Abstract
It is important to identify predictors of psychological health among breast cancer patients that can be relatively easily identified by medical care providers. This article investigates the role of one class of such potential predictors: easily identified demographics that have potential social and/ or practical implications. Specifically, we examined whether income, marital status, presence of children in the home, education, travel distance, age and rurality interact with time to predict psychological health over the first year post diagnosis. Two hundred and twenty five breast cancer patients receiving radiation treatment completed four surveys over the course of 13 months that included measures of both their physical health and depressive symptoms. The results revealed that women who were not married had children living in the home or had to travel long distances to receive radiation treatment reported higher levels of depressive symptoms across the entire study. Women with lower incomes reported increased depressive symptoms, but only after the completion of treatment. Younger women reported elevated depressive symptoms during initial treatment, but this effect dissipated after the completion of treatment. The current results suggest that demographic patient characteristics may indeed be useful in identifying both when and for whom depressive symptoms are particularly likely to be problematic.
Keywords: breast cancer, depression, income, marital status, distance, children
Introduction
A substantial number of women experience various forms of psychological distress following breast cancer diagnosis and treatment (e.g., Gallagher, Parle, & Cairns, 2002; Ganz et al., 2004; Hagedoorn, Sanderman, Bolks, Tunistra, & Coyne, 2008; Primo et al., 2000). Depressive symptoms are a particularly important form of distress to consider, given their association with a decreased willingness to comply with medical regimens, decreased immune functioning and increased mortality risk (Andersen, Kicolt-Glaser, & Glaser, 1994; Cohen & Hebert, 1996; DiMatteo, Lepper, & Croghan, 2000; Hjerl et al., 2003). Although studies typically report a linear decrease in depressive symptoms (and other forms of psychological distress) over time (Manne, Ostroff, Winkel, Grana, & Fox, 2005; Stommel, Kurtz, Kurtz, Given, & Given, 2004), a non-trivial number of breast cancer patients report elevated depressive symptoms for extended periods of time (Bower, 2008; Glanz & Lerman, 1992; Helgeson, Snyder, & Seltman, 2004; Hughes, 1982; Irvine, Brown, Crooks, Roberts, & Browne, 1991; Michael, Kawachi, Berkman, Holmes, & Coliditz, 2000). Thus, it is vital to identify risk factors that predict both who is at risk for experiencing depressive symptoms as well as when these persons are more likely to report depressive symptoms.
A number of previous studies have sought to address this aim by identifying psychosocial predictors of psychological health, such as coping (Alferi, Culver, Carver, Arena, & Antoni, 1999; Epping-Jordan et al., 1999; Stanton & Snider, 1993) and social support (Helgeson & Cohen, 1996). Undoubtedly, these factors are important; however, medical personnel are unlikely to have access to this type of information. In contrast, patient’s demographic information (such as socio-economic or marital status) is much more readily accessible. This is important because medical personnel are uniquely positioned to provide some forms of resources, aid and support to their patients. Thus, it seems critical to identify patient characteristics that predict both depressive symptoms and are readily accessible to medical personnel.
Perhaps the most intuitive predictors of psychological functioning that medical personnel can easily access would be those related to physical health, such as diagnosis and treatment. Surprisingly, however, studies show few, if any, associations between disease severity or treatments and depressive symptoms. For example, depressive symptoms do not differ between patients who receive breast conserving surgery and patients who receive mastectomies (Bardwell et al., 2006; Ganz, Schag, Lee, Polinsky, & Tan, 1992; Pozo et al., 1992; Scheier & Helgeson, 2006), between patients taking tamoxifen and patients who are not (Day, Ganz, & Costantino, 1999; Fallowfleld et al., 2001; Love, Cameron, Connel, & Leventhal, 1991), or between patients with different stages of cancer (Bardwell et al., 2006; DeShields, Tibbs, Fan, & Taylor, 2006; Helgeson et al., 2004; Scheier & Helgeson, 2006). Consistent with this, in a review of the literature, Bower (2008) concluded that severity of disease and treatment regimens likely have little to do with depressive symptoms compared to the influence of other factors, indicating that this knowledge might not be the most useful for discerning who might be most at risk of experiencing depressive symptoms.
Thus, we chose to explore an alternative group of potential predictors of depressive symptoms that could also be easily identified by medical personnel: demographics. Most previous research treats demographics as covariates or as descriptors of a sample (e.g., Hack et al., 2010; Schnoll, Harlow, Brandt, & Stolbach, 1998; Stanton, Danoff-Burg, & Huggins, 2002; Stanton & Snider, 1993), but it is also useful to consider them as independent predictors that have the potential to characterise the experiences of breast cancer patients. For example, a breast cancer patient with children living in the home may have a fundamentally different experience than her counterpart who does not. As another example, women with relatively high incomes may experience less distress than women with lower incomes faced with worries about how they will pay for mounting medical bills or cope with the loss of income than can result from lost time at work. These types of demographic characteristics may be useful in predicting the level of depressive symptoms a woman is likely to experience in response to breast cancer diagnosis, treatment and survivorship.
The extant literature suggests several demographics (each of which has a number of social/practical implications) that might be useful in this regard. Income (Shimozuma, Ganz, Peterson, & Hirji, 1999), education (Hack et al., 2010; Schnoll, Knowles, & Harlow, 2002; Shimozuma et al., 1999), age (Kroenke et al., 2004; Stanton & Snider, 1993; Stanton et al., 2000; Williamson, 2000) and being married (Schnoll et al., 2002) all appear to be inversely related to distress, whereas the presence of children in the home is associated with increased distress (Bloom, Stewart, Chang, & Banks, 2004; DeShields et al., 2006). Importantly, however, studies that examine more than one of these demographics in a single study are rare. Rarer still are studies that examine the influence of multiple demographics over time.1 This study aimed to extend our understanding of these patient characteristics by simultaneously examining multiple demographics and by examining their influence longitudinally. This is important because these demographics are likely to be correlated (e.g. education and income) and because their influence may change over time.
We also aimed to extend the findings in the current literature by investigating two often overlooked demographics with important implications for psychological health: rural residence and distance travelled for treatment. Living in a rural area has the potential to pose a number of unique obstacles for breast cancer patients, including decreased access to information and resources and increased potential for stigmatising experiences (Bettencourt, Schlegel, Talley, & Molix, 2007; Schlegel, Talley, Molix, & Bettencourt, 2009). Similarly, several (mostly qualitative) studies (Beaulieu, Massey, Tucker, Schoenberg, & Ross, 2003; Davis, Girgis, Williams, & Beeney, 1998; Davis, Williams, Redman, White, & King, 2003; Girgis, Boyes, Sanson-Fisher, & Burrows, 2000; Gray, James, Manthorne, Gould, & Fitch, 2004) have argued that there are a variety of social and economic costs (e.g. disruptions in family life and employment) associated with travelling long distances for breast cancer treatment. Importantly, distance travelled and rurality are not perfectly correlated. Some treatment facilities are located in smaller towns and rural areas, so it is possible to live in a rural area and still be relatively close to an oncologist and treatment facility. Thus, we thought it was important to examine both of these potential relationships with depressive symptoms.
