Abstract
Müller glia are the major glial component of the retina. They are one of the last retinal cell types to be born during development and they function to maintain retinal homeostasis and integrity. In mammals, Müller glia respond to retinal injury in a variety of ways that can be either protective or detrimental to retinal function. Although under special circumstances these cells can be coaxed to proliferate and generate neurons, these responses are meager and insufficient for repairing a damaged retina. By contrast, in teleost fish (such as zebrafish) the response of Müller glia to retinal injury involves a reprogramming event that imparts retinal stem cell characteristics and allows them to produce a proliferating population of progenitors that can regenerate all major retinal cell types and restore vision. Recent studies have revealed a number of important mechanisms underlying Müller glia reprogramming and retina regeneration in fish that may lead to new strategies for stimulating retina regeneration in mammals.
Introduction
Sight is one of our most precious senses and loss of sight extracts a large economic toll for both individuals and societies. Lost sight can result from traumatic injuries and disease, such as glaucoma, diabetic retinopathy and macular degeneration. A number of approaches for restoring sight to the blind are being pursued, including prosthetic devices, cell transplants and gene therapy1–3. Although each of these strategies has exhibited different degrees of success, they all rely on invasive surgeries and the introduction of foreign material into the eye. Ideally, one would like to develop a reparative strategy by which the retina could heal itself.
Although the idea of a self-healing retina may seem far-fetched, it is not unprecedented; teleost fish, such as zebrafish, have a remarkable capacity to regenerate their retina after damage and restore lost sight4–6. This regeneration relies on a single retinal cell type, the Müller glia, that is common to all vertebrate retinas. Müller glia are the major glial cell type in the retina and normally contribute to retinal structure and homeostasis7,8. However, after an injury to the retina, zebrafish Müller glia undergo a reprogramming event and acquire stem cell characteristics that allow them to generate progenitors for retinal repair9–14. Why zebrafish use this cell to regenerate a damaged retina and mammals do not remains unknown. It is possible that gaining a better understanding of retinal regeneration in teleost fish may hold the key for unlocking the regenerative potential of mammalian Müller glia.
In this review I summarize the responses of Müller glia to retinal injury in mammals, birds and fish. Because of the recent advances made in understanding how zebrafish Müller glia reprogram for retinal repair, I have focused this review on the signaling mechanisms underlying Müller glia reprogramming and the generation of Müller glia-derived progenitors in zebrafish. Finally, I describe future prospects for retina regeneration research in fish and mammals. The advances made in studying retina regeneration in fish are remarkable and I suspect that these advances will inspire new strategies for stimulating retina regeneration in mammals. I hope that this review helps to spur progress towards this goal.
Müller glia anatomy and function
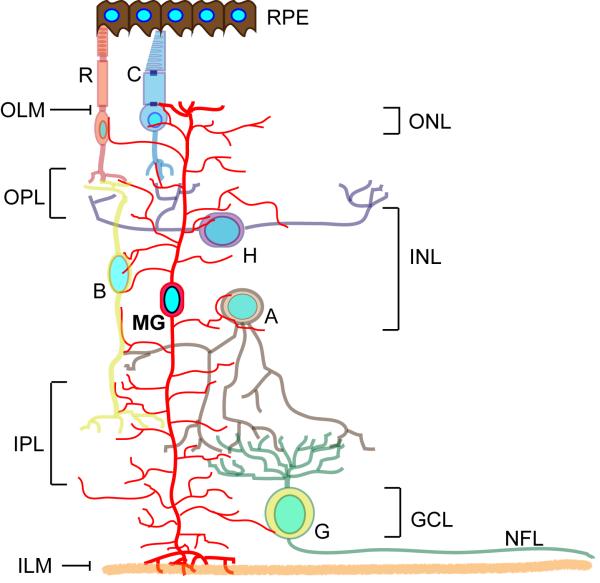
The retina is divided into 3 cellular layers, the outer nuclear layer (ONL), the inner nuclear layer (INL) and the ganglion cell layer (GCL) (Fig. 1). The ONL houses photoreceptors, which sense light and transduce this information to ganglion cells in the GCL via three types of interneurons (bipolar cells, amacrine cells and horizontal cells) that reside in the INL. Ganglion cells send their axons to the brain through the optic nerve and function to transfer visual information gathered in the eye to the brain.
Müller glia arise from multipotent progenitors 15 through a poorly understood process that includes Notch, Rax and Janus-activated kinase (Jak) signaling pathways16–20. Although their cell body resides in the INL, Müller glia are the only cell type to span all retinal layers and have processes that contact neighboring neurons and contribute to the outer and inner limiting membranes 21,22. Because of this, Müller glia are well positioned to monitor retinal homeostasis and contribute to retinal structure and function7,23. In doing so, they serve as barriers and conduits for the transfer of a wide variety of molecules between different retinal cells and compartments24–26. They also support neurons by releasing trophic factors, recycling neurotransmitters and controlling ionic balance in the extracellular space8,27–29. In addition, Müller glia phagocytize cone outer segments, contribute to outer segment assembly and participate in a cone-specific visual cycle that helps recycle the retinal chromophore for photodetection 30–32. Quite remarkably, it was recently found that, independent of their homeostatic function, Müller glia directly contribute to vision by acting as optical fibers to guide light to photoreceptors33. Although radial in structure, Müller glia differ from radial glia in the cortex in that they do not function as neural progenitors or serve as scaffolds for cell migration during retina development34. Nonetheless, progenitor characteristics have been noted in Müller glia; including the expression of progenitor-like genes, proliferative responses and the ability to generate neurons under special conditions35–44.
Müller glia response to injury
Müller glia are remarkably resilient to damage, a property that might be attributed to their unique physiology8,22,23,45,46. Müller glia respond to retinal injury and disease by changing their morphology, biochemistry and physiology23. This injury response is often referred to as reactive gliosis. Depending on the severity of damage, this response may include Müller glia proliferation. However, the triggers for proliferative gliosis are not well understood. Both proliferative and non-proliferative responses to injury are accompanied by changes in gene and protein expression and are often associated with Müller glia hypertrophy. This reactive gliosis can be beneficial to neurons by preventing glutamate neurotoxicity and releasing a variety of factors that protect neurons from cell death23; however, prolonged gliosis is detrimental because it interferes with retinal homeostasis and the ability of Müller glia to support retinal neurons and therefore often leads to neurodegeneration. Furthermore, the deposition of cell masses as a consequence of proliferative gliosis impedes normal retina function.
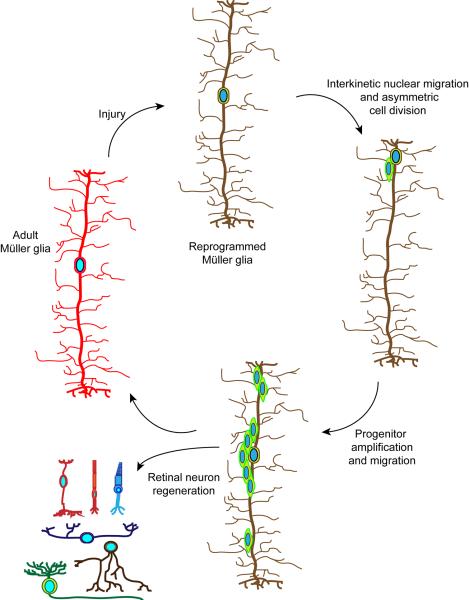
As noted above, Müller glia share some characteristics with retinal stem cells and in some species Müller glia can regenerate neurons. Thus if one could tip the balance from a gliotic response to one that is reparative, it might be possible to use Müller glia for endogenous repair. In order for Müller glia to participate in retinal repair, one can envisage 3 important steps that must occur (Fig. 2): Müller glia reprogramming to adopt stem cell characteristics, generation of a proliferating population of multipotent Müller glia-derived progenitors, and progenitor cell cycle exit and neuronal differentiation.
Figure 2. Generation of multipotent Müller glia-derived progenitors for retinal repair.
Adult Müller glia in zebrafish respond to retinal injury by reprogramming their genome (illustrated by a change in the colour of the cell) so that they can acquire stem cell properties9,10. This reprogramming results in interkinetic nuclear migration to the outer nuclear layer and an asymmetric cell division near the outer limiting membrane13. This asymmetric cell division generates a multipotent progenitor that transiently proliferates and restores the original Müller glia. Multipotent progenitors migrate to all cell layers, exit the cell cycle and regenerate all major retinal cell types11,80.
Models for studying retina regeneration
A number of model systems have provided important insights into the process of retina regeneration and the role that Müller glia play in this process (Table 1). Three animals have dominated the field: teleost fish, which naturally regenerate a damaged retina; postnatal chicks, which exhibit a limited regenerative capacity; and mice, which normally do not regenerate but are an important model for devising and testing strategies of mammalian retinal repair.
Table 1.
