Abstract
Objective
To assess whether obesity is an independent predictor of mortality in women with cervical cancer.
Methods
This retrospective cohort study of patients with stages IB1-IVA cervical cancer treated with curative intent at MD Anderson Cancer Center from 1980 through 2007 categorized these women as underweight, normal weight, overweight, obese, or morbidly obese according to National Institutes of Health definitions. In addition to weight category, known prognostic factors for survival after a diagnosis of cervical cancer were included in a multivariate model. These known prognostic factors included age, smoking status, race or ethnicity (self-reported), socioeconomic status, comorbidities, tumor histologic subtype, tumor stage, tumor size, presence or absence of hydronephrosis, radiologic evidence of nodal metastasis, and the addition of concurrent chemotherapy with definitive radiation.
Results
A total of 3,086 patients met the inclusion criteria. The median survival for the entire cohort was 81 months (range, 0–365). The presence of lymph node spread and advancing stage were the most significant predictors of survival. Compared to normal-weight women, morbidly obese women had a significantly higher hazard ratio for both all-cause death (hazard ratio, 1.26; 95% CI, 1.10–1.45) and disease-specific death (hazard ratio, 1.24; 95% CI, 1.06–1.47). Underweight, overweight, and obese women did not have an increased risk for death compared to normal-weight women.
Conclusions
After controlling for all previously known prognostic factors, morbid obesity remains an independent risk factor for death from cervical cancer. Overweight and obese women have the same prognosis as normal-weight women.
INTRODUCTION
Epidemiologic studies have linked obesity with the risk of developing certain cancers, including colorectal, postmenopausal breast, kidney, esophageal (adenocarcinoma), pancreatic, liver, gallbladder, and endometrial cancers.[1] Many of these cancers arise as a result of physiologically altered endocrine and/or metabolic functioning in obese people. Large, prospective, population-based studies have demonstrated that in addition to increasing the risk of developing cancer, obesity remains an independent risk factor for death from malignancy in both obesity-associated and non-obesity-associated tumors.
Although these large population-based studies attempted to control for covariates such as age, race, and smoking status, among others, they did not control for other general confounders of mortality such as medical comorbidities or socioeconomic status. More importantly, none of these studies attempted to account for cancer-specific prognostic factors such as stage, histologic subtype, tumor grade, and treatment modality. For women with cervical cancer, many of these cofactors may be related. For example, although no data exist correlating obesity with advanced stage at diagnosis, obese women are less likely to undergo cervical cancer screening, [2] and lack of access to screening typically correlates with advanced stage at diagnosis.[3]
One way to determine if obesity is an independent risk factor for death after a diagnosis of cervical cancer is to control for known prognostic factors for survival in a multivariate analysis. The objective of this study was to assess whether obesity is an independent predictor of mortality in women with cervical cancer. We hypothesized that after controlling for known prognostic confounders, obesity would not remain an independent risk factor for death from cervical cancer.
MATERIALS AND METHODS
After approval was received from The University of Texas MD Anderson Cancer Center Institutional Review Board, data were abstracted from a database of patients with cervical cancer treated definitively during the period from January 1, 1980, through December 31, 2007. This database was created retrospectively in 1987 and continuously updated over a 25-year period using a relational database (Filemaker Pro, version 11, Filemaker, Inc., Santa Clara, CA). The senior author (PJE) abstracted roughly half the data while the other half was input by data coordinators and trainees. For those patient not input by the senior author, 30% of the cases were subsequently audited for accuracy. Furthermore, the database is continuously tested for data consistency and errors. Patients with stages IB1-IVA primary invasive cervical carcinoma (1994 FIGO staging) [4] treated with curative intent were included. Patients with primary cervical melanoma, sarcoma or lymphoma or disease metastatic to the cervix were excluded
Multiple covariates were examined including demographic variables, tumor and treatment factors. Age, height, and weight were recorded at the time of initial diagnosis. Body mass index (BMI) was computed for each patient by dividing her weight in kilograms by her height in meters squared. Using definitions from the National Institutes of Health, patients were then classified as underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2), obese (BMI 30.0–34.9 kg/m2), or morbidly obese (BMI ≥ 35.0 kg/m2). Race/ethnicity and smoking status were recorded at initial visit. Socioeconomic status was determined on the basis of the median household income for the patient’s zip code according to U.S. Census data for the decade during which the patient was diagnosed. The median household income values were then divided into quintiles. Medical comorbidities were assigned a score in the manner described by Charlson et al.[5]
