Abstract
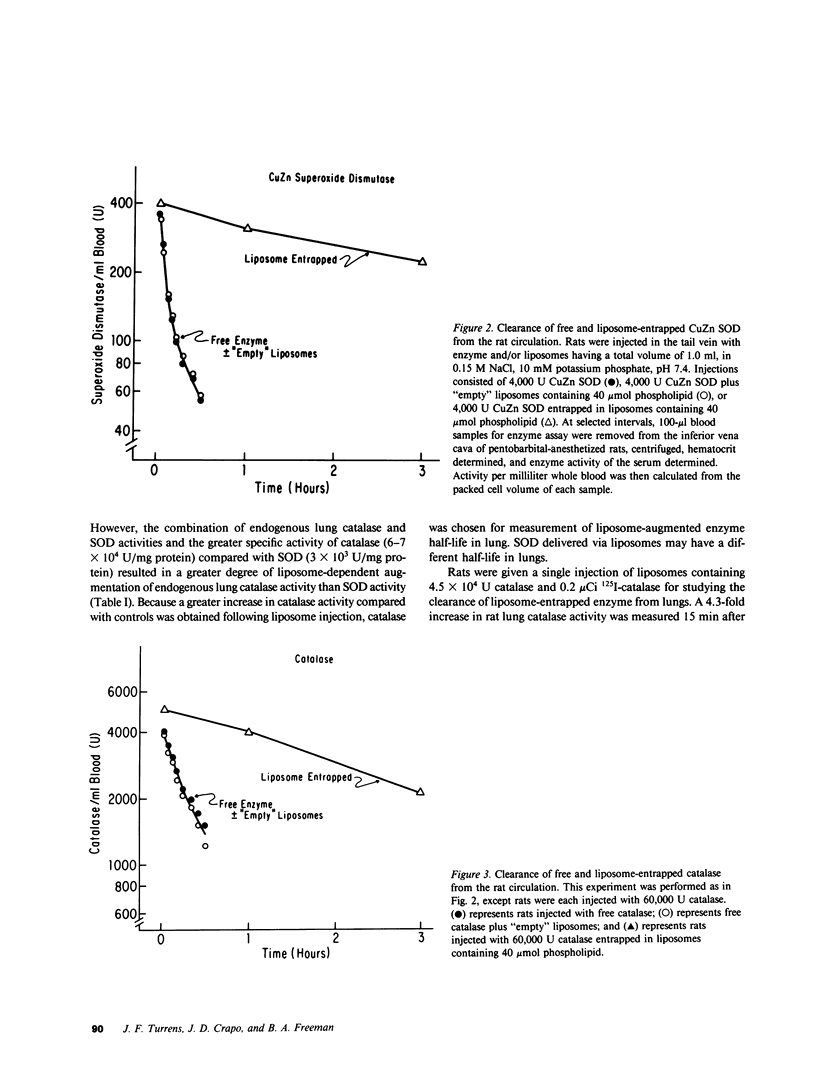
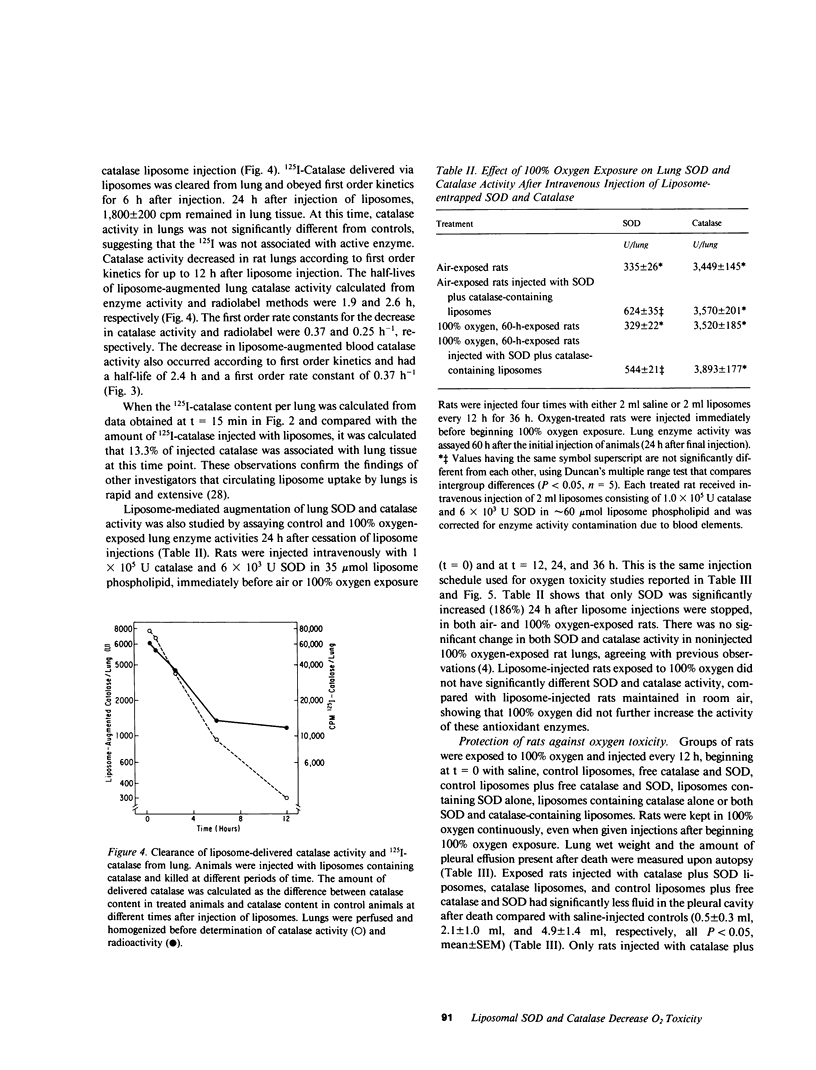
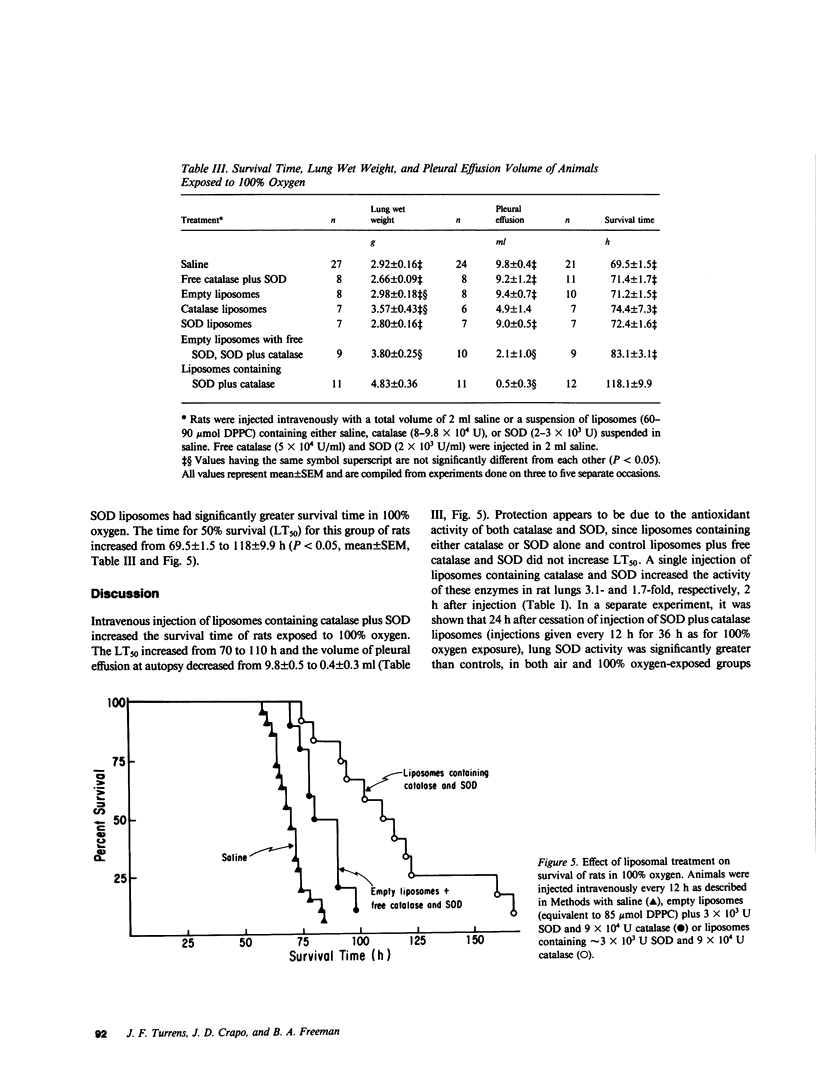
Survival of rats exposed to 100% oxygen was increased from 69.5 +/- 1.5 to 118.1 +/- 9.9 h (mean +/- SEM, P less than 0.05) when liposomes containing catalase and superoxide dismutase were injected intravenously before and during exposure. The increased survival time in 100% oxygen was also associated with significantly less fluid in the pleural cavity. Rats injected with catalase- and superoxide dismutase-containing liposomes, which had increased survival in 100% oxygen, had increased lung wet weight upon autopsy compared with saline-injected controls (2.9 +/- 0.2 g/lung vs. 4.8 +/- 0.4 g/lung, mean +/- SE, P less than 0.05). Intravenous injection of control liposomes along with catalase and superoxide dismutase in the suspending buffer decreased the mean pleural effusion volume 89% and had no significant effect on survival time. Lung catalase and superoxide dismutase activities were increased 3.1- and 1.7-fold, respectively, 2 h after a single intravenous injection of liposomes containing catalase or superoxide dismutase. Superoxide dismutase activity was also significantly greater than controls in both air- and 100% oxygen-exposed rat lungs, when enzyme activity was assayed 24 h after cessation of injection of control and oxygen-exposed rats with enzyme-containing liposomes every 12 h for 36 h. Free superoxide dismutase and catalase injected intravenously in the absence of liposomes did not increase corresponding lung enzyme activities, affect pleural effusion volume, lung wet weight, or extend the mean survival time of rats exposed to 100% oxygen. The clearance of liposome-augmented 125I-labeled catalase from lung and plasma obeyed first order kinetics according to a one-compartment model. When clearance of liposome-augmented catalase activity or radioactivity were the parameters used for pharmacokinetic studies, the half-life of augmented lung catalase was 1.9 and 2.6 h, respectively. The half-life of liposome-entrapped catalase and superoxide dismutase activity in the circulation was 2.5 and 4 h, respectively, while intravenously injected catalase and superoxide dismutase had a circulation half-life of 23 and 6 min, respectively.