Abstract
Otologic surgery often involves a mastoidectomy procedure, in which part of the temporal bone is milled away in order to visualize critical structures embedded in the bone and safely access the middle and inner ear. We propose to automate this portion of the surgery using a compact, bone-attached milling robot. A high level of accuracy is required to avoid damage to vital anatomy along the surgical path, most notably the facial nerve, making this procedure well-suited for robotic intervention. In this study, several of the design considerations are discussed and a robot design and prototype are presented. The prototype is a 4 degrees-of-freedom robot similar to a four-axis milling machine that mounts to the patient’s skull. A positioning frame, containing fiducial markers and attachment points for the robot, is rigidly attached to the skull of the patient, and a CT scan is acquired. The target bone volume is manually segmented in the CT by the surgeon and automatically converted to a milling path and robot trajectory. The robot is then attached to the positioning frame and is used to drill the desired volume. The accuracy of the entire system (image processing, planning, robot) was evaluated at several critical locations within or near the target bone volume with a mean free space accuracy result of 0.50 mm or less at all points. A milling test in a phantom material was then performed to evaluate the surgical workflow. The resulting milled volume did not violate any critical structures.
Keywords: Robotic surgery, otologic surgery, mastoidectomy, bone milling, acoustic neuroma
1. INTRODUCTION
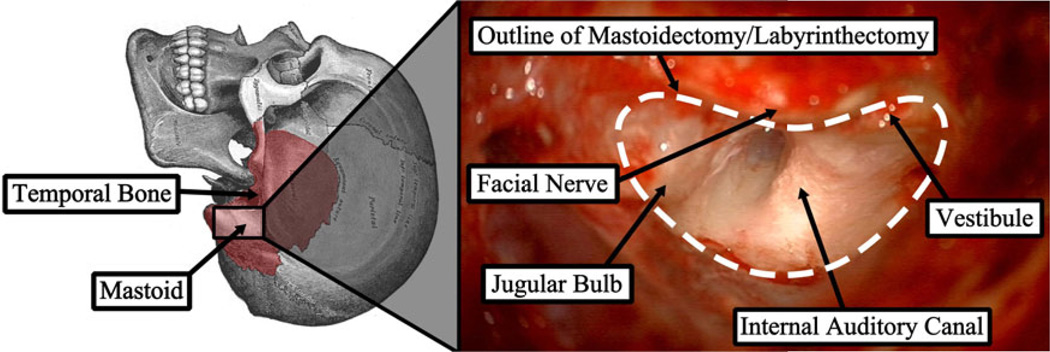
The complex anatomy and presence of many critical structures embedded within the bone makes inner ear surgery well-suited for image-guided and robotic intervention. A common procedure performed as part of most otologic surgeries is mastoidectomy (Figure 1), which is the removal of all or part of the mastoid portion of the temporal bone with a high speed surgical drill. A mastoidectomy by itself could be performed to treat various diseases and infections such as mastoiditis and cholesteotoma, but it is often a necessary component of other surgeries as a means to gain access to the middle and inner ear. The procedure is challenging since the surgeon must first identify the vital anatomy within the bone (e.g. facial nerve, chorda tympani, tegmen tympani, and sigmoid sinus) and then carefully remove the bone around these structures.
Figure 1.
Mastoid region of the temporal bone and photograph from surgical case showing the region after the target bone has been removed with several key anatomical structures identified.
A procedure that is being specifically targeted in the presented work is the translabyrinthine approach for acoustic neuroma (a.k.a. vestibular schwannoma) tumor removal. Acoustic neuromas are benign tumors located on the vestibular nerve in the internal auditory canal (IAC) that can cause hearing loss, increased intracranial pressure, and loss of balance, among other symptoms. To reach the IAC and remove the tumor, a mastoidectomy and labyrinthectomy must be performed. This extensive amount of bone removal can take several hours. Our approach is to perform the bone removal portion of this procedure robotically, preserving the surgeon for the more delicate work of removing the tumor.