Tumor histologic subtype was categorized as squamous carcinoma or adenocarcinoma. Rare histologic subtypes such as undifferentiated, neuroendocrine (small cell or large cell) and clear cell carcinomas were categorized as nonsquamous carcinoma/nonadenocarcinoma. Pretreatment tumor size was assessed clinically by a faculty gynecologic oncologist and/or a faculty radiation oncologist who specialized in gynecologic malignancies. Tumor stage was determined using the clinical staging system developed by the International Federation of Gynecology and Obstetrics in 1994. [4] The presence or absence of hydronephrosis was determined by either intravenous pyelography or computed tomography. Clinical evidence of nodal metastases was ascertained from imaging reports. For multivariate analysis, known prognostic factors such as tumor size > 4 cm and stage IIIB, IVA or tumor size > 7 cm were utilized.[6]
Currently, our institutional protocol is for most patients with stage IB1 disease to undergo radical hysterectomy with pelvic lymphadenectomy with or without postoperative adjuvant radiation therapy; however, early in the experience included in this study, many women with stage IB1 tumors were treated with definitive radiation therapy using a combination of external beam and intracavitary radiation.[7] Patients with stage IB2-IVA disease typically are treated with definitive radiation therapy. In addition, patients with early-stage disease (stage IB1) felt to be poor surgical candidates may be treated with definitive radiation therapy. In the 1980s, patients treated with radiation received radiation only; in the 1990s, patients treated with radiation received either radiation alone or chemoradiation depending on their enrollment in an ongoing protocol at that time.[8] After 2000, the majority of patients undergoing radiation therapy received concurrent chemotherapy. As the inclusion period spanned 27 years, analyses of outcomes by decades (1980–1989, 1990–1999, and 2000–2007) were also performed.
Data were analyzed using STATA statistical software, version 11.1. Associations between clinical variables were calculated using the chi-squared test. The Kruskal-Wallis test was used to compare median age in subgroups. The rates of overall survival and disease-specific survival were estimated using the Kaplan-Meier method with all-time intervals measured from the date of initiation of initial treatment. For calculations of disease-specific survival, the following were scored as events: deaths owing to disease, deaths resulting directly or indirectly from treatment-related complications, and deaths of unknown causes that occurred less than 5 years after completion of treatment (125 deaths). Deaths that were clearly unrelated to cervical cancer or its treatment and deaths of unknown causes that occurred at least 5 years after completion of treatment were censored from analyses of disease-specific survival. Survival times were compared using the log-rank method. Forward and backward stepwise Cox proportional hazards regression models were used to evaluate the relative importance of predictive factors for overall and disease-specific survival resulting in the same model; criteria for inclusion or exclusion from the model were P ≤ .05 and P > .051, respectively. The following variables met inclusion for the multivariate analysis: age, ethnicity, smoking, Charlson co-morbidity score, socioeconomic status, weight class, histologic subtype, tumor size, presence of hydronephrosis, node status, and utilization of concurrent chemotherapy with radiation therapy.
RESULTS
A total of 3,104 patients with stages IB1-IVA cervical cancer were treated at MD Anderson Cancer Center during the period from January 1, 1980, through December 31, 2007. Of these, 18 patients had missing data for height, weight, or both, and were therefore excluded from the study, leaving 3,086 patients for evaluation. The median age for the entire cohort was 45 years (range, 13–96), and the median BMI was 26.7 kg/m2 (range, 11.0–78.8). Five percent of patients were underweight, 35.5% were normal weight, 26.2% were overweight, 18.0% were obese, and 15.2% were morbidly obese. The median survival for the entire cohort was 81 months (range, 0–365).
Table 1 shows the demographic data for each weight category. Underweight and normal-weight women tended to be younger, Caucasian, White, or Anglo, and smokers. Heavier patients were more likely to have diabetes, have hypertension, have a high Charlson comorbidity score, and live in a zip code area with a low median household income. Table 2 shows the tumor factors for each weight category. As weight class increased, so did stage and the likelihood of nodal disease.
Table 1.
