Abstract
The purpose of this study was to describe 18F-FDG uptake across a spectrum of pediatric brain tumors and correlate 18F-FDG PET with MR imaging variables, progression-free survival (PFS), and overall survival (OS).
Methods
A retrospective analysis was conducted of children enrolled in phase I/II clinical trials through the Pediatric Brain Tumor Consortium from August 2000 to June 2010. PET variables were summarized within diagnostic categories using descriptive statistics. Associations of PET with MR imaging variables and PFS and OS by tumor types were evaluated.
Results
Baseline 18F-FDG PET was available in 203 children; 66 had newly diagnosed brain tumors, and 137 had recurrent/refractory brain tumors before enrolling in a Pediatric Brain Tumor Consortium trial. MR imaging was performed within 2 wk of PET and before therapy in all cases. The 18F-FDG uptake pattern and MR imaging contrast enhancement (CE) varied by tumor type. On average, glioblastoma multiforme and medulloblastoma had uniform, intense uptake throughout the tumor, whereas brain stem gliomas (BSGs) had low uptake in less than 50% of the tumor and ependymoma had low uptake throughout the tumor. For newly diagnosed BSG, correlation of 18F-FDG uptake with CE portended reduced OS (P = 0.032); in refractory/recurrent BSG, lack of correlation between 18F-FDG uptake and CE suggested decreased PFS (P = 0.023). In newly diagnosed BSG for which more than 50% of the tumor had 18F-FDG uptake, there was a suggestion of lower apparent diffusion coefficient (P = 0.061) and decreased PFS (P = 0.065).
Conclusion
18F-FDG PET and MR imaging showed a spectrum of patterns depending on tumor type. In newly diagnosed BSG, the correlation of 18F-FDG uptake and CE suggested decreased OS, likely related to more aggressive disease. When more than 50% of the tumor had 18F-FDG uptake, the apparent diffusion coefficient was lower, consistent with increased cellularity. In refractory/recurrent BSG, poor correlation between 18F-FDG uptake and CE was associated with decreased PFS, which may reflect concurrent tissue breakdown at sites of treated disease and development of new sites of 18F-FDG–avid malignancy.
Keywords: MR diffusion, 18F-FDG PET, pediatric, brain tumor
Brain tumors are the most common solid tumors (1) and second leading cause of cancer death in children (2). Classified by cell type and histologically graded using World Health Organization criteria (3), prognosis differs depending on histology, location, extent, and molecular subtypes (4). The Pediatric Brain Tumor Consortium (PBTC) was formed to conduct phase I/II clinical trials for children with primary brain tumors, characterize reliable biomarkers and predictors of therapy response, and develop and coordinate innovative neuroimaging techniques (5).
18F-FDG PET is helpful in evaluating pediatric brain tumors for which 18F-FDG uptake intensity and uniformity have been linked with tumor type, malignancy grade, and prognosis (6–13). Combining 18F-FDG PET and MR imaging allows for metabolic and anatomic evaluation of pediatric brain tumors (14,15). A better understanding of variations in 18F-FDG PET and MR imaging findings across the spectrum of pediatric tumors may clarify early markers of therapy response and survival, ultimately improving clinical management. Our aim was to describe patterns of tumor 18F-FDG uptake and correlate 18F-FDG PET with MR imaging variables, progression-free survival (PFS), and overall survival (OS).
MATERIALS AND METHODS
A retrospective analysis was conducted of all children with 1 baseline-interpretable 18F-FDG PET scan, enrolled in a PBTC phase I/II trial from August 2000 to June 2010. All children underwent pretherapy MR imaging within 2 wk of PET. The institutional review boards of all sites approved each trial before patient enrollment, and approval was maintained throughout the studies. MR and 18F-FDG PET imaging data acquired at participating institutions were electronically transferred to the PBTC Operations and Biostatistics Center and PBTC Neuroimaging Center for analysis (16). Investigators at the Neuroimaging Center were masked to patient outcome at the time of image evaluation.
18 F-FDG PET and MR Imaging Acquisition
18F-FDG PET was performed using a variety of scanners (AdvanceNXI, DiscoveryLS, and DiscoverySTE [GE Healthcare]; G-PET [Philips]; HR1 and HiRez Bioscan [Siemens]). Consistency was maintained by adherence to a standard acquisition protocol and quality assurance program including daily blank scans, quarterly normalization, calibration, and preventive maintenance (17). Patients fasted for 4 h before 18F-FDG administration. Baseline brain PET was acquired in 3-dimensional mode for 10 min, 40–60 min after intravenous administration of 18F-FDG (5.55 MBq/kg; minimum, 18 MBq; maximum, 370 MBq). Attenuation correction was performed using a 3-min segmented transmission scan with 68Ge/68Ga rods for PET-only scanners or a CT-based approach with PET/CT scanners. Data were reconstructed using Fourier rebinning, followed by a 2-dimensional ordered-subset expectation maximization reconstruction algorithm.
MR imaging was performed at each institution using 1.5-T scanners with axial T1, fluid-attenuated inversion recovery (FLAIR), and T2 sequences. T1 images were also acquired after gadolinium diethylenetriamine pentaacetic acid (Gd-DTPA) contrast administration. Gd-DTPA axial T1–weighted spin-echo images were 4-mm contiguous slices using a repetition time (TR)/echo time (TE) of 500–700 ms/minimum full. FLAIR images were 4-mm contiguous slices using a TR/time of inversion/TE of 10,000/2,200/162 ms. Diffusion images were single-shot echoplanar spin-echo images with a TR/TE of 2,000/80 ms, 128 × 128 matrix, b-factor of 5/1,000s/mm2, 3 directions (x,y,z) for trace imaging, receiver bandwidth of ±64kHz, frequency direction right to left (R/L), and slice thickness/gap of 4/0.