Robotic bone milling has been commercially successful for joint arthroplasty and resurfacing (e.g. RIO System by Mako Surgical Corp., Ft. Lauderdale, FL, USA; ROBODOC Surgical System by Curexo Technology Corp., Fremont, CA, USA; CASPAR by URS Ortho GMBH & Co. KG, Rastatt, Germany). Bone milling was one of the first tasks targeted in surgical robotics since the rigidity of the bone allows for pre-operative planning of the procedure in a manner similar to that of planning for computer-numeric-control (CNC) machining. Since the vital structures in the temporal bone region are embedded in the bone, the pre-operative images provide an accurate representation of the anatomy with minimal risk of variation during the surgical procedure. Thus, the combination of an accurate robot and an accurate representation of the patient anatomy allows for safe and precise execution of the surgical task, which requires sub-millimetric accuracy.1 The feasibility of robotic mastoidectomy has been shown by several research groups.2–4 These systems, like most of the orthopedic bone milling systems, use a large, free-standing robot to perform the milling. These robots are generally bulky, very expensive and provide a workspace much larger than that required for the mastoidectomy procedure. An exception is the work of Weber et al.5, 6 who use a small serial robot that mounts directly to the bed near the patient’s head to drill a direct path through the mastoid to the cochlea for inserting an electrode array. Additionally, the accuracy of milling in the skull has been shown to be improved by localizing the drill tip with a combination of a tracking system and local sensors.7
We propose to use a compact, bone-attached robot designed specifically for temporal bone milling. This design eliminates the need for a tracking system and the error associated with monitoring and aligning the patient with the robot as well as allows a robot workspace more suited for the task at hand. Bone-attached robots have been shown to be accurate enough for inner ear surgery,8 and orthopedic bone milling has been successfully performed using bone-attached robots.9–11 Our approach combines the attributes of the aforementioned systems in an effort to improve the mastoidectomy component of otologic surgery.
2. ROBOT DESIGN AND PROTOTYPE
Prior to designing the robot, two primary design criteria were considered: the forces required to safely mill temporal bone and the required workspace to reach a wide range of patients. The forces were measured while milling formalin-fixed temporal bone specimens using an industrial, serial robot under a variety of cutting conditions, including different burr types, cutting angles, cutting velocities and cutting depths12. Additionally, different types of bone in the temporal bone were evaluated: cortical bone on the surface and trabecular bone within the mastoid. Key findings of this study were: (1) Larger, fluted burrs should be used whenever possible to reduce forces at the drill tip. However, based on the specific anatomy, only certain sizes may be permissible in order to avoid contact with critical structures. (2) Lower drill angles should be used (i.e. the side of the burr should be used) as much as possible. (3) The milling trajectory should employ higher cutting velocities and shallower depths of cut rather than slower velocities and deeper cuts to minimize the forces at the tip and thus decrease the risk of deection of the burr off the desired path. (4) Higher speeds and deeper cuts can be used in the mastoid region compared to cortical bone for the same force thresholds.
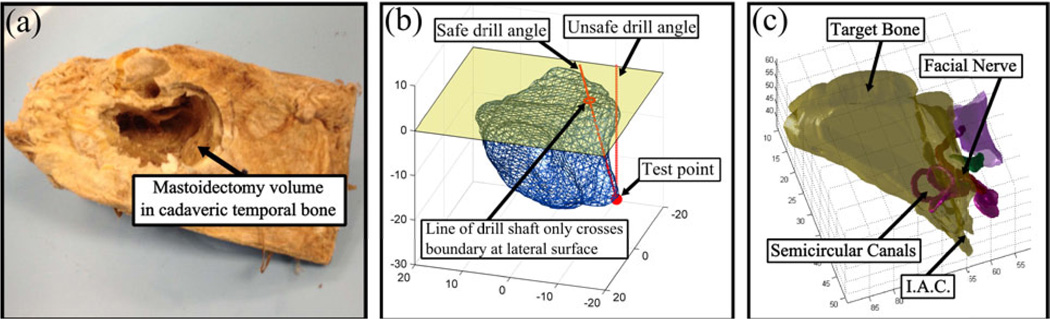
In order to determine the required workspace for the robot to perform mastoidectomy, two analyses were performed. First, a set of ten temporal bone specimens that had mastoidectomies drilled (Figure 2a) were scanned and analyzed. The drilled volume was segmented for each of the specimens using custom software. The workspace volume required for the robot to cover all specimens was determined by first aligning the specimens along their lateral surfaces and then calculating the total volume that includes the segmented volume for all specimens. This volume was expanded by 10% to account for some outlier patients that do not fit within this volume. The required workspace can be approximated as an inverted elliptical cone that is 41 mm deep with maximum cross-section at the surface with a major diameter of 52 mm and minor diameter of 45 mm.
Figure 2.
(a) One of ten mastoidectomy drilled cadaveric temporal bones scanned and analyzed in workspace analysis, (b) example test point showing safe and unsafe drill angles for angular workspace analysis, and (c) target volume to be removed for accessing the internal auditory canal (IAC) in acoustic neuroma surgery and critical structures in the vicinity of surgical workspace.
Next, the required drill angles to reach all target points safely were calculated. Two cases were considered: two rotational degrees-of-freedom (DOF) and one rotational DOF. For each target point in the segmented volume, the minimum drill angle(s) required to reach the point without any part of the drill touching an untargeted point or any critical structure was calculated (Figure 2b). It was determined that one DOF is required with a tilt range of 45°. In order for one angular DOF to safely access all target points, the robot must be oriented correctly on patient’s skull. This adds some additional complexity in the setup of the robot but we believe it is outweighed by the reduced complexity and thus increased accuracy of the robot.