Factors affecting Mtiller glia reprogramming and proliferation.
| Factor | Function | Animal tested | Expression following injury | Effect on Miiller glia regenerative response | Ref |
|---|---|---|---|---|---|
| Delta/Notch | Transmembrane ligand/receptor | Bird, Rodent, Fish | Induced | Stimulates in birds and mice; inhibits in fish | 40,47,48,83 |
| BMP/Smad | Secreted factor/transcription factor | Rodent | Induced | Stimulates | 62 |
| CNTF | Secreted factor | Fish | *No | Stimulates | 85 |
| Dkk | Secreted factor | Fish | Suppressed | Inhibits | 63 |
| EGF/EGFR | Secreted factor/receptor | Rodent | EGFR is induced | Stimulates | 40,60,62 |
| FGF2/FGFR/Mapk | Secreted factor, receptor, signaling | Bird, Rodent, Fish | Induced | Stimulates | 47,49–52,115,122,123 |
| Insulin/IGF1/PI3K | Secreted factor, signaling | Bird, Rodent, Fish | Induced | Stimulates | 49–51,122 |
| Glutamate | Secreted factor | Rodent | ? | Stimulates | 43 |
| Hbegf/Mapk | Secreted factor, signaling | Fish | Induced | Stimulates | 83 |
| Shh | Secreted factor | Rodent | ? | Stimulates | 58 |
| TGF β | Secreted factor | Rodent, Fish | Suppressed | Inhibits | 61,94 |
| Tnf α | Secreted factor | Fish | Induced | Stimulates | 84 |
| Hspd1 | Heat shock protein | Fish | Induced | Stimulates | 12 |
| let-7 | microRNA | Fish | Suppressed | Inhibits | 10 |
| Lin28 | RNA binding protein | Fish | Induced | Stimulates | 10 |
| Mps1 | Mitotic regulator | Fish | Induced | Stimulates | 12 |
| Apobec2 | Putative cytosine deaminase | Fish | Induced | Stimulates | 97 |
| Ascl1 | Transcription factor | Bird, Rodent, Fish | Induced in bird and fish | Stimulates | 10,63,64 |
| β-catenin | Signal transducer | Fish | Stabilized | Stimulates | 63,92 |
| Insm1a | Transcription factor | Fish | Induced | !stimulates | 96 |
| Pax6 | Transcription factor | Bird, Rodent, Fish | Induced | Stimulates in fish | 39,40,98 |
| Stat3 | Signal transducer and transcription factor | Fish | Induced | Stimulates | 88 |
| Six3b | Transcription factor | Fish | Induced | Stimulates | 94 |
| Tgif1 | Transcription factor | Fish | Induced | Stimulates | 94 |
a cntf gene has not been identified in fish.
stimulates MCillerglia reprogramming, but also stimulates progenitor cell cycle exit and differentiation.
Birds
Adult birds do not regenerate a damaged retina. However, postnatal chicks respond to retinal injury with Müller glia proliferation and a small amount of neural regeneration39. This proliferation is stimulated by Notch signaling47,48 and proliferating cells express progenitor markers, such as paired box 6 (Pax6), achaete-scute complex homologue 1 (Ascl1) and Ceh-10 homeodomain-containing homolog (Chx10)39. Although the endogenous factors mediating Müller glia proliferation remain unknown, candidates include growth factors, such as fibroblast growth factor 2 (FGF2), insulin and insulin-like growth factor-1 (IGF-1)49–51. Remarkably, these factors can also stimulate Müller glia proliferation in the uninjured chick retina49,50 and appear to act via Notch as well as the FGF receptor, mitogen-activated protein kinase (MAPK) and extracellular-signal-regulated kinase (Erk) signaling pathways47,52.
Mammals
Although mammalian Müller glia can respond to injury, proliferate and express genes associated with retinal stem cells16,37, they do not function as retinal progenitors in vivo. Nonetheless, these characteristics suggest that, under the right circumstances, Müller glia might be coaxed to adopt characteristics of a retinal progenitor that can be used for repair. Indeed, in rodent and human cell culture, Müller glia have been observed to generate both neurons and glia53,54. Importantly, primary human Müller glia cultures can generate photoreceptors and RGCs that have some reparative potential when transplanted into a damaged rodent retina55–57. These studies suggest that human Müller glia are capable of generating neurons under appropriate conditions and that they may be able to participate in repair.
A number of studies have attempted to coax mammalian Müller glia to mount a regenerative response in vivo with limited success. Pharmacological damage to ganglion and bipolar cells or photoreceptors can induce a small amount of Müller glia proliferation and neuronal regeneration in rodents, but these regenerative events are very rare41,44. Other pharmacological and genetic engineering approaches indicate that Wnt/β-catenin, sonic hedgehog, epidermal growth factor (EGF)/EGF receptor (EGFR), glutamate and Ascl1-dependent signaling events can stimulate some Müller glia proliferation and neural regeneration in the injured mammalian retina40–43,58,59.
One of the most potent methods for stimulating Müller glia proliferation in the mouse retina is a combination of N-methyl-D-aspartate (NMDA)-induced retinal damage and EGF treatment40. EGFR expression in Müller glia is suppressed during postnatal development and this, along with increased transforming growth factor-β (TGFβ) signaling, correlates with the reduced proliferative capacity of Müller glia60,61. Although it is not clear whether TGFβ signaling is suppressed in the NMDA damaged mouse retina, EGFR expression is observed following damage60. EGF and NMDA-treatment appears to stimulate Müller glia proliferation by activating MAPK, phosphatidylinositol 3-kinase (PI3K) and bone morphogenetic protein (BMP) signaling pathways62. Interestingly, sub retinal delivery of low non-toxic doses of glutamate also stimulates Müller glia proliferation and a small amount of neural regeneration43. This raises the intriguing possibility that glutamate itself may be a secreted factor that stimulates Müller glia proliferation in the injured retina.
Although Müller glia proliferate and activate the expression of progenitor genes in EGF or NMDA-treated mouse retinas40; there was a notable lack of expression of Ascl1, a gene previously shown to be induced in proliferating Müller glia in the injured chick retina39 and essential for Müller glia reprogramming and Müller glia proliferation in zebrafish10,63,64. Remarkably, forced overexpression of Ascl1 in combination with EGF treatment stimulated Müller glia reprogramming, proliferation and bipolar neuron generation in postnatal mouse retinal explants59.
Fish
Unlike birds and mammals, teleost fish such as zebrafish can regenerate a damaged retina that restores visually mediated behaviors4–6. This regenerative response, along with its amenability to genetic manipulation, has made zebrafish a favoured model for studying retina regeneration. Although the teleost retina shares structure and function with the mammalian retina, distinguishing features include: a ciliary marginal zone in which retinal progenitors reside and add new neurons and glia as the retina expands throughout the animals life65; rod precursors in the ONL that selectively generate rods as the retina grows66; and Müller glia that generate rod progenitors and can be stimulated to generate multipotent progenitors for retinal repair11,67–69. Together, these features suggest that teleost fish possess a unique retinal environment that supports progenitor cell formation and maintenance. Below I describe the mechanistic underpinnings of retina regeneration in fish.
Mechanisms of regeneration in fish
Early studies, which predominantly used goldfish as a model system, firmly established that retina regeneration stemmed from the actions of injury-responsive cells that are intrinsic to the central retina70–75. Although the nature and origin of these progenitors were unknown, rod precursors, Müller glia and neuroepithelial cells were considered72,73,76–78. Müller glia were finally identified as the source of these progenitors using transgenic zebrafish in which Müller glia or Müller glia-derived progenitors were specifically labeled with green fluorescent protein (GFP)11,14,67,69,79. By taking advantage of bromodeoxyuridine (BrdU) lineage tracing strategies and CreER/LoxP technology that enabled researchers to generate transgenic fish in which Müller glia-derived progenitors were permanently labelled, it was shown that these progenitors produced all major retinal cell types and remained stably integrated into the retinal architecture11,80. When thinking about how Müller glia respond to retinal injury, we need to consider not only how injury signals are sensed and transmitted to the genome to reprogram Müller glia, but also how Müller glia-derived progenitors exit the cell cycle and differentiate.
How do Müller glia sense injury?
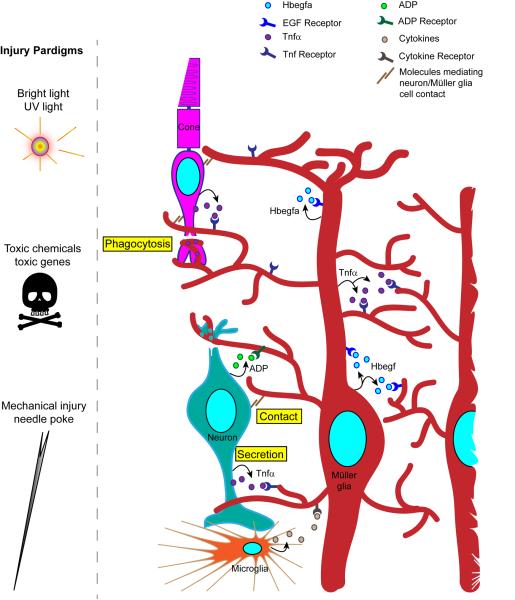
Fish Müller glia undergo reprogramming that enables regeneration in response to a variety of retinal injuries including those caused by intense light67,81, chemicals69, mechanical damage11,79 and cell type-specific expression of toxic genes82 (Fig. 3). It is likely that these different onslaughts converge on similar signaling pathways to stimulate Müller glia reprogramming. Although photoreceptor damage was previously thought to be necessary to stimulate a regenerative response72; more recent studies suggest that this is not the case39,69.
Figure 3. Injury paradigms and the communication of injury to Müller glia.
Various injury paradigms have been used to induce retinal damage and stimulate regeneration in zebrafish. These include prolonged exposure to intense bright light, short exposure to ultraviolet (UV) light; intravitreal injection of toxins (such as oubain and N-methyl-D-aspartate (NMDA)); expression of a toxic gene (such as bacterial nitroreductase, which, in combination with a prodrug generates a cytotoxic product); and mechanical injury (such as that resulting from a needle poke)11,12,69,81,82. Light-based damage paradigms generally destroy a population of photoreceptors, whereas toxins can cause wide-spread damage. Cytotoxic gene products can be directed to specific retinal cell types using appropriate promoters to drive their expression. Mechanical injury generally destroys all retinal cell types in a circumscribed region of the retina. The figure illustrates the ways in which injured cells might communicate with Müller glia to stimulate their reprogramming. These include secretion of signaling molecules (arrows) from damaged cells, Müller glia or infiltrating microglia; altered contact between damaged cells and Müller glia; and phagocytosis of injured cells by Müller glia. Recent studies have suggested that growth factors, such as heparin-binding epidermal growth factor (Hbegf), and cytokines, such as tumor necrosis factor- α (Tnfα), are necessary for Müller glia reprogramming and progenitor formation in the injured retina83,84. These factors are produced in Müller glia at the injury site and therefore, may act in an autocrine and paracrine fashion. Tnfα and ADP are also released from injured retinal neurons84,87.