Image Analysis
Tumor extent was evaluated by a pediatric neuroradiologist using axial T1 post–Gd-DTPA, FLAIR, and T2-weighted MR imaging. Apparent diffusion coefficient (ADC) image/region-of-interest (ROI) analyses were performed using ImageJ (U.S. National Institutes of Health). From the ADC map, an ROI (diameter, 3–5 mm) within the solid part of the tumor was determined using T1, FLAIR, T2, and post–Gd-DTPA T1 as a reference. The mean ADC of the ROI was divided by the mean ADC from an ROI in normal frontal white matter (WM) to obtain the normalized ADC within the tumor.
18F-FDG PET and MR imaging data were registered using a mutual-information algorithm. The registration quality was evaluated subjectively, and the data were analyzed by consensus among a neuroradiologist, nuclear medicine physician, and nuclear medicine physicist (11,15). The data analysis has been previously described (14). 18F-FDG uptake uniformity was subjectively graded as percentage of tumor (on FLAIR) demonstrating 18F-FDG uptake using a 4-point scale (0, <25%; 1, 26%–50%; 2, 51%–75%; and 3, 76%–100%) reached as a consensus between the neuroradiologist and the PET experts. 18F-FDG uptake intensity was subjectively categorized using a 5-point scale (1, <WM; 2, similar to WM; 3, >WM but <gray matter [GM]; 4, similar to GM; and 5, >GM). Grade 0 uniformity indicated negligible tumor 18F-FDG uptake. The axial image through the tumor containing the highest 18F-FDG uptake was identified, and a 2-dimensional ROI was manually drawn based on tumor 18F-FDG definition. Because of the wide range of 18F-FDG uptake across all tumor types, a set threshold could not be used and ROIs were defined subjectively. Because PET data were from a multicenter trial, ROI values were standardized by normalizing values by a comparison region (contralateral WM or GM) as previously reported (14). Continuous PET variables including mean or maximum tumor 18F-FDG uptake normalized by WM or GM were derived.
Linear associations between continuous variables of interest were described using the Spearman rank correlation coefficient, and χ2 (or Armitage Trend tests, when appropriate) was used to describe the association between categoric variables of interest. To compare the distribution of a continuous imaging marker between 2 groups of patients, the Wilcoxon–Mann–Whitney test was used. Distributions of PFS and OS were estimated using the Kaplan–Meier method, and survival distributions were compared between 2 or more groups using the log-rank test. Cox proportional hazards models were used to investigate associations of continuous 18F-FDG PET variables, PFS, and OS. OS was defined as the interval between initiation of treatment and death on study. PFS was defined as the interval between initiation of treatment and the earliest of either progressive disease or death during the study for patients whose treatment failed. Patients without failure for PFS or OS were censored at the off-study date. When statistically appropriate, log-transformation of covariates was used in survival models.
RESULTS
An interpretable baseline 18F-FDG PET scan was available in 203 children (104 girls, 99 boys; age, 0.4–21 y): 66 newly diagnosed (ND) brain tumors and 137 refractory/recurrent (RR) disease (Table 1) enrolled in a PBTC phase I/II trial between August 2000 and June 2010. Baseline MR imaging was performed within 2 wk of baseline PET in all cases. Astrocytoma included juvenile astrocytoma, pilocytic astrocytoma, and astrocytoma not otherwise specified (NOS); medulloblastoma included desmoplastic medulloblastoma and medulloblastoma NOS; and ependymoma included anaplastic ependymoma and ependymoma NOS.
TABLE 1.
Pediatric Brain Tumor Types with Baseline 18F-FDG PET
| Diagnosis | ND
|
RR
|
||||
|---|---|---|---|---|---|---|
| No. of patients | Death | Progression | No. of patients | Death | Progression | |
| BSG | 53 | 46 | 50 | 18 | 8 | 15 |
|
| ||||||
| GBM | 9 | 7 | 9 | 15 | 6 | 14 |
|
| ||||||
| Anaplastic astrocytoma | 2 | 1 | 2 | 28 | 10 | 24 |
|
| ||||||
| Astrocytoma/astrocytic glioma | — | — | — | 23 | 3 | 15 |
|
| ||||||
| Ependymoma | — | — | — | 15 | 2 | 12 |
|
| ||||||
| Medulloblastoma | — | — | — | 10 | 0 | 7 |
|
| ||||||
| Primitive neuroectodermal tumor/pineoblastoma | — | — | — | 5 | 3 | 5 |
|
| ||||||
| Others* | 2 | 2 | 2 | 23 | 5 | 14 |
Others: anaplastic ganglioglioma, ganglioglioma, glioblastoma (NOS), malignant glioma, malignant glioma (NOS except nasal glioma, not neoplastic), gliomatosis cerebri, gliosarcoma, mixed oligoastrocytoma, myxopapillary ependymoma, pleomorphic xanthoastrocytoma, rhabdoid sarcoma.
PET Variables
The intensity and uniformity of 18F-FDG uptake varied by brain tumor type. Because of the small sample size, analysis was possible only for a subgroup of types (Tables 2–4). In Table 2, the column categories are based on subjective 18F-FDG uptake grading.
TABLE 2.