The above analysis was then repeated specifically for acoustic neuroma cases, which involves mastoidectomy and labyrinthectomy. An experienced surgeon manually segmented on six patient scans the bone region to be removed to reach the IAC for accessing the tumor (Figure 2c). These segmented volumes were analyzed in the same manner as the mastoidectomy volumes. The required workspace for this procedure is similar to that of mastoidectomy with the exception that the depth requirement is 49 mm.
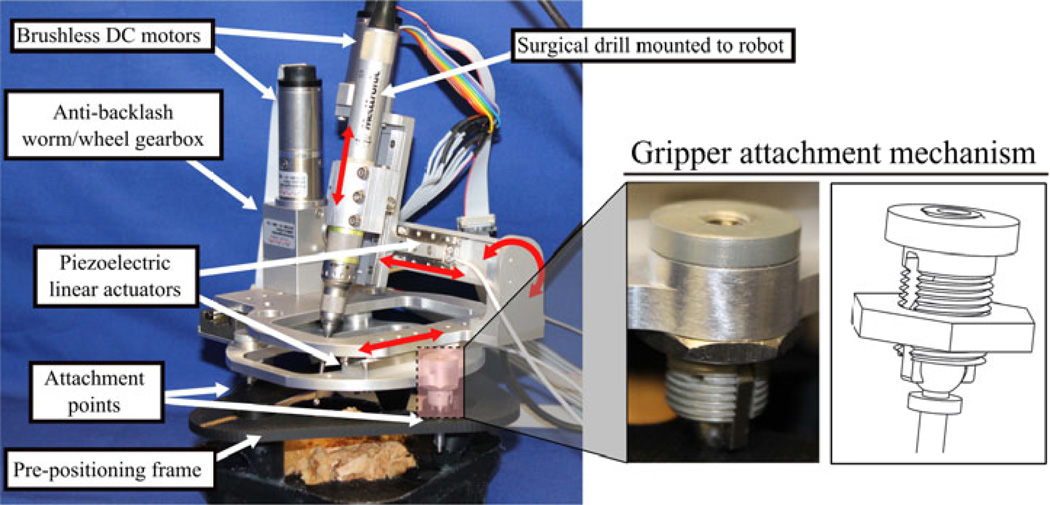
Based on the analysis outlined above, a robot prototype was designed (Figure 3). The chosen kinematic architecture is a 4 DOF robot analogous to a four-axis milling machine. The robot can move along the x-, y-, and z-directions and rotate about the x-direction. Piezoelectric linear actuators (SmarAct GmbH, Oldenburg, Germany) are used for the×and y directions (motion in the directions along the surface of the skull), a brushless DC motor (Maxon Precision Motor, Inc., Fall River, MA, USA) with a lead screw is used for the z-direction (into the skull), and a brushless DC motor and anti-backlash worm-wheel gearbox (Gysin AG, Itingen, Switzerland) is used for the rotational direction. The robot is attached to three titanium spheres on a pre-positioning frame (PPF), which is rigidly attached to the head of the patient. The PPF is CT scanned with the patient and the spheres serve as both fiducial markers to easily register the patient to their CT image and as attachment points to attach the robot to the patient.
Figure 3.
Robot prototype attached to test specimen via pre-positioning frame (PPF). The robot is attached to the PPF using spherical gripper mechanisms that lock to the fiducial marker spheres.