In summary, we examined a number of demographic characteristics that had the potential to identify who is most likely to experience depressive symptoms following a breast cancer diagnosis as well as when in the first year following treatment these depressive symptoms are most likely to occur. Although these analyses do not examine the influence of disease and treatment factors, per se, it is important to note that all of the analyses controlled for self-reported physical health (i.e. physical symptoms), thereby ensuring that the findings could not be explained by underlying associations with poorer physical health.
Method
Participants and procedure
Two hundred and twenty five participants were recruited from nine radiation clinics in Missouri. An oncology nurse at participating clinics provided the baseline survey (Wave 1) to eligible patients during their first week of radiation treatment. Eligible patients were (a) diagnosed with breast cancer, (b) 18 years of age or older, (c) female and (d) English speaking. Three follow-up surveys were mailed directly to participants approximately three (Wave 2), seven (Wave 3) and 13 months (Wave 4) later. Participants were paid $25 for each completed survey. Two hundred and seven (92% of the initial sample) participants returned the second survey, 205 (91.1%) returned the third survey and 203 (90.2%) returned the fourth survey. Analyses were conducted that compared participants who completed all of the surveys (n=190) to participants who failed to complete at least one survey assessment (n = 35) on all of the demographic and symptom variables. By and large, these analyses suggested that there were no differences between completers and non-completers on these variables. There was, however, one trend that suggested participants who failed to complete all four surveys tended to have lower incomes than those who completed all four waves of the study (t(223) = −1.94, p < 0.06). Notably, however, the non-completers did not differ from completers in depressive symptoms at Wave 1. Also, less than half of the 35 ‘non-completers’ failed to complete all three of the follow up surveys (n = 14), suggesting that the vast majority of participants contributed multiple data points to the data set.
The broader purpose of this longitudinal study was to identify psychosocial predictors of rural women’s physical and mental health during the first year of survivorship. As such, the surveys also included other measures that are outside the scope of the current report such as social support, coping strategies, health locus of control and patient satisfaction. Other reports using the data from these measures has have been published previously (Bettencourt, Talley, Molix, & Schlegel, 2008; Schlegel et al., 2009; Talley, Molix, Schlegel, & Bettencourt, 2010).
Measures
Demographic and medical information
Table 1 includes a summary of the demographic variables for the sample as well as information about medical/treatment variables. Annual household income was reported on a 12-point categorical scale (percentages are reported on a 6-point scale for ease of presentation in Table 1). Participants self-reported their relationship status, the distance between their home and radiation clinic in miles (one way), and the number of children living in the home (which was coded as a dichotomous variable for the primary analyses; Bloom et al., 2004).
Table 1.
Baseline characteristics of participants
| Variable | n | % | M | SD | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Age | 57.04 | 12.49 | 27 | 91 | ||
| Rurality | 0.003 | 0.93 | −1.11 | 2.32 | ||
| Miles travelled | 29.79 | 35.25 | 1 | 244 | ||
| Marital status | ||||||
| Married/Cohabitating | 158 | 70.2 | ||||
| Unmarried | 67 | 29.8 | ||||
| Race/Ethnicity | ||||||
| White | 204 | 92.9 | ||||
| Black | 7 | 3.1 | ||||
| American Indian | 5 | 2.2 | ||||
| Other | 2 | 0.8 | ||||
| Income | ||||||
| Less than $15,000 | 40 | 17.8 | ||||
| 15–35K | 66 | 29.4 | ||||
| 35–55K | 44 | 19.5 | ||||
| 55–75K | 27 | 11.7 | ||||
| 75–95K | 22 | 9.8 | ||||
| Over 95,000 | 26 | 11.5 | ||||
| Children in household | ||||||
| Yes | 84 | 34.1 | ||||
| No | 156 | 63.4 | ||||
| Stage | ||||||
| 0 | 43 | 19.6 | ||||
| I | 99 | 43.6 | ||||
| II | 46 | 20.4 | ||||
| III–IVa | 37 | 15.1 | ||||
| Tamoxifen | ||||||
| Yes | 156 | 63.4 | ||||
| No | 89 | 36.2 | ||||
| Chemotherapy | ||||||
| Yes | 186 | 75.6 | ||||
| No | 59 | 24.0 | ||||
| Depressive symptoms | ||||||
| Wave 1 | 12.37 | 8.98 | ||||
| Wave 2 | 10.70 | 9.86 | ||||
| Wave 3 | 10.09 | 8.94 | ||||
| Wave 4 | 10.82 | 10.22 | ||||
| Physical symptoms | ||||||
| Wave 1 | 2.17 | 0.74 | ||||
| Wave 2 | 2.14 | 0.73 | ||||
| Wave 3 | 2.01 | 0.62 | ||||
| Wave 4 | 2.14 | 0.73 | ||||
Notes:
There were only four women in the sample diagnosed with stage IV; thus, we combined these into one group. Due to missing data, total for some variables is not equal to full sample n (225). Wave 1 = beginning of radiation treatment, Wave 2 = 3 months later, Wave 3 = 7 months later, Wave 4 = 13 months later.
Rurality was quantified with a continuous variable that we have used in our previous research (Schlegel et al., 2009). It is a composite of the population of a participant’s hometown (recorded from 2000 census data) and the rural-urban continuum code for the corresponding county (from the United States Department of Agriculture). County continuum codes are based on a county’s population, degree of urbanisation and adjacency to a metropolitan area. Both indices of rurality (county code and city population) were standardised and then averaged. To give a sense of what the values on this index mean, the most rural participant (i.e. the participant with the highest score) lived in a town with a population of 145 and county with the most rural continuum code. The most urban participants (i.e. those with the lowest score) lived in a city of 441,545 and a county with the most urban continuum code. Women at the median lived in a town of 17,757 and a county with a continuum code in the middle of the scale. More details about this variable can be found in our previous research (Schlegel et al., 2009).
Physical symptoms
The measure of physical symptoms included 16 items chosen for their appropriateness for the sample. A 7-point scale was used, ranging in severity from 0 (not at all) to 6 (severe; α = 0.90). The symptoms included: fatigue, appetite loss, nausea, breast pain, hot flashes, shoulder pain, hair loss, arm pain, decreased movement of the arm, weight gain, itchiness or discomfort of the skin, chest wall pain, weight loss, redness of the skin, blistering or draining of the skin and swelling of the arm. The average of all 16 items was used as our measure of physical symptoms (descriptive statistics are reported in Table 1).