18F-FDG Uptake Intensity by Tumor Type
| Tumor type |
18F-FDG uptake intensity
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <WM (no further data)
|
Similar to WM
|
>WM and <GM
|
Similar to GM
|
> GM
|
All patients (n) | ||||||
| n | Row % | n | Row % | n | Row % | n | Row % | n | Row % | ||
| Anaplastic astrocytoma RR | 3 | 10.7 | 3 | 10.7 | 10 | 35.7 | 4 | 14.3 | 8 | 28.6 | 28 |
|
| |||||||||||
| Astrocytoma/astrocytic glioma RR | 3 | 13.0 | 4 | 17.4 | 5 | 21.7 | 7 | 30.4 | 4 | 17.4 | 23 |
|
| |||||||||||
| Ependymoma RR | 6 | 40.0 | 2 | 13.3 | 6 | 40.0 | 1 | 6.7 | — | — | 15 |
|
| |||||||||||
| Medulloblastoma RR | 2 | 20.0 | — | — | 3 | 30.0 | 1 | 10.0 | 4 | 40.0 | 10 |
|
| |||||||||||
| BSG ND | 8 | 15.1 | 7 | 13.2 | 29 | 54.7 | 3 | 5.7 | 6 | 11.3 | 53 |
|
| |||||||||||
| BSG RR (P = 0.40)* | 2 | 11.1 | 1 | 5.6 | 14 | 77.8 | 1 | 5.6 | — | — | 18 |
|
| |||||||||||
| GBM ND | 1 | 11.1 | . | — | 2 | 22.2 | 2 | 22.2 | 4 | 44.4 | 9 |
|
| |||||||||||
| GBM RR (P = 0.76)* | — | — | 1 | 6.7 | 5 | 33.3 | 4 | 26.7 | 5 | 33.3 | 15 |
|
| |||||||||||
| All patients | 25 | 14.6 | 18 | 10.5 | 74 | 43.3 | 23 | 13.5 | 31 | 18.1 | 171 |
Comparing ND with RR patients using Cochran–Armitage Trend test.
Data for ND (anaplastic astrocytoma, primitive neuroectodermal tumor/pineoblastoma, and other categories) and for RR (primitive neuroectodermal tumor/pineoblastoma, and other categories) in Table 1 have not been included because of small sample size. Column categories are based on subjective grading of intensity of 18F-FDG uptake.
TABLE 4.
Descriptive Statistics of Continuous PET Variables by Tumor Types
| Tumor type | n | Minimum | Median | Maximum | P* |
|---|---|---|---|---|---|
| Mean tumor 18F-FDG uptake/GM | |||||
| Anaplastic astrocytoma RR | 25 | 0.38 | 0.85 | 2.35 | |
| Astrocytoma/astrocytic glioma RR | 20 | 0.35 | 0.70 | 2.68 | |
| BSG RR | 16 | 0.13 | 0.49 | 0.88 | 0.067 |
| BSG ND | 45 | 0.38 | 0.61 | 1.23 | |
| Ependymoma RR | 9 | 0.48 | 0.59 | 0.80 | |
| GBM RR | 15 | 0.55 | 0.68 | 2.59 | 0.23 |
| GBM ND | 8 | 0.55 | 1.02 | 1.45 | |
| Medulloblastoma RR | 8 | 0.62 | 0.84 | 1.29 | |
| Maximum tumor 18F-FDG uptake/GM | |||||
| Anaplastic astrocytoma RR | 25 | 0.45 | 0.97 | 3.44 | |
| Astrocytoma/astrocytic glioma RR | 20 | 0.41 | 0.81 | 3.69 | |
| BSG RR | 16 | 0.33 | 0.51 | 0.90 | 0.053 |
| BSG ND | 45 | 0.38 | 0.63 | 1.48 | |
| Ependymoma RR | 9 | 0.48 | 0.65 | 0.84 | |
| GBM RR | 15 | 0.60 | 0.80 | 3.14 | 0.25 |
| GBM ND | 8 | 0.72 | 1.15 | 2.50 | |
| Medulloblastoma RR | 8 | 0.63 | 0.92 | 1.37 | |
| Mean tumor 18F-FDG uptake/WM | |||||
| Anaplastic astrocytoma RR | 25 | 0.82 | 1.42 | 3.15 | |
| Astrocytoma/astrocytic glioma RR | 20 | 0.87 | 1.70 | 4.37 | |
| BSG RR | 16 | 0.97 | 1.18 | 1.71 | 0.23 |
| BSG ND | 45 | 0.71 | 1.28 | 2.89 | |
| Ependymoma RR | 9 | 0.85 | 1.59 | 2.43 | |
| GBM RR | 15 | 1.26 | 1.55 | 6.36 | 0.042 |
| GBM ND | 8 | 1.51 | 2.93 | 5.77 | |
| Medulloblastoma RR | 8 | 1.18 | 2.40 | 3.91 | |
| Maximum tumor 18F-FDG uptake/WM | |||||
| Anaplastic astrocytoma RR | 25 | 0.77 | 1.54 | 6.45 | |
| Astrocytoma/astrocytic glioma RR | 20 | 0.81 | 1.75 | 5.47 | |
| BSG RR | 16 | 0.63 | 1.07 | 1.65 | 0.26 |
| BSG ND | 45 | 0.67 | 1.23 | 2.85 | |
| Ependymoma RR | 9 | 0.89 | 1.42 | 2.25 | |
| GBM RR | 15 | 1.00 | 1.55 | 7.17 | 0.032 |
| GBM ND | 8 | 1.36 | 2.92 | 8.76 | |
| Medulloblastoma RR | 8 | 1.27 | 2.04 | 4.18 | |
P value is from Wilcoxon–Mann–Whitney test comparing ND vs. RR.