3. SURGICAL WORKFLOW
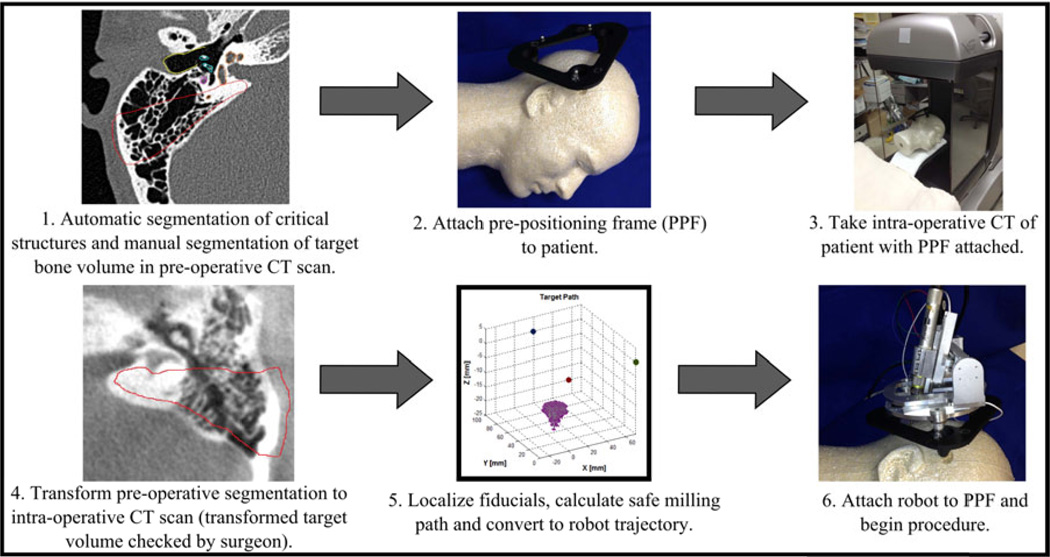
Prior to surgery, preoperative planning is performed by first automatically segmenting various anatomical structures, including the facial nerve, chorda tympani, external auditory canal, semicircular canals and ossicles13 in the pre-operative CT scan of the patient. The target volume to mill out is then manually segmented by the surgeon around the automatically segmented critical structures. In the operating room after the patient is anaesthetized, the PPF is attached to the patient using small screws via stab incisions and an intraoperative CT scan of the patient with the PPF attached is acquired. The CT scan could be performed in the operating room using a scanner such as the xCAT ENT mobile CT scanner (Xoran Technologies, Ann Arbor, MI). The intraoperative CT is then registered to the preoperative CT and the segmentations from the preoperative CT are transformed to the intraoperative CT. Fiducial markers (three spheres in the PPF) are then localized in the image. The locations of these markers relative to the robot are known from calibration of the robot prior to surgery so the target bone volume can be transformed to the robot coordinate system. Automatic computation of the milling path using a custom path planning software (written in Matlab, The Mathworks, Natick, MA, USA) is then performed (see Section 4 for details on the path planning algorithm). This path is then converted to a robot trajectory incorporating cutting velocity and drill angle considerations. Finally, after the targeted region of bone is exposed on the patient via skin incision, the robot is attached to the PPF and the procedure begins. The surgeon monitors the robot throughout the procedure and has the ability to pause or stop the milling at any time (Figure 4).
Figure 4.
Proposed steps in surgical workflow.
4. PATH PLANNING
Our path planning algorithm is an extension of an algorithm by Danilchenko used in earlier robotic mastoidectomy trials.14 This algorithm takes as input a three-dimensional array in which each element represents one voxel and each voxel has one of four values indicating that it contains air, targeted bone, forbidden bone, or the start/end point. The algorithm finds a path that begins at the starting point, visits all accessible target voxels while avoiding all forbidden voxels and returns to the start/end point.
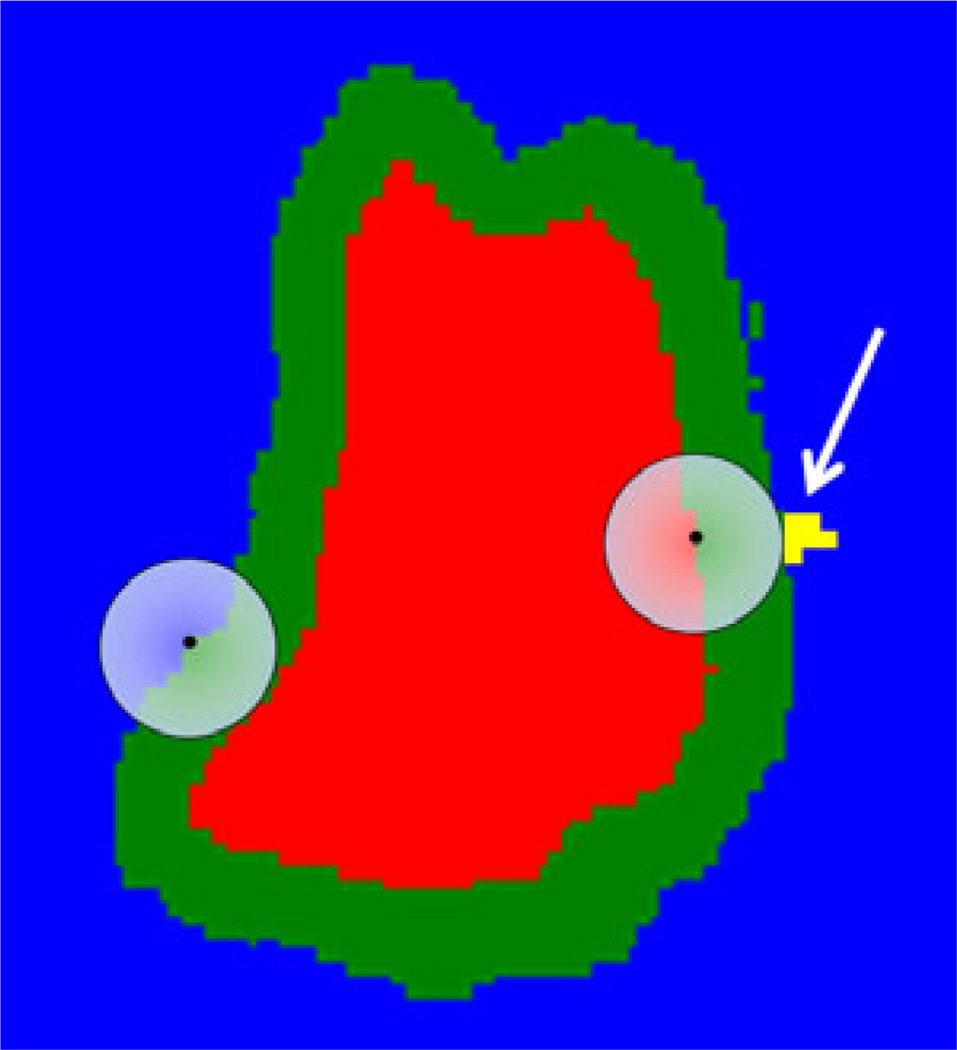
We have improved the Danilchenko algorithm to model the physical dimensions of the drill bit. That original algorithm treated the drill bit as if it were exactly the size of one voxel, whereas a standard surgical bit is in fact a sphere that spans many voxels. Thus, if the center of the bit follows a given path, it will ablate not only the bone on the path but all bone within one radius of the bit center. To accommodate the size and shape of the bit, we modify the voxel array by means of the morphological image-processing operation known as dilation.15 Dilation of a region expands it beyond its original boundary into its surroundings. The extent of the expansion depends on the size and shape of a specified “structuring element”. Figure 5 is a two-dimensional illustration of dilation, as it is used in our application. The combination of the red, green, and yellow (arrow) regions is the targeted region; blue is the forbidden region. The semi-transparent circular disk on the left represents the structuring element during preprocessing; the identical disk on the right represents the drill bit during ablation. The structuring element is chosen to have the same shape and size as the bit, and dilation is applied to the forbidden (blue) region by placing the center of the element on every voxel in the forbidden region and forcing all voxels within the element have the forbidden value. The green and yellow voxels are voxels that were marked as targeted before dilation and are marked as forbidden after dilation.