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGMEYER H. U. Zur Messung von Katalase-Aktivitäten. Biochem Z. 1955;327(4):255–258. [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Foscue H. A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980 Jul;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., DeLong D. M., Sjostrom K., Hasler G. R., Drew R. T. The failure of aerosolized superoxide dismutase to modify pulmonary oxygen toxicity. Am Rev Respir Dis. 1977 Jun;115(6):1027–1033. doi: 10.1164/arrd.1977.115.6.1027. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Tierney D. F. Superoxide dismutase and pulmonary oxygen toxicity. Am J Physiol. 1974 Jun;226(6):1401–1407. doi: 10.1152/ajplegacy.1974.226.6.1401. [DOI] [PubMed] [Google Scholar]
- Cross C. E., Watanabe T. T., Hasegawa G. K., Goralnik G. N., Roertgen K. E., Kaizu T., Reiser K. M., Gorin A. B., Last J. A. Biochemical assays in lung homogenates: artifacts caused by trapped blood after perfusion. Toxicol Appl Pharmacol. 1979 Mar 30;48(1 Pt 1):99–109. doi: 10.1016/s0041-008x(79)80012-5. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Fox R. B., Hoidal J. R., Brown D. M., Repine J. E. Pulmonary inflammation due to oxygen toxicity: involvement of chemotactic factors and polymorphonuclear leukocytes. Am Rev Respir Dis. 1981 May;123(5):521–523. doi: 10.1164/arrd.1981.123.5.521. [DOI] [PubMed] [Google Scholar]
- Frank L., Yam J., Roberts R. J. The role of endotoxin in protection of adult rats from oxygen-induced lung toxicity. J Clin Invest. 1978 Feb;61(2):269–275. doi: 10.1172/JCI108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981 Nov 10;256(21):10986–10992. [PubMed] [Google Scholar]
- Freeman B. A., Topolosky M. K., Crapo J. D. Hyperoxia increases oxygen radical production in rat lung homogenates. Arch Biochem Biophys. 1982 Jul;216(2):477–484. doi: 10.1016/0003-9861(82)90236-3. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Young S. L., Crapo J. D. Liposome-mediated augmentation of superoxide dismutase in endothelial cells prevents oxygen injury. J Biol Chem. 1983 Oct 25;258(20):12534–12542. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hunt C. A., Rustum Y. M., Mayhew E., Papahadjopoulos D. Retention of cytosine arabinoside in mouse lung following intravenous administration in liposomes of different size. Drug Metab Dispos. 1979 May-Jun;7(3):124–128. [PubMed] [Google Scholar]