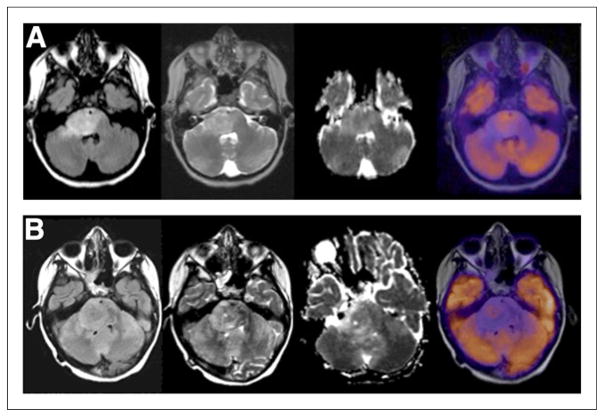
ND brain stem gliomas (BSGs) (Fig. 1A) typically had 18F-FDG uptake intensity between WM and GM in <25% to >75% of the tumor, whereas RR BSGs (Fig. 1B) had 18F-FDG uptake intensity between WM and GM in <25% of the tumor. There was no significant difference between 18F-FDG uptake intensity and uniformity in ND versus RR BSG (P = 0.40 and 0.10, respectively). There was the suggestion that a small subpopulation of ND BSG had intense or uniform 18F-FDG uptake, whereas this was rare in RR BSG. In ND BSG, if 18F-FDG uptake intensity was similar to or >GM (~10% of cases), 18F-FDG uptake was often spread throughout >50% of the tumor (~80% of cases). If 18F-FDG uptake intensity was <GM, 18F-FDG uptake was seen in 50% or less of the tumor (P = 0.06). There was no significant relationship between 18F-FDG uptake intensity and uniformity in RR BSG (P = 1.0).
FIGURE 1.
18F-FDG uptake intensity and uniformity in BSG. (A) An 11-y-old boy with ND BSG showing intensity of 18F-FDG uptake between WM and GM in 50%–75% of tumor as defined on FLAIR. From left to right are FLAIR, T2, ADC, and PET images. In region of uptake within BSG there is lower ADC value. (B) An 8-y-old girl with RR BSG showing intensity of 18F-FDG uptake between WM and GM in <25% of tumor (FLAIR). From left to right are FLAIR, T2, ADC, and PET images.
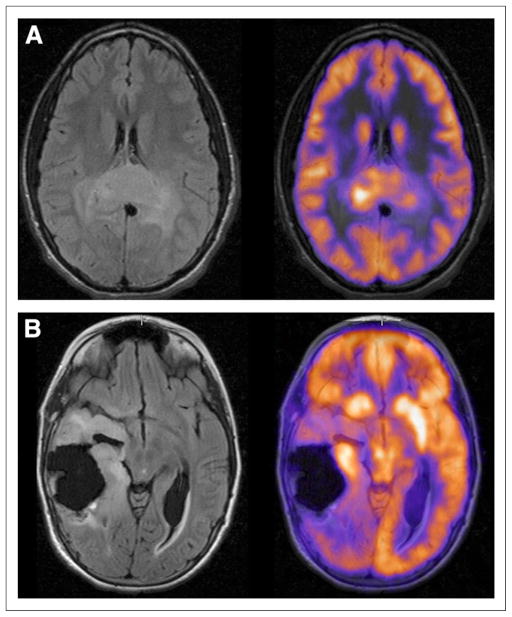
ND glioblastoma multiforme (GBM) (Fig. 2A) often had 18F-FDG uptake intensity similar to or >GM in >75% of the tumor, whereas RR GBM (Fig. 2B) often had 18F-FDG uptake intensity similar to or >GM in >50% of the tumor. There was no significant difference between 18F-FDG uptake intensity or uniformity in ND versus RR GBM (P = 0.76 and 0.91, respectively) and no significant relationship between 18F-FDG uptake intensity and uniformity in RR GBM (P = 1.0).
FIGURE 2.
18F-FDG uptake intensity and uniformity in GBM. (A) An 18-y-old boy with ND GBM showing intensity of 18F-FDG uptake similar to GM in >75% of tumor (FLAIR). (B) An 11-y-old girl with RR GBM showing intensity of 18F-FDG uptake >GM in <25% of tumor (FLAIR).
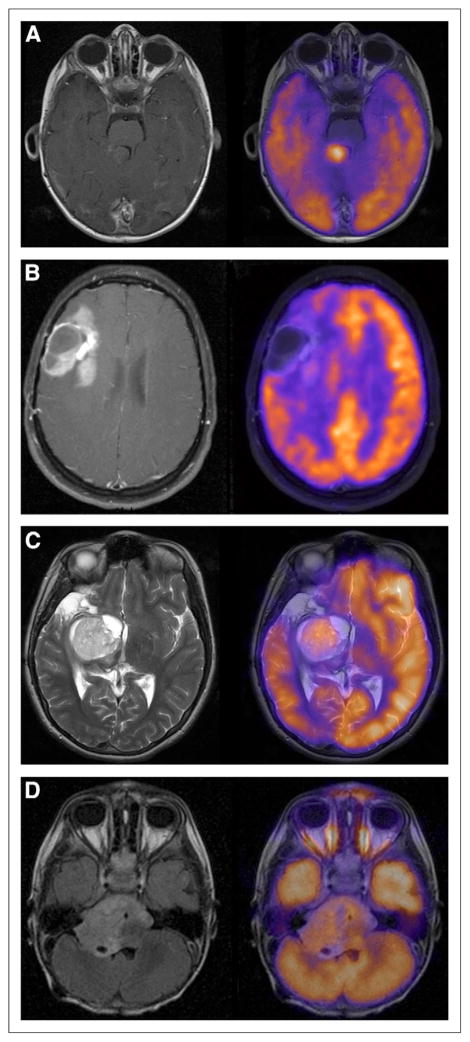
RR medulloblastoma typically had intense, diffuse 18F-FDG uptake throughout the tumor (Fig. 3A), whereas RR anaplastic astrocytoma had mild-moderate 18F-FDG uptake in a smaller amount of the tumor (Fig. 3B). In RR astrocytoma/astrocytic glioma, the appearance was often mild-moderate 18F-FDG uptake in most of the tumor (Fig. 3C). There was no significant relationship between 18F-FDG uptake intensity and uniformity in RR anaplastic astrocytoma or astrocytoma/astrocytic glioma. 18F-FDG uptake in ependymoma was often mild and spread throughout the tumor (Fig. 3D). More than half the children had tumor 18F-FDG uptake similar to or <WM, whereas this was less than a third in almost all other tumor types.