Secreted factors, such as heparin binding epidermal-like growth factor (Hbegf), tumour necrosis factor-α (TNFα), Wnts and ciliary neurotrophic factor (Cntf) have been reported to contribute to injury-induced Müller glia reprogramming and progenitor formation in fish63,83–86. However, a cntf gene has not been identified in zebrafish, perhaps suggesting that other interleukin-6 (IL-6) family cytokines contribute to retina regeneration in this species. Remarkably, some of these secreted factors have been found to stimulate Müller glia reprogramming and proliferation in the uninjured retina83,85. In the injured retina most of the genes encoding these secreted factors are induced in injury-responsive Müller glia and their products may contribute to Müller glia reprogramming and retina regeneration in an autocrine and paracrine fashion (Fig. 3). Two factors that regulate Müller glia proliferation, ADP and Tnfα, are not only produced in Müller glia, but also appear to be released by dying retinal neurons84,87; perhaps suggesting that they play a role as injury signals that initiate a Müller glia regenerative response. Tnfα contributes to injury-dependent induction of Ascl1a and Stat384, two transcription factors whose expression is necessary for the generation of Müller glia-derived progenitors10,63,64,88. However, it is not yet clear whether the Tnfα that promotes this expression is released from dying cells and/or Müller glia. Finally, microglia and other immune-related cells respond to retinal injury by migrating to the injury site where they may release factors that influence Müller glia reprogramming and proliferation (Fig. 3)89,90.
When considering additional mechanisms that may contribute to the transmission of injury signals to Müller glia, the fact that Müller glia make contact with neighboring cells and can participate in phagocytosis (Fig. 3), should not be ignored23. Indeed, Müller glia phagocytize injured photoreceptors and inhibitors of phagocytosis suppress progenitor formation in the injured zebrafish retina91. It can also be hypothesized that altered contact between Müller glia and their injured neighbors may be sensed by integrins, cadherins, Notch and other signaling components which may contribute to initiating an injury response, although this has yet to be tested (Fig. 4).
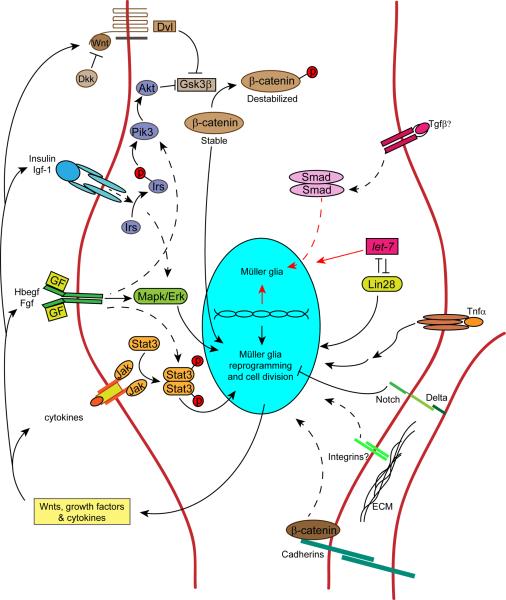
Figure 4. Signaling cascades contributing to Müller glia reprogramming and progenitor proliferation in zebrafish.
Retina regeneration requires the activation of a variety of signaling cascades. This diversity of signaling may reflect the variety of injuries and signaling molecules that stimulate retina regeneration. Signaling pathways that have been shown to regulate retina regeneration are indicated by solid arrows, while those indirectly implicated or hypothesized to be involved are indicated by dashed arrows. Secreted factors that regulate Müller glia proliferation are indicated outside the cell (those impacting Müller glia proliferation in birds/mammals49–51, but not yet tested in zebrafish are annotated with a question mark). Arrows pointing to the top half of the nucleus represent pathways that stimulate/maintain Müller glia differentiation/quiescence, while arrows pointing to the bottom half of the nucleus represent pathways that impact Müller glia reprogramming and proliferation. Wnts are secreted lipid-modified glycoproteins that bind Frizzled family receptors to regulate β-catenin stabilization. Dkk (Dickkopf) is a secreted Wnt signaling antagonist. Dvl (Dishevled) is a cytoplasmic phosphoprotein acting downstream of Wnt receptors. Gsk3β (glycogen synthase kinase 3β) regulates β-catenin stabilization by phosphorylation. β-catenin regulates cell adhesion and gene expression. Insulin and Igf-1 (insulin-like growth factor 1) are secreted proteins that bind tyrosine kinase receptors that signal via Irs (insulin receptor signaling protein), an adapter protein that couples insulin and Igf-1 receptor to PI3K (phosphoinositide 3-kinase) and Akt (protein kinase B) activation. Hbegf (heparin binding epidermal-like growth factor) is a transmembrane protein that undergoes ectodomain shedding. It is a member of the Egf family of growth factor ligands and acts via epidermal growth factor receptors. Fgfs (Fibroblast growth factors) are secreted growth factors that bind to fibroblast growth factor receptors. Egf and Fgf receptors are tyrosine kinase receptors that signal via Mapk (mitogen activated protein kinase) and Erk (extracellular signal regulated kinase). Cytokines are secreted proteins that often signal through receptors that lack intrinsic tyrosine kinase activity. Cytokine receptors are often coupled to Jak (Janus kinase) activation. Jak proteins are non-receptor tyrosine kinases that transduce cytokine-mediated signals by phosphorylating Stat proteins (signal transducers and activators of transcription). Tgfβ (Transforming growth factor-beta) is a secreted protein that signals via the Smad pathway to alter gene expression. let-7 is a microRNA that is a posttranscriptional regulator of RNA expression. Lin28 is a RNA binding protein that regulates let-7 microRNA expression. Tnfα (tumor necrosis factor alpha) is a secreted cytokine that acts via TNF receptors to regulate cell signaling and gene expression. Delta-Notch signaling is mediated by single pass trans-membrane proteins expressed on adjacent cells. ECM (extracellular matrix) can signal via transmembrane integrin receptors to regulate cell function.
Signal transduction in injury-responsive Müller glia
The diversity of signaling molecules communicating with Müller glia in the injured retina (Table 1 and Fig. 3) suggests that injuries activate multiple signaling cascades (Fig. 4). A major challenge in evaluating the roles of these signaling pathways is determining when and where they are activated following retinal injury. Pharmacological inhibition, knockdown strategies and genetic manipulations of gene expression often lack cellular resolution. In addition, even when signal transduction pathways can be identified in specific cells, one is limited by the sensitivity of the detection method. With these caveats in mind, pharmacological inhibition and genetic manipulations suggest that glycogen synthase kinase 3β (Gsk3β)/β-catenin, Notch, Mapk/Erk and Jak/signal transducer, activator of transcription (Stat) signaling pathways regulate zebrafish retina regeneration (Fig. 4)63,83,88,92. All of these pathways can couple extracellular events with the gene expression changes that drive Müller glia reprogramming. However, of these pathways, only β-catenin activation has been confirmed to occur in Müller glia-derived progenitors63,92.
In zebrafish, retinal injury results in wnt gene expression and β-catenin stabilization in Müller glia-derived progenitors63,92. β-catenin is a multifunctional protein that, in collaboration with t-cell factor (TCF)/lymphoid enhancer-binding factor (LEF) family members, links changes in Wnt and cadherin signaling on the cell surface to gene expression93. Inhibition of Wnt signaling or conditional expression of dominant-negative TCF suppresses progenitor formation in the injured retina63,92. Furthermore, pharmacological activation of the β-catenin signaling pathway in the uninjured zebrafish retina with lithium or a Gsk3β inhibitor, stimulates Müller glia reprogramming and progenitor formation63,92. These treatments bypass an inhibitory retinal environment that results, in part, from pan-retinal expression of the Wnt antagonist, Dickkopf (Dkk)63.
Although growth factors, such as HB-EGF, can stimulate progenitor formation in the uninjured zebrafish retina83, their mechanism of action is poorly understood. Injury-dependent induction of Hbegfa appears to be necessary for retina regeneration following a mechanical injury83; however, it may not be necessary for regeneration following photoreceptor damage84. In HB-EGF-treated uninjured retinas or mechanically injured retinas, the generation of Müller glia-derived progenitors is mediated by EGFR and MAPK/Erk signaling (Fig. 4)83.
The finding that cytokines, such as CNTF, can stimulate Müller glia to generate progenitors in the uninjured fish retina, suggests that Jak/Stat signaling may also be involved in Müller glia reprogramming and retina regeneration85. Indeed, retinal injury stimulates Stat3 expression in both quiescent Müller glia and in Müller glia-derived progenitors and Stat3 knockdown inhibits progenitor formation14,88. Taken together the above studies suggest that it is the combinatorial action of cytokines, Wnts and growth factors that stimulate Müller glia reprogramming and retina regeneration in the injured zebrafish retina.
A Müller glial cell acquires the properties of a retinal stem cell by reprogramming its genome to express genes that allow it to generate multipotent progenitors for retinal repair. The above discussion suggests that activation of Mapk/Erk, Gsk3β/β-catenin and Jak/Stat signaling cascades may be critical for this genomic reprogramming. However, changes in the levels of activated/stabilized β-catenin lag behind the earliest changes in gene expression noted after retinal injury and correlate best with the production of progenitors from reprogrammed Müller glia63. This discrepancy between β-catenin activation and Müller glia reprogramming may simply reflect the limits of immunofluorescence detection or may indicate that other signaling pathways act earlier, perhaps to control both Müller glia reprogramming and β-catenin stabilization.
Retina regeneration is not only driven by activation of signaling pathways that stimulate Müller glia reprogramming and progenitor formation, but also by suppression of pathways that drive Müller glia differentiation and quiescence (Fig. 4). let-7 miRNA and Dkk signaling are two such inhibitory pathways that help maintain zebrafish Müller glia in a quiescent state (Fig. 4)10,63. TGFβ signaling inhibitors, TGFβ-induced factor homeobox 1 (Tgif1) and sine oculus homeobox 3b (Six3b), enhance progenitor proliferation in the injured zebrafish retina94. However, these inhibitors are transcriptional corepressors that have multiple targets and their effect on TGFβ signaling in the injured zebrafish retina remains untested. Notch signaling also appears to play an inhibitory role during zebrafish retina regeneration (Fig. 4). However, unlike most inhibitory pathways, which are suppressed following retinal injury, Notch signaling components, such as deltaA, deltaB, deltaC and notch1 and Notch target genes, such as her4, are induced by injury83. Furthermore, unlike Notch's pro-proliferative effects in the chick retina48, Notch signaling in the injured fish retina suppresses the number of Müller glia recruited to an injury response83. In this way Notch signaling seems to help match the number of injury-responsive Müller glia with the extent of retinal damage83. It is likely that additional inhibitory pathways help to maintain Müller glia quiescence in the uninjured retina and their identification remains an important area of study.
