2;363(23):2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambrosini G, Musi E, Ho AL, de Stanchina E, Schwartz GK. Inhibition of mutant GNAQ signaling in uveal melanoma induces AMPK-dependent autophagic cell death. Molecular cancer therapeutics. 2013 May;12(5):768–776. doi: 10.1158/1535-7163.MCT-12-1020. [DOI] [PubMed] [Google Scholar]
- 10.Ambrosini G, Pratilas CA, Qin LX, et al. Identification of unique MEK-dependent genes in GNAQ mutant uveal melanoma involved in cell growth, tumor cell invasion, and MEK resistance. Clin Cancer Res. 2012 Jul 1;18(13):3552–3561. doi: 10.1158/1078-0432.CCR-11-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khalili JS, Yu X, Wang J, et al. Combination small molecule MEK and PI3K inhibition enhances uveal melanoma cell death in a mutant GNAQ- and GNA11-dependent manner. Clin Cancer Res. 2012 Aug 15;18(16):4345–4355. doi: 10.1158/1078-0432.CCR-11-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh TC, Marsh V, Bernat BA, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007 Mar 1;13(5):1576–1583. doi: 10.1158/1078-0432.CCR-06-1150. [DOI] [PubMed] [Google Scholar]
- 13.Banerji U, Camidge DR, Verheul HM, et al. The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): a phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res. 2010 Mar 1;16(5):1613–1623. doi: 10.1158/1078-0432.CCR-09-2483. [DOI] [PubMed] [Google Scholar]
- 14.Patel M, Smyth E, Chapman PB, et al. Therapeutic implications of the emerging molecular biology of uveal melanoma. Clin Cancer Res. 2011 Apr 15;17(8):2087–2100. doi: 10.1158/1078-0432.CCR-10-3169. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) European journal of cancer. 2009 Jan;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Schwartz GK, DeAngelis LM, Kaley T, Carvajal RD. Dropped head syndrome: report of three cases during treatment with a MEK inhibitor. Neurology. 2012 Oct 30;79(18):1929–1931. doi: 10.1212/WNL.0b013e318271f87e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedikian AY, Papadopoulos N, Plager C, Eton O, Ring S. Phase II evaluation of temozolomide in metastatic choroidal melanoma. Melanoma Res. 2003 Jun;13(3):303–306. doi: 10.1097/00008390-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Huang MH, Lee JH, Chang YJ, et al. MEK inhibitors reverse resistance in epidermal growth factor receptor mutation lung cancer cells with acquired resistance to gefitinib. Molecular oncology. 2013 Feb;7(1):112–120. doi: 10.1016/j.molonc.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatzivassiliou G, Haling JR, Chen H, et al. Mechanism of MEK inhibition determines efficacy in mutant KRAS- versus BRAF-driven cancers. Nature. 2013 Sep 12;501(7466):232–236. doi: 10.1038/nature12441. [DOI] [PubMed] [Google Scholar]
- 20.Nissan MH, Pratilas CA, Jones AM, et al. Loss of NF1 in Cutaneous Melanoma Is Associated with RAS Activation and MEK Dependence. Cancer research. 2014 Apr 15;74(8):2340–2350. doi: 10.1158/0008-5472.CAN-13-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. The lancet oncology. 2013 Mar;14(3):249–256. doi: 10.1016/S1470-2045(13)70024-X. [DOI] [PubMed] [Google Scholar]
- 22.Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. The New England journal of medicine. 2013 Feb 14;368(7):623–632. doi: 10.1056/NEJMoa1209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. The lancet oncology. 2013 Jan;14(1):38–47. doi: 10.1016/S1470-2045(12)70489-8. [DOI] [PubMed] [Google Scholar]
- 24.Robert C, Dummer R, Gutzmer R, et al. Selumetinib plus dacarbazine versus placebo plus dacarbazine as first-line treatment for BRAF-mutant metastatic melanoma: a phase 2 double-blind randomised study. The lancet oncology. 2013 Jul;14(8):733–740. doi: 10.1016/S1470-2045(13)70237-7. [DOI] [PubMed] [Google Scholar]
- 25.Lev DC, Ruiz M, Mills L, McGary EC, Price JE, Bar-Eli M. Dacarbazine causes transcriptional up-regulation of interleukin 8 and vascular endothelial growth factor in melanoma cells: a possible escape mechanism from chemotherapy. Molecular cancer therapeutics. 2003 Aug;2(8):753–763. [PubMed] [Google Scholar]
- 26.Infante JR, Papadopoulos KP, Bendell JC, et al. A phase 1b study of trametinib, an oral Mitogen-activated protein kinase kinase (MEK) inhibitor, in combination with gemcitabine in advanced solid tumours. European journal of cancer. 2013 Jun;49(9):2077–2085. doi: 10.1016/j.ejca.2013.03.020. [DOI] [PubMed] [Google Scholar]