FIGURE 3.
18F-FDG uptake intensity and uniformity in medulloblastoma, anaplastic astrocytoma, astrocytoma, and ependymoma. (A) A 22-mo-old boy with RR medulloblastoma showing intensity of 18F-FDG uptake >GM in >75% of tumor (T1 post–Gd-DTPA). (B) A 15-y-old girl with RR anaplastic astrocytoma showing intensity of 18F-FDG uptake between WM and GM in 50%–75% of tumor (T1 post–Gd-DTPA). (C) A 15-y-old girl with RR astrocytoma showing intensity of 18F-FDG uptake similar to GM in >75% of tumor (T2). (D) A 2-y-old boy with RR ependymoma showing intensity of 18F-FDG uptake between WM and GM in >75% of tumor (FLAIR).
Median maximum tumor 18F-FDG uptake/GM was highest in ND GBM, followed by RR: anaplastic astrocytoma, medulloblastoma, astrocytoma/astrocytic glioma, GBM and ependymoma, and finally ND and RR BSG. Median maximum tumor 18F-FDG uptake/WM was highest in ND GBM, followed by RR: medulloblastoma, astrocytoma/astrocytic glioma, GBM, anaplastic astrocytoma, and ependymoma, and finally ND and RR BSG. ND GBM had higher mean and maximum tumor 18F-FDG uptake/WM than RR GBM (P = 0.042 and 0.032, respectively), suggesting ND BSG had higher mean and maximum tumor 18F-FDG uptake/GM than RR BSG (P = 0.067 and 0.053, respectively). When ND and RR cohorts were combined, GBM had higher tumor 18F-FDG uptake–to–comparative tissue ratios (P < 0.001) than BSG.
PET and MR Imaging Variable Correlation
Evaluation of both tumor MR imaging contrast enhancement (CE) and PET 18F-FDG uptake was possible in 120 children (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org). Near-complete or complete correlation was most common in GBM and medulloblastoma and least common in BSG and anaplastic astrocytoma. There was no significant difference in correlation of CE and 18F-FDG uptake between ND and RR BSG (P = 0.75), although GBM had higher correlation of CE with 18F-FDG uptake than BSG (P = 0.0011).
In ND BSG, intense 18F-FDG uptake (uptake similar to or >GM) suggested lower ADC (P = 0.075), and uniform 18F-FDG uptake (uptake in >50% of tumor) suggested lower ADC (P = 0.061). In general, ND BSG had less CE (P < 0.0001) and higher ADC (P = 0.03) than RR BSG.
In ND BSG (Supplemental Table 2), maximum tumor 18F-FDG uptake/WM correlated with ADC. Mean tumor 18F-FDG uptake/WM correlated with CE and ADC. In RR GBM, there was correlation of maximum tumor 18F-FDG uptake/GM, mean tumor 18F-FDG uptake/GM, maximum tumor 18F-FDG uptake/WM, and mean tumor 18F-FDG uptake/WM with CE.
In ND BSG, there was a suggestion that 18F-FDG uptake in >50% of the tumor was associated with decreased PFS (P = 0.065), although not with OS (P = 0.17). There was no association of 18F-FDG uptake intensity with PFS or OS (Supplemental Table 3).
In RR BSG, GBM, and astrocytoma, there was no association of 18F-FDG uptake uniformity or intensity with survival. There was correlation of mean tumor 18F-FDG uptake/GM with OS for anaplastic astrocytoma (P = 0.014).
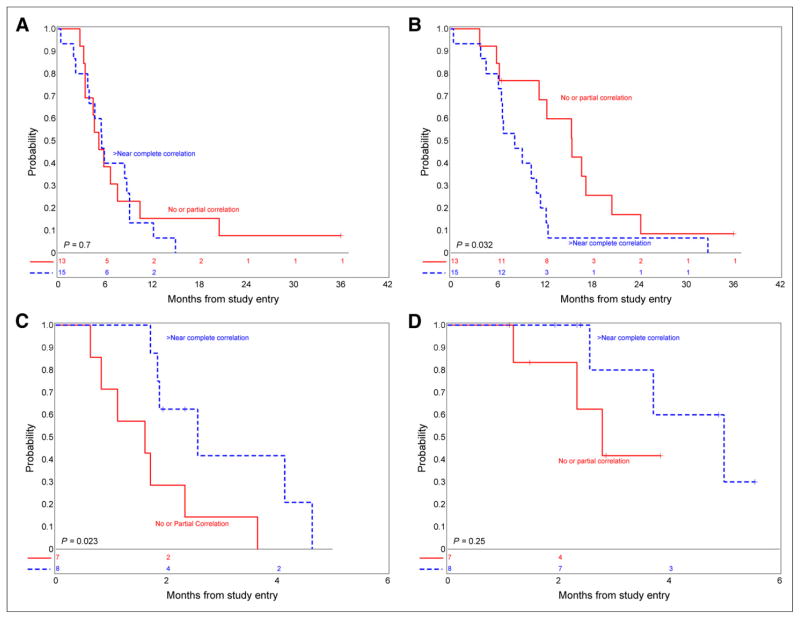
In ND BSG, higher correlation of MR imaging CE and PET 18F-FDG uptake was not associated with PFS (P = 0.7) but suggested worse OS (P = 0.032) (Figs. 4A and 4B). In RR BSG, increased CE suggested lower PFS (P = 0.0081, Supplemental Table 3), and higher correlation of MR imaging CE and PET 18F-FDG uptake suggested more favorable PFS (P = 0.023) but was not associated with OS (P = 0.25) (Figs. 4C and 4D). The association between correlation of MR imaging CE and PET 18F-FDG uptake intensity and uniformity with PFS and OS could not be calculated for other tumor types given the small sample size.
