Abstract
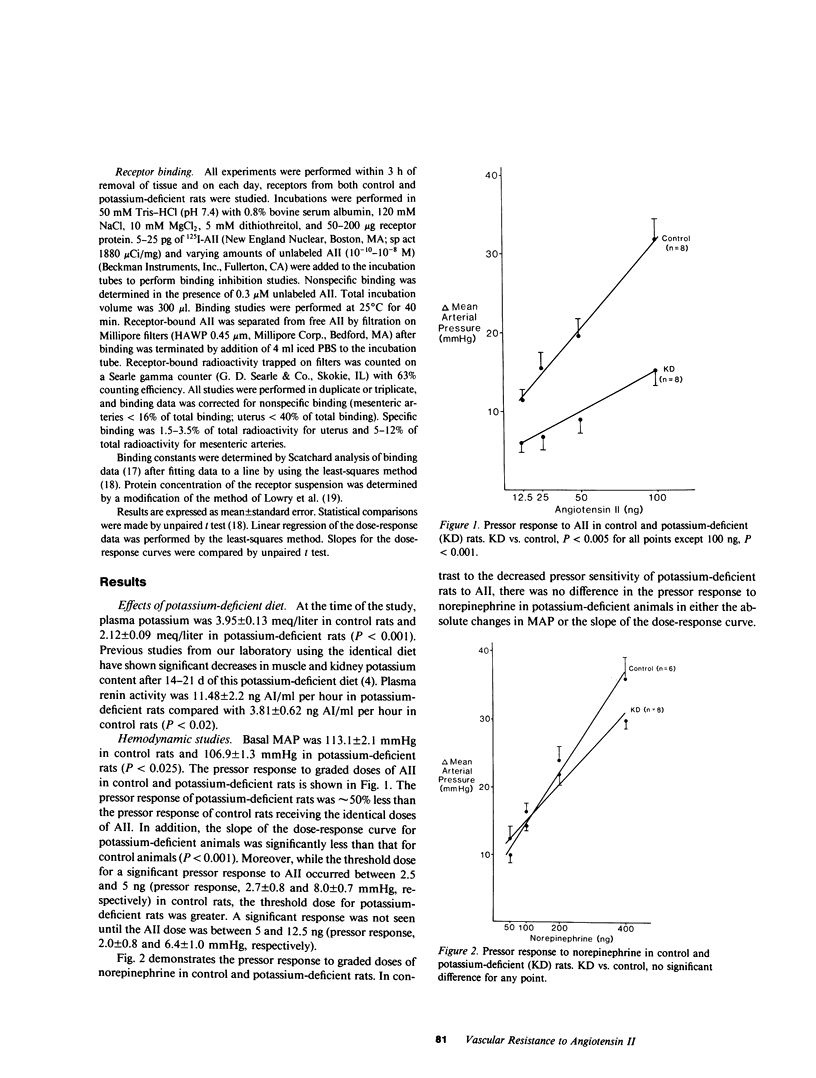
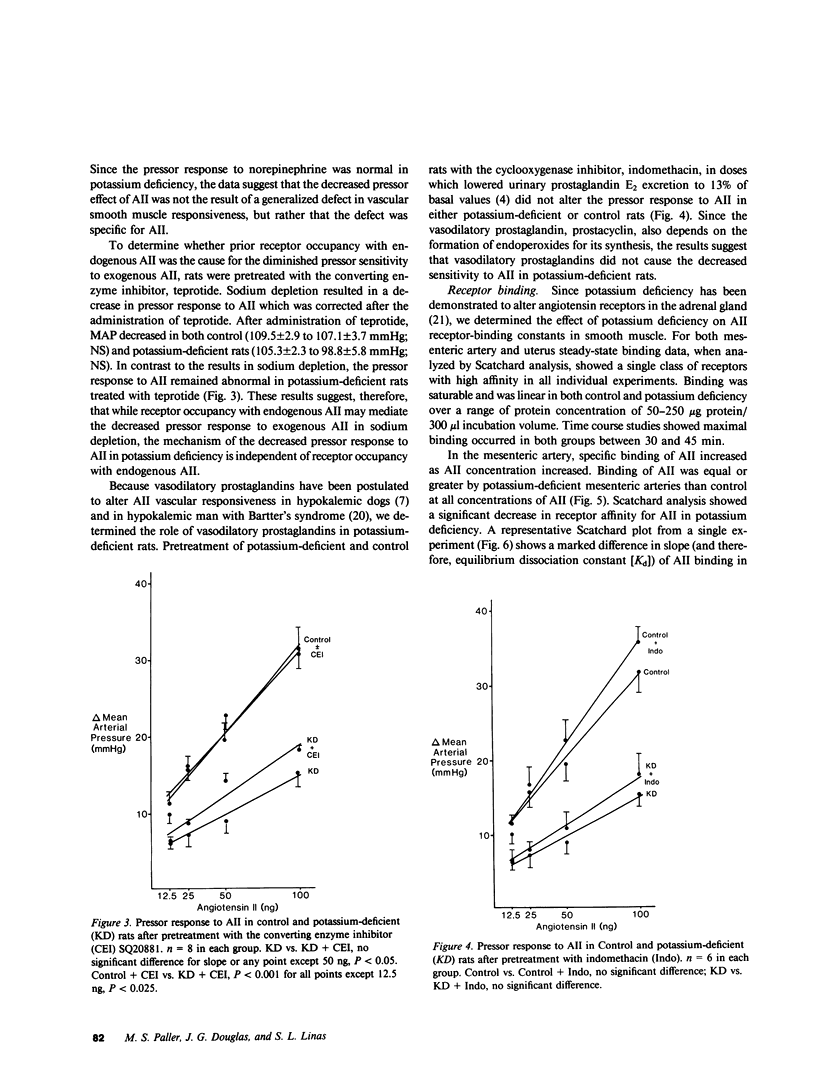
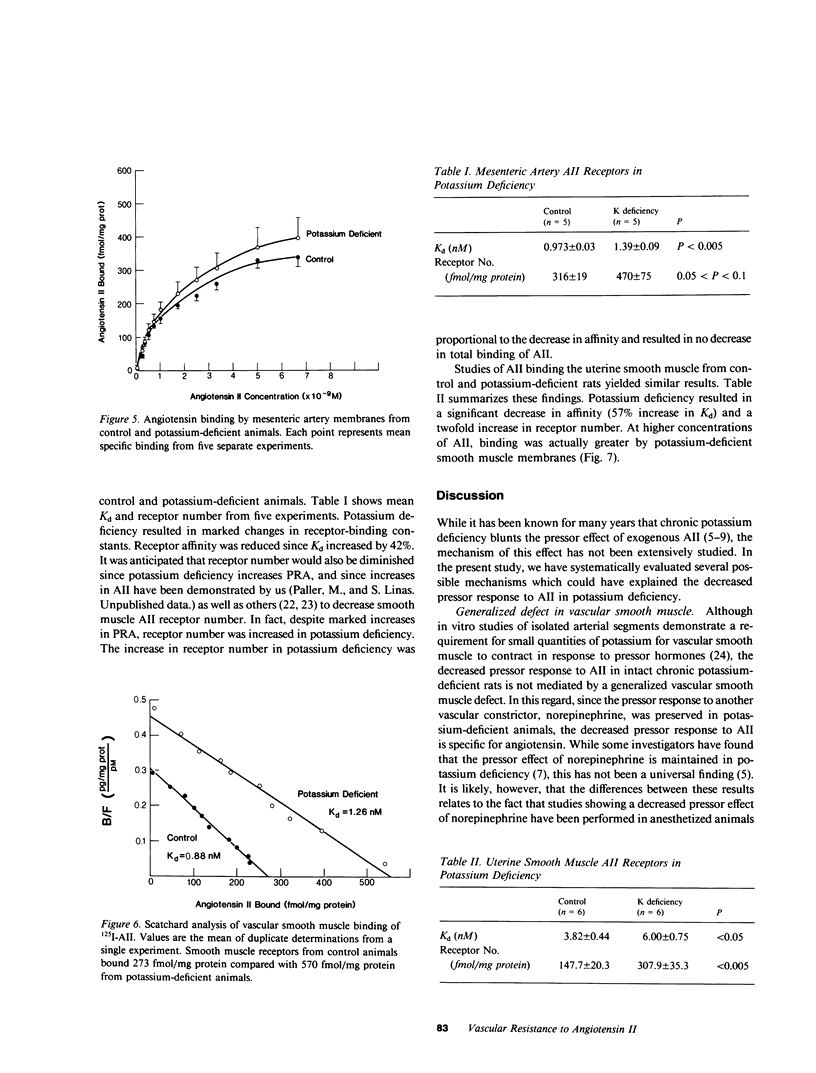
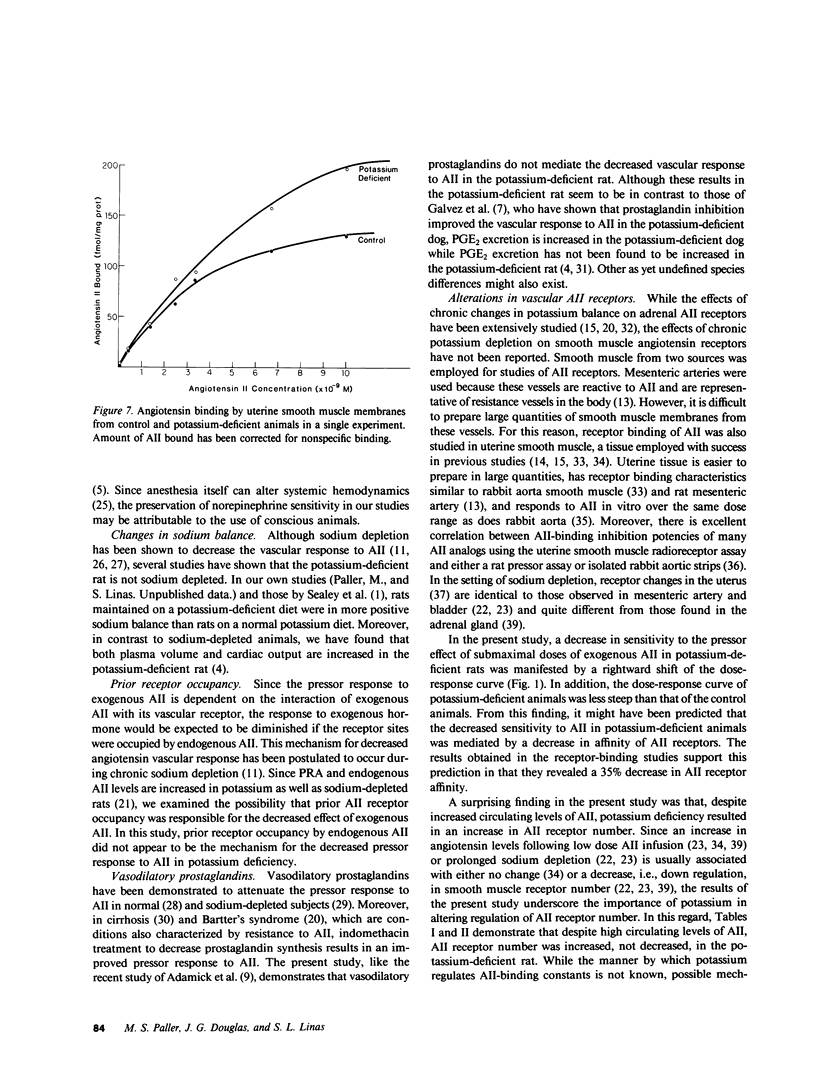
Chronic potassium deficiency in the rat results in a decrease in the pressor sensitivity to exogenous angiotensin II (AII). To define the mechanism of this resistance to AII, studies were performed in conscious rats after 14-21 d of dietary potassium deficiency. The pressor response to graded doses of AII was 50% less in potassium-deficient than control animals. In contrast, the pressor response to graded doses of norepinephrine was preserved in potassium-deficient rats; therefore, the decreased response to AII was not due to a generalized defect in vascular reactivity. Pretreatment with either the converting enzyme inhibitor, teprotide, or the prostaglandin synthesis inhibitor, indomethacin, failed to normalize the response to AII. Thus, neither prior receptor occupancy with endogenous AII nor the presence of vasodilatory prostaglandins caused the decreased AII response in potassium deficiency. Since the pressor response to AII involves angiotensin interaction with its vascular receptor, binding studies of mesenteric artery and uterine smooth muscle AII receptors were performed. Scatchard analysis showed that potassium deficiency resulted in a decrease in binding affinity (50% increase in Kd) in both uterine (6.00 vs. 3.82 nM; P less than 0.05) and vascular (1.39 vs. 0.973 nM; P less than 0.005) smooth muscle. Furthermore, despite increased circulating AII, there was an increase in AII receptor number in potassium-deficient uterine (308 vs. 147 fmol/mg protein; P less than 0.005) and vascular (470 vs. 316 fmol/mg protein; 0.05 less than P less than 0.1) smooth muscle. Although potassium deficiency resulted in alterations in receptor-binding parameters, the changes in binding affinity and number were