Figure 5.
Illustration of the use of dilation to accommodate the finite size of the drill bit. Input targeted region is red, green, and yellow (arrow). Blue is the forbidden region. Output targeted region is red. The semi-transparent circular disk on the left represents the structuring element during preprocessing; the disk on the right represents the drill bit during ablation. The yellow region (arrow) is unreachable because of the bluntness of the bit.
The resultant modified targeted region, which is the red region, serves as input to the path-planning algorithm, which determines a path for the center of the drill bit that causes that center to pass through all the red voxels. As a result, both the red and green regions will be ablated. The bit is shown at one position during its progress through the path. That position has been chosen to show a problem with the bit. Its spherical shape renders unreachable those targeted voxels like the yellow ones (arrow) that lie within nooks in the forbidden region into which the bit does not fit. This problem is inherent to the bit and is present whether drilling is done by hand or via automatic means. Were such voxels contacted by any part of the bit, at least one forbidden voxel would be contacted as well, but the dilation pre-processing step allows the algorithm to adhere to the rule, “first do no harm”, protecting all forbidden voxels at the cost of allowing some targeted voxels to remain undrilled. A second aspect of the arrangement of targeted voxels that can render them unreachable is an overhang. An overhang is the situation in which a targeted voxel lies below a forbidden voxel, where below means in the direction perpendicular to the robot base and into the skull. In our current implementation, the path is calculated without consideration of drill orientation. Therefore in order for the center to reach a given voxel, there must be no forbidden voxels above it. Such voxels will, like the targeted voxels in nooks described above, remain untouched. The drill angle associated with each target voxel is determined after the path is generated based on the direction of cutting and the location of the burr within the target volume.
If the shape of the targeted region were convex, then the number of steps on the path would be one less than the number of reachable voxels. This is a perfect path in terms of efficiency. However, convexity is rare, and as a result, some degree of backtracking can be expected, where backtracking means moving through targeted voxels after they have already been ablated. Our algorithm includes heuristics to find short backtracks and can provide information to the robot that allows it to speed up during backtracking, which will increase the time efficiency of the robot, but we measure path efficiency simply as the number of reachable targeted bone voxels divided by the number of voxels in the path. This definition gives 100% for a perfect path. Efficiencies of close to 90% are routinely achieved by our algorithm.