Early response genes associated with reprogramming
By identifying the earliest changes in gene expression during retina regeneration one gains insight into how Müller glia reprogram from a differentiated support cell into one that produces progenitors for retinal repair. Microarray-based analysis of the zebrafish regeneration-associated transcriptome has identified over 1500 genes exhibiting differential expression between Müller glia and Müller glia-derived progenitors12,14,89,95,96. This is probably an underestimate since these studies only interrogated ~60% or less of the zebrafish genome. Nonetheless, this analysis has already facilitated the identification and characterization of a number of regeneration-associated genes that contribute to Müller glia's transition from a fully differentiated support cell to one with stem cell characteristics10,12,14,63,64,83,84,88,94,96–101.
One set of genes that are rapidly induced in zebrafish Müller glia following retinal injury are those encoding secreted growth factors and cytokines, such as hbegfa and tnfα83,84. Their expression by Müller glia suggests they may act in an autocrine/paracrine fashion to stimulate progenitor formation and proliferation. The mechanism underlying injury-dependent induction of these genes has not been studied in detail and remains an important area of investigation.
Injury-dependent induction of ascl1a gene expression results in suppression of genetic programs that promote cellular differentiation and the activation of programs that promote proliferation. ascl1a gene induction is under control of Hbegfa in the mechanically injured zebrafish retina83. Ascl1a stimulates lin28 gene expression, which has been shown to contribute to both let-7 miRNA suppression and further Ascl1a induction (Figs. 4 and 5)10,88. Lin28 is an RNA-binding protein that is highly expressed in embryonic stem cells and is associated with stem cell self-renewal102,103. It has been used to reprogram somatic cells into induced pluripotent stem cells (iPSCs) 104 and is an important regulator of tissue regeneration in mammals105. let-7 miRNAs are small regulatory RNAs associated with cellular differentiation103. Lin28 and let-7 regulate each other's expression; as Lin28 levels rise, let-7 miRNA levels fall and vice versa102,103,106. Because Lin28 and let-7 can regulate a large proportion of the cellular transcriptome103,107, they are important players in Müller glia reprogramming, proliferation and differentiation10. In addition to activating lin28 gene expression, Ascl1a also impacts Müller glia reprogramming and proliferation by regulating the Wnt signaling pathway, where it inhibits dkk gene expression and activates expression of wnt genes (Fig. 5)63,96. Finally, Ascl1a regulates the expression of insulinoma-associated 1a (insm1a), a transcriptional repressor that affects both Müller glia reprogramming and progenitor cell cycle exit (Fig. 5)96.
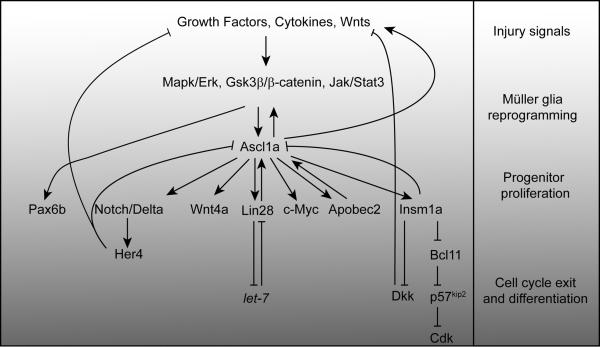
Figure 5. Regeneration-associated transcriptional cascades in zebrafish.
Growth factors, Wnts and cytokines appear to impinge on Mapk/Erk, Gsk3β/β-catenin and Jak/Stat signaling pathways to stimulate Müller glia reprogramming in response to retinal injury63,83,88,92. These pathways participate in injury-dependent ascl1a gene expression63,83,88. Ascl1a is a bHLH (basic helix-loop-helix) transcription factor impinging on almost all aspects of retina regeneration. It regulates genes responsible for generating Müller glia-derived progenitors, such as those encoding Wnts, growth factors, Lin28, c-Myc, Apobec2b, Insm1a and Stat310,63,64,83,88,96,97. Ascl1a also controls the expression of proteins and microRNAs that inhibit progenitor formation and proliferation, such as Notch, Dkk, Insm1a (this protein contributes to both progenitor formation and differentiation), p57kip2 and let-710,63,83,96. Gsk3β inhibition stimulates pax6b expression in an Ascl1a-independent fashion63. The regenerative steps outlined on the right-hand side, along with the gray gradient, illustrate the gradual transition of Müller glia to progenitors and their differentiation during retina regeneration.
In the light damaged zebrafish retina, stat3 gene expression may precede that of ascl1a88. Stat3 is a signal transducer and activator of transcription that links membrane events with changes in gene expression. Following retinal injury, Stat3 protein is increased in both quiescent and proliferating Müller glia, whereas injury-dependent Ascl1a expression is restricted to reprogrammed Müller glia and Müller glia-derived progenitors10,64,88. Importantly, the retinal cell types expressing activated pStat3 remain uncharacterized and further research may reveal a progenitor-specific action of this signaling molecule. Surprisingly, Stat3 knockdown only reduced progenitor formation by ~40%, which may suggest that this protein has a modulatory role88. However, it may be that residual pStat3 remaining after knockdown was sufficient to drive progenitor formation. Alternatively, these findings may indicate that multiple Stat proteins are activated by injury and that knockdown of any individual Stat is insufficient to robustly suppress progenitor formation. Finally, it has been suggested that there is a pStat3/Ascl1a signaling loop: not only does Stat3 knockdown reduce Ascl1a expression, but Ascl1a knockdown also suppresses Stat3 expression84,88.
Pax6b is necessary for the proliferation of Müller glia-derived progenitors in zebrafish and it is induced just before Müller glia begin dividing10,98. Interestingly, pax6b gene expression is not controlled by Ascl1a expression, but rather appears to be under control of Gsk3β/β-catenin signaling (Fig. 5)63.
Epigenetic changes during retina regeneration
Injury-dependent reprogramming of Müller glia shares some features with the process of somatic cell reprogramming and the generation of iPSCs. Indeed, many of the genes used to stimulate pluripotency in somatic cells are induced in Müller glia as they reprogram to a stem cell10. During iPSC formation, pluripotency genes undergo a demethylation event that allows their chromatin to assume a more “open” accessible state that is permissive for gene expression108,109. Interestingly, forced hypomethylation of DNA in zebrafish Müller glia-derived progenitors with 5-aza-2'-deoxycytidine (5-dAza), stimulated expression of the transgenic reporter gene, 1016 tuba1a:gfp, whose expression reflects injury-dependent Müller glia reprogramming9. Furthermore, this hypomethylation reduced progenitor amplification, migration and differentiation9. These data are consistent with the idea that DNA demethylation contributes to Müller glia reprogramming, whereas DNA methylation may be necessary for the migration and differentiation of Müller glia-derived progenitors. Indeed, reduced representation bisulfite sequencing identified a small proportion of the zebrafish Müller glia genome that changed its methylation pattern during retina regeneration9. Demethylation predominated early after injury when Müller glia were being reprogramed to adopt the properties of retinal stem cells and methylation regained prominence later when progenitors were migrating and differentiating9. Furthermore, this analysis revealed a correlation between numbers of genes induced and DNA demethylation9. Importantly, the promoters of a number of regeneration-associated genes, including ascl1a, lin28, hbegfa and insm1a, exhibited a low basal level of methylation in Müller glia that remained unchanged in progenitors9. Interestingly, these same genes exhibit a similar low basal methylation in Müller glia from mammals9; perhaps contributing to the noted progenitor-like characteristics and plasticity of these cells 16,37,40–44,53,54,58.
DNA methylation reflects the capacity for gene expression, whereas histone modifications can distinguish active from repressed genes110. A striking feature of the chromatin present in iPSCs is the presence of bivalent domains that harbor histones with both active and repressive modifications111. These bivalent domains may indicate a transcriptionally poised state that can rapidly change under different cellular demands and thus enhance cellular plasticity. It would not be too surprising if chromatin from zebrafish Müller glia exhibit histone modifications that contribute to their plasticity. Although this has not yet been studied in zebrafish, it is noteworthy that forced overexpression of Ascl1 in mouse Müller glia impacts histone modification, gene expression and progenitor formation59.
Cell cycle exit and differentiation
Reprogrammed Müller glia in zebrafish divide asymmetrically near the ONL to generate a population of transient amplifying progenitors that contribute to retinal repair (Fig. 2)13. Gsk3β inhibition prevents this asymmetric division and encourages a symmetric division resulting in depletion of the differentiated Müller glia pool92. Mapk/Erk, Gsk3β/β-catenin and Stat signaling contribute to formation of the progenitor pool (Fig. 5)63,83,88,92. Pax6b controls the earliest division of the first Müller glia-derived progenitor98 and Pax6a98, heat shock 60kDa protein 1 (Hspd1)12 and many of the gene products described above contribute to their expansion (Table 1). Progenitors are born apically near the ONL and migrate into the INL in an N-cadherin-dependent fashion (Fig. 2)13. Furthermore, monopolar spindle 1 (Mps1) may enhance the proliferation of photoreceptor progenitors in the ONL12.
Although we know very little about the mechanisms that drive progenitors out of the cell cycle, the transcriptional repressor, Insm1a appears to play an important role (Fig. 5)96. Insm1a drives cell cycle exit by inhibiting expression of cell cycle-associated genes and enhancing the expression of p57kip2, a gene encoding a cyclin-dependent kinase (Cdk) inhibitor (Fig. 5)96. Insm1a appears to stimulate p57kip2 expression by inhibiting the expression of the p57kip2 gene repressor, Bcl11 (Fig. 5)96. Remarkably, Ascl1a also contributes to this regulation by enhancing insm1a promoter activity (Fig. 5)96.