directionally opposite, so that in potassium deficiency there was either no change or an increase in total AII binding. We conclude that the decrease in angiotensin pressor sensitivity in potassium-deficient rats is mediated by a postreceptor defect since it occurs subsequent to the binding of AII to its vascular smooth muscle receptor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamick R., Gold M. E., Hayes S., Coleman R., McCreary J. T., Sabatini S., Arruda J. A., Kurtzman N. A. Factors influencing vascular hyporesponsiveness to angiotensin II. Circ Res. 1981 Oct;49(4):932–939. doi: 10.1161/01.res.49.4.932. [DOI] [PubMed] [Google Scholar]
- Aguilera G., Catt K. Regulation of vascular angiotensin II receptors in the rat during altered sodium intake. Circ Res. 1981 Sep;49(3):751–758. doi: 10.1161/01.res.49.3.751. [DOI] [PubMed] [Google Scholar]
- Aguilera G., Hauger R. L., Catt K. J. Control of aldosterone secretion during sodium restriction: adrenal receptor regulation and increased adrenal sensitivity to angiotensin II. Proc Natl Acad Sci U S A. 1978 Feb;75(2):975–979. doi: 10.1073/pnas.75.2.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. M., Gimbrone M. A., Jr, Alexander R. W. Angiotensin II stimulates phosphorylation of the myosin light chain in cultured vascular smooth muscle cells. J Biol Chem. 1981 May 25;256(10):4693–4696. [PubMed] [Google Scholar]
- Baxter J. D., Funder J. W. Hormone receptors. N Engl J Med. 1979 Nov 22;301(21):1149–1161. doi: 10.1056/NEJM197911223012104. [DOI] [PubMed] [Google Scholar]
- Bohr D. F. Vascular smooth muscle updated. Circ Res. 1973 Jun;32(6):665–672. doi: 10.1161/01.res.32.6.665. [DOI] [PubMed] [Google Scholar]
- Burks T. F., Spalding C. T., Jones V. D. Influence of sodium and potassium content on arterial responsiveness. Circ Res. 1971 Nov;29(5):525–533. doi: 10.1161/01.res.29.5.525. [DOI] [PubMed] [Google Scholar]