Depressive symptoms
Depressive symptoms were measured using the Center for Epidemiologic Studies-Depression Scale (Radloff, 1977). On this 20-item scale, participants rated the intensity and frequency of depressive symptoms they had experienced in the previous week on a 4-point scale ranging from 0 (rarely or none of the time) to 3 (most or all of the time; α = 0.87). Example items include, ‘I felt sad’ and ‘I had crying spells’. Following previous research, all participants’ scores were summed. Participants missing more than four items on the scale were excluded and person mean imputation was performed for the participants who were missing between 1 and 3 items (Callahan & Wolinsky, 1994; Ried, Tueth, Handberg, Kupfer, & Pepine, 2005, descriptive statistics are reported in Table 1). At Wave 1 (during treatment), 27.9% of the participants reported clinically significant levels of depressive symptoms (CES-D score >/= 16, Myers & Weissman, 1980), at Waves 2 (3 months later), 3 (7 months later) and 4 (13 months later), 25.7%, 23.2% and 24.2% of patients, respectively, reported clinically significant levels of depressive symptoms.
Analytic plan
Given that scores for depression were not independent within persons, multilevel modelling (MLM) was used to account for within-person dependence and between-person change over time in depression. MLM also accommodates unequal numbers of observations and unequal time intervals between the waves of data collection. MLM fixed-effect estimates can be interpreted similar to regression coefficients in OLS regression (Raudenbush & Bryk, 2002; Snijders & Bosker, 1999).
Level 1 of the MLM (within-person level) included the depression measure at each wave predicted from linear and quadratic components of time and from physical symptoms. The physical symptoms variable was centred on its grand mean (mean physical symptoms across women over all waves). For the model to be estimated, the unexplained variance in depression (rij) at each wave was fixed using an estimate calculated from its variance and reliability (1-Cronbach α) x var. The equations at level 1 assumed the following form:
In interpreting the coefficients, β0j represents mean depression at the first wave (i.e. during treatment) for patients reporting average levels of physical symptoms, β1j represents mean linear rate of change in depression, β2j represents mean quadratic rate of change in depression and β3j represents the mean effect of physical symptoms on depression over the four waves.
Level 2 of the MLM (between-person level) included the patient demographic characteristics hypothesised to affect depression trajectories. To arrive at a final model, model fitting was completed in the following order. First, a baseline model was fit that included level 1 predictors only. Next, all level 2 predictors were specified to predict mean depression (β0j) at Wave 1, with each level 2 predictor added to the linear (β1j) and quadratic (β2j) components of the trajectory in a separate series of models to see whether they were predictors of the trajectory on their own; where predictors were significant, they were included in the final model. This strategy was adopted as some variables occluded significant relationships between other variables and the outcomes when they were all simultaneously included in the model; however, given that the coefficients for the linear and quadratic components of time define a single trajectory, if a variable was a significant predictor of one component, it was included as a predictor of the other component. All analyses were conducted in HLM 6.02 (Raudenbush, Bryk, & Congdon, 2000).
Results
Baseline model
The coefficients for both components (linear and quadratic) of the trajectory and the coefficient for physical symptoms were significant. As shown in Figure 1, the baseline model closely resembled the plot of the actual data. The nature of this trajectory suggested that women reported decreased depressive symptoms over time. However, there was some suggestion that women reported depressive symptoms that were slightly elevated at the final wave (i.e. 13-month follow up) of data collection, rather than decreased symptoms from Wave 3 (as a simple linear trend would suggest).
Figure 1.
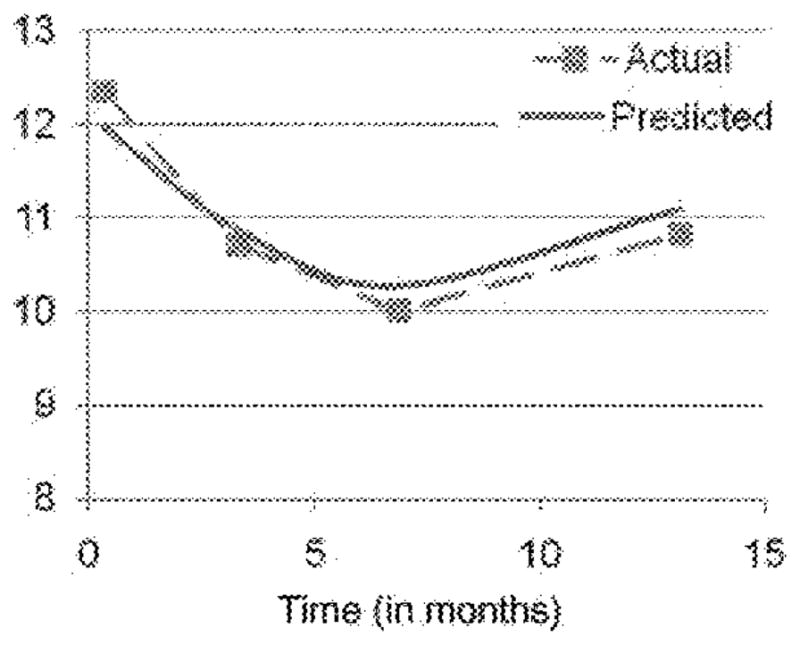
The average predicted trajectories of depressive symptoms over the course of the study compared to the observed means at each wave.
Final model
As indicated by the difference in deviance statistics, the final model was a significant improvement over the baseline model (Δχ2(13) = 49.64, p < 0.001). Level 2 of the final model assumed the following form:
Parameters will be interpreted for the final model only. The parameters for the final model are presented in Table 2.
Table 2.
