Abstract
Background
BCR-ABL1 tyrosine kinase inhibitors (TKIs) improve the outcome of childhood Philadelphia-chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) when incorporated into post-remission induction chemotherapy. No data is available on the impact of TKIs on end of induction minimal residual disease (MRD) in patients with poor early response to 2 weeks of induction therapy not containing TKIs.
Methods
We analyzed early response to TKIs during remission induction in children with Ph+ALL treated at St Jude Children’s Research Hospital. MRD was measured on Day 15 and Day 42 of induction. TKIs were incorporated into induction therapy on Day 22 in the post TKIs era.
Results
TKIs produced a marked drop in MRD levels: at the end of remission induction, 9 of 11 patients treated with imatinib or dasatinib and conventional induction chemotherapy achieved MRD-negative status compared to only 2 of 16 patients treated with chemotherapy alone (P <0.001). Five-year event-free survival (±SD) was 68.6%±19.2% for the 11 patients who received TKIs versus 31.6%±9.9% for the 19 who did not (P=0.022); notably, 2 of the former group received hematopoietic stem cell transplantation versus 15 of the latter (P=0.002). MRD levels and outcome were not significantly different among 498 patients with standard/high risk Ph-negative ALL treated in the pre-TKIs or post-TKIs eras.
Conclusion
TKIs administered in the early phases of therapy can dramatically reduce MRD and improve outcome of childhood Ph+ ALL.
Keywords: TKIs, Ph+, MRD, pediatric, acute lymphoblastic leukemia
INTRODUCTION
The t(9;22) (q34;q11.2) chromosomal translocation, i.e., the Philadelphia (Ph) chromosome, and the resulting BCR-ABL1 fusion protein (1), occurs in 20%–30% of adult acute lymphoblastic leukemia (ALL) cases and in 3%–5% of childhood ALL (2;3). Due to its high rate of relapse with chemotherapy, allogeneic hematopoietic stem cell transplant (HSCT) in first complete remission (CR) is often recommended but the survival benefits resulting from this intervention in childhood Ph+ ALL have been modest (4). Over the past decade, tyrosine kinase inhibitors (TKIs) targeting BCR-ABL1 have been added to chemotherapy regimens; a Children Oncology Group (COG) study that combined imatinib with intensive post remission induction chemotherapy yielded a significant improvement in event-free survival (EFS) rates for childhood Ph+ALL (5). The impact of TKIs on early response was not evaluated in that study as imatinib was introduced after completion of induction therapy.
The optimal way to integrate TKIs, conventional chemotherapy and HSCT in Ph+ ALL is still unclear (5–7). Introduction of TKIs early during therapy might produce significant gains in initial leukemia cytoreduction (7), which could result in less induction failures and, perhaps, reduce the need for HSCT. However, it is uncertain whether early attainment of minimal residual disease (MRD)-negative status warrants omission of HSCT. In this study, we measured levels of MRD during and at the end of remission induction therapy to quantify the impact of TKIs on the reduction of leukemia burden in childhood Ph+ ALL. We compared response rates and overall clinical outcome of this cohort treated with TKIs with those of Ph+ ALL patients treated in the pre-TKIs era, and in parallel cohorts of standard/high risk Ph-negative ALL patients.
PATIENTS AND METHODS
Patients
From December 1991 to October 2012, 1035 patients (aged 1–18 years) with newly diagnosed B-lineage ALL were enrolled in the St Jude Children’s Research Hospital frontline ALL studies Total 13–16 (8;9). Of the 1035 patients, 507 were treated on the low risk and 528 on the standard/high risk arm, including 30 patients with Ph+ ALL. The TKIs imatinib (340 mg/m2 daily on Total 15 July 2004–September 2007) or dasatinib (40 mg/m2 BID on Total 16 October 2007–October 2012) were administered continuously through all phases of treatment starting on day 22–26 of remission induction therapy to patients with Ph+ ALL diagnosed after an amendment to Total 15 (July 27, 2004; n=11). Diagnosis of Ph+ ALL was based on conventional cytogenetics/FISH and detection of the BCR-ABL1 fusion transcript by reverse transcriptase-polymerase chain reaction (RT-PCR). MRD studies were performed by flow cytometry between day 15 and 19 of remission induction (“Day 15”), and on hematopoietic recovery at end of induction (between days 42 and 46; “Day 42”). Some patients (8 in the pre-TKI and 5 in the post-TKI group) also had their MRD tested by PCR amplification of immunoglobulin and T-cell receptor (Ig/TCR) genes (9). The sensitivity levels that we can achieved routinely by both flow cytometry and PCR is 1 in 10,000 leukemic cells in bone marrow mononucleated cells (i.e., 0.01%)(10). Hence, the cut-off level that defined MRD positivity by either method was 0.01%. These studies were approved by the institutional review board, and written informed consent or assent, as appropriate, was obtained for all patients.