Demographic Characteristics of Study Participants
| Characteristic | Under-weight | Normal weight | Over-weight | Obese | Morbidly Obese | P Value |
|---|---|---|---|---|---|---|
|
| ||||||
| Total, no. (%) | 155 (5.0) | 1096 (35.5) | 809 (26.2) | 557 (18.0) | 469 (15.2) | |
|
| ||||||
| Median age (range), years | 43 (20–93) | 43 (13–96) | 48 (18–90) | 47 (19–90) | 46 (21–88) | <.0001 |
|
| ||||||
| Age group, no. (%) | <.0001 | |||||
| < 40 years | 60 (38.7) | 445 (40.6) | 237 (29.3) | 158 (28.4) | 132 (28.1) | |
| 40–50 years | 37 (23.9) | 257 (23.4) | 204 (25.2) | 152 (27.3) | 154 (32.8) | |
| 51–60 years | 26 (16.8) | 182 (16.6) | 155 (19.2) | 111 (19.9) | 101 (21.5) | |
| 61–70 years | 17 (11.0) | 122 (11.1) | 129 (15.9) | 92 (16.5) | 58 (12.4) | |
| >70 years | 15 (9.7) | 90 (8.2) | 84 (10.4) | 44 (7.9) | 24 (5.1) | |
|
| ||||||
| Race/ethnicity, no. (%) | <.0001 | |||||
| Caucasian/ White/Anglo | 99 (63.9) | 695 (63.4) | 387 (47.8) | 258 (46.3) | 211 (45.0) | |
| Hispanic | 15 (21.3) | 199 (13.5) | 233 (20.4) | 179 (19.7) | 115 (30.2) | |
| Black | 33 (9.7) | 148 (18.2) | 165 (28.8) | 110 (32.1) | 142 (24.5) | |
| Asian | 8 (5.2) | 48 (4.4) | 21 (2.6) | 6 (1.1) | 0 (0) | |
| Other/Unknown | 0 (0) | 6 (0.5) | 3 (0.4) | 4 (0.7) | 1 (0.2) | |
|
| ||||||
| Median household income by quintile, no. (%) | <.001 | |||||
| Lowest | 2 (1.4) | 34 (3.3) | 39 (5.0) | 23 (4.3) | 16 (3.5) | |
| Second | 60 (41.1) | 436 (41.8) | 385 (49.2) | 250 (46.4) | 239 (52.2) | |
| Third | 49 (33.6) | 302 (29.0) | 203 (25.9) | 165 (30.6) | 121 (26.4) | |
| Fourth | 10 (6.9) | 112 (10.6) | 57 (7.3) | 29 (5.4) | 24 (5.2) | |
| Highest | 3 (2.1) | 14 (1.3) | 8 (1.0) | 6 (1.1) | 1 (0.2) | |
| Unknown | 22 (15.1) | 144 (13.8) | 91 (11.6) | 66 (12.2) | 57 (12.5) | |
|
| ||||||
| Smoker, no. (%) | <.0001 | |||||
| No | 65 (41.9) | 620 (56.6) | 550 (68.0) | 364 (65.4) | 329 (70.2) | |
| Yes | 88 (56.8) | 453 (41.3) | 246 (30.4) | 178 (32.0) | 132 (28.1) | |
| Unknown | 2 (1.3) | 23 (2.1) | 13 (1.6) | 15 (2.7) | 8 (1.7) | |
|
| ||||||
| Charlson score, no. (%) | <.0001 | |||||
| 1 | 141 (91.0) | 1005 (91.7) | 700 (86.5) | 469 (84.2) | 371 (79.1) | |
| 2 | 11 (7.1) | 82 (7.5) | 92 (11.4) | 72 (12.9) | 76 (16.2) | |
| 3 | 3 (1.9) | 9 (0.8) | 17 (2.1) | 16 (2.9) | 22 (4.7) | |
|
| ||||||
| Diabetes, no. (%) | <.0001 | |||||
| No | 153 (98.8) | 1056 (96.4) | 732 (90.5) | 490 (87.8) | 391 (83.4) | |
| Yes | 2 (1.2) | 40 (3.6) | 77 (9.5) | 67 (12.2) | 78 (16.6) | |
|
| ||||||
| Hypertension, no. (%) | <.0001 | |||||
| No | 133 (85.8) | 938 (85.6) | 615 (76.0) | 389 (69.8) | 298 (63.5) | |
| Yes | 22 (14.2) | 158 (14.4) | 194 (24.0) | 168 (30.2) | 171 (36.5) | |
Table 2.