5. EXPERIMENTAL METHODS
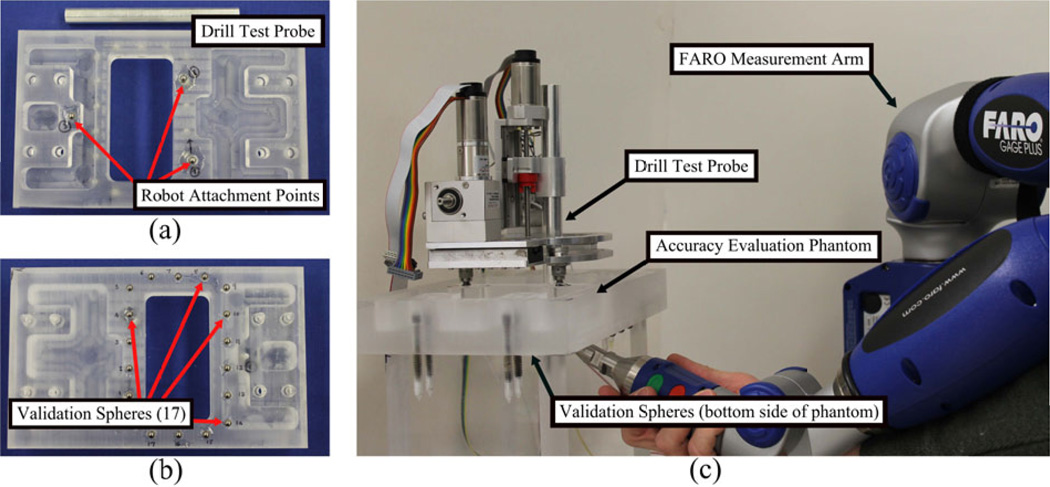
To evaluate the free space targeting accuracy of the robot prototype, the virtual target method described by Balachandran et al. was used.16 A custom phantom and drill probe were built for this experiment (Figure 6). The top of the phantom contains three titanium spheres that serve as fiducial markers and robot attachment points - similar to the spheres on the PPF on which the robot would be attached for the patient in a surgical setting. A FARO GagePlus coordinate measuring machine (FARO Technologies, Inc., Lake Mary, FL) was used to accurately measure the position of a drill probe attached to the robot. The bottom of the phantom contains seventeen titanium spheres to serve as validation spheres for registering the CT scan of the phantom to the coordinate system of the FARO GagePlus. Multiple scans of the phantom were taken using a xCAT mobile CT scanner. A manually segmented mastoidectomy/labyrinthectomy volume from patient data to perform acoustic neuroma surgery was superimposed onto each of the scans and several target points were selected from these volumes to serve as “virtual targets” in the experiment. The target points were chosen such that they represent critical points in standard mastoidectomies and acoustic neuroma procedures. These targets were located in the segmented volume on the bone surface (2 points), and regions close to the facial nerve (2 points), the vestibule (1 point), and the IAC (3 points). Additionally, each of the seventeen validations spheres and the three robot fiducial spheres were automatically localized in the CT scan. The position of the three robot fiducial markers and the eight target points were given as inputs to the robot control software.
Figure 6.
(a) Top of accuracy test phantom and probe used to measure drill tip position, (b) bottom of test phantom showing validation spheres, and (c) measurement of drill tip position for a given target point using Faro measurement arm.
The robot was then mounted onto the test phantom. The phantom and the FARO were rigidly fixed to the same table to minimize any relative motion during the measurements. The centers of the seventeen validation spheres were then localized using the FARO. Next, the robot was programmed to move to each of the eight target points. At each point, the location of the tip of the drill probe was measured with the FARO. The drill probe has a conical divot at the end of it making it easy to accurately and consistently measure the tip of the probe with the spherical tip of the FARO end-effector.
Using the validation spheres, a rigid transformation was calculated between the FARO coordinate system and the image coordinate system with least-squares error.17 Then, the measured locations of the drill tip in FARO space were transformed to image space and compared with the specified target points. The distance between the target point in the image space and transformed point from FARO space was computed as the target registration error (TRE) at that target location.
This error measurement includes errors in image localization as well as robot kinematics. This procedure was repeated for three different scans of the phantom with each scan using a different segmented mastoidectomy volume. Additionally, the measurements for each scan/mastoidectomy target points were repeated three times for a total of 72 measurements (3 measurements per scan × 3 scans × 8 targets per scan). Between each set of measurements, the robot was removed from the phantom and powered-down then re-attached, turned on and run through its standard initialization procedure. Root-mean-square (RMS), mean, and standard deviation values were computed.