In zebrafish, Müller glia-derived progenitors can regenerate all major retinal cell types. This multipotency distinguishes them from rod precursors in fish and Müller glia-derived progenitors in birds and mammals which have a severely limited ability to regenerate multiple cell types39,40,77. This difference in multipotency may reflect intrinsic differences in gene expression and extrinsic differences in the progenitor's environment. Although Muller glia-derived progenitors in zebrafish can regenerate all types of damaged retinal neurons; it is not clear whether the identity of the dying cells can influence progenitor differentiation in order to generate replacements. Interestingly, when Müller glia in the uninjured retina are forced to reprogram and generate progenitors, these progenitors have the capacity to make all retinal cell types63,83,96. Experimental paradigms that result in damage to particular cell types (such as photoreceptors or bipolar cells) have demonstrated that progenitors can replace the lost cell types12,14,67,82,101,112–114. However, because these studies used only antibodies or transgenic reporter lines that detect the damaged cell type, it was not possible to determine if progenitors also made other cell types that were only transiently maintained.
The mechanisms controlling the differentiation of Müller glia-derived progenitors in the adult fish retina are poorly understood. In regeneration models in which photoreceptors are selectively damaged, Mps1 appears to promote proliferation of photoreceptor progenitors and controls their differentiation into cones (Table 2)12. It is not known whether Mps1 also affects differentiation of other cell types. Interestingly, in the photoreceptor damage model, Fgf signaling and galectin Drgal1-L2 are necessary for regeneration of rod, but not cone photoreceptors (Table 2)99,115. In a mechanical injury model in which all retinal cell types are damaged, Notch activity seems to impact differentiation of all cell types with Notch inhibition increasing Müller glia differentiation and suppressing neuronal differentiation, while notch intracellular domain (NICD) overexpression stimulated photoreceptor differentiation at the expense of Müller glia, bipolar and ganglion cell differentiation (Table 2)83. Finally, cell adhesion or progenitor migration also impacts progenitor differentiation since inhibition of N-cadherin expression reduced progenitor migration into the INL and suppressed regeneration of inner retinal neurons (Table 2)13. Understanding the mechanisms underlying progenitor differentiation and choices of cell fate in the adult retina may suggest ways of enhancing and directing differentiation of progenitors in the adult mammalian retina.
Table 2.
Factors affecting progenitor cell cycle exit and differentiation in zebrafish.
| Factor | Normal function | Method of reduction | Effect on progenitor cell cycle exit | Effect on progenitor migration | Effect on progenitor differentiation | Ref |
|---|---|---|---|---|---|---|
| Drgal1-L2 (Igals2a) | Galectin family member, β-galactoside binding protein | Morpholino knockdown | None noted | None noted | Stimulates rod regeneration | 99 |
| FGFR | Signal transduction | Dominant/negative | *None noted | None noted | *Photoreceptor maintenance and rod regeneration | 115,123 |
| Insmla | Transcriptional repressor | Morpholino Knockdown | Stimulates cell cycle exit | Stimulates migration into ONL | Stimulates differentiation | 96 |
| Mps1 | Mitotic check point regulation | Temperature sensitive mutation | None noted | None noted | Stimulates cone differentiation | 12 |
| N-cadherin | Cell adhesion | Semi-dominant mutation | None noted | Progenitors accumulate in ONL | Necessary for regeneration of inner retinal neurons | 13 |
| Notch | Signal transduction | Pharmacological inhibition; NICD overexpression | !None noted | None noted | Stimulates photoreceptor differentiation at the expense of MCillerglia differentiation | 83 |
One study reports no effect of dnfgfr on progenitor proliferation following light damage and that fgf signaling is necessary for rod maintenance115, while the other study shows dnfgfr suppresses progenitor proliferation following light damage and that fgf signaling is necessary for rod and cone maintenance123.
Notch signaling inhibits progenitor formation and proliferation in the injured retina83.
Future prospects
Some of the most pressing questions that remain concerning retina regeneration center on the differences noted between fish and mammalian Müller glia. Why do zebrafish Müller glia readily reprogram in response to injury, whereas those in mammals do not? Even when Müller glia are coaxed to divide in mammals, why do they only rarely regenerate neurons? There are a number of possible answers to these questions that span intrinsic differences between fish and mammalian Müller glia and progenitors to the different environments (niches) that nurture them.
High throughput sequencing allows us to discern differences in the transcriptomes and epigenomes of zebrafish and mammalian Müller glia/progenitors. Differences in the transcriptomes of human Müller glia in the retina and those that generate progenitors in culture may help define signals that stimulate their conversion to multipotency. Zebrafish provide a convenient system for testing the significance of specific genes and regulatory events on regeneration. Those found to be important for regeneration can then be tested in mammals to determine if regeneration can be enhanced.
Although injured cells appear to provide the initial stimulus that initiates Müller glia reprogramming and retina regeneration; it is not clear whether other cell types may also participate. In particular microglia and infiltrating immune cells may play a role. These cells can respond to injury by migration, phagocytosis and release of factors that may act on Müller glia to initiate/enhance their reprogramming and/or affect the proliferation and differentiation of Müller glia-derived progenitors. Microglia play an important role in zebrafish brain regeneration and macrophages impact limb regeneration in salamanders116,117. Following retinal injury, microglia become activated and migrate to the injury site89,90,118, perhaps suggesting a role in retina regeneration. Further analysis of their contribution to retina regeneration in zebrafish is warranted and if they play an important role, comparing their injury response with that of mammals may suggest strategies for improving mammalian retina regeneration.
The variety of injury paradigms and secreted factors that stimulate zebrafish retina regeneration and converge on Mapk/Erk, Gsk3β/β-catenin and Jak/Stat signaling is remarkable and suggest activation of these pathways is critical for retina regeneration. However, it is still unclear whether these pathways drive all aspects of Müller glia reprogramming and progenitor proliferation or are more restricted in their action. Further analysis of their role in Müller glia reprogramming in fish is crucial for understanding their significance in controlling retina regeneration. These pathways have not been well characterized in the injured mammalian retina and may represent good targets for enhancing retinal repair.
Even if mammalian Müller glia could be coaxed to reprogram, their environment may be hostile to progenitor formation and differentiation. Interestingly, Ephrins, bone morphogenetic proteins (Bmps) and secreted frizzled-related protein 2 (Sfrp2) negatively regulate stem cells and are expressed in the adult mammalian retina119–121. Whether these signals collaborate with others to inhibit Müller glia reprogramming and progenitor formation remains to be determined. Retinal injury stimulates a series of events that overcomes an environment that maintains Müller glia quiescence in the uninjured fish retina. Thus, zebrafish provide an ideal system for identifying these quiescence promoting factors and developing strategies for their suppression. A combination of neutralizing quiescence-promoting factors and stimulating activators of regeneration may be the most successful strategy for restoring a regenerative response to the mammalian retina.
The goal of scientists studying retina regeneration is to apply regenerative strategies to blinding eye diseases and injuries. The ability to use endogenous stem cells for repair has a lot of appeal and avoids many concerns associated with prosthetic devices and cell transplants. In particular, endogenous repair will not stimulate an immune response and does not require cell infiltration. Because of their robust regenerative response and ease of genetic manipulation, fish have led the way in identifying mechanisms underlying retina regeneration. Recent progress in characterizing these mechanisms suggests strategies for stimulating retinal repair in mammals. However, the problem is complex and many questions remain. Fish will continue to be an important model for uncovering mechanisms controlling retina regeneration and other species like birds and mice will serve as important models for testing these mechanisms and revealing others that restore a regenerative response to animals blinded by injury or disease.
Online `at-a-glance' summary.
-
1.
Müller glia from fish, birds and mammals share structure and function.
-
2.
A key difference between Müller glia from fish and mammals is their ability to participate in retinal repair. Unlike birds and mammals, Müller glia from fish respond to retinal injury by undergoing a reprogramming event that allows them to acquire properties of a retinal stem cell so they can generate multipotent progenitors for repair.
-
3.
A variety of secreted growth factors, cytokines and Wnts from injured cells and Müller glia themselves appear drive Müller glia reprogramming in fish by activating a variety of signaling cascades that include Mapk/Erk, Gsk3β/β-catenin and Jak/Stat signaling.
-
4.
Growth factors and cytokines can stimulate Müller glia proliferation in damaged retinas of birds and mice, but these proliferating cells exhibit a very limited ability to regenerate new neurons and generally do not survive.
-
5.
In fish, factors like Tnfα, Hbegf, Ascl1a, Stat3 and Lin28 appear to regulate the earliest stages of Müller glia reprogramming, while Pax6a and Pax6b drive progenitor expansion and Insm1a drives progenitors out of the cell cycle.
-
6.
In addition to activation of gene expression programs that drive Müller glia reprogramming, zebrafish also suppress gene expression programs that inhibit Müller glia reprogramming like those controlled by let7 miRNAs, Dickkopf, Tgif1 and Six3b.
-
6.
Notch signaling stimulates the formation of Müller glia-derived progenitors in birds, but inhibits the zone of injury-responsive Müller glia in fish.
-
7.
Forced Ascl1 overexpression along with EGF treatment can stimulate Müller glia in postnatal mouse retinal explants to reprogram and generate bipolar neurons.
-
8.
Müller glia reprogramming and retina regeneration is associated with changes in DNA methylation in fish; however, many key reprogramming genes exhibit a low basal level of methylation in the uninjured retinas of both fish and mice suggesting they may be poised for expression.
-
9.
Unravelling mechanisms underlying Müller glia reprogramming and retina regeneration in fish along with studies of Muller glia in other species like birds and mammals may reveal novel strategies for stimulating retina regeneration in humans.
Figure 1. Retinal anatomy.