Tumor Factors for Study Participants*
| Factor | Under-weight | Normal weight | Over-weight | Obese | Morbidly Obese | P Value |
|---|---|---|---|---|---|---|
|
| ||||||
| Total, no. (%) | 155 (5.0) | 1096 (35.5) | 809 (26.2) | 557 (18.0) | 469 (15.2) | |
|
| ||||||
| Tumor histologic subtype, no. (%) | .53 | |||||
| Squamous | 124 (80.0) | 849 (77.5) | 626 (77.4) | 436 (78.2) | 365 (77.8) | |
| Adenocarcinoma | 30 (19.4) | 215 (19.8) | 160 (19.8) | 105 (18.9) | 78 (16.6) | |
| Neuroendocrine/Undifferentiated/ Other | 1 (0.6) | 30 (2.7) | 22 (2.7) | 16 (2.9) | 26 (5.5) | |
|
| ||||||
| Clinical stage, no. (%) | .023 | |||||
| IB1 | 60 (38.7) | 464 (42.3) | 305 (37.7) | 215 (38.6) | 177 (37.7) | |
| IB2 | 33 (21.3) | 260 (23.7) | 209 (25.8) | 160 (28.7) | 116 (24.7) | |
| IIA | 15 (9.7) | 73 (6.7) | 62 (7.7) | 47 (8.4) | 31 (6.6) | |
| IIB | 14 (9.0) | 121 (11.0) | 110 (13.6) | 66 (11.9) | 64 (13.7) | |
| IIIA | 1 (0.7) | 17 (1.6) | 14 (1.7) | 11 (2.0) | 12 (2.6) | |
| IIIB | 29 (18.7) | 142 (13.0) | 104 (12.9) | 54 (9.7) | 67 (14.3) | |
| IVA | 3 (1.9) | 19 (1.7) | 5 (0.6) | 4 (0.7) | 1 (0.2) | |
|
| ||||||
| Stage III or IV, no. (%) | .038 | |||||
| No | 122 (78.7) | 918 (83.8) | 686 (84.8) | 488 (87.6) | 388 (82.7) | |
| Yes | 33 (21.3) | 178 (16.2) | 123 (15.2) | 69 (12.4) | 81 (17.3) | |
|
| ||||||
| Hydronephrosis, no. (%) | .24 | |||||
| No | 138 (89.0) | 1005 (91.7) | 744 (92.0) | 526 (94.4) | 430 (91.7) | |
| Yes | 17 (11.0) | 91 (8.3) | 65 (8.0) | 31 (5.6) | 39 (8.3) | |
|
| ||||||
| Nodal status, no. (%) | .013 | |||||
| Negative | 131 (84.5) | 891 (81.3) | 619 (76.5) | 442 (79.4) | 351 (74.8) | |
| Positive | 24 (15.5) | 205 (18.7) | 190 (23.5) | 115 (20.6) | 118 (25.2) | |
Table 3 shows the treatments for each weight category. Patients in higher weight classes were more likely to be treated with initial radiation as opposed to surgery (p<.0001). For those patients who received surgery first, patients in the higher weight classes were more likely to get adjuvant radiation therapy (p = .002). Finally, for patients who received initial radiation therapy, there was no difference among weight classes as to whom then underwent a completion hysterectomy (p=.89)
Table 3.
Treatment for Study Participants*
| Under-weight | Normal weight | Over-weight | Obese | Morbidly Obese | p-value | |
|---|---|---|---|---|---|---|
|
| ||||||
| Total | 155 | 1096 | 809 | 557 | 469 | |
|
| ||||||
| Radical hysterectomy | 37 (23.9) | 284 (25.9) | 164 (20.3) | 94 (16.9) | 51 (10.9) | < .0001 |
| Primary radiation therapy | 108 (69.7) | 753 (68.7) | 616 (76.1) | 443 (79.5) | 405 (86.4) | |
| Other** | 10 (6.4) | 59 (5.4) | 29 (3.6) | 20 (3.6) | 13 (2.7) | |
|
| ||||||
| Radical hysterectomy | 37 | 284 | 164 | 94 | 51 | .002 |
| Adjuvant radiation | 9 (24.3) | 53 (18.7) | 58 (35.4) | 26 (27.7) | 16 (31.4) | |
| No adjuvant radiation | 28 (75.7) | 231 (81.3) | 106 (64.6) | 68 (72.3) | 35 (68.6) | |
|
| ||||||
| Primary radiation therapy | 108 | 753 | 616 | 443 | 405 | .89 |
| Adjuvant Surgery | 3 (2.8) | 13 (1.7) | 10 (1.6) | 10 (2.3) | 8 (2.0) | |
| No adjuvant surgery | 105 (97.2) | 740 (98.3) | 606 (98.4) | 433 (97.7) | 397 (98.0) | |
Values in table are number of patients (percentage).