- Campbell W. B., Schmitz J. M. Effect of alterations in dietary potassium on the pressor and steroidogenic effects of angiotensins II and III. Endocrinology. 1978 Dec;103(6):2098–2104. doi: 10.1210/endo-103-6-2098. [DOI] [PubMed] [Google Scholar]
- Deth R., van Breemen C. Agonist induced release of intracellular Ca2+ in the rabbit aorta. J Membr Biol. 1977 Jan 28;30(4):363–380. doi: 10.1007/BF01869677. [DOI] [PubMed] [Google Scholar]
- Devynck M. A., Meyer P. Angiotensin receptors in vascular tissue. Am J Med. 1976 Nov;61(5):758–767. doi: 10.1016/0002-9343(76)90157-1. [DOI] [PubMed] [Google Scholar]
- Devynck M. A., Rouzaire-Dubois B., Chevillotte E., Meyer P. Variations in the number of uterine angiotensin receptors following changes in plasma angiotensin levels. Eur J Pharmacol. 1976 Nov;40(1):27–37. doi: 10.1016/0014-2999(76)90350-2. [DOI] [PubMed] [Google Scholar]
- Douglas J. G., Brown G. P. Effect of prolonged low dose infusion of angiotensin II and aldosterone on rat smooth muscle and adrenal angiotensin II receptors. Endocrinology. 1982 Sep;111(3):988–992. doi: 10.1210/endo-111-3-988. [DOI] [PubMed] [Google Scholar]
- Douglas J. G., Michailov M., Khosla M. C., Bumpus F. M. Comparative receptor-binding properties of heptapeptide and octapeptide antagonists of angiotensin II in rat adrenal glomerulosa and uterine smooth muscle. Endocrinology. 1980 Jan;106(1):120–124. doi: 10.1210/endo-106-1-120. [DOI] [PubMed] [Google Scholar]
- Douglas J. G. Potassium ion as a regulator of adrenal angiotensin II receptors. Am J Physiol. 1980 Nov;239(5):E317–E321. doi: 10.1152/ajpendo.1980.239.5.E317. [DOI] [PubMed] [Google Scholar]
- Douglas J., Catt K. J. Regulation of angiotensin II receptors in the rat adrenal cortex by dietary electrolytes. J Clin Invest. 1976 Oct;58(4):834–843. doi: 10.1172/JCI108536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J., Hansen J., Catt K. J. Relationships between plasma renin activity and plasma aldosterone in the rat after dietary electrolyte changes. Endocrinology. 1978 Jul;103(1):60–65. doi: 10.1210/endo-103-1-60. [DOI] [PubMed] [Google Scholar]