Statistical methods
Fisher’s exact test was used to compare MRD status on days 15 and 42 among patient groups. EFS and overall survival (OS) distributions were compared with the Mantel-Haenszel test and the associated standard errors calculated according to Peto and Pike.
RESULTS
There were no significant differences in presenting features between the 11 patients with Ph+ ALL who received TKIs treatment (5 imatinib, 6 dasatinib) and the 19 treated in the pre-TKIs era (Table 1). Three of the 19 patients who were treated in the pre-TKIs era failed to achieve morphologic remission at the end of remission induction therapy, whereas all 11 patients who received TKIs achieved remission.
Table 1.
Characteristics of Ph+ ALL patients according to TKI administration
| Features at diagnosis | No. of pts. treated with no TKI |
No. of pts. treated with TKI |
P value | |
|---|---|---|---|---|
| Age (years) | 1–10 | 10 | 5 | 1.0000 |
| >10 | 9 | 6 | ||
| WBC (× 109/L) | WBC<50 | 12 | 5 | 0.4539 |
| WBC ≥50 | 7 | 6 | ||
| Race | White | 13 | 7 | 0.5828 |
| Black | 4 | 4 | ||
| Other | 2 | 0 | ||
| Gender | Male | 10 | 8 | 0.4425 |
| Female | 9 | 3 | ||
| DNA index | ≥1.16 | 2 | 2 | 0.6111 |
| other | 17 | 9 | ||
| CNS Status | CNS1 | 13 | 8 | 0.3248 |
| CNS2 | 4 | 1 | ||
| CNS3 | 0 | 1 | ||
| traumatic puncture with blasts | 2 | 0 | ||
Day 42 MRD measurements by flow cytometry and/or PCR were available for 15 of the 19 Ph+ patients treated without TKIs (Table 2). As shown in Table 3, 13 were MRD+, and 8 of them had MRD levels ≥1%; one patient did not have MRD studies but had 17% blasts detectable by morphology, and Ph chromosome detectable by cytogenetics. By contrast, only 2 of the 11 patients who received TKIs (1 imatinib and 1 dasatinib) were MRD+, both at <0.1% (P<0.0001). Thus, all 9 patients classified to have very high-risk ALL according to our risk stratification because of MRD ≥1% and/or or remission induction failure were among the 19 patients treated with chemotherapy alone.
Table 2.
Day 42 MRD levels among patients with Ph+ ALL treated in the pre- and post-TKI era
| ID number |
Tyrosine kinase inhibitor |
MRD level (%) by | |
|---|---|---|---|
| Flow cytometry | PCR amplification of Ig/TCR genes |
||
| 1 | None | 19 | 23 |
| 2 | None | 0.74 | |
| 3 | None | 4.76 | 3.6 |
| 4 | None | Not tested* | |
| 5 | None | 0.61 | 2.3 |
| 6 | None | 2.9 | |
| 7 | None | <0.01 | |
| 8 | None | 1.51 | |
| 9 | None | 0.05 | |
| 10 | None | 5 | |
| 11 | None | 0.21 | 0.23 |
| 12 | None | 0.29 | 0.023 |
| 13 | None | <0.01 | <0.01 |
| 14 | None | 4.52 | 5.20 |
| 15 | None | 12.57 | |
| 16 | None | 1.34 | |
| 17 | Imatinib | 0.06 | 0.09 |
| 18 | Imatinib | <0.01 | <0.01 |
| 19 | Imatinib | <0.01 | |
| 20 | Imatinib | <0.01 | <0.01 |
| 21 | Imatinib | <0.01 | <0.01 |
| 22 | Dasatinib | <0.01 | <0.01 |
| 23 | Dasatinib | <0.01 | |
| 24 | Dasatinib | <0.01 | |
| 25 | Dasatinib | <0.01 | |
| 26 | Dasatinib | 0.02 | |
| 27 | Dasatinib | <0.01 | |
17% blasts by morphology and t(9;22) (q34;q11.2) detectable by cytogenetic analysis
Table 3.