Other includes radiation after a cut-through hysterectomy, brachytherapy only, radiation therapy changed from curative intent to palliative, simple hysterectomy only, radiation or surgery for cancer in a cervical stump
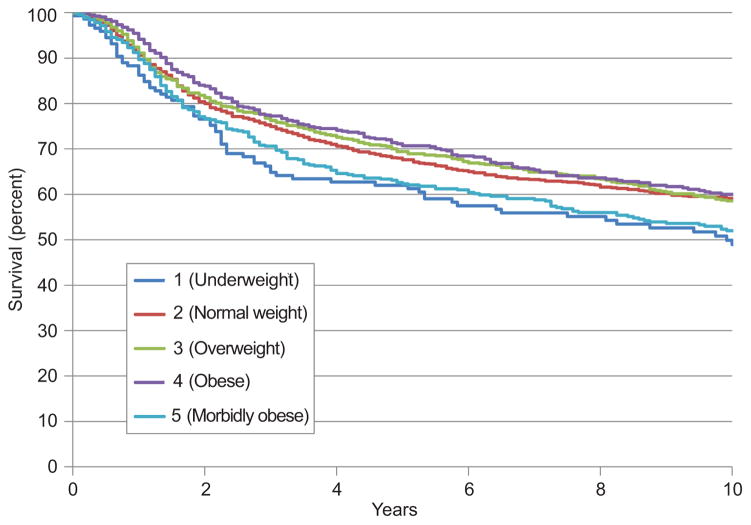
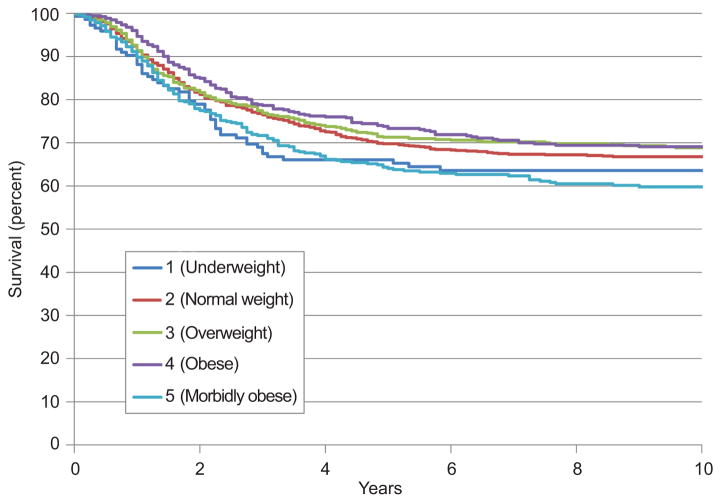
The median follow-up time for patients who did not experience recurrence was 133 months (range, 0–366). Figure 1 shows the Kaplan-Meyer curves for overall survival for each of the five weight categories. The five-year overall survival rate was 61% for underweight patients, 67% for normal weight patients, 68% for overweight patients, 70% for obese patients, and 61% for morbidly obese patients. Figure 2 shows the Kaplan-Meyer curves for disease-specific survival for each of the five weight categories. The five-year disease-specific survival rate was 65% for underweight patients, 69% for normal weight patients, 70% for overweight patients, 72% for obese patients, and 63% for morbidly obese patients.
Figure 1.
Overall survival by weight class.
Figure 2.
Disease-specific survival by weight class.