- FREED S. C., FRIEDMAN M. Depressor effect of potassium restriction on blood pressure of the rat. Proc Soc Exp Biol Med. 1951 Oct;78(1):74–77. doi: 10.3181/00379727-78-18978. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Baukal A., Catt K. J. Angiotensin II receptors in bovine adrenal cortex. Modification of angiotensin II binding by guanyl nucleotides. J Biol Chem. 1974 Jan 25;249(2):664–666. [PubMed] [Google Scholar]
- Gunther S., Gimbrone M. A., Jr, Alexander R. W. Identification and characterization of the high affinity vascular angiotensin II receptor in rat mesenteric artery. Circ Res. 1980 Aug;47(2):278–286. doi: 10.1161/01.res.47.2.278. [DOI] [PubMed] [Google Scholar]
- Gunther S., Gimbrone M. A., Jr, Alexander R. W. Regulation by angiotensin II of its receptors in resistance blood vessels. Nature. 1980 Sep 18;287(5779):230–232. doi: 10.1038/287230a0. [DOI] [PubMed] [Google Scholar]
- Halushka P. V., Wohltmann H., Privitera P. J., Hurwitz G., Margolius H. S. Bartter's syndrome: urinary prostaglandin E-like material and kallikrein; indomethacin effects. Ann Intern Med. 1977 Sep;87(3):281–286. doi: 10.7326/0003-4819-87-3-281. [DOI] [PubMed] [Google Scholar]
- Hood V. L., Dunn M. J. Urinary excretion of prostaglandin E2 and prostaglandin F2alpha in potassium-deficient rats. Prostaglandins. 1978 Feb;15(2):273–280. doi: 10.1016/0090-6980(78)90166-1. [DOI] [PubMed] [Google Scholar]
- Kwan C. Y., Garfield R., Daniel E. E. An improved procedure for the isolation of plasma membranes from rat mesenteric arteries. J Mol Cell Cardiol. 1979 Jul;11(7):639–659. doi: 10.1016/0022-2828(79)90378-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linas S. L., Berl T., Aisenbrey G. A., Better O. S., Anderson R. J. The effect of anesthesia on hemodynamics and renal function in the rat. Pflugers Arch. 1980 Mar;384(2):135–141. doi: 10.1007/BF00584429. [DOI] [PubMed] [Google Scholar]