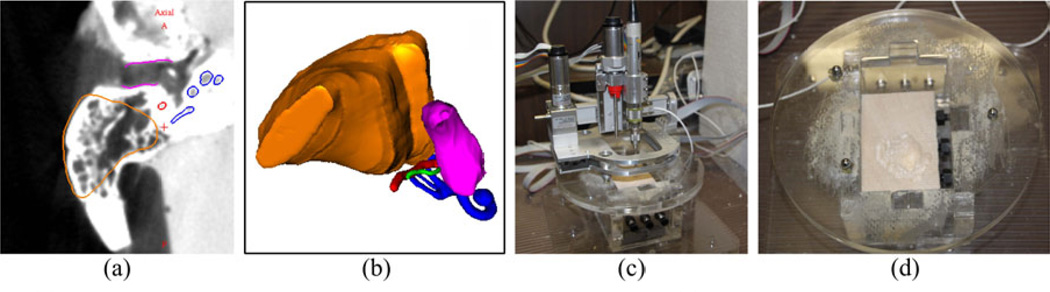
Next, a milling experiment was performed to test the surgical workflow of the system. Wood was used as a surrogate material for bone and a testing mount was constructed to place the material in a similar position relative to the robot as the bone would be in a surgical case. The wood was fixed relative to the robot to simulate the bone-attached approach. A mastoidectomy segmentation was used for the target volume and superimposed onto the testing mount. From this volume, a path and robot trajectory were calculated. The robot was mounted to the three spheres on the testing mount and performed the milling (Figure 7). To evaluate the milling experiment, a post-operative CT scan was acquired for the testing mount. This scan was registered to the pre-operative plan using the three fiducial markers. The removed volume of bone was manually segmented and compared with the planned milling trajectory and critical structures.
Figure 7.
(a) Segmented slice of target volume to be milled in experiment, (b) 3D representation of target volume along with other anatomy of the ear, (c) test setup with robot attached to testing mount and material fixed rigidly in mount, and (d) drilled volume in test material after procedure.
6. RESULTS
The results of the free space accuracy evaluation experiments are summarized in Table 1. For all four locations tested, the mean TRE and root mean square TRE were 0.50 millimeteres or less, which is within the range that we believe to be suitable for otologic surgery. In scans 2 and 3 of the phantom, all of the validation spheres were used to register the FARO coordinate system to the image coordinate system; however, in scan 1, only 12 of the validation spheres were visible and used to register to two coordinate systems.
Table 1.
Target registration error (TRE) for free space accuracy evaluation.
| Skull Surface | Facial Nerve | Vestibule | IAC | |
|---|---|---|---|---|
| Root Mean Square TRE (mm) | 0.47 | 0.49 | 0.50 | 0.48 |
| Mean TRE (mm) | 0.38 | 0.42 | 0.43 | 0.42 |
| Standard Deviation (mm) | 0.28 | 0.26 | 0.26 | 0.24 |
In the mililng experiment, the input to path planning was a 25.6 × 25.6 × 14.2 cm CT image with cubic voxels that were 0.8 mm on a side (downsampled from 0.4 mm). The dimensions of the input array were 320 × 320 × 178, which comprises 18.2 million cubic voxels, or 9,332 cm3. Of this volume, only 12,854 voxels, or 6.6 cm3, were targeted. The structuring element, which is a digital representation of the bit shape constructed of 0.8 mm cubes, comprised a set of 275 voxels approximating the shape of a 5 mm sphere. Among the targeted voxels, 8.3 percent, or 1,074 are unreachable in this volume (yellow region in Figure 5) because of the shape and size of the bit. Another 87 voxels are unreachable because of overhangs. On our phantom, the algorithm, implemented in MATLAB, required 80 minutes. The robot successfully removed the target volume (Figure 7d). After aligning the post-operative CT scan with the pre-operative CT scan and segmented structures, it was determined that the experimentally removed volume did not overlap with the critical structures.
7. CONCLUSIONS
We have described a bone-attached robotic system and its proposed surgical workflow for performing the mastoidectomy and labyrinthectomy portion of otologic surgery. Several of the design considerations were discussed and a four degree-of-freedom robot prototype was presented. The free space accuracy of the system was shown to be within the tolerance required for otologic surgery and workflow was verified with an experiment in phantom material.
Future work on this project will focus on expanding the experiments to include cadaveric temporal bones and further evaluation of the dynamic milling accuracy. It is interesting to note that the error in the free space accuracy evaluation was primiarly in the direction along the drill shaft. An improved calibration procedure for this drill length is essential to decreasing the overall error. Furthermore, since the drill may have to be swapped out mid-procedure for larger milling volumes, this calibration must be simple and fully automated. Additionally, the path planning and trajectory planning should be improved to incorporate drill shaft angles to alow for reaching overhung areas and ensuring that the drill is cutting with the side of the burr.
These initial studies are promising and indicate that a bone-attached robot of this design may one day be able to automate a common component of otologic surgery, improving patient safety and preserving surgeons for the more delicate portions of surgery.
ACKNOWLEDGEMENTS
This work was supported by the National Center for Research Resources, Grant UL1 RR024975-01, and is now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06 as well as award number R01 DC012593 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the offcial views of the National Institutes of Health.