On univariate analysis, worse overall survival was related to increased age (P < .0001), African American race (P < .0001), smoking (P < .0001), decreased median household income (P < .0001), increased Charlson comorbidity index score (P < .0001), weight category (P = .003), morbid obesity (P = .0006), tumor >4 cm (P < .0001), tumor >7 cm (P < .0001), presence of hydronephrosis (P < .0001), and node positivity (P < .0001).
Table 4 shows the variables that remained significant for disease-specific and overall survival in multivariate analysis. Morbid obesity remained a significant predictor of both disease-specific (HR, 1.24; 95% CI, 1.06–1.47) and overall survival (HR, 1.26; 95% CI, 1.10–1.45) even after correction for other predictors of poor outcome. None of the other weight categories was significantly correlated with disease-specific or overall survival. In addition, there were no correlations with survival when patients were analyzed based on decade of treatment. As we did not have median household income data for 380 (12%) patients, this variable was not included in the multivariate analysis presented in Table 4. However, when we did perform the analysis with median household income included, low median household income was an independent risk factor for poor survival. All of the other variables presented in table 4 also remained significant prognostic factors for survival.
Table 4.
Variables Significant for Disease-Specific and Overall Survival in the Cox Multivariate Proportion Hazards Regression Model
| Variable | HR for Disease-Specific Death (95% CI) | HR for Death (95% CI) |
|---|---|---|
| Morbid obesity | 1.24 (1.06–1.47) | 1.26 (1.10–1.45) |
| Age | NS | 1.03 (1.02–1.03) |
| Smoking | 1.20 (1.06–1.37) | 1.24 (1.08–1.43) |
| Charlson score > 1 | 1.26 (1.03–1.53) | 1.37 (1.18–1.60) |
| Charlson score > 2 | 1.60 (1.06–2.40) | 1.58 (1.15–2.15) |
| Nonsquamous histologic subtypes | 1.49 (1.29–1.74) | 1.18 (1.04–1.34) |
| Tumor > 4 cm | 2.49 (2.05–3.03) | 1.59 (1.39–1.81) |
| Stage IIIB, IVA or Tumor > 7 cm | 1.76 (1.45–2.13) | 1.61 (1.41–1.84) |
| Hydronephrosis | 1.34 (1.06–1.68) | 1.45 (1.22–1.73) |
| Positive nodes | 2.02 (1.75–2.33) | 1.75 (1.55–1.99) |
| Concurrent chemotherapy with radiation therapy | 0.64 (0.54–0.75) | 0.72 (0.62–0.84) |
DISCUSSION
In this study, after controlling for known confounders, morbidly obese women with cervical cancer had a risk of death from disease that was 1.26 times the risk of death in their normal-weight counterparts. Although underweight women also had a higher risk of death, being underweight was not an independent risk factor for death in the multivariate analysis. Overweight and obese women had the same mortality as normal-weight women. Finally, known prognostic factors also remained significant on multivariate analysis, including demographic endpoints (age, smoking, and comorbidities), tumor factors (nonsquamous histologic subtypes, tumor size, hydronephrosis, and nodal status), and treatment paradigms (concurrent chemoradiation).
Many investigators have found obesity and morbid obesity to be risk factors for death from cancer in both population-based [9, 10] and site-specific studies.[11–13] For example, Calle et al found that morbidly obese women were more likely than normal-weight women to die from cancer in the gallbladder, liver, breast, esophagus, pancreas, kidney, ovary, uterus, and non-Hodgkin lymphoma as well as disease in the cervix (relative risk, 3.20).[9] The mechanism by which obesity confers a worse prognosis in virtually every malignancy is certainly multifactorial and may differ from site to site. For patients with cervical cancer, most likely both treatment-related and biological factors contribute to decreased disease-specific survival for the morbidly obese.
Patients with early-stage cervical cancer typically undergo radical surgery, whereas patients with advanced-stage cervical cancer usually are treated with initial radiation therapy. In our study, morbidly obese patients were more likely to undergo primary radiation therapy, even when they had relatively early disease. Surgeons may have had reservations operating on the morbidly obese even though surgical outcomes are equivalent across weight categories in patients who do undergo surgery.[14]
The delivery of radiation therapy can also be more challenging in morbidly obese patients. Initial diagnostic imaging is often of poor quality, potentially leading to errors in the assessment of the extent of disease specified for treatment planning. The computed tomography scanners typically used for radiation treatment simulation may not provide a full representation of a morbidly obese patient’s external contour, complicating treatment planning.[15] Also, in morbidly obese patients, even high-energy radiation beams may have trouble penetrating to the center of the pelvis without overdosing peripheral soft tissues. Furthermore, laxity of pannicular and other skin folds can lead to inaccuracies in day-to-day set-up.[16] For example, 21% of morbidly obese men with prostate cancer have a left-right and an anterior-posterior shift of more than 1 cm during external beam radiation therapy due to deviations in daily set-up.[17] In some cases, the lateral movement may be over 4 cm.[18] Although modern daily on-board imaging techniques may reduce this uncertainty, the poor quality of images acquired in obese patients may still compromise the accuracy of set-up.