Fixed and random effects
| Fixed effect | Coefficient | SE | t | df | p | |
|---|---|---|---|---|---|---|
| Intercept | ||||||
| Intercept | γ00 | 14.42 | 1.66 | 8.70 | 211 | 0.00 |
| Marital status | γ01 | −2.89 | 1.32 | −2.20 | 211 | 0.03 |
| Rurality | γ02 | 0.43 | 0.61 | 0.71 | 211 | 0.48 |
| Income | γ03 | −0.15 | 0.24 | −0.64 | 211 | 0.52 |
| Education | γ04 | −0.18 | 0.44 | −0.41 | 211 | 0.69 |
| Children in home | γ05 | 3.14 | 1.20 | 2.61 | 211 | 0.01 |
| Miles from radiation clinic | γ06 | 0.94 | 0.55 | 1.71 | 211 | 0.09 |
| Age | γ07 | −1.94 | 0.70 | −2.77 | 211 | 0.01 |
| Month (linear component) | ||||||
| Intercept | γ10 | −0.32 | 0.39 | −0.82 | 215 | 0.41 |
| Income | γ11 | −0.13 | 0.06 | −2.33 | 215 | 0.02 |
| Education | γ12 | 0.17 | 0.11 | 1.47 | 215 | 0.14 |
| Age | γ13 | 0.38 | 0.17 | 2.26 | 215 | 0.03 |
| Month squared (quadratic component) | ||||||
| Intercept | γ20 | 0.03 | 0.03 | 1.00 | 215 | 0.32 |
| Income | γ21 | 0.01 | 0.00 | 1.80 | 215 | 0.07 |
| Education | γ22 | −0.01 | 0.01 | −1.42 | 215 | 0.16 |
| Age | γ23 | −0.02 | 0.01 | −1.55 | 215 | 0.12 |
| Physical symptoms | ||||||
| Intercept | γ30 | 2.43 | 0.62 | 3.93 | 218 | 0.00 |
| Random effect | ||||||
| SD | Variance | χ2 | df | p | ||
| Intercept | U0 | 7.69 | 59.19 | 656.74 | 72 | 0.00 |
| Month (linear component) | U1 | 1.75 | 3.06 | 316.89 | 76 | 0.00 |
| Month squared (quadratic component) | U2 | 0.13 | 0.02 | 347.96 | 76 | 0.00 |
| Physical symptoms | U3 | 5.87 | 34.47 | 354.49 | 79 | 0.00 |
Sustained differences
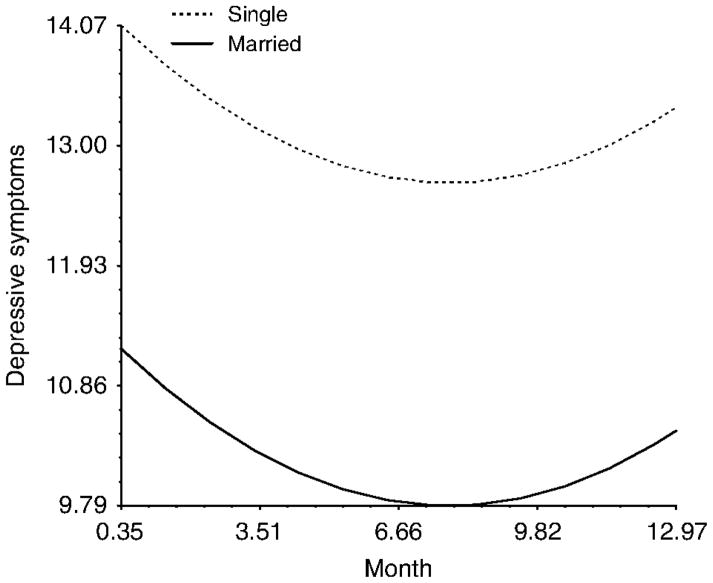
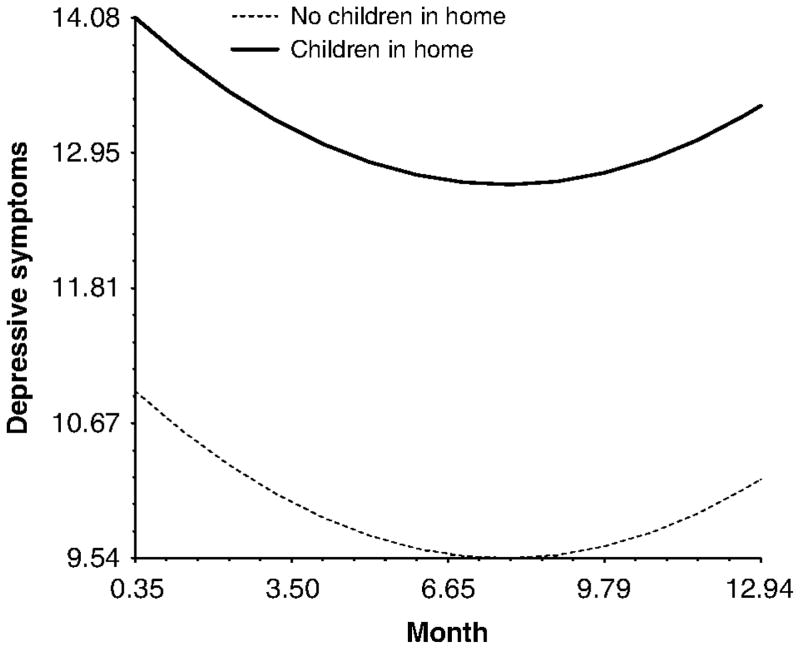
There were three predictors that influenced levels of depressive symptoms at Wave 1 (i.e. during treatment), but did not influence the trajectory of depressive symptoms. This group of predictors may be thought of as predictors of ‘sustained differences’ in depressive symptoms in that they lead to group differences in the initial values while having no effect on the trajectory of depressive symptoms (i.e. disparities were maintained across time between groups). An example of this type of pattern is shown in Figure 2 for marital status. As can be seen in the figure, married women reported less depressive symptoms than single women during treatment (i.e. at Wave 1), and this initial relative difference was sustained over the course of study. A similar pattern emerged for the presence of children in the home (Figure 3). Women who had at least one child living in the home experienced more depressive symptoms than those women who did not and these differences were sustained over time. Finally, there was a marginal effect of miles from radiation clinic that suggested that women who live farther from their radiation clinics are more likely to experience elevated depressive symptoms across the course of the study.
Figure 2.
The effect of marital status on depressive symptoms over the course of the study.
Figure 3.
The effect of children in the home on depressive symptoms over the course of the study.
Trajectory differences
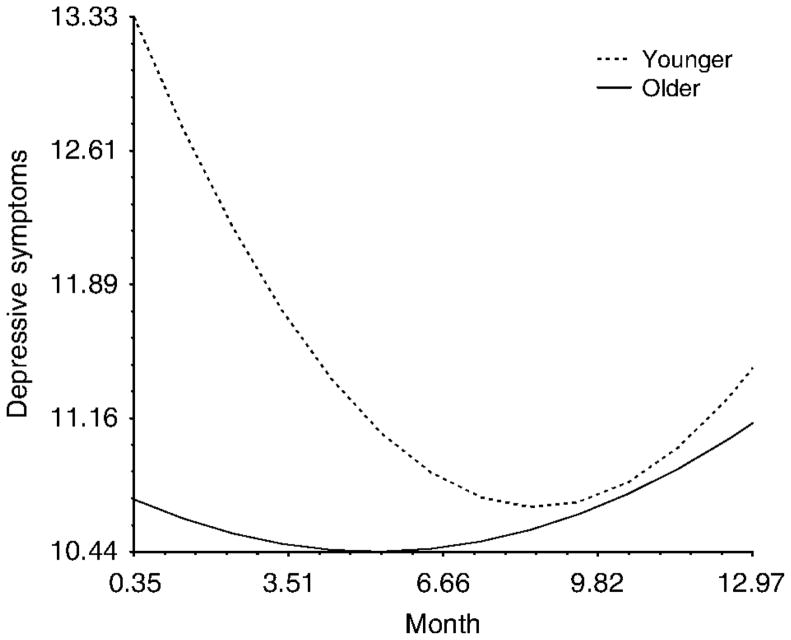
Two variables predicted differences in trajectories. First, age predicted initial values of depressive symptoms such that younger women reported elevated depressive symptoms during radiation treatment. However, the interaction between age and time suggested that younger women recovered from these early experiences with depressive symptoms and reported levels of depressive symptoms that were similar to their older counterparts in the final waves of the study (Figure 4).