- Juliano R. L., Stamp D. Pharmacokinetics of liposome-encapsulated anti-tumor drugs. Studies with vinblastine, actinomycin D, cytosine arabinoside, and daunomycin. Biochem Pharmacol. 1978 Jan 1;27(1):21–27. doi: 10.1016/0006-2952(78)90252-6. [DOI] [PubMed] [Google Scholar]
- Kao Y. J., Juliano R. L. Interactions of liposomes with the reticuloendothelial system. Effects of reticuloendothelial blockade on the clearance of large unilamellar vesicles. Biochim Biophys Acta. 1981 Nov 5;677(3-4):453–461. doi: 10.1016/0304-4165(81)90259-2. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Tracy T. F., Jr, Biddlecome S. M., Bourke R. S. The effect of entrapment in liposomes on the in vivo distribution of [3H]methotrexate in a primate. Cancer Res. 1976 Aug;36(8):2949–2957. [PubMed] [Google Scholar]
- Kuhn S. H., Gemperli B., Shephard E. G., Joubert J. R., Weidemann P. A., Weissmann G., Finkelstein M. C. Interaction of liposomes with human leukocytes in whole blood. Biochim Biophys Acta. 1983 Feb 16;762(1):119–127. doi: 10.1016/0167-4889(83)90124-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavelle D., Paxton W. B., Blaustein D. I., Ostro M. J., Giacomoni D. Improved methods for the delivery of liposome-sequestered RNA into eucaryotic cells. Arch Biochem Biophys. 1982 May;215(2):486–497. doi: 10.1016/0003-9861(82)90107-2. [DOI] [PubMed] [Google Scholar]
- Machy P., Leserman L. D. Small liposomes are better than large liposomes for specific drug delivery in vitro. Biochim Biophys Acta. 1983 May 5;730(2):313–320. doi: 10.1016/0005-2736(83)90348-6. [DOI] [PubMed] [Google Scholar]
- Majewska M. D., Strosznajder J., Lazarewicz J. Effect of ischemic anoxia and barbiturate anesthesia on free radical oxidation of mitochondrial phospholipids. Brain Res. 1978 Dec 15;158(2):423–434. doi: 10.1016/0006-8993(78)90685-6. [DOI] [PubMed] [Google Scholar]
- Poste G., Bucana C., Raz A., Bugelski P., Kirsh R., Fidler I. J. Analysis of the fate of systemically administered liposomes and implications for their use in drug delivery. Cancer Res. 1982 Apr;42(4):1412–1422. [PubMed] [Google Scholar]
