Abstract
Visual short-term memory (VSTM) is a capacity-limited system for maintaining visual information across brief durations. Limits in the amount of information held in memory reflect processing constraints in the intraparietal sulcus (IPS), a region of the fronto-parietal network also involved in visual attention. During VSTM and visual attention, areas of IPS demonstrate hemispheric asymmetries. While the left hemisphere represents information in only the right-hemifield, the right hemisphere represents information across the visual field. In visual attention, hemispheric asymmetries are associated with differences in behavioral performance across the visual field. In order to assess the degree of hemifield asymmetries in VSTM, we measured memory performance across the visual field for both single- and two- feature objects. Consistent with theories of right hemisphere dominance, there was a memory benefit for single-feature items in the left visual hemifield. However, when the number of features increased, the behavioral bias reversed, demonstrating a benefit for remembering two-feature objects in the right-hemifield. On an individual basis, the cost of remembering an additional feature in the hemifields was correlated, suggesting that the shift in hemifield biases reflected a redistribution of resources across the visual field. Furthermore, we demonstrate that these results cannot be explained by differences in perceptual or decision making load. Our results are consistent with a flexible resource model of VSTM in which attention and/or working memory demands result in representation of items in the right hemifield by both the left and right hemispheres.
Introduction
In dynamically changing environments, it is important to represent information for brief durations in the presence of distraction and incoming sensory stimulation. In the visual system, visual short-term memory (VSTM) serves as a short-term buffer, allowing the system to maintain information over several seconds (Phillips, 1974). However, the amount of information held in VSTM is severely limited, reflecting constraints in the processing capabilities of underlying brain networks (Phillips, 1974; Cowan, 2001).
The amount of information stored in memory is directly related to limits in neural resources in brain activity underlying VSTM (Todd and Marois, 2004; Vogel and Machizawa, 2004; Xu and Chun, 2006). A presumptive neural marker of VSTM, the contralateral delay activity (CDA), assumes that brain activity in each hemisphere represents memory items in the contralateral visual field (e.g., right hemisphere represents the left visual field) (Vogel and Machizawa, 2004; Vogel et al., 2005) and is argued to reflect the number of items held during VSTM, with activity increasing with the number of items presented and plateauing when memory resources are fully distributed (Vogel and Machizawa, 2004; Vogel et al., 2005).
Studies using functional magnetic resonance imaging (fMRI) have demonstrated that activity within the intraparietal sulcus (IPS) is central to VSTM (Todd and Marois, 2004; Xu and Chun, 2006). Similar to the CDA, thought to be elicited by sources in posterior parietal and lateral occipital cortex, activity in IPS strongly corresponds to the amount of information held in memory. Areas of IPS involved in VSTM overlap fronto-parietal networks (Sheremata et al., 2010), known to be involved in perception and attention (Corbetta and Shulman, 2002). However, contrary to the simple contralateral nature of CDA, recent fMRI studies demonstrate hemispheric asymmetries in regions of IPS. Specifically, activity in right IPS reflects VSTM items presented across the entire visual field, resulting in a decreased contralateral bias in the right as compared to the left hemisphere (Sheremata et al., 2010).
Consistent with anatomical overlap of VSTM and attention, these apparent hemispheric asymmetries in IPS observed during VSTM mirror asymmetries observed during visual attention. Behavioral performance biases across the visual field on visuo-spatial attentional tasks correlate with anatomical and functional measures of hemispheric asymmetries. For example, greater white matter volume in right than left hemisphere has been associated with an attentional bias to the left visual field (Thiebaut de Schotten et al., 2011), supporting right hemisphere dominance theories of visual attention (Mesulam, 1981). Right hemisphere dominance theories have been associated with biases toward the left visual field, a phenomenon known as pseudoneglect (Bowers and Heilman, 1980). Consistent with right hemisphere dominance for visual attention, a recent VSTM study documented a similar behavioral benefit in the left-hemifield during an orientation change detection task (Gamble and Somers, 2012).
In contrast, behavioral benefits for items presented in the right visual field have been argued to reflect the right hemisphere representing the right, or ipsilateral, visual field to a greater extent than the left hemisphere can represent the left visual field. Behavioral bias toward the right visual field is greater in participants with stronger hemispheric asymmetries across the fronto-parietal attention network (Szczepanski and Kastner, 2013). Evidence therefore suggests that hemispheric asymmetries in fronto-parietal networks can result in both left- and right-hemifield behavioral biases.
Hemispheric asymmetries observed in VSTM using fMRI, as well as mirrored asymmetries observed in attentional selection, set up directly testable predictions as to how VSTM performance should vary across the visual field. We hypothesized that if, as has been assumed, each hemisphere directs resources toward the contralateral visual field, there should be no difference in performance for items presented in the left and right visual field regardless of memory load. In contrast, if VSTM demonstrates right hemisphere dominance, there should be a benefit for items presented in the left, or contralateral, visual hemifield. However, if hemispheric asymmetries result in representation of the right visual field by both the left and right hemispheres, then dual representation should result in behavioral benefits of items for the right, as compared to the left, hemifield. Finally, behavioral (Wilson et al., 2012) and neural (Xu and Chun, 2006; Luria et al., 2010; Luria and Vogel, 2011) measures of VSTM have documented differences in memory for single- and multi-feature VSTM, suggesting additional resources are necessary for remembering multi-feature items. As asymmetries have been shown to be memory load-dependent (Sheremata et al., 2010), behavioral hemifield asymmetries may vary for single- and multi-feature items.
In order to investigate the effect of hemispheric asymmetries on VSTM performance across the visual field, we measured VSTM performance in two sets of behavioral experiments using single- and two- feature objects. In the first experiment, results showed set-size dependent memory enhancement for single-feature objects in the left hemifield, consistent with a right hemisphere benefit for VSTM (Gamble and Somers, 2012). In addition, increasing the number of object features caused a change in the behavioral bias, with a benefit for items presented in the right visual hemifield. In a second set of experiments, each individual trial was cued to rule out the possibility that memory load dependent hemifield asymmetries were caused by perceptual or encoding strategies (Experiment 2a) or decision-making factors (Experiment 2b). We found that increasing the number of features biased performance across the visual field when participants were given a cue before the memory trial, but not after the memory probe, suggesting that hemifield asymmetries result from memory-related demands and not decision-making processes. Together these experiments demonstrate that hemifield asymmetries are evident in VSTM and emerge in a resource-dependent manner. Mechanisms that could potentially give rise to such hemifield asymmetries are proposed and discussed.