Figure 4.
The interaction between age and time predicting depressive symptoms over the course of the study.
Note: While the difference in the linear rate of change was statistically significant, there was no difference in the quadratic component (i.e. the curve in the trajectory).
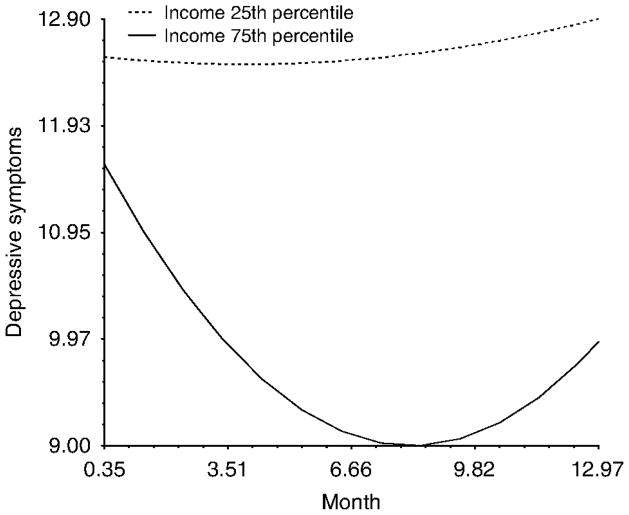
Income had no influence on depressive symptoms while women were in treatment (i.e. at Wave 1); however, income levels did predict differences in trajectories over time. Women with relatively lower incomes never experienced the expected decrease in depressive symptoms and reported a similar level of elevated depressive symptoms across the course of the entire study. Conversely, women with relatively higher incomes reported the expected trajectory of generally decreasing depressive symptoms (Figure 5).
Figure 5.
The interaction between income and time predicting depressive symptoms over the course of the study.
Note: While the difference in the linear rate of change was statistically significant, there was only a marginal difference in the quadratic component (i.e. the curve in the trajectory).
Discussion
This study explored the ways in which demographic patient characteristics could be used to identify both when and who is most likely to experience distress following a breast cancer diagnosis. In particular, we focused on patient characteristics that could be relatively easily identified by medical personnel, but were not related to disease or treatment.
Prior to exploring potential predictors of depressive symptoms, we examined the ‘average’ trajectory of depressive symptoms over time. This analysis revealed an overall quadratic trend. The nature of this curve suggested a decrease in depressive symptoms between the beginning of treatment and 3 months later (i.e. between Waves 1 and 2), with more similar levels of depressive symptoms for the following 10 months. This finding suggests that the completion of active treatment reduces psychological distress among breast cancer survivors. The shape of the trajectory also suggested that depressive symptoms were somewhat elevated at 13 months, especially when compared to the pattern that would be observed if the relationship between time and depression was simply linear. A variety of factors might explain this pattern. For example, even women with initially strong social support may find that this support falters as time since diagnosis increases (Lethborg, Kissane, Burns, & Snyder, 2000). Fear of cancer recurrence may also be experienced by breast cancer survivors after the initial sense of relief after the completion of treatment fades (Maher, 1982; Ward, Viergutz, Tormey, deMuch, & Pualen, 1992).2
Importantly, however, the results showed variability in this trajectory across individuals and several of the demographic variables were useful predictors of deviations from this pattern. First, both marital status and the presence of children in the home predicted sustained disparities in depressive symptoms over time. Specifically, single women reported elevated depressive symptoms throughout the year, compared to their married counterparts, and women with at least one child in the home reported elevated depressive symptoms relative to their counterparts who had no children in the home. Both of these patterns are consistent with previous findings (Bloom et al., 2004; DeShields et al., 2006; Schnoll et al., 2002). However, the current findings offer an advance in our understanding. Whereas previous studies have typically examined these relationships at one time point, this study demonstrates that these effects may not dissipate after active treatment is complete and may last as long as 1 year post treatment (if not more). In the prior literature, the lasting influence of these variables on depressive symptoms has not been documented over such a long period of time. These findings suggest that both single women and women with children in the home may be in need of additional support across the entire year following breast cancer diagnosis and treatment. Though future research is needed to elucidate the exact reasons for these disparities, it may be that single women are in particular need of emotional support (i.e. communicating care and concern, Helgeson & Cohen, 1996) and their providers could suggest available outlets for receiving this type of social support (i.e. support groups, online discussion boards) or encourage them to reach out to family members and friends. Regarding women with children in the home, these women may be in particular need of instrumental support (i.e. providing material goods or practical assistance; Helgeson & Cohen, 1996) to help them manage the variety of demands associated with raising children. It may be that the physical and emotional strain of cancer survivorship exacerbates the pressures of the parental role over time. Accordingly, care providers could help women identify sources of practical support services for getting parental support in the homes.
Moreover, the results provided initial, tentative evidence that the number of miles a breast cancer patient travels to receive care influences her course of depressive symptoms. Specifically, women who lived further from their oncology providers reported somewhat elevated depressive symptoms throughout the entire study compared to their counterparts who lived closer to their oncology services. This finding is somewhat surprising because these differences would likely be expected to dissipate over time as breast cancer patients are no longer faced with the burden of frequent travel for treatment. Perhaps these findings suggest that breast cancer survivors’ perceptions of the relative accessibility of medical personnel who are cancer experts (e.g., Dunkel-Schetter, 1984) directly influence their experiences of depressive symptoms. Alternatively, some patients who travel farther to receive care may experience a loss of social networks that are established in the city in which treatment is received (e.g., Gray et al., 2004). Of course, some caution should be used in interpreting this finding, considering that the effect of distance did not quite meet typical cutoffs of statistical significance. Nonetheless, this finding is important in that this is the first study to quantitatively examine the effect of distance on psychological health among breast cancer patients. Future research is critical to determine the reliability of this pattern as well as the underlying explanations for it, as these findings have potentially immensely important practical implications. For example, breast cancer patients who live far from their oncology providers could be afforded regular access to their oncology care providers (i.e. through more frequent follow-up appointments or online/telephone conferencing). Alternatively, support groups (whether in person or online) specifically focused on the experience of having to receive cancer treatment far from home might be helpful. At the very least, patients could be made aware of the potential ‘risks’ to their mental health of living far from their treatment centre to help normalise their concerns or experiences.