- Schaefer-Ridder M., Wang Y., Hofschneider P. H. Liposomes as gene carriers: efficient transformation of mouse L cells by thymidine kinase gene. Science. 1982 Jan 8;215(4529):166–168. doi: 10.1126/science.7053567. [DOI] [PubMed] [Google Scholar]
- Shephard E. G., Joubert J. R., Finkelstein M. C., Kühn S. H. Phagocytosis of liposomes by human alveolar macrophages. Life Sci. 1981 Dec 28;29(26):2691–2698. doi: 10.1016/0024-3205(81)90528-2. [DOI] [PubMed] [Google Scholar]
- Sjostrom K., Crapo J. D. Structural and biochemical adaptive changes in rat lungs after exposure to hypoxia. Lab Invest. 1983 Jan;48(1):68–79. [PubMed] [Google Scholar]
- Straubinger R. M., Hong K., Friend D. S., Papahadjopoulos D. Endocytosis of liposomes and intracellular fate of encapsulated molecules: encounter with a low pH compartment after internalization in coated vesicles. Cell. 1983 Apr;32(4):1069–1079. doi: 10.1016/0092-8674(83)90291-x. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu Rev Biophys Bioeng. 1980;9:467–508. doi: 10.1146/annurev.bb.09.060180.002343. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada G., Onodera H., Tada K. Delivery of fungal beta-galactosidase to rat brain by means of liposomes. Tohoku J Exp Med. 1982 Feb;136(2):219–229. doi: 10.1620/tjem.136.219. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Freeman B. A., Crapo J. D. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch Biochem Biophys. 1982 Sep;217(2):411–421. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Freeman B. A., Levitt J. G., Crapo J. D. The effect of hyperoxia on superoxide production by lung submitochondrial particles. Arch Biochem Biophys. 1982 Sep;217(2):401–410. doi: 10.1016/0003-9861(82)90518-5. [DOI] [PubMed] [Google Scholar]
- Tyrrell D. A., Heath T. D., Colley C. M., Ryman B. E. New aspects of liposomes. Biochim Biophys Acta. 1976 Dec 14;457(3-4):259–302. doi: 10.1016/0304-4157(76)90002-2. [DOI] [PubMed] [Google Scholar]
- Yam J., Roberts R. J. Pharmacological alteration of oxygen-induced lung toxicity. Toxicol Appl Pharmacol. 1979 Feb;47(2):367–375. doi: 10.1016/0041-008x(79)90332-6. [DOI] [PubMed] [Google Scholar]