In addition to potentially receiving inadequate radiation therapy, morbidly obese patients may receive insufficient doses of chemotherapy. There remains concern among oncologists that utilizing actual body weight in obese and morbidly obese patients to calculate chemotherapy prescriptions may lead to increased toxicity. In fact, as many as 40% of obese patients receive smaller doses of chemotherapy than would be predicted by their actual body weight.[19] For women with cervical cancer, cisplatin given as part of combination chemotherapy may be “capped” at 70 mg weekly.[20] This correlates with a body surface area of only 1.75 m2, significantly less than the true body surface area of obese and morbidly obese women. Furthermore, chemotherapy given for recurrent disease is often “capped” at a body surface area of 2.0 m2, also considerably less than the calculated body surface area for these women. In tumor types other than cervical cancer, weight-based reductions in the chemotherapy dose administered to obese patients have been shown to correlate with decreased survival.[21] Furthermore, there are no data to support increased toxicity of chemotherapy when patients are given doses based on actual body weight. For that reason, the American Society of Clinical Oncology has recently recommended that actual body weight be used to determine the chemotherapy dose in obese and morbidly obese patients and has indicated that selecting reduced doses in these patients may lead to poorer survival rates.[19]
Although compromised treatment may help to explain poorer outcomes in morbidly obese women with cervical cancer, biological factors may also contribute to their decreased survival. For example, obese people typically have chronic hyperinsulinemia. High circulating levels of insulin, in turn, promote production of insulin-like growth factor 1 (IGF-1).[1] IGF-1 is a regulator of cellular proliferation and has many oncologic effects, including stimulation of cancer cells. This peptide hormone has been shown to adversely affect prognosis in a wide variety of tumors.[22] In cervical cancer cell cultures and mouse models, IGF-1 has been implicated in both the development and progression of the disease.[23] Furthermore, high levels of the IGF-1 receptor in tissue specimens have been correlated with worse prognosis in women with cervical cancer.[24]
Although this was a retrospective study with all the bias inherent in that type of design, we believe this study has some remarkable strengths. First, in addition to the traditional demographic and tumor factors included in most retrospective studies, we were able to account for socioeconomic status and medical comorbidities, both of which are important potential confounders of mortality in women with cervical cancer. In addition, the study had the benefit of a very large cohort of patients with 1,512 deaths. This large number of events allowed for a vigorous multivariate analysis in an effort to determine whether BMI had an independent influence on mortality. This is in contrast to many of the previous studies evaluating obesity and cancer survival. For example, in the excellent study by Calle et al, [9] although over 900,000 people were evaluated, the analysis of mortality in women with cervical cancer included only 164 deaths. Finally, in our study, the multivariate analysis showed that not only was morbid obesity an adverse prognostic factor for survival; so too were all other known risk factors for mortality in women with cervical cancer.