Illustration of the major retinal cell types and their organization in the retina. The retina is divided into 3 laminar layers; the outer nuclear layer (ONL), inner nuclear layer (INL) and ganglion cell layer (GCL). Six different neuronal cell types and one glia cell type are distributed among these layers with rod and cone photorecptors in the ONL; bipolar, horizontal and amacrine interneurons, along with the Müller glia cell bodies, in the INL; and ganglion cells in the GCL. Ganglion cell axons run just beneath the GCL and comprise a nerve fiber layer (NFL). Synapses between photoreceptors and interneurons take place in the outer plexiform layer (OPL) and synapses between interneurons and ganglion cells take place in the inner plexiform layer (IPL). MG processes span all retinal layers and contribute to the formation of the inner limiting membrane (ILM) and outer limiting membrane (OLM). The retinal pigment epithelium (RPE) consists of pigmented cells that absorb light and make contact with photoreceptors.
Acknowledgements
Research in the Goldman lab is supported by NEI grants RO1 EY 018132 and 1R21 EY022707 from the NIH, an Innovative Ophthalmic Research Award from Research to Prevent Blindness and a gift from the Marjorie and Maxwell Jospey Foundation. D. G. thanks Goldman lab members Curtis Powell, Jin Wan and Xiao-Feng Zhao for helpful comments and suggestions on this review.
Glossary Terms
- Multipotent progenitors
Cells that have the potential to differentiate into more than a single cell type, but are more restricted in their fate than embryonic stem cells.
- Limiting membranes
The boundary between the retina and the vitreous is referred to as the inner limiting membrane and is composed of Müller glia endfeet and astrocytes. The outer limiting membrane forms a barrier between the neural retina and the subretinal space. The outer limiting membrane is formed by adherens junctions between Müller glia and photoreceptor inner segments.
- Outer segments
The part of photoreceptors that are adjacent to the retinal pigment epithelial cell layer that contain membrane discs filled with opsin.
- Radial glia
Cells that span the radial axis of the developing cortex and serve as precursors or guides for newly born postmitotic neurons on their way into the mantle zone.
- Glial scarring
A reactive cellular process that results in Müller glia proliferation and formation of cell masses that are detrimental to retina function.
- Neuroepithelial cells
A neural stem cell that can self-renew and give rise to all neural cell types.
- Transcriptome
The complete set of RNA molecules produced by a cell or a population of cells at a given time point.
- Induced pluripotent stem cells (iPSCs)
A type of pluripotent stem cell generated from fully differentiated cells.
- Reduced representation bisulfite sequencing
A high throughput technique for analyzing DNA methylation at the nucleotide level on a genome-wide scale.
- Transient amplifying progenitors
Cells that arise from adult stem cells and divide a finite number of times until they become differentiated. They are committed progenitor cells.
- Chromophore
In the retina, retinal is the chromophore attached to opsins that allow light absorption by photoreceptors.
Biography
Daniel Goldman is the Bernard W. Agranoff Collegiate Professor of Neuroscience, Professor of Biological Chemistry and Research Professor, Molecular & Behavioral Neuroscience Institute at the University of Michigan, Ann Arbor, USA. He received his Ph.D. in Biochemistry from the University of Illinois where he studied bacterial chemotaxis under the direction of George Ordal. He did his postdoctoral training at the Salk Institute in the laboratory of Steve Heinemann where he studied activity-dependent control of neuromuscular synapse formation. His current research interests focus on neuromuscular regeneration, optic nerve regeneration and retina regeneration.
References
- 1.Shepherd RK, Shivdasani MN, Nayagam DA, Williams CE, Blamey PJ. Visual prostheses for the blind. Trends Biotechnol. 2013;31:562–571. doi: 10.1016/j.tibtech.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Pearson RA, et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485:99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first demonstration of vision recovery following rod precursor transplants into a genetic model of blindness.
- 3.Boye SE, Boye SL, Lewin AS, Hauswirth WW. A comprehensive review of retinal gene therapy. Mol Ther. 2013;21:509–519. doi: 10.1038/mt.2012.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherpa T, et al. Ganglion cell regeneration following whole-retina destruction in zebrafish. Dev Neurobiol. 2008;68:166–181. doi: 10.1002/dneu.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindsey AE, Powers MK. Visual behavior of adult goldfish with regenerating retina. Vis Neurosci. 2007;24:247–255. doi: 10.1017/S0952523806230207. [DOI] [PubMed] [Google Scholar]
- 6.Mensinger AF, Powers MK. Visual function in regenerating teleost retina following cytotoxic lesioning. Vis Neurosci. 1999;16:241–251. doi: 10.1017/s0952523899162059. [DOI] [PubMed] [Google Scholar]
- 7.Reichenbach A, Bringmann A. New functions of Muller cells. Glia. 2013;61:651–678. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]; An excellent review of recently discovered functions of Müller glia.
- 8.Bringmann A, et al. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochem Int. 2009;54:143–160. doi: 10.1016/j.neuint.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Powell C, Grant AR, Cornblath E, Goldman D. Analysis of DNA methylation reveals a partial reprogramming of the Muller glia genome during retina regeneration. Proc Natl Acad Sci U S A. 2013;110:19814–19819. doi: 10.1073/pnas.1312009110. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper identifies a changing DNA methylation landscape as Müller glia transition to a stem cell.
- 10.Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Muller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol. 2010a;12:1101–1107. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper identifies pluripotency factors participating in Müller glia reprogramming and retina regeneration. It reveals a lin28/let7 miRNA signaling loop that is common to Müller glia reprogramming, embryonic stem cells and induced pluripotent stem cells.
- 11.Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26:6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first paper to use trangenic zebrafish and BrdU lineage tracing to show Müller glia are the source of retinal progenitors for retina regeneration in zebrafish and that these cells can regenerate all major retinal cell types.
- 12.Qin Z, Barthel LK, Raymond PA. Genetic evidence for shared mechanisms of epimorphic regeneration in zebrafish. Proc Natl Acad Sci U S A. 2009;106:9310–9315. doi: 10.1073/pnas.0811186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagashima M, Barthel LK, Raymond PA. A self-renewing division of zebrafish Muller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development. 2013;140:4510–4521. doi: 10.1242/dev.090738. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates interkinetic nuclear migration, assymetric division and the participation of N-cadherin in the generation of a Müller glia-derived progenitor.
- 14.Kassen SC, et al. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev Neurobiol. 2007;67:1009–1031. doi: 10.1002/dneu.20362. [DOI] [PubMed] [Google Scholar]
- 15.Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- 16.Jadhav AP, Roesch K, Cepko CL. Development and neurogenic potential of Muller glial cells in the vertebrate retina. Prog Retin Eye Res. 2009;28:249–262. doi: 10.1016/j.preteyeres.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jadhav AP, Cho SH, Cepko CL. Notch activity permits retinal cells to progress through multiple progenitor states and acquire a stem cell property. Proc Natl Acad Sci U S A. 2006;103:18998–19003. doi: 10.1073/pnas.0608155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furukawa T, Mukherjee S, Bao ZZ, Morrow EM, Cepko CL. rax, Hes1, and notch1 promote the formation of Muller glia by postnatal retinal progenitor cells. Neuron. 2000;26:383–394. doi: 10.1016/s0896-6273(00)81171-x. [DOI] [PubMed] [Google Scholar]
- 19.Goureau O, Rhee KD, Yang XJ. Ciliary neurotrophic factor promotes muller glia differentiation from the postnatal retinal progenitor pool. Dev Neurosci. 2004;26:359–370. doi: 10.1159/000082278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattacharya S, Das AV, Mallya KB, Ahmad I. Ciliary neurotrophic factor-mediated signaling regulates neuronal versus glial differentiation of retinal stem cells/progenitors by concentration-dependent recruitment of mitogen-activated protein kinase and Janus kinase-signal transducer and activator of transcription pathways in conjunction with Notch signaling. Stem Cells. 2008;26:2611–2624. doi: 10.1634/stemcells.2008-0222. [DOI] [PubMed] [Google Scholar]
- 21.Reichenbach A, Reichelt W. Postnatal development of radial glial (Muller) cells of the rabbit retina. Neurosci. Lett. 1986;71:125–130. doi: 10.1016/0304-3940(86)90545-8. [DOI] [PubMed] [Google Scholar]
- 22.Magalhaes MM, Coimbra A. The rabbit retina Muller cell. A fine structural and cytochemical study. J Ultrastruct Res. 1972;39:310–326. doi: 10.1016/s0022-5320(72)90026-3. [DOI] [PubMed] [Google Scholar]
- 23.Bringmann A, et al. Cellular signaling and factors involved in Muller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res. 2009;28:423–451. doi: 10.1016/j.preteyeres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Tout S, Chan-Ling T, Hollander H, Stone J. The role of Muller cells in the formation of the blood-retinal barrier. Neuroscience. 1993;55:291–301. doi: 10.1016/0306-4522(93)90473-s. [DOI] [PubMed] [Google Scholar]
- 25.Shen W, et al. Conditional Mullercell ablation causes independent neuronal and vascular pathologies in a novel transgenic model. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:15715–15727. doi: 10.1523/JNEUROSCI.2841-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagelhus EA, et al. Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Muller cells is mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane domains. Glia. 1999;26:47–54. doi: 10.1002/(sici)1098-1136(199903)26:1<47::aid-glia5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Pow DV, Crook DK. Direct immunocytochemical evidence for the transfer of glutamine from glial cells to neurons: use of specific antibodies directed against the d-stereoisomers of glutamate and glutamine. Neuroscience. 1996;70:295–302. doi: 10.1016/0306-4522(95)00363-n. [DOI] [PubMed] [Google Scholar]
- 28.Schutte M, Werner P. Redistribution of glutathione in the ischemic rat retina. Neurosci. Lett. 1998;246:53–56. doi: 10.1016/s0304-3940(98)00229-8. [DOI] [PubMed] [Google Scholar]
- 29.Seki M, Nawa H, Fukuchi T, Abe H, Takei N. BDNF is upregulated by postnatal development and visual experience: quantitative and immunohistochemical analyses of BDNF in the rat retina. Invest. Ophthalmol. Vis. Sci. 2003;44:3211–3218. doi: 10.1167/iovs.02-1089. [DOI] [PubMed] [Google Scholar]
- 30.Long KO, Fisher SK, Fariss RN, Anderson DH. Disc shedding and autophagy in the cone-dominant ground squirrel retina. Exp. Eye Res. 1986;43:193–205. doi: 10.1016/s0014-4835(86)80087-2. [DOI] [PubMed] [Google Scholar]
- 31.Wang JS, Kefalov VJ. The cone-specific visual cycle. Prog. Retin. Eye Res. 2011;30:115–128. doi: 10.1016/j.preteyeres.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Iannaccone A, Jablonski MM. Contribution of Muller cells toward the regulation of photoreceptor outer segment assembly. Neuron Glia Biol. 2005;1:1–6. doi: 10.1017/S1740925X05000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franze K, et al. Muller cells are living optical fibers in the vertebrate retina. Proc. Natl. Acad. Sci. U. S. A. 2007;104:8287–8292. doi: 10.1073/pnas.0611180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weissman T, Noctor SC, Clinton BK, Honig LS, Kriegstein AR. Neurogenic radial glial cells in reptile, rodent and human: from mitosis to migration. Cereb Cortex. 2003;13:550–559. doi: 10.1093/cercor/13.6.550. [DOI] [PubMed] [Google Scholar]
- 35.Blackshaw S, et al. Genomic analysis of mouse retinal development. PLoS Biol. 2004;2:E247. doi: 10.1371/journal.pbio.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trimarchi JM, Stadler MB, Cepko CL. Individual retinal progenitor cells display extensive heterogeneity of gene expression. PLoS One. 2008;3:e1588. doi: 10.1371/journal.pone.0001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roesch K, et al. The transcriptome of retinal Muller glial cells. J Comp Neurol. 2008;509:225–238. doi: 10.1002/cne.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]; An analysis of the mouse Müller glia transcriptome at the single cell level suggests some similarity with retinal progenitors and that there may be heterogeneity among Müller glia.