Experiment 1
Method
Participants
Twenty-four participants (12 female), naïve to the purpose of the study, completed the experiment for course credit. Participants ranged in age from 19 to 32 years, had normal or corrected-to-normal vision, and gave informed consent approved by the George Washington Institutional Review Board. Four participants were excluded due to poor performance (failure to remember at least 2 memory items in any condition) or incomplete data collection.
Visual Stimuli and experimental procedure
Experiments were run on a desktop PC using the Psychopy software package (Peirce, 2009). Stimuli were presented on a Dell monitor (1280×1024 pixels, 75 Hertz refresh rate) at a viewing distance of approximately 62 cm.
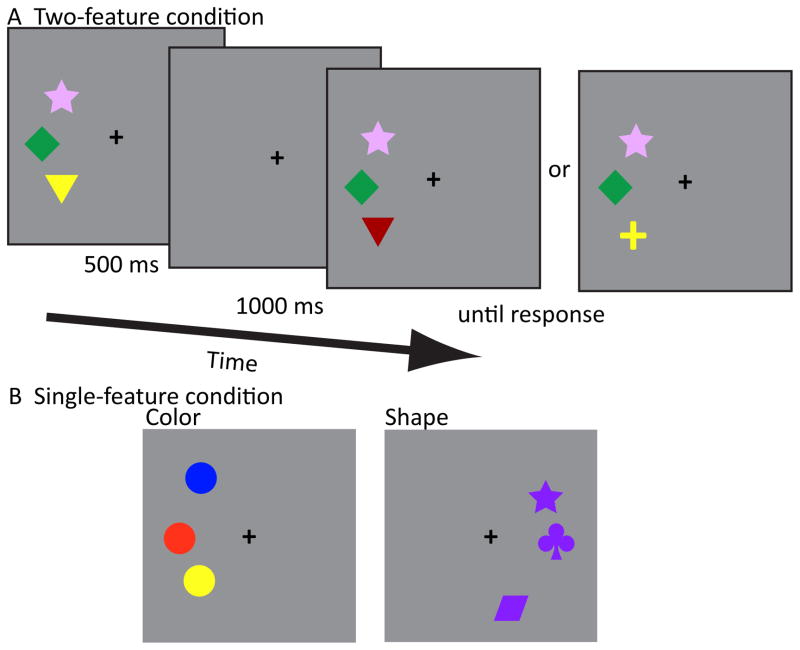
Participants performed a change detection task in which colored shapes were presented against a mean gray luminance background. Maximally discriminable, common colors (dark blue, orange red, green, yellow, purple, plum, and maroon) and shapes (rhombus, cross, triangle, diamond, circle, club, and star) were pseudo-randomly chosen without repeat (Figure 1).
Figure 1.
Stimuli and VSTM trial structure. (A) In the two-feature condition participants encoded a set of briefly presented items and determined whether there was a change. In half of the trials, a change in color or shape could occur. (B) Sample stimuli used in the single-feature condition. Note that irrelevant feature dimension was fixed (e.g., shape was identical for all color items, while color was identical for all shape items).
Stimuli subtended 1.2° visual angle along the longest dimension and were presented on a perimeter of an imaginary circle with a radius of 5° from fixation, rendering all items equidistant from the fixation point. During each block, a single set size was chosen depending upon the feature condition (see below). In half of the blocks, items were presented left of fixation, and in the other half of the blocks items were presented right of fixation, with hemifield order counterbalanced across participants in both the single- and two- feature conditions. Participants were instructed to maintain fixation, and before each trial the fixation cross blinked off and then on to redirect participants gaze toward fixation.
Stimuli were presented for 500 ms followed by a 1000 ms memory delay period (Figure 1). After the memory delay, the items were again presented. In half of the trials one of the items changed in the relevant dimension (color, shape, or color or shape), and participants responded to indicate whether all items remained the same or if there was a change in one of the item’s relevant dimension. Auditory feedback was given after each trial to indicate whether the participant answered correctly. Feature (color/shape) and visual field location (left/right hemifield) order were counterbalanced across participants.
The number of to-be-remembered features was manipulated in blocks. In the single-feature condition, an instruction screen indicated which feature dimension to monitor. All items were presented in a single value of the irrelevant feature dimension, pseudo-randomly chosen for each participant (e.g., dark blue objects in the shape blocks and triangles in the color blocks). In the two-feature condition, either color or shape could change and participants were instructed to detect a change in either dimension.
Capacity was initially calculated using Cowan’s formula K = SS*(H+C-1) (Cowan, 2001), where SS represents set size, H represents hit rate, and C represents correct rejection rate. Because the set sizes for the two-feature condition were dependent on participants’ individual capacities, we calculated K from VSTM performance during the first hemifield presented in the single-feature condition, with the order of the first hemifield presentation counterbalanced across participants (i.e., half the participants performed the single-set size in the left hemifield first and the right hemifield last). Maximum capacity (K) was defined as the maximum number of items remembered for any set size (3–5 items) in the first presented hemifield. In cases where a participant’s calculated capacity was not an integer, capacity was rounded to the nearest integer. Set sizes in the two-feature condition in both the left and right hemifield were based upon the maximum number of items remembered at any set size. After estimating each participant’s maximum capacity (K) for the single-feature condition, participants were then tested at set sizes of K, K+1, and K+2. This procedure allowed us to reduce inter-subject variability by controlling task difficulty across participants. Following the multiple-feature condition, participants performed the single-feature task in the opposite visual hemifield.
A recent report demonstrates that Pashler’s formula (Pashler, 1988) for measuring capacity (Kp) more accurately reflects the number of items held in VSTM when memory is probed using a whole display (Rouder et al., 2011). This is due to the fact that, as the number of probed items increases beyond subjects’ capacity, the rate of informed guessing also increases. Even though the small set sizes used here might result in minimal differences between K and Kp, in order to be conservative, data were also analyzed using Kp. We therefore re-categorized participants’ data using Pashler’s formula, Kp = SS*((H+FA)/(1-FA)), where SS represents set size, H represents hit rate, and FA represents the false alarm rate, and analyzed the data after recategorizing based upon participants’ Kp.
Results
Cowan’s K
On average, participants’ memory capacity, measured by Cowan’s K, was approximately 3 items for color and 2 items for shape in the single-feature condition (Kcolor=3.3, Kshape=2.4). Therefore, in the two-feature condition, individualized set sizes K, K+1, and K+2 corresponded to 2, 3, and 4 items, respectively, for 13 participants, and 3, 4, and 5 items for 7 participants. For these set sizes, participants performed at 92±1, 86±1, 77±2% correct and 87±2, 79±1, 73±2% (mean ± std error) correct, for the single and two feature conditions, respectively. Change detection performance was measured using d′.