The findings for household income suggest that women with lower incomes may be especially in need of aid after treatment is complete. Although women with different incomes reported similar levels of depressive symptoms during active treatment, those with higher incomes reported a decrease in those depressive symptoms over time. Women with lower incomes, however, did not experience this decrease and reported a level of depressive symptoms across the entire 13 months that was similar to what they reported during active treatment. Whereas previous studies have shown a detrimental effect of income (e.g., Shimozuma et al., 1999), this study is the first to reveal that the effects of income on depressive symptoms vary over time. Perhaps, for the lower income women, the termination of treatment is associated with heightened financial concerns about medical bills, the impact of lost income during active treatment or both. The stark difference in recovery trajectories between women with different incomes is alarming and highlights the utility of examining this practical factor in identifying the women who may be most in need of help at various points of recovery. Also, the influence of income on the course of depressive symptoms seems important in light of current political discussions regarding the financial impact of serious and chronic illness.
The observed patterns for women at different ages painted a slightly more optimistic picture. Although younger women experienced significantly more distress during active treatment, this distress seemed to dissipate after treatment was complete. It seems that the disruptions younger women are likely to experience closer to diagnosis do not have a lasting effect on their overall psychological health. This is not to say that all women are likely to recover as easily as the pattern suggests. However, it is encouraging to see that, on average, younger women are experiencing at least some resilience as time since diagnosis progresses. Simply making younger women aware of these patterns of change may provide some relief during the stressful time of treatment. A message that ‘things are likely to get better’ may be a helpful source of comfort and normalise the experience of depressive symptoms during treatment for these younger women.
Finally, one of the often overlooked variables we investigated, rurality, was unrelated to depressive symptoms. In some ways, we were somewhat surprised by this finding considering the potential obstacles that rural women face, such as social role disruption and stigma (see Bettencourt et al., 2007 for a review). The current findings, however, may suggest that a simplistic view of rurality is misguided. Rurality may be better thought of as a set of cultural values and a ‘way of life’ (e.g. Woods, 2005) than as a set of obstacles to be faced. In this way, rurality may still influence the qualitative experience of being a cancer survivor without significantly influencing people’s general level of psychological adjustment. Indeed, some of our previous findings support this notion and show that rurality interacts with other psychological variables such as coping strategies (Schlegel et al., 2009) and locus of control (Bettencourt et al., 2008) to predict depressive symptoms. We believe these findings of these latter studies suggest that rurality is important in the context of psychology and health and that the experiences of rural people warrant further investigation.
This study was limited in several ways. First, because of the sampling strategy, the sample is relatively homogeneous. Most of the patients were white and all were breast cancer patients who underwent radiation treatment. Nonetheless, the sample is more diverse than others in rurality, stage of breast cancer and income. Future research with more diverse samples is critical, however, for understanding the generalisability of the observed relationships. Another potential limitation is that we used a subjective measure of physical health, (i.e. self-reported severity of physical symptoms). While we believe that the subjective experience of physical symptoms is important, an objective measure of physical functioning would provide a more robust test of the influence of our demographic variables on depressive symptoms. Finally, although the study had a relatively low attrition rate, the initial recruitment rate was relatively low and it is possible that this influenced the nature of the final sample.
Despite these limitations, this study offers a number of significant and previously undocumented contributions to our understanding of the complex patterns of psychological recovery that follow breast cancer diagnosis and treatment. Firstly, given that two of the demographic variables interacted with time to predict depressive symptoms, the findings suggest that cross-sectional studies of breast cancer patients may sometimes fail to provide a sufficient picture of the nature of recovery in the year following diagnosis and treatment. We hope our findings prompt future research on the course of psychological health during survivorship. Secondly, and importantly, the current findings also suggest that relatively easily known demographic characteristics can be used to predict differences in these patterns of recover over time. Although future research is needed to replicate these findings in more diverse samples, over longer periods of time and to explore the underlying reasons for differences in trajectories, this study offers promise that medical practitioners can better identify when and who is likely to experience psychological distress.
Acknowledgments
Data collection and manuscript preparation were supported by a grant from the National Cancer Institute (CA97916-01). We gratefully acknowledge the assistance of the staff at the Radiation Oncology Clinics, especially Linda Robb. This study was conducted in accord with APA ethical guidelines and was approved by the institutional review board of the University of Missouri.
Footnotes
One notable exception can be found in an empirical study by Helgeson et al. (2004), who examined age and education (in addition to disease variables and several psychosocial variables such as social support) as predictors of psychological and physical adjustment in a longitudinal study. This study differs from this report however in that we take a different analytic approach by allowing our predictors to potentially interact with time. This approach allows for a more nuanced view of when depressive symptoms are likely to be elevated for different patients.
It is worth noting that it is unlikely that actual cancer recurrence in the sample explains the current results. The Wave 4 survey included a question about cancer recurrence and 10 women indicated that their cancer had either recurred or spread to another site by that time. As such, we re-ran the baseline model to assess whether these 10 women were possibly driving the quadratic effect of time. The results revealed that the quadratic effect of time remained significant when these women were not included in the analysis (b = 0.02, p< 0.05).