FIGURE 4.
(A) Association between PFS and CE in ND BSG. (B) Association between OS and CE in ND BSG. (C) Association between PFS and CE in RR BSG. (D) Association between OS and CE in RR BSG.
DISCUSSION
18F-FDG PET and MR imaging provide complementary evaluation of pediatric brain tumors. Kwon et al. (6) studied 20 children with BSG and stereotactic biopsy or surgery. Twelve underwent preoperative 18F-FDG PET and MR imaging. The authors concluded that metabolism on 18F-FDG PET and enhancement on MR imaging differed by histology and were complementary. Borgwardt et al. (7) evaluated 38 children with a spectrum of primary brain tumors, pretherapy 18F-FDG PET, and MR imaging. They found improved tumor characterization when PET and MR imaging were coregistered. Also 18F-FDG uptake positively correlated with malignancy grade.
There is evidence suggesting that 18F-FDG uptake is associated with histologic grade and provides prognostic information in the pediatric population. Hoffman et al. (8) reviewed 18F-FDG PET in 17 children with posterior fossa tumors and found that increased uptake suggested more aggressive tumor types. Holthoff et al. (9) studied 18F-FDG PET in 15 children and found increased 18F-FDG uptake in medulloblastoma, compared with other posterior fossa tumors. Williams et al. (11) showed 3-dimensional maximum and mean tumor 18F-FDG uptake was associated with PFS in pediatric supratentorial anaplastic astrocytomas. Gururangan et al. (18) found a significant negative correlation between 18F-FDG uptake and survival in 22 children with ND or recurrent medulloblastoma. Kruer et al. (13) reported 18F-FDG PET was an independent measure of disease risk stratification in 46 children with low-grade astrocytomas.
There is the suggestion that 18F-FDG PET improves management of pediatric brain tumors. Pirotte et al. (12) reviewed 18F-FDG PET in 20 children. Higher tumor 18F-FDG uptake suggested higher grade and shorter survival. PET guidance improved diagnostic yield at biopsy, compared with MR imaging. Pirotte et al. (19) also suggested that 18F-FDG PET helps differentiate indolent from active disease; improves lesion delineation, compared with MR imaging; and impacts intervention.
The association of 18F-FDG PET and MR imaging in pediatric brain tumors provides both metabolic and anatomic information. In our review of 40 children with ND BSG, when tumor 18F-FDG uptake uniformity was higher, ADC was lower, suggesting an increased number of metabolically viable tumor cells associated with increased tumor cellularity and restricted ADC (14). We also found that higher 18F-FDG uptake suggested increased tumor CE and hypothesized this could be due to more aggressive disease because most BSGs do not enhance (20). In a subsequent exploratory evaluation, we suggested that the correlation of tumor 18F-FDG uptake on PET with MR CE and perfusion metrics provides a more complete understanding of tumor biology and aggressiveness (15).
In this study, 18F-FDG PET and MR imaging associations were evaluated across a spectrum of pediatric brain tumors. Hypermetabolic tumor types were typically GBM, anaplastic astrocytoma, and medulloblastoma whereas BSG and ependymoma tended to be less 18F-FDG–avid. 18F-FDG uptake was commonly intense and uniform in GBM and medulloblastoma, consistent with often aggressive, locally invasive, extensive disease. Low-grade lesions such as pilocytic astrocytoma can be hypermetabolic, contrary to the supposition that high uptake dictates aggressiveness (21,22).
A subset of children with ND BSG had intense, uniform 18F-FDG uptake, although most had 18F-FDG uptake between WM and GM in <50% of the tumor. Also in ND BSG, intense or uniform 18F-FDG uptake suggested increased MR CE and lower ADC, possibly reflecting a subset of aggressive baseline pediatric BSG with increased viable cellularity. ND BSG had less CE and higher ADC than RR BSG. Without a biopsy, the histologic milieu is unknown; however, BSGs at baseline often have increased ADC likely due to a combination of low tumor cellularity and vasogenic edema (23).
BSG was the only tumor type for which a statistical analysis correlating 18F-FDG uptake with MR CE and survival (PFS and OS) could be performed for both ND and RR disease. We found a suggestive association of 18F-FDG uptake uniformity with PFS similar to prior results; however, given the heterogeneity of 18F-FDG uptake across all children with BSG, the small sample size, and the dismal prognosis, a statistically meaningful correlation of 18F-FDG uptake pattern with PFS and OS was not reached. A larger prospective study is needed to verify the hypothesis that 18F-FDG uptake pattern predicts survival. Also, in ND BSG, correlation of tumor CE and 18F-FDG uptake suggested worse OS (P = 0.032), whereas in RR BSG, correlation of CE and 18F-FDG uptake suggested more favorable PFS (P = 0.023). Thus, correlation of 18F-FDG uptake and CE in pediatric BSG at baseline suggests decreased survival, likely related to more aggressive disease. After therapy, this relationship is reversed, possibly reflecting decreased survival with development of new sites of 18F-FDG–avid malignancy.