A within-subjects analysis of variance (ANOVA) was conducted with d′ as a measure, and main factors of hemifield (left/right), condition (single-feature/two- feature), set size (K/K+1/K+2), and feature (color/shape). The ANOVA revealed a main effect of: set size [F(2,38)=59.18, p<0.001], with higher discriminability for smaller set sizes; feature [F(1,19)=75.61, p<0.001], reflecting better performance in VSTM for color than shape; and condition (F(1,19)=11.85, p<0.01), driven by poorer performance in the two- feature than single-feature condition. The main effect of condition confirmed previous reports of increased demand for remembering objects with two-features (Xu and Chun, 2006; Luria et al., 2010; Luria and Vogel, 2011; Wilson et al., 2012). The ANOVA also revealed a two-way interaction between feature and condition [F(1,19)=12.93, p<0.01], reflecting a greater feature-cost for detecting changes in color than shape.
Crucially, there was a significant three-way interaction between hemifield, condition, and set size [F(2,38)=5.86, p<0.01]. Planned comparisons indicated that this interaction was driven by a change in performance at set size K+1 in the left- and right-hemifields: performance was better in the left hemifield in the single-feature condition [t(19)=3.08, p < 0.01], consistent with previous reports of working memory benefits in the left, as compared to the right, visual hemifield (Gamble and Somers, 2012). In the two- feature condition, however, significantly better performance was seen in the right-hemifield [t(19)=2.38, p<0.05] (Figure 2). These results demonstrate that VSTM performance across the visual field is dependent upon the number of features held in memory, suggesting that remembering a second feature changed how resources across the visual field are distributed.
Figure 2.
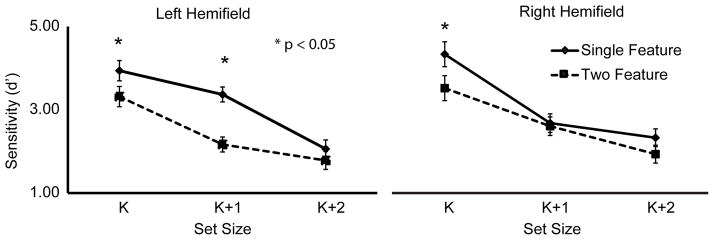
VSTM performance as measured by d′. Performance differed between the single-feature and two-feature conditions in a hemifield- and set size- dependent manner, with the greatest hemifield difference observed at set size K+1. Asterisks signify a significant difference between single-feature and two-feature conditions in each hemifield.
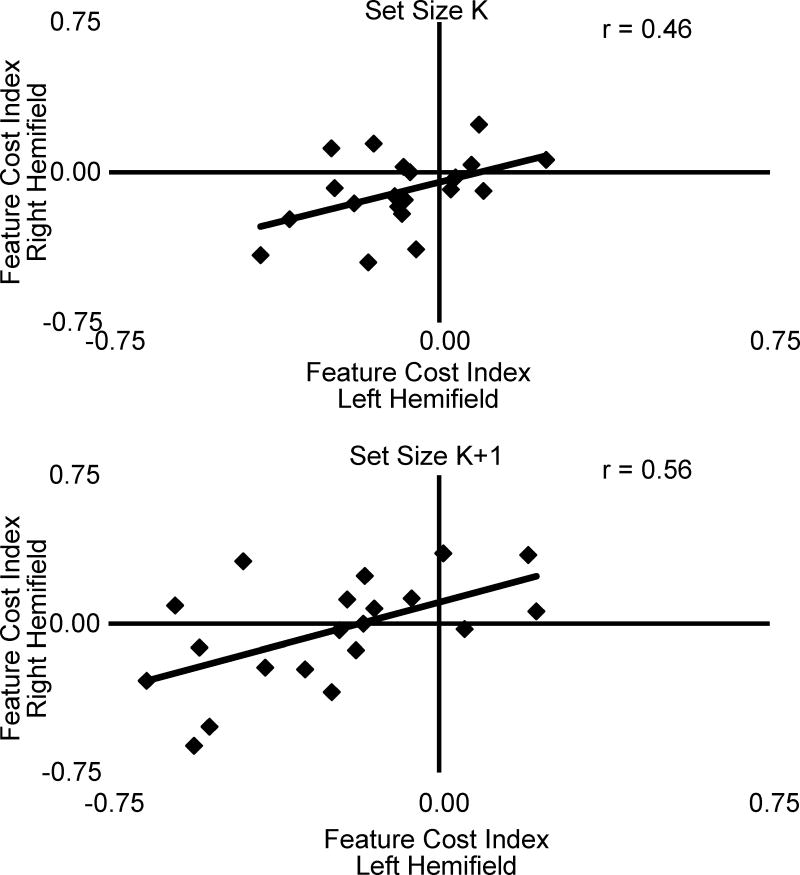
Performance on visuospatial tasks has been shown to reflect differences in fronto-parietal networks at the individual level (Thiebaut de Schotten et al., 2011; Szczepanski and Kastner, 2013). We reasoned that if the difference across the visual field for single- and two- feature memory trials reflects a shift in resources from the left-to the right-hemifield, an individual’s overall amount of resources should remain constant. Because the cost for remembering an additional feature in the left visual field should correlate with the cost in the right visual field across participants, we measured the relationship between the cost of remembering two features in the left- and right-hemifields. A feature-cost index was created to measure the cost of remembering two features for each hemifield for each participant:
At the group level, there was a significant cost for remembering two features in both hemifields at set size K [left, t(19)=2.68, p<0.05; right t(19)=2.51, p<0.05] while there was a significant cost for remembering two features in the left, but not right, hemifield at set size K+1 [left, t(19)=4.88, p<0.001; right, t(19)=0.25, p=0.80]. However, at both set sizes K and K+1 there was a significant correlation between the feature-cost index in the left and right visual field [K, r(18)=0.46, p<0.05; K+1, r(18)=0.56, p<0.01] (Figure 3). Therefore, although individuals vary in the degree of performance bias across the visual field, participants with greater feature-cost indices in the left hemifield also had greater feature-cost indices in the right hemifield. In contrast, at set size K+2, there was no significant difference in memory performance between the single- and two-feature conditions [left, t(19)=1.26, p=0.22; right t(19)=1.21, p=0.24], suggesting that performance did not reflect the number of features held in memory. At this set size there was not a significant relationship between feature-cost across hemifields. This suggests that behavioral asymmetries reflect a shift within a constant set of resources for memory performance across the visual field.