References
- Alferi S, Culver JL, Carver CS, Arena PL, Antoni MH. Religiousity, religious coping, and distress: A prospective study of Catholic and Evangelical Hispanic women in treatment for early-stage breast cancer. Health Psychology. 1999;43:343–356. doi: 10.1177/135910539900400304. [DOI] [PubMed] [Google Scholar]
- Andersen BL, Kiecolt-Glaser JK, Glaser R. A biobehavioral model of cancer stress and disease course. The American Psychologist. 1994;49:389–404. doi: 10.1037//0003-066x.49.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell WA, Natarajan L, Joel DE, Rock CL, Mortimer JE, Hollenbach K, Pierce JP. Objective cancer-related variables are not associated with depressive symptoms in women treated for early-stage breast cancer. Journal of Clinical Oncology. 2006;24:2420–2427. doi: 10.1200/JCO.2005.02.0081. [DOI] [PubMed] [Google Scholar]
- Beaulieu JE, Massey CS, Tucker TC, Schoenberg N, Ross F. Rural-urban variation in breast-conserving surgery in Kentucky. Kentucky Medical Association Journal. 2003;101:455–59. [PubMed] [Google Scholar]
- Bettencourt BA, Schlegel RJ, Talley A, Molix LA. The breast cancer experience of rural women: A literature review. Psycho-Oncology. 2007;6:875–887. doi: 10.1002/pon.1235. [DOI] [PubMed] [Google Scholar]
- Bettencourt BA, Talley AE, Molix LA, Schlegel RJ. Rural and urban breast cancer patients: Health locus of control and psychological adjustment. Psycho-Oncology. 2008;17:932–939. doi: 10.1002/pon.1315. [DOI] [PubMed] [Google Scholar]
- Bloom JR, Stewart SL, Chang S, Banks PJ. Then and now: Quality of life of young breast cancer survivors. Psycho-Oncology. 2004;13:147–160. doi: 10.1002/pon.794. [DOI] [PubMed] [Google Scholar]
- Bower JE. Behavioral symptoms in patients with breast cancer and survivors. Journal of Clinical Oncology. 2008;26:768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan CM, Wolinsky FD. The effect of gender and race on the measurement properties of the CES-D in older adults. Medical Care. 1994;32:341–356. doi: 10.1097/00005650-199404000-00003. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hebert TB. Psychological factors and physical disease from the perspective of psychoimmunology. Annual Review of Psychology. 1996;47:113–142. doi: 10.1146/annurev.psych.47.1.113. [DOI] [PubMed] [Google Scholar]
- Davis C, Girgis A, Williams P, Beeney L. Needs assessment of rural and remote women traveling to the city for breast cancer treatment. Australian and New Zealand Journal of Public Health. 1998;22:525–527. doi: 10.1111/j.1467-842x.1998.tb01431.x. [DOI] [PubMed] [Google Scholar]
- Davis C, Williams P, Redman S, White K, King E. Assessing the practical and psychological needs of rural women with early breast cancer in Australia. Social Work in Health Care. 2003;36:25–36. doi: 10.1300/j010v36n03_02. [DOI] [PubMed] [Google Scholar]
- Day R, Ganz PA, Costantino JP. Tamoxifen and depression: More evidence from the national surgical adjuvant breast and bowel project’s breast cancer prevention (P-l) randomized study. Journal of the National Cancer Institute. 1999;93:1615–1623. doi: 10.1093/jnci/93.21.1615. [DOI] [PubMed] [Google Scholar]
- DeShields T, Tibbs T, Fan M, Taylor M. Differences in patterns of depression after treatment for breast cancer. Psycho-Oncology. 2006;15:398–406. doi: 10.1002/pon.962. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for non-compliance with medical treatment: Meta-analysis of the effects of anxiety and depression inpatient adherence. Archives of Internal Medicine. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- Dunkel-Schetter C. Social support and cancer: Findings based on patient interviews and their implications. Journal of Social Issues. 1984;40:77–98. [Google Scholar]
- Epping-Jordan J, Compas B, Osowiecki D, Oppedisano G, Gerhardt C, Primo K, Krag D. Psychological adjustment in breast cancer: Processes of emotional distress. Health Psychology. 1999;18:315–326. doi: 10.1037//0278-6133.18.4.315. [DOI] [PubMed] [Google Scholar]
- Fallowfield L, Fleissig A, Edwards R, West A, Powles TJ, Howell A, Cuzick J. Tamoxifen for the prevention of breast cancer: Psychosocial impact on women participating in two randomized control trials. Journal of Clinical Oncology. 2001;19:1885–1892. doi: 10.1200/JCO.2001.19.7.1885. [DOI] [PubMed] [Google Scholar]
- Gallagher J, Parle M, Cairns D. Appraisal and psychological distress six months after diagnosis of breast cancer. British Journal of Health Psychology. 2002;7:365–376. doi: 10.1348/135910702760213733. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Kwan L, Stanton AL, Krupnick JL, Rowland JH, Meyerowitz BE, Belin TR. Quality of life at the end of primary treatment of breast cancer: First results from the moving beyond cancer randomized trial. Journal of the National Cancer Institute. 2004;96:376–387. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Schag CAC, Lee JJ, Polinsky ML, Tan SJ. Breast conservation versus mastectomy: Is there a difference in psychological adjustment or quality of life in the year after surgery? Cancer. 1992;69:1729–1738. doi: 10.1002/1097-0142(19920401)69:7<1729::aid-cncr2820690714>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Girgis A, Boyes A, Sanson-Fisher RW, Burrows S. Perceived needs of women diagnosed with breast cancer: Rural versus urban location. Australian and New Zealand Journal of Public Health. 2000;24:166–173. doi: 10.1111/j.1467-842x.2000.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Glanz K, Lerman C. Psychosocial impact of breast cancer: A critical review. Annals of Behavioral Medicine. 1992;14:204–212. [Google Scholar]
- Gray RE, James P, Manthorne J, Gould J, Fitch MI. A consultation with Canadian rural women with breast cancer. Health Expectations. 2004;7:40–50. doi: 10.1046/j.1369-6513.2003.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack TF, Pickles T, Ruether JD, Weir L, Butlz BD, Mackey J, Degner LF. Predictors of distress and quality of life in patients undergoing cancer therapy: Impact of treatment type and decisional role. Psycho-Oncology. 2010;19:606–616. doi: 10.1002/pon.1590. [DOI] [PubMed] [Google Scholar]
- Hagedoorn M, Sanderman R, Bolks HN, Tunistra J, Coyne JC. Distress in couples coping with cancer: A meta-analysis and critical review of role and gender effects. Psychological Bulletin. 2008;134:1–30. doi: 10.1037/0033-2909.134.1.1. [DOI] [PubMed] [Google Scholar]
- Helgeson VS, Cohen S. Social support and adjustment to cancer: Reconciling descriptive, correlational and intervention research. Health Psychology. 1996;15:135–148. doi: 10.1037//0278-6133.15.2.135. [DOI] [PubMed] [Google Scholar]