The main limitations of this study are small sample size and poor survival, making it difficult to draw statistically meaningful inferences. Our sample size, however, is significantly larger than previously published studies and will contribute to the growing literature on 18F-FDG PET and MR imaging associations across the spectrum of pediatric brain tumors. The study was also performed on a variety of tumor types over a span of almost a decade. For this analysis, it was decided to use a consistent approach to image analysis involving the manual definition of a 2-dimensional ROI based on 18F-FDG uptake. Future analyses of similar data may involve an automated 3-dimensional voxel-based approach. In the correlation of MR and 18F-FDG PET, the ROIs on the 2 studies were defined independently and, thus, may not have coincided. The correlation of areas of highest signal for each modality is a reasonable approach, although we have also investigated the use of a common ROI in these analyses (15). Future studies could evaluate PET/MR or additional PET radiopharmaceuticals such as 18F-fluorothymidine, 18F-fluoroazomycin arabinoside, or 18F-fluoroethyl-L-tyrosine in pediatric brain tumors. PET/MR has the potential for simultaneous acquisition of PET and MR imaging with real-time metabolic and anatomic image registration on a voxel-by-voxel basis. Further, PET/MR would likely require a single visit to the imaging department, improving convenience with lower radiation exposure than is needed for PET/CT plus MR imaging. Finally, novel PET tracers may result in improved sensitivity or insight into tumor biology by studying aspects of proliferation and hypoxia.
CONCLUSION
18F-FDG PET uptake patterns vary depending on pediatric brain tumor type, with GBM and medulloblastoma commonly showing uniformly hypermetabolic disease, whereas BSG and ependymoma are not significantly 18F-FDG–avid. At baseline, NG BSGs often have less CE and increased ADC, suggesting decreased baseline aggressiveness and cellularity. Although the correlation of 18F-FDG uptake and CE in ND BSG suggests decreased survival, after therapy this relationship is reversed, likely reflecting poor survival in children with concurrent blood–brain barrier breakdown at sites of treated disease and development of new sites of 18F-FDG–avid malignancy.
Supplementary Material
TABLE 3.
18F-FDG Uptake Uniformity by Tumor Type
| Tumor type | Uniformity
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| <25%
|
25%–50%
|
51%–75%
|
76%–100%
|
All patients (n) | |||||
| n | Row % | n | Row % | n | Row % | n | Row % | ||
| Anaplastic astrocytoma RR | 9 | 36.0 | 7 | 28.0 | 4 | 16.0 | 5 | 20.0 | 25 |
|
| |||||||||
| Astrocytoma/astrocytic glioma RR | 1 | 5.3 | 1 | 5.3 | 6 | 31.6 | 11 | 57.9 | 19 |
|
| |||||||||
| Ependymoma RR | 2 | 25.0 | 1 | 12.5 | 1 | 12.5 | 4 | 50.0 | 8 |
|
| |||||||||
| Medulloblastoma RR | — | — | — | — | 1 | 12.5 | 7 | 87.5 | 8 |
|
| |||||||||
| BSG ND | 12 | 26.7 | 12 | 26.7 | 6 | 13.3 | 15 | 33.3 | 45 |
|
| |||||||||
| BSG RR (P = 0.10)* | 9 | 56.3 | 2 | 12.5 | 3 | 18.8 | 2 | 12.5 | 16 |
|
| |||||||||
| GBM ND | 2 | 25.0 | 1 | 12.5 | 1 | 12.5 | 4 | 50.0 | 8 |
|
| |||||||||
| GBM RR (P = 0.91)* | 2 | 13.3 | 4 | 26.7 | 3 | 20.0 | 6 | 40.0 | 15 |
|
| |||||||||
| All patients | 37 | 25.7 | 28 | 19.4 | 25 | 17.4 | 54 | 37.5 | 144† |
ND vs. RR compared using Cochran–Armitage Trend test.
Uniformity data missing for 27 patients.
Acknowledgments
We acknowledge Cynthia Dubé for help with manuscript preparation.
Footnotes
DISCLOSURE
This study was supported by NIH grant U01 CA81457 for the Pediatric Brain Tumor Consortium (PBTC), Pediatric Brain Tumor Consortium Foundation (PBTCF), Pediatric Brain Tumor Foundation of the United States (PBTFUS), and American Lebanese Syrian Associated Charities (ALSAC). No other potential conflict of interest relevant to this article was reported.