- 38.Del Debbio CB, et al. Notch and Wnt signaling mediated rod photoreceptor regeneration by Muller cells in adult mammalian retina. PLoS One. 2010;5:e12425. doi: 10.1371/journal.pone.0012425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer AJ, Reh TA. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]; An important paper that focused the field of retina regeneration on Müller glia as a source of retinal progenitors that could be used for repair.
- 40.Karl MO, et al. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci U S A. 2008;105:19508–19513. doi: 10.1073/pnas.0807453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ooto S, et al. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U S A. 2004;101:13654–13659. doi: 10.1073/pnas.0402129101. [DOI] [PMC free article] [PubMed] [Google Scholar]; First demonstration of Müller glia as a source of new neurons in the mammalian retina.
- 42.Osakada F, et al. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007;27:4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first report that Wnt signaling may control the generation of Müller glia-derived progenitors in mammals.
- 43.Takeda M, et al. alpha-Aminoadipate induces progenitor cell properties of Muller glia in adult mice. Invest Ophthalmol Vis Sci. 2008;49:1142–1150. doi: 10.1167/iovs.07-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan J, et al. Preferential regeneration of photoreceptor from Muller glia after retinal degeneration in adult rat. Vision Res. 2008;48:223–234. doi: 10.1016/j.visres.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Gohdo T, Ueda H, Ohno S, Iijima H, Tsukahara S. Heat shock protein 70 expression increased in rabbit muller cells in the ischemia-reperfusion model. Ophthalmic Res. 2001;33:298–302. doi: 10.1159/000055684. [DOI] [PubMed] [Google Scholar]
- 46.Paasche G, Huster D, Reichenbach A. The glutathione content of retinal Muller (glial) cells: the effects of aging and of application of free-radical scavengers. Ophthalmic Res. 1998;30:351–360. doi: 10.1159/000055495. [DOI] [PubMed] [Google Scholar]
- 47.Ghai K, Zelinka C, Fischer AJ. Notch signaling influences neuroprotective and proliferative properties of mature Muller glia. J Neurosci. 2010;30:3101–3112. doi: 10.1523/JNEUROSCI.4919-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayes S, Nelson BR, Buckingham B, Reh TA. Notch signaling regulates regeneration in the avian retina. Dev Biol. 2007;312:300–311. doi: 10.1016/j.ydbio.2007.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischer AJ, Reh TA. Exogenous growth factors stimulate the regeneration of ganglion cells in the chicken retina. Dev Biol. 2002;251:367–379. doi: 10.1006/dbio.2002.0813. [DOI] [PubMed] [Google Scholar]
- 50.Fischer AJ, McGuire CR, Dierks BD, Reh TA. Insulin and fibroblast growth factor 2 activate a neurogenic program in Muller glia of the chicken retina. J Neurosci. 2002;22:9387–9398. doi: 10.1523/JNEUROSCI.22-21-09387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ritchey ER, Zelinka CP, Tang J, Liu J, Fischer AJ. The combination of IGF1 and FGF2 and the induction of excessive ocular growth and extreme myopia. Exp Eye Res. 2012;99:1–16. doi: 10.1016/j.exer.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fischer AJ, Scott MA, Tuten W. Mitogen-activated protein kinase-signaling stimulates Muller glia to proliferate in acutely damaged chicken retina. Glia. 2009;57:166–181. doi: 10.1002/glia.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Das AV, et al. Neural stem cell properties of Muller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006;299:283–302. doi: 10.1016/j.ydbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 54.Lawrence JM, et al. MIO-M1 cells and similar muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007;25:2033–2043. doi: 10.1634/stemcells.2006-0724. [DOI] [PubMed] [Google Scholar]
- 55.Singhal S, et al. Human Muller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem Cells Transl Med. 2012;1:188–199. doi: 10.5966/sctm.2011-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jayaram H, et al. Transplantation of photoreceptors derived from human muller glia restore rod function in the P23H rat. Stem Cells Transl Med. 2014;3:323–333. doi: 10.5966/sctm.2013-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giannelli SG, Demontis GC, Pertile G, Rama P, Broccoli V. Adult human Muller glia cells are a highly efficient source of rod photoreceptors. Stem Cells. 2011;29:344–356. doi: 10.1002/stem.579. [DOI] [PubMed] [Google Scholar]
- 58.Wan J, Zheng H, Xiao HL, She ZJ, Zhou GM. Sonic hedgehog promotes stem-cell potential of Muller glia in the mammalian retina. Biochem Biophys Res Commun. 2007;363:347–354. doi: 10.1016/j.bbrc.2007.08.178. [DOI] [PubMed] [Google Scholar]
- 59.Pollak J, et al. ASCL1 reprograms mouse Muller glia into neurogenic retinal progenitors. Development. 2013;140:2619–2631. doi: 10.1242/dev.091355. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report shows Ascl1 overexpresssion can drive Müller glia reprogramming in postnatal mice. Interestingly, this protein also drives Müller glia reprogramming in zebrafish (see references 10, 63 and 64).
- 60.Close JL, Liu J, Gumuscu B, Reh TA. Epidermal growth factor receptor expression regulates proliferation in the postnatal rat retina. Glia. 2006;54:94–104. doi: 10.1002/glia.20361. [DOI] [PubMed] [Google Scholar]
- 61.Close JL, Gumuscu B, Reh TA. Retinal neurons regulate proliferation of postnatal progenitors and Muller glia in the rat retina via TGF beta signaling. Development. 2005;132:3015–3026. doi: 10.1242/dev.01882. [DOI] [PubMed] [Google Scholar]
- 62.Ueki Y, Reh TA. EGF stimulates Muller glial proliferation via a BMP-dependent mechanism. Glia. 2013;61:778–789. doi: 10.1002/glia.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramachandran R, Zhao XF, Goldman D. Ascl1a/Dkk/{beta}-catenin signaling pathway is necessary and glycogen synthase kinase-3{beta} inhibition is sufficient for zebrafish retina regeneration. Proc Natl Acad Sci U S A. 2011;108:15858–15863. doi: 10.1073/pnas.1107220108. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that Wnt signaling is necessary for retina regeneration in the injured zebrafish retina and that GSK3β inhibition suffices to drive Müller glia reprogramming in the uninjured retina.
- 64.Fausett BV, Gumerson JD, Goldman D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J Neurosci. 2008;28:1109–1117. doi: 10.1523/JNEUROSCI.4853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johns PR. Growth of the adult goldfish eye. III. Source of the new retinal cells. The Journal of comparative neurology. 1977;176:343–357. doi: 10.1002/cne.901760304. [DOI] [PubMed] [Google Scholar]
- 66.Johns PR, Fernald RD. Genesis of rods in teleost fish retina. Nature. 1981;293:141–142. doi: 10.1038/293141a0. [DOI] [PubMed] [Google Scholar]
- 67.Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thummel R, Kassen SC, Montgomery JE, Enright JM, Hyde DR. Inhibition of Muller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev Neurobiol. 2008;68:392–408. doi: 10.1002/dneu.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27:1712–1724. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hitchcock PF, Lindsey Myhr KJ, Easter SS, Jr., Mangione-Smith R, Jones DD. Local regeneration in the retina of the goldfish. J Neurobiol. 1992;23:187–203. doi: 10.1002/neu.480230209. [DOI] [PubMed] [Google Scholar]
- 71.Braisted JE, Raymond PA. Regeneration of dopaminergic neurons in goldfish retina. Development. 1992;114:913–919. doi: 10.1242/dev.114.4.913. [DOI] [PubMed] [Google Scholar]
- 72.Raymond PA, Reifler MJ, Rivlin PK. Regeneration of goldfish retina: rod precursors are a likely source of regenerated cells. J Neurobiol. 1988;19:431–463. doi: 10.1002/neu.480190504. [DOI] [PubMed] [Google Scholar]
- 73.Braisted JE, Essman TF, Raymond PA. Selective regeneration of photoreceptors in goldfish retina. Development. 1994;120:2409–2419. doi: 10.1242/dev.120.9.2409. [DOI] [PubMed] [Google Scholar]
- 74.Hitchcock PF, Vanderyt JT. Regeneration of the dopamine-cell mosaic in the retina of the goldfish. Vis Neurosci. 1994;11:209–217. doi: 10.1017/s0952523800001577. [DOI] [PubMed] [Google Scholar]
- 75.Braisted JE, Raymond PA. Continued search for the cellular signals that regulate regeneration of dopaminergic neurons in goldfish retina. Brain Res Dev Brain Res. 1993;76:221–232. doi: 10.1016/0165-3806(93)90210-2. [DOI] [PubMed] [Google Scholar]
- 76.Wu DM, et al. Cones regenerate from retinal stem cells sequestered in the inner nuclear layer of adult goldfish retina. Invest. Ophthalmol. Vis. Sci. 2001;42:2115–2124. [PubMed] [Google Scholar]
- 77.Otteson DC, D'Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev. Biol. 2001;232:62–76. doi: 10.1006/dbio.2001.0163. [DOI] [PubMed] [Google Scholar]
- 78.Yurco P, Cameron DA. Responses of Muller glia to retinal injury in adult zebrafish. Vision Res. 2005;45:991–1002. doi: 10.1016/j.visres.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 79.Senut MC, Gulati-Leekha A, Goldman D. An element in the alpha1-tubulin promoter is necessary for retinal expression during optic nerve regeneration but not after eye injury in the adult zebrafish. J Neurosci. 2004;24:7663–7673. doi: 10.1523/JNEUROSCI.2281-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramachandran R, Reifler A, Parent JM, Goldman D. Conditional gene expression and lineage tracing of tuba1a expressing cells during zebrafish development and retina regeneration. J Comp Neurol. 2010;518:4196–4212. doi: 10.1002/cne.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J Neurobiol. 2000;44:289–307. doi: 10.1002/1097-4695(20000905)44:3<289::aid-neu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 82.Montgomery JE, Parsons MJ, Hyde DR. A novel model of retinal ablation demonstrates that the extent of rod cell death regulates the origin of the regenerated zebrafish rod photoreceptors. J Comp Neurol. 2010;518:800–814. doi: 10.1002/cne.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wan J, Ramachandran R, Goldman D. HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration. Dev Cell. 2012;22:334–347. doi: 10.1016/j.devcel.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]; HB-EGF was the first secreted factor identified to drive Müller glia to form progenitors in the uninjured retina. This paper also demonstrated a role for Notch signaling in restricting the zone of injury-responsive Müller glia in the injured retina.