Acknowledgments
Supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant, CA016672.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 2.Wee CC, McCarthy EP, Davis RB, Phillips RS. Screening for cervical and breast cancer: is obesity an unrecognized barrier to preventive care? Ann Intern Med. 2000;132:697–704. doi: 10.7326/0003-4819-132-9-200005020-00003. [DOI] [PubMed] [Google Scholar]
- 3.Akers AY, Newmann SJ, Smith JS. Factors underlying disparities in cervical cancer incidence, screening, and treatment in the United States. Curr Probl Cancer. 2007;31:157–81. doi: 10.1016/j.currproblcancer.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Quinn MA, Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, et al. Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95 (Suppl 1):S43–103. doi: 10.1016/S0020-7292(06)60030-1. [DOI] [PubMed] [Google Scholar]
- 5.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 6.Eifel PJ, Jhingran A, Levenback CF, Tucker S. Predictive value of a proposed subclassification of stages I and II cervical cancer based on clinical tumor diameter. Int J Gynecol Cancer. 2009;19:2–7. doi: 10.1111/IGC.0b013e318197f185. [DOI] [PubMed] [Google Scholar]
- 7.Eifel PJ. The uterine cervix. In: Cox JD, Ang KK, editors. Radiation Oncology. Rationale, Technique, Results. St Louis: Mosby; 2003. pp. 681–723. [Google Scholar]
- 8.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–43. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 9.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 10.Lew EA, Garfinkel L. Variations in mortality by weight among 750,000 men and women. J Chronic Dis. 1979;32:563–76. doi: 10.1016/0021-9681(79)90119-x. [DOI] [PubMed] [Google Scholar]
- 11.Freedland SJ, Platz EA. Obesity and prostate cancer: making sense out of apparently conflicting data. Epidemiol Rev. 2007;29:88–97. doi: 10.1093/epirev/mxm006. [DOI] [PubMed] [Google Scholar]
- 12.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370–8. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 13.Dignam JJ, Polite BN, Yothers G, Raich P, Colangelo L, O’Connell MJ, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006;98:1647–54. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- 14.Frumovitz M, Sun CC, Jhingran A, Schmeler KM, Dos Reis R, Milam MR, et al. Radical hysterectomy in obese and morbidly obese women with cervical cancer. Obstet Gynecol. 2008;112:899–905. doi: 10.1097/AOG.0b013e3181863280. [DOI] [PubMed] [Google Scholar]
- 15.Fisher C, Fortenberry C, Jhingran A, Eifel P. Novel technique for simulation and external beam treatment planning for obese patients. Practical Radiation Oncology. 2011;1:152–155. doi: 10.1016/j.prro.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Sweigart KD. A simple method of alignment for pelvic irradiation in obese patients. Med Dosim. 2002;27:269–70. doi: 10.1016/s0958-3947(02)00151-6. [DOI] [PubMed] [Google Scholar]
- 17.Wong JR, Gao Z, Merrick S, Wilson P, Uematsu M, Woo K, et al. Potential for higher treatment failure in obese patients: correlation of elevated body mass index and increased daily prostate deviations from the radiation beam isocenters in an analysis of 1,465 computed tomographic images. Int J Radiat Oncol Biol Phys. 2009;75:49–55. doi: 10.1016/j.ijrobp.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 18.Millender LE, Aubin M, Pouliot J, Shinohara K, Roach M., 3rd Daily electronic portal imaging for morbidly obese men undergoing radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;59:6–10. doi: 10.1016/j.ijrobp.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Griggs JJ, Mangu PB, Anderson H, Balaban EP, Dignam JJ, Hryniuk WM, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30:1553–61. doi: 10.1200/JCO.2011.39.9436. [DOI] [PubMed] [Google Scholar]
- 20.Monk BJ, Tewari KS, Koh WJ. Multimodality therapy for locally advanced cervical carcinoma: state of the art and future directions. J Clin Oncol. 2007;25:2952–65. doi: 10.1200/JCO.2007.10.8324. [DOI] [PubMed] [Google Scholar]
- 21.Rosner GL, Hargis JB, Hollis DR, Budman DR, Weiss RB, Henderson IC, et al. Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: results from Cancer and Leukemia Group B study 8541. J Clin Oncol. 1996;14:3000–8. doi: 10.1200/JCO.1996.14.11.3000. [DOI] [PubMed] [Google Scholar]
- 22.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–69. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 23.Shen MR, Hsu YM, Hsu KF, Chen YF, Tang MJ, Chou CY. Insulin-like growth factor 1 is a potent stimulator of cervical cancer cell invasiveness and proliferation that is modulated by alphavbeta3 integrin signaling. Carcinogenesis. 2006;27:962–71. doi: 10.1093/carcin/bgi336. [DOI] [PubMed] [Google Scholar]
- 24.Huang YF, Shen MR, Hsu KF, Cheng YM, Chou CY. Clinical implications of insulin-like growth factor 1 system in early-stage cervical cancer. Br J Cancer. 2008;99:1096–102. doi: 10.1038/sj.bjc.6604661. [DOI] [PMC free article] [PubMed] [Google Scholar]