References
- 1.National Cancer Institute. National Cancer Institute fact sheet: childhood cancers. NCI website; Jun, 2008. [Accessed July 5, 2014]. http://www.cancer.gov/cancertopics/factsheet/Sites-Types/childhood. [Google Scholar]
- 2.American Brain Tumor Association. Brain tumor statistics. ABTA website; [Accessed July 5, 2014]. http://www.abta.org/news/brain-tumor-fact-sheets/ [Google Scholar]
- 3.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pediatric Brain Tumor Consortium. Introduction to PBTC. PBTC website; [Accessed July 5, 2014]. http://www.pbtc.org/public/gen_info.htm. [Google Scholar]
- 6.Kwon JW, Kim I, Cheon J, et al. Paediatric brainstem gliomas: MRI, 18F-FDG-PET and histological grading correlation. Pediatr Radiol. 2006;36:959–964. doi: 10.1007/s00247-006-0256-5. [DOI] [PubMed] [Google Scholar]
- 7.Borgwardt L, Højgaard L, Carstensen H, et al. Increased fluorine-18 2-fluoro-2-deoxy-D-glucose (FDG) uptake in childhood CNS tumors is correlated with malignancy grade: a study with FDG positron emission tomography/magnetic resonance imaging coregistration and image fusion. J Clin Oncol. 2005;23:3030–3037. doi: 10.1200/JCO.2005.02.074. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman JM, Hanson MW, Friedman HS, et al. FDG–PET in pediatric posterior fossa brain tumors. J Comput Assist Tomogr. 1992;16:62–68. doi: 10.1097/00004728-199201000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Holthoff VA, Herholz K, Berthold F, et al. In vivo metabolism of childhood posterior fossa tumors and primitive neuroectodermal tumors before and after treatment. Cancer. 1993;72:1394–1403. doi: 10.1002/1097-0142(19930815)72:4<1394::aid-cncr2820720440>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 10.Utriainen M, Metsahonkala L, Salmi T, et al. Metabolic characterization of childhood brain tumors comparison of 18F-fluorodeoxyglucose and 11C-methionine positron emission tomography. Cancer. 2002;95:1376–1386. doi: 10.1002/cncr.10798. [DOI] [PubMed] [Google Scholar]
- 11.Williams G, Fahey F, Treves ST, et al. Exploratory evaluation of two-dimensional and three-dimensional methods of 18F-FDG PET quantification in pediatric anaplastic astrocytoma: a report from the Pediatric Brain Tumor Consortium (PBTC) Eur J Nucl Med Mol Imaging. 2008;35:1651–1658. doi: 10.1007/s00259-008-0780-7. [DOI] [PubMed] [Google Scholar]
- 12.Pirotte BJ, Lubansu A, Massager N, Wikler D, Goldman S, Levivier M. Results of positron emission tomography guidance and reassessment of the utility of and indications for stereotactic biopsy in children with infiltrative brainstem tumors. J Neurosurg. 2007;107(5 suppl):392–399. doi: 10.3171/PED-07/11/392. [DOI] [PubMed] [Google Scholar]
- 13.Kruer MC, Kaplan AM, Etzl MM, et al. The value of positron emission tomography and proliferation index in predicting progression in low-grade astrocytomas of childhood. J Neurooncol. 2009;95:239–245. doi: 10.1007/s11060-009-9922-4. [DOI] [PubMed] [Google Scholar]
- 14.Zukotynski KA, Fahey FH, Kocak M, et al. Evaluation of 18F-FDG PET and MRI associations in pediatric diffuse intrinsic brain stem glioma: a report from the Pediatric Brain Tumor Consortium. J Nucl Med. 2011;52:188–195. doi: 10.2967/jnumed.110.081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zukotynski KA, Fahey FH, Vajapeyam S, et al. Exploratory evaluation of MRI permeability with 18F-FDG PET mapping in pediatric brain tumors: a report from the Pediatric Brain Tumor Consortium. J Nucl Med. 2013;54:1237–1243. doi: 10.2967/jnumed.112.115782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poussaint TY, Philips PC, Vajapeyam S, et al. The Neuroimaging Center of the Pediatric Brain Tumor Consortium: collaborative neuroimaging in pediatric brain tumor research—a work in progress. AJNR. 2007;28:603–607. [PMC free article] [PubMed] [Google Scholar]
- 17.Fahey FH, Kinahan PE, Doot RK, et al. Variability in PET quantitation within a multicenter consortium. Med Phys. 2010;37:3660–3666. doi: 10.1118/1.3455705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gururangan S, Hwang E, Herendon JE, II, Fuchs H, George T, Coleman RE. [18F] fluorodeoxyglucose-positron emission tomography in patients with medulloblastoma. Neurosurgery. 2004;55:1280–1288. doi: 10.1227/01.neu.0000143027.41632.2b. [DOI] [PubMed] [Google Scholar]
- 19.Pirotte BJ, Lubansu A, Massager N, et al. Clinical impact of integrating positron emission tomography during surgery in 85 children with brain tumors. J Neurosurg Pediatr. 2010;5:486–499. doi: 10.3171/2010.1.PEDS09481. [DOI] [PubMed] [Google Scholar]
- 20.Fischbein NJ, Prados M, Wara W, Russo C, Edwards M, Barkovich A. Radiologic classification of brain stem tumors: correlation of magnetic resonance imaging appearance with clinical outcome. Pediatr Neurosurg. 1996;24:9–23. doi: 10.1159/000121010. [DOI] [PubMed] [Google Scholar]
- 21.Borgwardt L, Larsen HJ, Pedersen K, et al. Practical use and implementation of PET in children in a hospital PET centre. Eur J Nucl Med Mol Imaging. 2003;30:1389–1397. doi: 10.1007/s00259-003-1263-5. [DOI] [PubMed] [Google Scholar]
- 22.Patil S, Biassoni L, Borgwardt L. Nuclear medicine in pediatric neurology and neurosurgery: epilepsy and brain tumors. Semin Nucl Med. 2007;37:357–381. doi: 10.1053/j.semnuclmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Chen HJ, Panigraphy A, Dhall G, Finlay JL, Nelson MD, Jr, Blüml S. Apparent diffusion and fractional anisotropy of diffuse intrinsic brain stem gliomas. AJNR. 2010;31:1879–1885. doi: 10.3174/ajnr.A2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.