- Linas S. L., Dickmann D. Mechanism of the decreased renal blood flow in the potassium-depleted conscious rat. Kidney Int. 1982 May;21(5):757–764. doi: 10.1038/ki.1982.94. [DOI] [PubMed] [Google Scholar]
- McCaleb M. L., Donner D. B. Affinity of the hepatic insulin receptor is influenced by membrane phospholipids. J Biol Chem. 1981 Nov 10;256(21):11051–11057. [PubMed] [Google Scholar]
- Negus P., Tannen R. L., Dunn M. J. Indomethacin potentiates the vasoconstrictor actions of angiotensin II in normal man. Prostaglandins. 1976 Aug;12(2):175–180. doi: 10.1016/0090-6980(76)90111-8. [DOI] [PubMed] [Google Scholar]
- ROSENMAN R. H., FREED C., FRIEDMAN M. The peripheral vascular reactivity of potassium-deficient rats. Circulation. 1952 Mar;5(3):412–414. doi: 10.1161/01.cir.5.3.412. [DOI] [PubMed] [Google Scholar]
- Reid W. D., Laragh J. H. Sodium and potassium intake, blood pressure, and pressor response to angiotensin. Proc Soc Exp Biol Med. 1965 Oct;120(1):26–29. doi: 10.3181/00379727-120-30434. [DOI] [PubMed] [Google Scholar]
- Rioux F., Park W. K., Regoli D. Application of drug-receptor theories to angiotensin. Can J Physiol Pharmacol. 1973 Sep;51(9):665–672. doi: 10.1139/y73-100. [DOI] [PubMed] [Google Scholar]
- Rouzaire-Dubois B., Devynck M. A., Chevillotte E., Meyer P. Angiotensin receptors in rat uterine membranes. FEBS Lett. 1975 Jul 15;55(1):168–172. doi: 10.1016/0014-5793(75)80985-9. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Clark I., Bull M. B., Laragh J. H. Potassium balance and the control of renin secretion. J Clin Invest. 1970 Nov;49(11):2119–2127. doi: 10.1172/JCI106429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack B. L., Ledingham J. M. The influence of sodium intake on the pressor response to angiotensin II in the unanaesthetized rat. Clin Sci Mol Med. 1976 Apr;50(4):285–291. doi: 10.1042/cs0500285. [DOI] [PubMed] [Google Scholar]