Figure 3.
Relationship between feature cost-indices in the left and right hemifields for set sizes K and K+1. The feature cost-index is a normalized measure of the change in performance for remembering 2 features vs. 1 feature, and was measured in both the left and right visual fields. Correlation between the feature-cost index in left and right hemifields reflects the relationship between resource demand across the visual field.
Pashler’s K
Given a recent report demonstrating that Pashler’s measure of K (Kp)(Pashler, 1988) accounts more accurately for guess rates when using a whole-display probe (Rouder et al., 2011), we reclassified subjects using Kp. Five subjects (25%) were reclassified from having a K of 2 items to having a Kp of 3 memory items while there was no change in estimated memory capacity for the remaining 15 participants. Because set sizes in the two-feature condition were estimated using Cowan’s estimation of K, leaving the participants subjects with incomplete data at each set size, we restricted our analysis to the set size Kp+1, which demonstrated hemifield differences in the original analysis. A within-subjects analysis of variance (ANOVA) was conducted with main factors of hemifield (left/right) and condition (single-feature/two- feature). After reclassifying participants’ capacity based upon Kp, an ANOVA demonstrated a significant main effect of condition [F(1,19)=5.94, p < 0.05], reflecting an overall cost for remembering a second feature. Importantly, and replicating our finding using Cowan’s K, a significant interaction between condition and hemifield [F(1,19)=6.39, p < 0.05] reflected better memory performance for single-feature items in the left hemifield [t(19)=2.21, p < 0.05] and better memory performance for two-feature items in the right hemifield [t(19)=2.13, p < 0.05], resulting in a greater cost for remembering two-features in the left than in the right visual field at Kp +1 [t(19)=2.53, p < 0.05]. Furthermore, after reclassifying participants’ data according to Kp, there was a significant correlation between feature-cost indices in the left and right visual fields [r(18)=0.46, p<0.05], mirroring the relationship using Cowan’s K. This suggests that, across K+1 and KP+1, hemifield asymmetries occur in a feature-load dependent manner.
Experiments 2A and 2B
In Experiment 1 we demonstrated that behavioral performance for items presented across the visual field varied in a feature-load dependent manner. However, it is possible that performance differences were modulated not only by memory demands, but also by perceptual and decision processes that varied between the conditions. In Experiment 2A we replicated results from Experiment 1 using cues for each trial to enable the use of identical memory arrays to investigate feature-load effects on hemifield asymmetries. By using identical stimuli in single- and two-feature conditions, we were able to rule out the possibility that hemifield differences reflected better perceptual representations across the visual field. Furthermore, by randomizing trials within a single block, we minimized the possibility that participants utilized different encoding strategies for the two conditions.
Experiment 2A
Methods
The methods in Experiments 2A were identical to those in Experiment 1 except as described below. Thirty-one additional participants (23 female), naïve to the purpose of the study, completed the experiment for course credit. Three participants were excluded because they were unable to remember a minimum of 2 items in the single-feature condition. On each trial, color and shape varied for each memory item, and a cue indicated the relevant feature-dimension. On single feature trials, a written cue “color” or “shape” directed subjects to detect a change in the relevant feature dimension, and in two-feature trials, the cue “either” directed subjects to detect a change in either color or shape. Cues were presented for 500 ms followed by a blank 200ms interval. In each block, trials for each condition were presented in a randomized order. In both the single-feature conditions (color and shape), memory performance was measured at set sizes 3, 4, and 5 to determine subjects’ maximum capacity. Maximum capacity was defined as the maximum number of items reached for both single feature conditions (color and shape) across both hemifields at any set size, using both Cowan’s and Pashler’s formulas (K and Kp, respectively). Therefore, if a subject had a capacity of 2.4 in the left hemifield and 2.6 in the right hemifield, then the subject’s maximum capacity would be estimated to be 2, because capacity in the left hemifield never reached 3. We restricted all further analyses to set size K+1 and Kp+1, the set size at which hemifield asymmetries were observed in Experiment 1.
Results
Cowan’s K
At set size K+1, participants’ performance was similar to performance in Experiment 1 (accuracy rate: single feature 82±1%, two feature 80±1%). A within subjects ANOVA with factors of hemifield (left, right), condition (single-feature/two-feature), and feature (one, two) and d′ as a measure revealed a main effect of: feature [F(1,27)=121.74, p<0.001], reflecting better performance in VSTM for color than shape; and condition [F(1,27)=7.00, p<0.05], driven by poorer performance in the two- feature condition. Importantly, the ANOVA also revealed a two-way interaction between hemifield and condition [F(1,27)=4.62, p<0.05], replicating the Experiment 1 finding of feature-load dependent changes in VSTM performance across the visual field (Figure 4A). Paired-comparisons demonstrated that this interaction reflected significantly better performance in the single-feature condition than the two-feature condition in the left hemifield [t(27)=4.12, p<0.001], but not in the right hemifield [t(27)=0.38, p=0.71].
Figure 4.
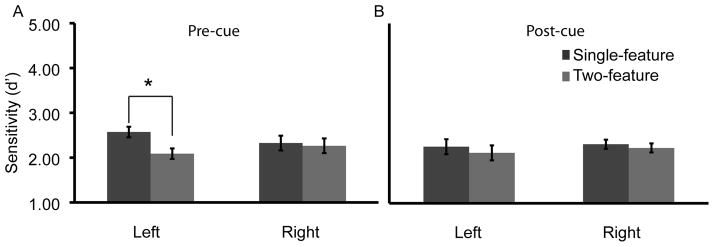
Performance for single- and two-feature conditions for Experiments 2A and 2B. (A) When participants were cued to remember either a single-or two-features before the memory trial, there was a significant cost for remembering a second feature in the left, but not right, hemifield. (B) When participants were cued after the memory probe (post-cue) to detect a change in a single- or in two-features, there was no significant cost for detecting a change in both features in either hemifield.
We hypothesized that the increased memory demand for two-feature as compared to single-feature memory items resulted in a shift in hemifield asymmetries toward a right hemifield bias. In order to test this hypothesis, we calculated a feature cost index as in Experiment 1 (see methods). The feature cost index was significantly higher in the left than right visual hemifield [t(27)=2.15, p<0.05], demonstrating a greater cost for remembering an additional feature in the left hemifield than in the right hemifield.