REFERENCES
- 1.Labadie RF, Majdani O, Fitzpatrick JM. Image-guided technique in neurotology. Otolaryngologic Clinics of North America. 2007;40(3):611–624. doi: 10.1016/j.otc.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Danilchenko A, Balachandran R, Toennis JL, Baron S, Munkse B, Fitzpatrick JM, Withrow TJ, Webster RJ, III, Labadie RF. Robotic Mastoidectomy. Otology & Neurotology. 2010;(32):11–16. doi: 10.1097/MAO.0b013e3181fcee9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Federspil PA, Geisthoff UW, Henrich D, Plinkert PK. Development of the first force-controlled robot for otoneurosurgery. The Laryngoscope. 2003 Mar.113:465–471. doi: 10.1097/00005537-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Lim H, Han J-M, Hong J, Yi B-J, Lee SH, Jeong JH, Matsumoto N, Oka M, Komune S, Hashizume M. Mechatronics and Automation (ICMA), 2011 International Conference on. IEEE; 2011. Image-guided robotic mastoidectomy using human-robot collaboration control; pp. 549–554. [Google Scholar]
- 5.Stieger C, Caversaccio M, Arnold A, Zheng G, Salzmann J, Widmer D, Gerber N, Thurner M, Nauer C, Mussard Y, et al. Development of an auditory implant manipulator for minimally invasive surgical insertion of implantable hearing devices. The Journal of Laryngology & Otology. 2011;125(03):262–270. doi: 10.1017/S0022215110002185. [DOI] [PubMed] [Google Scholar]
- 6.Williamson T, Bell B, Gerber N, Salas L, Zysset P, Caversaccio M, Weber S. Estimation of tool pose based on force density correlation during robotic drilling. Biomedical Engineering, IEEE Transactions on. 2013;60(4):969–976. doi: 10.1109/TBME.2012.2235439. [DOI] [PubMed] [Google Scholar]
- 7.Stolka PJ, Henrich D. Intelligent Robots and Systems, 2006 IEEE/RSJ International Conference on. IEEE; 2006. Improving navigation precision of milling operations in surgical robotics; pp. 2351–2357. [Google Scholar]
- 8.Kratchman LB, Blachon GS, Withrow TJ, Balachandran R, Labadie RF, Webster RJ. Design of a bone-attached parallel robot for percutaneous cochlear implantation. Biomedical Engineering, IEEE Transactions on. 2011 Oct.58:2904–2910. doi: 10.1109/TBME.2011.2162512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoham M, Burman M, Zehavi E, Joskowicz L, Batkilin E, Kunicher Y. Bone-mounted miniature robot for surgical procedures: concept and clinical applications. Robotics and Automation, IEEE Transactions on. 2003;19(5):893–901. [Google Scholar]
- 10.Wolf A, Jaramaz B, Lisien B, DiGioia A. Mbars: mini bone-attached robotic system for joint arthroplasty. The International Journal of Medical Robotics and Computer Assisted Surgery. 2005;1(2):101–121. doi: 10.1002/rcs.20. [DOI] [PubMed] [Google Scholar]
- 11.Plaskos C, Cinquin P, Lavallée S, Hodgson A. Praxiteles: a miniature bone-mounted robot for minimal access total knee arthroplasty. The International Journal of Medical Robotics and Computer Assisted Surgery. 2005;1(4):67–79. doi: 10.1002/rcs.59. [DOI] [PubMed] [Google Scholar]
- 12.Dillon NP, Kratchman LB, Dietrich MS, Labadie RF, Webster RJ, III, Withrow TJ. An experimental evaluation of the force requirements for robotic mastoidectomy. Otology & Neurotology. 2013 doi: 10.1097/MAO.0b013e318291c76b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noble JH, Warren FM, Labadie RF, Dawant BM. Automatic segmentation of the facial nerve and chorda tympani in ct images using spatially dependent feature values. Medical physics. 2008;35:5375. doi: 10.1118/1.3005479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danilchenko A. PhD thesis. Vanderbilt University; 2011. Fiducial-Based Registration with Anisotropic Localization Error. [Google Scholar]
- 15.Sonka M, Hlavac V, Boyle R. Image processing, analysis, and machine vision. Thomson-Engineering; 2007. [Google Scholar]
- 16.Balachandran R, Mitchell JE, Dawant BM, Fitzpatrick JM. Accuracy evaluation of microtargeting platforms for deep-brain stimulation using virtual targets. Biomedical Engineering, IEEE Transactions on. 2009;56(1):37–44. doi: 10.1109/TBME.2008.2002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonka M, Fitzpatrick JM, Masters BR. Handbook of medical imaging, volume 2: Medical image processing and analysis. Optics & Photonics News. 2002;13:50–51. [Google Scholar]