- Song C. S., Bodansky O. Subcellular localization and properties of 5'-nucleotidase in the rat liver. J Biol Chem. 1967 Feb 25;242(4):694–699. [PubMed] [Google Scholar]
- Speckart P., Zia P., Zipser R., Horton R. The effect of sodium restriction and prostaglandin inhibition on the renin-angiotensin system in man. J Clin Endocrinol Metab. 1977 May;44(5):832–837. doi: 10.1210/jcem-44-5-832. [DOI] [PubMed] [Google Scholar]
- Stockigt J. R., Collins R. D., Biglieri E. G. Determination of plasma renin concentration by angiotensin I immunoassay. Diagnotic import of precise measurement of subnormal renin in hyperaldosteronism. Circ Res. 1971 May;28(5 Suppl):175–191. doi: 10.1161/01.res.28.5.ii-175. [DOI] [PubMed] [Google Scholar]
- Strewler G. J., Hinrichs K. J., Guiod L. R., Hollenberg N. K. Sodium intake and vascular smooth muscle responsiveness to norepinephrine and angiotensin in the rabbit. Circ Res. 1972 Nov;31(5):758–766. doi: 10.1161/01.res.31.5.758. [DOI] [PubMed] [Google Scholar]
- Thurston H., Laragh J. H. Prior receptor occupancy as a determinant of the pressor activity of infused angiotensin II in the rat. Circ Res. 1975 Jan;36(1):113–117. doi: 10.1161/01.res.36.1.113. [DOI] [PubMed] [Google Scholar]
- Vesely D. L. Angiotensin II stimulates guanylate cyclase activity in aorta, heart, and kidney. Am J Physiol. 1981 Apr;240(4):E391–E393. doi: 10.1152/ajpendo.1981.240.4.E391. [DOI] [PubMed] [Google Scholar]
- Zipser R. D., Hoefs J. C., Speckart P. F., Zia P. K., Horton R. Prostaglandins: modulators of renal function and pressor resistance in chronic liver disease. J Clin Endocrinol Metab. 1979 Jun;48(6):895–900. doi: 10.1210/jcem-48-6-895. [DOI] [PubMed] [Google Scholar]