Pashler K
In order to confirm that the observed results were not due to inaccuracies in the calculation of participants’ capacity, we again reclassified participants’ data based on Kp. One participant was reclassified from having a K of 2 items to having a Kp of 3 memory items while there was no change in estimated memory capacity for the remaining 27 participants. An ANOVA revealed a main effect of: feature [F(1,27)=122.4, p<0.001], reflecting better performance in VSTM for color than shape; and condition (F(1,27)=11.14, p<0.01), driven by poorer performance in the two- feature than single-feature condition. Importantly, the main result was replicated, showing a marginally significant interaction between condition and hemifield [F(1,27)=3.98, p=0.056], providing strong evidence for a change in hemifield biases with feature-load.
We conducted the same correlation in feature cost index across the hemifield analysis as in Experiment 1, however this analysis did not yield a significant correlation. As is the case with a null result, there are many possible explanations as to why this analysis did not yield significance. Here, we focus on two possible explanations. While it has been argued that all of an objects’ features are encoded and stored in memory (Luck and Vogel, 1997), participants can selectively control which features are stored in memory (Woodman and Vogel, 2008). Because this selection is under participant’s control, it is likely that it requires additional resources. The effect on performance in the left and right hemifield may vary because of different costs for remembering an additional feature. Alternatively, consistent with individual differences in controlling which objects are encoded into memory (Vogel et al., 2005), individuals may vary in the extent to which they can selectively encode single features. In either case, this additional step of selecting a feature would affect our behavioral measure, making it harder to measure a relationship in feature cost across the visual field.
Experiment 2B
In Experiments 1 and 2A we demonstrated that hemifield asymmetries occurred in a feature-load dependent manner. However, it could be argued that decision making costs, higher in the two-feature than single-feature condition, modulated memory performance across the visual field in an apparent feature-load dependent manner. Therefore, in Experiment 2B we utilized post-cues to test whether post-perceptual decision making processes resulted in hemifield asymmetries. We predicted that, because dynamic reallocation of resources requires preparation before the trial, then a cue given after the memory probe would be insufficient to bias memory, and no hemifield asymmetries should occur.
Methods
Twenty-eight additional participants (18 female), naïve to the purpose of the study, completed the experiment for course credit. Three participants were excluded due to poor performance (failure to remember at least 2 memory items in any condition) or incomplete data collection. The methods in Experiment 2B were identical to those in Experiment 2A except the cue was presented after the memory probe (a post-cue), just before participants’ response. In each trial, the cue was presented after a 500ms presentation of the memory probe and a 200ms blank interval. Therefore, while participants had to encode and remember both features, the post-cue allowed us to investigate the effect of detecting a change in a single feature dimension as compared to in two separate feature dimensions.
Results
Cowan’s K
At set size K+1, participants’ accuracy was 81±1% for the single feature condition and 80±1% for the two feature condition. As in Experiments 1 and 2A with d′ as a measure, a within-subjects ANOVA revealed a main effect of feature [F(1,24)=121.73, p<0.001], reflecting better performance in VSTM for color than shape. However, unlike in Experiments 1 and 2A, there was no significant effect of condition nor a significant interaction between condition and hemifield [all Fs <1], demonstrating that differences in decision-making processes could not account for the changes in performance across the hemifields reported in Experiments 1 and 2A (Figure 4B).
In order to directly compare the effect of feature-load on memory vs. decision making costs, we compared the feature-cost indices in Experiments 2A and 2B in the left and right hemifields, respectively. The feature-cost index was significantly lower in Experiment 2B than in 2A in the left hemifield [feature cost-index 2A=0.11±0.03, 2B = 0.02±0.04, t(51)=2.15, p<0.05], but not in the right hemifield [feature cost-index 2A= 0.001±0.04, 2B=0.01±0.0, t(51)=2.15, p<0.05]. We therefore conclude that decision-making differences do not account for the feature-cost demonstrated in the left hemifield in the previous experiments.
Pashler’s K
Classifying subjects using Kp, 3 participants were reclassified from having a K of 2 items to having a Kp of 3 memory items while there was no change in estimated memory capacity for the remaining 22 participants. An ANOVA revealed a main effect of: feature [F(1,24)=60.65, p<0.001], reflecting better performance in VSTM for color than shape, but no significant effect of condition (F(1,24)=0.37, p=0.55) nor an interaction between condition and hemifield (F(1,24) = 1.04, p = 0.32). Comparing Experiment 2B to Experiment 2A, we also found a marginally significant decrease in feature cost in the left [feature cost-index 2A=0.12±0.03, 2B= 0.03±0.04, t(24)=1.97, p = 0.06] but not the right [feature cost-index 2A=0.12±0.03, 2B= 0.03±0.04, t(51)=0.53 p = 0.52] hemifield, demonstrating that the feature-cost differences across the visual field were not related to differences in decision making.
Discussion
Here, we asked whether hemispheric asymmetries seen in VSTM result in behavioral asymmetries across the visual field depending upon the number of features to be remembered. In a set of three experiments, we show an advantage for items held in VSTM in the left visual field when encoding and storing a single-feature. These results are consistent with a right hemisphere bias resulting in better performance for items in the left visual field during both visual attention (Bowers and Heilman, 1980; Thiebaut de Schotten et al., 2011) and working memory (Gamble and Somers, 2012). However, when memory items had more than one feature, performance was better in the right-hemifield.
What mechanism can explain this change in behavioral biases? One possibility is that increased VSTM demands, associated with maintaining single- vs. multiple-feature items, result in the dynamic reallocation of resources from the left to the right hemifield. Recent studies demonstrate greater demand for VSTM resources for representing multiple- as compared to single- feature objects using both behavioral (Wilson et al., 2012) and neural (Xu and Chun, 2006; Luria et al., 2010; Luria and Vogel, 2011) measures of VSTM. It has also been demonstrated that with increasing memory load, the right hemisphere tends to represent items in both the left and right visual fields (Sheremata et al., 2010). If we assume that resources can flexibly shift between items stored in memory (Bays et al., 2009), then resources in the right hemisphere may shift from the left- to right- hemifield with increasing memory load. Similar hemispheric asymmetries have been shown to result in right hemifield biases in visual attention (Szczepanski and Kastner, 2013). Therefore, remembering multiple-feature objects may tax VSTM resources, resulting in a shift toward the right hemifield. Representation of the right hemifield by both the left and the right hemisphere would result in a behavioral benefit in the right hemifield.
Further supporting this dynamic reallocation of resources hypothesis, is evidence within our data indicating that the cost of remembering an additional feature in one visual hemifield predicted a significant amount of variability in the opposite visual hemifield (i.e., participants showing a relatively large cost for remembering an additional feature in the left hemifield also demonstrated a relatively large cost for remembering an additional feature in the right hemifield), strongly suggesting that performance reflected an overall limited amount of resources for an individual regardless of how those resources were distributed across the visual field.