- 84.Nelson CM, et al. Tumor necrosis factor-alpha is produced by dying retinal neurons and is required for Muller glia proliferation during zebrafish retinal regeneration. J Neurosci. 2013;33:6524–6539. doi: 10.1523/JNEUROSCI.3838-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper suggests TNFα may initiate the injury response in Müller glia since it is produced by injured cells and necessary for stimulating Müller glia proliferation.
- 85.Kassen SC, et al. CNTF induces photoreceptor neuroprotection and Muller glial cell proliferation through two different signaling pathways in the adult zebrafish retina. Exp Eye Res. 2009;88:1051–1064. doi: 10.1016/j.exer.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 86.Faillace MP, Julian D, Korenbrot JI. Mitotic activation of proliferative cells in the inner nuclear layer of the mature fish retina: regulatory signals and molecular markers. J Comp Neurol. 2002;451:127–141. doi: 10.1002/cne.10333. [DOI] [PubMed] [Google Scholar]
- 87.Battista AG, Ricatti MJ, Pafundo DE, Gautier MA, Faillace MP. Extracellular ADP regulates lesion-induced in vivo cell proliferation and death in the zebrafish retina. J. Neurochem. 2009;111:600–613. doi: 10.1111/j.1471-4159.2009.06352.x. [DOI] [PubMed] [Google Scholar]
- 88.Nelson CM, et al. Stat3 defines three populations of Muller glia and is required for initiating maximal muller glia proliferation in the regenerating zebrafish retina. J Comp Neurol. 2012;520:4294–4311. doi: 10.1002/cne.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper identifies a variety of Müller glia cell populations that increase Stat3 expression following retinal injury and suggests multiple roles for Stat3 in the injured retina.
- 89.Craig SE, Calinescu AA, Hitchcock PF. Identification of the molecular signatures integral to regenerating photoreceptors in the retina of the zebra fish. J Ocul Biol Dis Infor. 2008;1:73–84. doi: 10.1007/s12177-008-9011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zelinka C, Scott M, Fischer A. Reactive microglia and the formation of Muller glia-derived retinal progenitors. ARVO Meeting Abstracts. 2013;54:5583. doi: 10.1002/glia.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bailey TJ, Fossum SL, Fimbel SM, Montgomery JE, Hyde DR. The inhibitor of phagocytosis, O-phospho-l-serine, suppresses Muller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp Eye Res. 2010 doi: 10.1016/j.exer.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meyers JR, et al. beta-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina. Neural Dev. 2012;7:30. doi: 10.1186/1749-8104-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. The EMBO journal. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lenkowski JR, et al. Retinal regeneration in adult zebrafish requires regulation of TGFbeta signaling. Glia. 2013;61:1687–1697. doi: 10.1002/glia.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cameron DA, Gentile KL, Middleton FA, Yurco P. Gene expression profiles of intact and regenerating zebrafish retina. Mol Vis. 2005;11:775–791. [PubMed] [Google Scholar]
- 96.Ramachandran R, Zhao XF, Goldman D. Insm1a-mediated gene repression is essential for the formation and differentiation of Muller glia-derived progenitors in the injured retina. Nat Cell Biol. 2012;14:1013–1023. doi: 10.1038/ncb2586. [DOI] [PMC free article] [PubMed] [Google Scholar]; A remarkable demonstration of how a single transcription factor can stimulate the formation and differentiation of Muller glia-derived progenitors depending on the cellular environment it acts in.
- 97.Powell C, Elsaeidi F, Goldman D. Injury-dependent Muller glia and ganglion cell reprogramming during tissue regeneration requires Apobec2a and Apobec2b. J Neurosci. 2012;32:1096–1109. doi: 10.1523/JNEUROSCI.5603-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thummel R, et al. Pax6a and Pax6b are required at different points in neuronal progenitor cell proliferation during zebrafish photoreceptor regeneration. Exp Eye Res. 2010;90:572–582. doi: 10.1016/j.exer.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; An important paper demonstrating the role of Pax6 proteins in the first divisions of Müller glia-derived progenitors.
- 99.Craig SE, et al. The zebrafish galectin Drgal1-l2 is expressed by proliferating Muller glia and photoreceptor progenitors and regulates the regeneration of rod photoreceptors. Invest Ophthalmol Vis Sci. 2010;51:3244–3252. doi: 10.1167/iovs.09-4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Calinescu AA, Vihtelic TS, Hyde DR, Hitchcock PF. Cellular expression of midkine-a and midkine-b during retinal development and photoreceptor regeneration in zebrafish. The Journal of comparative neurology. 2009;514:1–10. doi: 10.1002/cne.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shyh-Chang N, Daley GQ. Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell. 2013;12:395–406. doi: 10.1016/j.stem.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;463:621–626. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 105.Shyh-Chang N, et al. Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell. 2013;155:778–792. doi: 10.1016/j.cell.2013.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rybak A, et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol. 2008;10:987–993. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 107.Peng S, et al. Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells. 2011;29:496–504. doi: 10.1002/stem.591. [DOI] [PubMed] [Google Scholar]
- 108.Mikkelsen TS, et al. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Polo JM, et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell. 2012;151:1617–1632. doi: 10.1016/j.cell.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Karlic R, Chung HR, Lasserre J, Vlahovicek K, Vingron M. Histone modification levels are predictive for gene expression. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2926–2931. doi: 10.1073/pnas.0909344107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bernstein BE, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 112.Morris AC, Scholz TL, Brockerhoff SE, Fadool JM. Genetic dissection reveals two separate pathways for rod and cone regeneration in the teleost retina. Dev Neurobiol. 2008;68:605–619. doi: 10.1002/dneu.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fraser B, DuVal MG, Wang H, Allison WT. Regeneration of cone photoreceptors when cell ablation is primarily restricted to a particular cone subtype. PLoS One. 2013;8:e55410. doi: 10.1371/journal.pone.0055410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ariga J, Walker SL, Mumm JS. Multicolor time-lapse imaging of transgenic zebrafish: visualizing retinal stem cells activated by targeted neuronal cell ablation. J Vis Exp. 2010 doi: 10.3791/2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qin Z, et al. FGF signaling regulates rod photoreceptor cell maintenance and regeneration in zebrafish. Exp Eye Res. 2011;93:726–734. doi: 10.1016/j.exer.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kyritsis N, et al. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science. 2012;338:1353–1356. doi: 10.1126/science.1228773. [DOI] [PubMed] [Google Scholar]
- 117.Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc. Natl. Acad. Sci. U. S. A. 2013 doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zelinka CP, Scott MA, Volkov L, Fischer AJ. The reactivity, distribution and abundance of Non-astrocytic Inner Retinal Glial (NIRG) cells are regulated by microglia, acute damage, and IGF1. PLoS One. 2012;7:e44477. doi: 10.1371/journal.pone.0044477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fang Y, et al. Ephrin-A3 suppresses Wnt signaling to control retinal stem cell potency. Stem Cells. 2013;31:349–359. doi: 10.1002/stem.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jiao JW, Feldheim DA, Chen DF. Ephrins as negative regulators of adult neurogenesis in diverse regions of the central nervous system. Proc. Natl. Acad. Sci. U. S. A. 2008;105:8778–8783. doi: 10.1073/pnas.0708861105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Balenci L, Wonders C, Coles BL, Clarke L, van der Kooy D. Bone morphogenetic proteins and secreted frizzled related protein 2 maintain the quiescence of adult mammalian retinal stem cells. Stem Cells. 2013;31:2218–2230. doi: 10.1002/stem.1470. [DOI] [PubMed] [Google Scholar]
- 122.Fischer AJ, Scott MA, Ritchey ER, Sherwood P. Mitogen-activated protein kinase-signaling regulates the ability of Muller glia to proliferate and protect retinal neurons against excitotoxicity. Glia. 2009;57:1538–1552. doi: 10.1002/glia.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hochmann S, et al. Fgf Signaling is Required for Photoreceptor Maintenance in the Adult Zebrafish Retina. PLoS One. 2012;7:e30365. doi: 10.1371/journal.pone.0030365. [DOI] [PMC free article] [PubMed] [Google Scholar]