- Helgeson VS, Snyder P, Seltman H. Psychological and physical adjustment to breast cancer over 4 years: Identifying distinct trajectories of change. Health Psychology. 2004;23:3–15. doi: 10.1037/0278-6133.23.1.3. [DOI] [PubMed] [Google Scholar]
- Hjerl K, Andersen EW, Keiding N, Mouridsen HT, Mortensen PB, Jorgenson T. Depression as a prognostic factor for breast cancer mortality. Psychosomatics. 2003;44:24–30. doi: 10.1176/appi.psy.44.1.24. [DOI] [PubMed] [Google Scholar]
- Hughes J. Emotional reactions to the diagnosis and treatment of early breast cancer. Journal of Psychosomatic Research. 1982;26:277–283. doi: 10.1016/0022-3999(82)90047-2. [DOI] [PubMed] [Google Scholar]
- Irvine DM, Brown B, Crooks D, Roberts J, Browne G. Psychosocial adjustment in women with breast cancer. Cancer. 1991;67:1097–1117. doi: 10.1002/1097-0142(19910215)67:4<1097::aid-cncr2820670438>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kroenke CH, Rosner B, Chen WY, Kawachi I, Colditz GA, Holmes MD. Functional impact of breast cancer by age at diagnosis. Journal of Clinical Oncology. 2004;22:1849–1856. doi: 10.1200/JCO.2004.04.173. [DOI] [PubMed] [Google Scholar]
- Lethborg CE, Kissane D, Burns WI, Snyder R. ‘Cast Adrift’: The experience of completing treatment among women with early stage breast cancer. Journal of Psychosocial Oncology. 2000;18:73–90. [Google Scholar]
- Love RR, Cameron L, Connel BI, Leventhal H. Symptoms associated with tamoxifen treatment in postmenopausal women. Archives of Internal Medicine. 1991;151:1842–1847. [PubMed] [Google Scholar]
- Maher E. Anomic aspects of recovery from cancer. Social Science & Medicine. 1982;16:907–912. doi: 10.1016/0277-9536(82)90210-6. [DOI] [PubMed] [Google Scholar]
- Manne SL, Ostroff J, Winkel G, Grana G, Fox K. Partner unsupportive responses, avoidant coping, and distress among women with early stage breast cancer: Patient and partner perspectives. Health Psychology. 2005;24:635–641. doi: 10.1037/0278-6133.24.6.635. [DOI] [PubMed] [Google Scholar]
- Michael YL, Kawachi I, Berkman LF, Holmes MD, Coliditz GA. The persistent impact of breast carcinoma on functional health status: Prospective evidence from the Nurses’ Health Study. Cancer. 2000;89:2176–2186. doi: 10.1002/1097-0142(20001201)89:11<2176::aid-cncr5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Myers JK, Weissman MM. Use of self-report symptom scale to detect depression in a community sample. American Journal of Psychiatry. 1980;137:1081–1084. doi: 10.1176/ajp.137.9.1081. [DOI] [PubMed] [Google Scholar]
- Pozo C, Carver CS, Noriega V, Harris SD, Robinson DS, Ketcham AS, Clark KC. Effects of mastectomy vs lumpectomy on emotional adjustment to breastcancer: A prospective study of the first year postsurgery. Journal of Clinical Oncology. 1992;10:1292–1298. doi: 10.1200/JCO.1992.10.8.1292. [DOI] [PubMed] [Google Scholar]
- Primo K, Compas B, Oppedisano G, Howell D, Epping-Jordan J, Krag D. Intrusive thoughts and avoidance in breast cancer: Individual differences and associations with psychological distress. Psychological Health. 2000;14:1141–1153. doi: 10.1080/08870440008407372. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:185–401. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2. Newbury Park, CA: Sage; 2002. [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 6 hierarchical linear and nonlinear modeling. Lincolnwood, IL: Scientific Software International, Inc; 2000. [Google Scholar]
- Ried LD, Tueth MJ, Handberg E, Kupfer S, Pepine CJ. A study of antihypertensive drugs and depressive symptoms (SADD-Sx) in patients treated with a calcium antagonist versus an atenolol hypertension treatment strategy in the International Verapamil SR-Trandolapril Study. Psychosomatic Medicine. 2005;67:398–406. doi: 10.1097/01.psy.0000160468.69451.7f. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Helgeson VS. Commentary on Bardwell et al. (2006) Journal of Clinical Oncology. 2006;24:2407–2408. doi: 10.1200/JCO.2005.05.5244. [DOI] [PubMed] [Google Scholar]
- Schlegel RJ, Talley AE, Molix LA, Bettencourt BA. Rural breast cancer patients, coping and depression: A prospective comparison study. Psychology and Health. 2009;24:933–948. doi: 10.1080/08870440802254613. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Harlow LL, Brandt U, Stolbach LL. Using two factor structures of the mental adjustment to cancer (MAC) scale for assessing adaptation to breast cancer. Psycho-Oncology. 1998;7:424–435. doi: 10.1002/(SICI)1099-1611(1998090)7:5<424::AID-PON322>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Knowles JC, Harlow L. Correlates of adjustment among breast cancer survivors. Journal of Psychosocial Oncology. 2002;20:37–59. [Google Scholar]
- Shimozuma K, Ganz PA, Peterson L, Hirji K. Quality of life in the first year after breasts cancer surgery: Rehabilitation needs and patterns of recovery. Breast Cancer Research and Treatment. 1999;56:45–57. doi: 10.1023/a:1006214830854. [DOI] [PubMed] [Google Scholar]
- Snijders T, Bosker R. Multilevel analysis. London: Sage; 1999. [Google Scholar]
- Stanton AL, Danoff-Burg S, Cameron CL, Bishop MM, Collins CA, Kirk SB, Twillman R. Emotionally expressive coping predicts psychological and physical adjustment to breast cancer. Journal of Consulting Clinical Psychology. 2000;68:875–882. [PubMed] [Google Scholar]
- Stanton AL, Danoff-Burg S, Huggins M. The first year after breast cancer diagnosis: Hope and coping strategies as predictors of adjustment. Psycho-Oncology. 2002;11:93–102. doi: 10.1002/pon.574. [DOI] [PubMed] [Google Scholar]
- Stanton AL, Snider PR. Coping with a breast cancer diagnosis: A prospective study. Health Psychology. 1993;12:16–23. doi: 10.1037//0278-6133.12.1.16. [DOI] [PubMed] [Google Scholar]
- Stommel M, Kurtz ME, Kurtz JC, Given CW, Given BA. A longitudinal analysis of the course of depressive symptomatology in geriatric patients with cancer of the breast, colon, lung, or prostate. Health Psychology. 2004;24:564–573. doi: 10.1037/0278-6133.23.6.564. [DOI] [PubMed] [Google Scholar]
- Talley AE, Molix L, Schlegel R, Bettencourt BA. The influence of breast cancer survivors’ perceived partner social support and need satisfaction on depressive symptoms: A longitudinal analysis. Psychology and Health. 2010;25:433–449. doi: 10.1080/08870440802582682. [DOI] [PubMed] [Google Scholar]
- Ward SE, Viergutz G, Tormey D, deMuch J, Pualen A. Patients’ reactions to completion of adjuvant breast cancer therapy. Nursing Research. 1992;41:362–366. [PubMed] [Google Scholar]
- Williamson GM. Extending the activity restriction model of depressed affect: Evidence from a sample of breast cancer patients. Health Psychology. 2000;19:339–347. [PubMed] [Google Scholar]
- Woods M. Rural geography. London: Sage; 2005. [Google Scholar]