The feature-load dependence of hemifield asymmetries in VSTM is consistent with the role of IPS in VSTM. For single feature items we demonstrated a left visual field benefit, consistent with right hemisphere dominance theories in visual attention. At least one previous study has demonstrated a left visual field benefit for single feature memory items (Gamble and Somers, 2012), suggesting that VSTM also exhibits right hemisphere dominance. However, there is also evidence that, as memory load increases, right IPS represents memory items not only in the left, but also the right, visual hemifield (Sheremata et al., 2010). IPS areas that show hemispheric asymmetries have been shown to represent memory items in a feature-load dependent manner (Xu and Chun, 2006), suggesting that increasing feature load for items held in VSTM increases the overall memory load, therefore causing the right hemisphere to represent both the left and right visual hemifields. Furthermore, similar visual field asymmetries have been shown to result in right visual field biases in visual attention (Szczepanski et al., 2013).
While hemispheric asymmetries and visual feature load suggest IPS involvement, it is possible that additional cortical areas may underlie or contribute to our effects, for example frontal eye fields (FEF). Szczepanski et al. (2013), using an attentional task, demonstrated spatial asymmetries in FEF, an area within the same fronto-parietal network as IPS. In addition, at least one paper has demonstrated FEF involvement in VSTM (Offen et al., 2010), though not in a memory-load dependent manner.
Another possible mechanism that could account for differences in hemifield biases in single vs. multi-feature VSTM could be remembering multiple features utilizes attentional demands that modulate memory representations in a hemifield-specific manner. A recent study demonstrated that, across fronto-parietal networks, attention modulates object-based memory activity when multiple features were monitored in a dual memory-attention task (Santangelo and Macaluso, 2011). Furthermore, remembering multiple items in memory results in relatively poorer attentional performance in the left as compared to right visual field, a phenomenon know as transient neglect (Emrich et al., 2011). Similar to deficits seen in hemispatial neglect (Behrmann and Tipper, 1999), transient neglect can also be demonstrated not only in retinotopic (Emrich et al., 2011), but also in object-based coordinates (Gozli et al., 2013), with poorer performance for detecting a target on the left, as compared to right, side of an object. Together these studies demonstrate that attention and VSTM can interact in hemifield specific manner and strongly suggest that understanding how attention modulates the demands of VSTM will be crucial for understanding hemifield asymmetries in VSTM.
Contrary to our results, Szczepanski et al., (2013) did not find overall hemifield asymmetries across participants in the attention domain. There are many possibilities that may account for the divergence between the two studies. First, it is possible that the asymmetries we demonstrate are specific to VSTM. In contrast, Szczepanski et al., (2013) used a landmark version of a line bisection task to measure behavioral biases. The landmark task requires perceptual and attentional, but not memory, resources. Second, in our VSTM task, participants had to select, encode, and maintain multiple items. Therefore, it is possible that hemifield asymmetries emerged from dividing attention and/or memory resources across multiple items. Third, set-size and feature-load dependence of hemifield asymmetries suggest hemifield asymmetries emerge only when resources are taxed. Differences in task difficulty, therefore, may account for differences in asymmetries in the two studies. Finally, by comparing asymmetries across single- and two-feature memory conditions, we measured asymmetries in a within-subjects manner. It is possible that this measure of hemifield asymmetries is more sensitive than comparing asymmetries across participants.
Our findings raise a number of intriguing questions about how VSTM memory resources are distributed. Why were hemifield asymmetries only evident for a single, K+1, set size? Previously, we demonstrated set size dependent hemispheric asymmetries in IPS that emerge only when memory resources are taxed (Sheremata et al., 2010). During a VSTM task performed at near ceiling (e.g., at set size K), memory resources in the left hemisphere may be sufficient to encode and store all the memory items presented in the right visual field, and the task may not necessitate additional resources be directed toward the right visual field. At another extreme, set size K+2, participants were presented with 2 memory items above their estimated memory capacity (Cowan, 2001), which we estimated as 2–3 items for our study design. Therefore at the highest set size participants were presented with approximately twice as many items as they could remember. If participants remembered all of the items presented, memory resources may be distributed amongst all of the items, resulting in poor resolution of memory item representations (Wilken and Ma, 2004; Bays et al., 2009). Alternatively, if participants can successfully ignore or forget items beyond their memory capacity, performance might reflect not only memory resources, but also a number of cognitive processes including distractor filtering that may show differences between individuals (Vogel et al., 2005). Significantly, Gamble & Somers (2012) only found behavioral differences across the visual field when memory items were presented in isolation but not when memory items were presented with distractor items. In either case, a more complete picture of how resources are allocated across the visual field may require more sensitive measurements of VSTM such as precision of individual memory representations. Hemifield asymmetries were apparent at set size at K+1 (Cowan’s K) as well as at Kp+1 (Pashler’s K), suggesting that the set size dependence is relatively resilient to minor modifications in the computation of K for individual participants. In addition, we suggest using these individual set sizes serve as an advantage in that it reduces variability between participants as compared to set sizes based upon a total number of objects.
While our data strongly support data limits in the capacity of visual working memory, it could be argued that the observed hemifield asymmetries in our data reflect limitations in visual or attentional processing rather than VSTM. We argue that this interpretation is unlikely, given that the memory array was presented for 500 ms and such long presentation times are well within the time required to detect and identify several visual items. This argument is supported by a particularly rigorous study, suggesting that at the upper limit for detecting items even the most inefficient visual search was estimated to be 150 ms/item (Wolfe, 1998). Given that our participants could remember a maximum of 3 items in the single-feature memory condition, it is unlikely that visual- or attention limits in encoding memory items constrained memory performance.
It is also possible that hemifield asymmetries reflect differences in encoding or consolidation rates across the hemispheres. One previous study argued that the consolidation rate for colored-squares was approximately 50ms/item (Vogel et al., 2006). While it is possible that VSTM consolidation rate is slower for two-feature than single-feature memory items, a consolidation rate account of hemispheric asymmetries would require that a consolidation rate for two-feature items is less than half as compared to single- feature objects. Therefore, we suggest that it is unlikely that differences in consolidation rates across the visual field led to memory load hemifield asymmetries.
An alternative, arguably simpler, account of our findings could be suggested in which the left hemisphere demonstrates a benefit for remembering multiple feature objects, possibly through the binding of features in memory. However, this explanation is at odds with the neuropsychological and brain stimulation literature. Feature binding has typically been measured using conjunction search, a type of visual search in which discrimination of a target from distractors requires recognizing a specific combination of individual features (Treisman and Gelade, 1980). Patients with left hemispatial neglect, precipitated by right hemisphere damage, display longer reaction times and poorer performance detecting targets defined by a conjunction of features than by an individual feature (Esterman, 2000). Brain stimulation studies using transcranial magnetic stimulation have induced this effect in healthy populations by transiently inducing lesions in right, but not left, posterior parietal cortex (Ashbridge et al., 1997). Several studies have demonstrated that stimulation of right parietal cortex causes interference of feature binding not only during visual search (Muggleton et al., 2008), but also by inducing illusory conjunctions of features from different objects (Koivisto and Silvanto, 2012) and disrupting binding across sensory modalities (Kamke et al., 2012). Feature binding deficits have also been demonstrated after stimulation of the right FEF (Muggleton et al., 2003). Therefore, while it is possible that our results stem from hemispheric asymmetries in feature binding, these asymmetries point to right, not left, hemisphere involvement, putatively in the areas of posterior parietal cortex including IPS.
What could initiate a change in hemifield biases from a left to a right hemifield benefit? In VSTM, attention demands increase with the number of items remembered. Therefore, it is unclear whether the shift in performance across the visual field is driven by attention or memory demands. In spatial neglect, damage to ventral parietal networks results in the inability to attend to objects in the contralateral visual hemifield. However spatial neglect is more prevalent after damage to the right as compared to the left hemisphere (Kinsbourne, 1977; Mesulam, 1981; Shomstein et al., 2010). This hemispheric asymmetry has been argued to reflect coding of left- and right- hemifield representations by the right hemisphere (Heilman and Van Den Abell, 1979; Mesulam, 1981), or by inter-hemispheric inhibition that is stronger in the right than left hemisphere (Kinsbourne, 1977). Inter-hemispheric inhibition argues that while increased attentional load increases attentional modulation symmetrically, inter-hemispheric inhibition by the right, but not left, hemisphere increases. Although our results suggest that the right hemifield is benefitting from dual representation by the left and right hemispheres, they also suggest that these whole field representations emerge only with sufficient memory or attention load. Therefore, further studies are needed to clarify the role of memory load on whole field representations.
Our results also have implications for using the Contralateral Delay Activity (CDA) to measure VSTM. It has been argued that the difference in activity for items presented to the contralateral vs. ipsilateral hemifield reflects resources underlying VSTM and that this activity correlates with measures of capacity (Vogel and Machizawa, 2004). It is likely, therefore, that hemispheric asymmetries may also occur in the CDA. Indeed, at least one study found greater CDA activity in the left than right hemisphere (Machizawa et al., 2012). However, it is important to keep differences between behavioral, fMRI, and EEG studies in mind when considering asymmetries across the three methodologies. For example, it is possible that our results occur due to hemifield asymmetries during encoding. Contrary to fMRI, where the relatively poor temporal resolution can confound encoding, maintenance, and retrieval, the CDA reflects only delay activity. Therefore, if asymmetries occur during encoding, asymmetries in the CDA may not be evident. Furthermore, in comparison to fMRI methods for measuring VSTM, which can separate out activity in the parietal and occipital lobes, the CDA likely measures activity in both parietal and occipital cortex (Vogel and Machizawa, 2004). We demonstrated that asymmetries were only apparent in parietal, but not occipital cortices (Sheremata et al., 2010). Therefore, it is unclear to what degree asymmetries in the CDA would be expected to occur. Further studies are needed to determine whether and to what degree the CDA is weighted toward one hemisphere or the other.
In summary, our results demonstrate that hemifield asymmetries in VSTM vary in a feature load dependent manner. Our results are inconsistent with a purely contralateral, slot-based model of memory in which memory is limited purely by a fixed number of objects regardless of the amount of information present in each object (Luck and Vogel, 1997). In contrast, our results give further support to flexible resource models of memory, in which the amount of information remembered is dependent on the content of the objects remembered as well as the task demands (Alvarez and Cavanagh, 2004; Bays and Husain, 2008).
In addition to increasing the understanding of resource allocation during VSTM, our findings have important implications for studying VSTM in spatial neglect. While the defining characteristic of spatial neglect is the inability to attend to the contralateral visual field, spatial memory deficits have been documented along the vertical meridian (Malhotra et al., 2005) as well as in the intact hemifield (Ferber and Danckert, 2006). In VSTM for objects, one study failed to find impaired memory for memory for a single-feature (Pisella et al., 2004). It is unknown, however, whether patients with hemispatial neglect are impaired for VSTM for multiple feature objects. It remains to be seen whether patients with neglect also have memory deficits for visual objects, and, if so, whether memory deficits are dependent on the number of features per remembered object.
Acknowledgments
This work was supported by grants from the National Science Foundation (BCS-1059523) and National Institute of Health (R21-EY021644) to S. Shomstein.
Footnotes
The authors declare no competing financial interests.
References
- Alvarez GA, Cavanagh P. The capacity of visual short-term memory is set both by visual information load and by number of objects. Psychol Sci. 2004;15:106–111. doi: 10.1111/j.0963-7214.2004.01502006.x. [DOI] [PubMed] [Google Scholar]
- Ashbridge E, Walsh V, Cowey A. ScienceDirect.com - Neuropsychologia - Temporal aspects of visual search studied by transcranial magnetic stimulation. Neuropsychologia. 1997 doi: 10.1016/s0028-3932(97)00003-1. [DOI] [PubMed] [Google Scholar]
- Bays PM, Catalao RFG, Husain M. The precision of visual working memory is set by allocation of a shared resource. Journal of Vision. 2009;9:7–7. doi: 10.1167/9.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays PM, Husain M. Dynamic Shifts of Limited Working Memory Resources in Human Vision. Science. 2008;321:851–854. doi: 10.1126/science.1158023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Tipper SP. Attention accesses multiple reference frames: Evidence from visual neglect. Journal of Experimental Psychology: Human. 1999;25:83–101. doi: 10.1037//0096-1523.25.1.83. [DOI] [PubMed] [Google Scholar]
- Bowers D, Heilman KM. Pseudoneglect: Effects of hemispace on a tactile line bisection task. Neuropsychologia. 1980;18:491–498. doi: 10.1016/0028-3932(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and brain sciences. 2001;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Emrich SM, Burianová H, Ferber S. Transient perceptual neglect: Visual working memory load affects conscious object processing. Journal of Cognitive Neuroscience. 2011;23:2968–2982. doi: 10.1162/jocn_a_00028. [DOI] [PubMed] [Google Scholar]
- Esterman M. Preattentive and attentive visual search in individuals with hemispatial neglect. Neuropsychology. 2000;14:599–611. doi: 10.1037//0894-4105.14.4.599. [DOI] [PubMed] [Google Scholar]
- Ferber S, Danckert J. Lost in space—The fate of memory representations for non-neglected stimuli. Neuropsychologia. 2006;44:320–325. doi: 10.1016/j.neuropsychologia.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Gamble CM, Somers DC. An Emergent Hemifield Asymmetry for Visual Short-Term Memory Capacity. Journal of Vision. 2012;12:347–347. [Google Scholar]
- Gozli DG, Wilson KE, Ferber S. The Spatially Asymmetric Cost of Memory Load on Visual Perception: Transient Stimulus-Centered Neglect. J Exp Psychol Hum Percept Perform. 2013 doi: 10.1037/a0034276. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Van Den Abell T. Right hemispheric dominance for mediating cerebral activation. Neuropsychologia. 1979 doi: 10.1016/0028-3932(79)90077-0. [DOI] [PubMed] [Google Scholar]
- Kamke MR, Vieth HE, Cottrell D, Mattingley JB. Parietal disruption alters audiovisual binding in the sound-induced flash illusion. NeuroImage. 2012;62:1334–1341. doi: 10.1016/j.neuroimage.2012.05.063. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. Hemi-neglect and hemisphere rivalry. Advances in neurology. 1977;18:41–49. [PubMed] [Google Scholar]
- Koivisto M, Silvanto J. ScienceDirect.com - NeuroImage - Visual feature binding: The critical time windows of V1/V2 and parietal activity. NeuroImage. 2012;59:1608–1614. doi: 10.1016/j.neuroimage.2011.08.089. Available at: http://www.sciencedirect.com/science/article/pii/S1053811911010214. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Luria R, Sessa P, Gotler A, Jolicœur P, Dell’Acqua Visual short-term memory capacity for simple and complex objects. Journal of Cognitive Neuroscience. 2010;22:496–512. doi: 10.1162/jocn.2009.21214. [DOI] [PubMed] [Google Scholar]
- Luria R, Vogel EK. Shape and color conjunction stimuli are represented as bound objects in visual working memory. Neuropsychologia. 2011;49:1632–1639. doi: 10.1016/j.neuropsychologia.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machizawa M, Goh C, Driver J, Husain M. Hemispheric differences in visual working memory maintenance indexed by contralateral delay activity. Journal of Vision. 2012;12:180–180. [Google Scholar]
- Malhotra PP, Jäger HRH, Parton AA, Greenwood RR, Playford EDE, Brown MMM, Driver JJ, Husain MM. Spatial working memory capacity in unilateral neglect. CORD Conference Proceedings. 2005;128:424–435. doi: 10.1093/brain/awh372. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Muggleton N, Cowey A, Walsh V. The role of the angular gyrus in visual conjunction search investigated using signal detection analysis and transcranial magnetic stimulation. Neuropsychologia. 2008 doi: 10.1016/j.neuropsychologia.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Muggleton NG, Juan C-H, Cowey A, Walsh V. Human Frontal Eye Fields and Visual Search. 2003. [DOI] [PubMed] [Google Scholar]
- Offen S, Gardner JL, Schluppeck D, Heeger DJ. Differential roles for frontal eye fields (FEFs) and intraparietal sulcus (IPS) in visual working memory and visual attention. Journal of Vision. 2010;10:28–28. doi: 10.1167/10.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H. Percept Psychophys. Springer; 1988. Familiarity and visual change detection. [DOI] [PubMed] [Google Scholar]
- Peirce JW. Generating stimuli for neuroscience using PsychoPy. Frontiers in Neuroinformatics. 2009;2 doi: 10.3389/neuro.11.010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips W. On the distinction between sensory storage and short-term visual memory. Percept Psychophys. 1974;16:283–290. [Google Scholar]
- Pisella L, Berberovic N, Mattingley JB. Impaired Working Memory for Location but not for Colour or Shape in Visual Neglect: a Comparison of Parietal and Non-Parietal Lesions. Cortex. 2004;40:379–390. doi: 10.1016/s0010-9452(08)70132-1. [DOI] [PubMed] [Google Scholar]
- Rouder JN, Morey RD, Morey CC, Cowan N. How to measure working memory capacity in the change detection paradigm. Psychon Bull Rev. 2011;18:324–330. doi: 10.3758/s13423-011-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo V, Macaluso E. The contribution of working memory to divided attention. Hum Brain Mapp. 2011;34:158–175. doi: 10.1002/hbm.21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheremata SL, Bettencourt KC, Somers DC. Hemispheric asymmetry in visuotopic posterior parietal cortex emerges with visual short-term memory load. Journal of Neuroscience. 2010;30:12581–12588. doi: 10.1523/JNEUROSCI.2689-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomstein S, Lee J, Behrmann M. Top-down and bottom-up attentional guidance: investigating the role of the dorsal and ventral parietal cortices. Experimental Brain Research. 2010;206:197–208. doi: 10.1007/s00221-010-2326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski SM, Kastner S. Shifting Attentional Priorities: Control of Spatial Attention through Hemispheric Competition. The Journal of Neuroscience. 2013;33:5411–5421. doi: 10.1523/JNEUROSCI.4089-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski SM, Pinsk MA, Douglas MM, Kastner S, Saalmann YB. Functional and structural architecture of the human dorsal frontoparietal attention network. Proceedings of the …; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell’Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DGM, Catani M. A lateralized brain network for visuospatial attention. Nature Neuroscience. 2011;14:1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cogn Psychol. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Woodman GF, Luck SJ. The time course of consolidation in visual working memory. J Exp Psychol Hum Percept Perform. 2006;32:1436–1451. doi: 10.1037/0096-1523.32.6.1436. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=17154783&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- Wilken P, Ma WJ. A detection theory account of change detection. Journal of Vision. 2004 doi: 10.1167/4.12.11. [DOI] [PubMed] [Google Scholar]
- Wilson KE, Adamo M, Barense MD, Ferber S. To bind or not to bind: Addressing the question of object representation in visual short-term memory. Journal of Vision. 2012;12:14–14. doi: 10.1167/12.8.14. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. What Can 1 Million Trials Tell Us about Visual Search? Psychological Science. 1998;9:33–39. [Google Scholar]
- Woodman GF, Vogel EK. Selective storage and maintenance of an object’s features in visual working memory. Psychon Bull Rev. 2008;15:223–229. doi: 10.3758/pbr.15.1.223. [DOI] [PubMed] [Google Scholar]
- Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]