Day 42 MRD among patients with Ph+ ALL or Ph− ALL treated in the pre- and post-TKI era
| Day 42 MRD status (level) |
Ph+ ALL | Ph− ALL | ||
|---|---|---|---|---|
| Pre TKI (n = 16) |
Post TKI (n = 11) |
Pre TKI (n = 167) |
Post TKI (n= 204) |
|
| Negative (<0.01%) | 2 | 9 | 113 | 134 |
| Positive (≥0.01%) | 14* | 2 | 54 | 70 |
| (0.01%–<0.1%) | 1 | 2 | 26 | 37 |
| (0.1%–<1%) | 4 | 0 | 20 | 22 |
| (≥1% or remission failure) | 9* | 0 | 8 | 11 |
In one of the 14 patients MRD was not tested by flow cytometry or PCR; this patient had 17% blasts by morphology and t(9;22) (q34;q11.2) detectable by cytogenetic analysis.
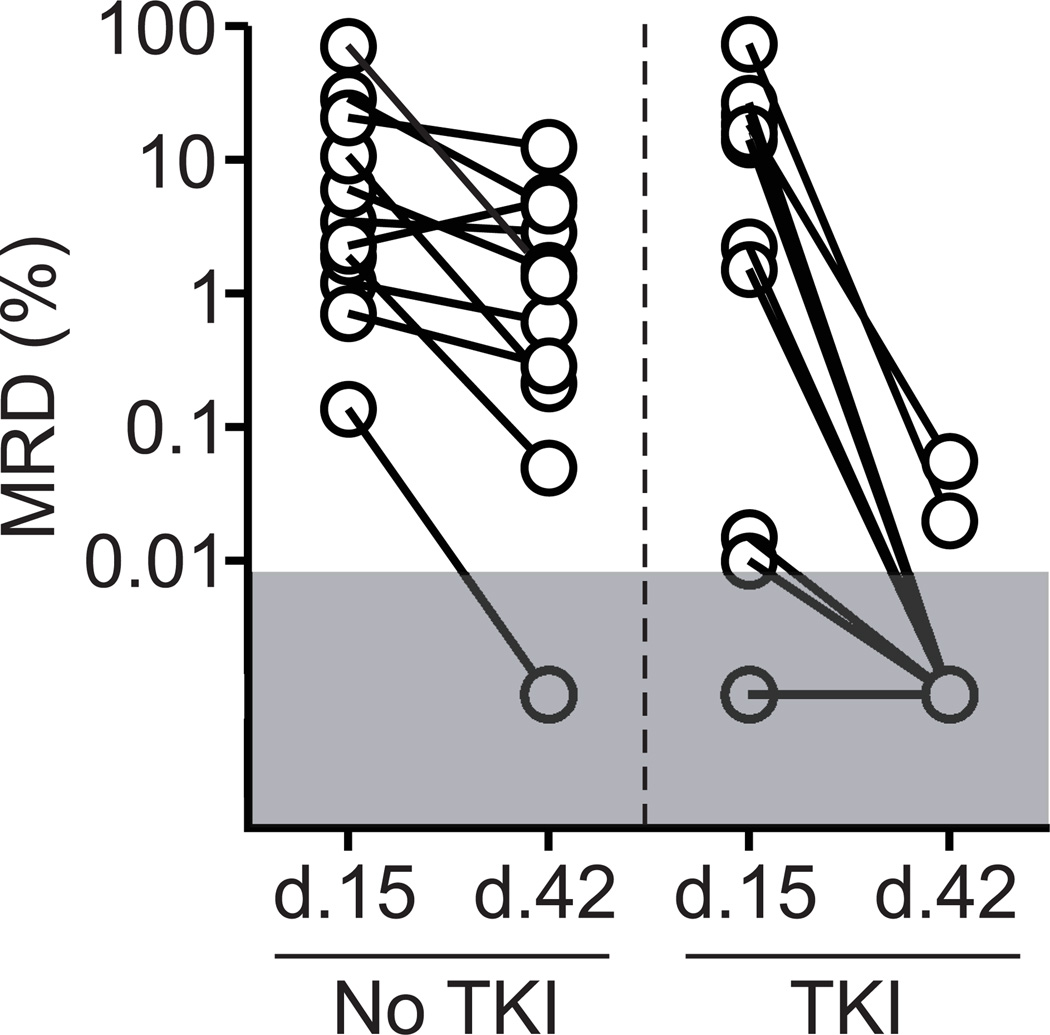
In contrast to differences observed for day 42 MRD, MRD levels on day 15, prior to TKI administration, were similar between the two groups. Thus, among the 22 patients with available day 15 measurements, all 11 treated with chemotherapy alone and 10 of the 11 who subsequently received TKIs were MRD-positive on day 15 (P = 0.58). Figure 1 illustrates the kinetics of leukemia cytoreduction between days 15 and 42 produced by the two types of treatment; the addition of TKIs reduced MRD levels in all patients whereas chemotherapy alone was largely ineffective.
Figure 1.
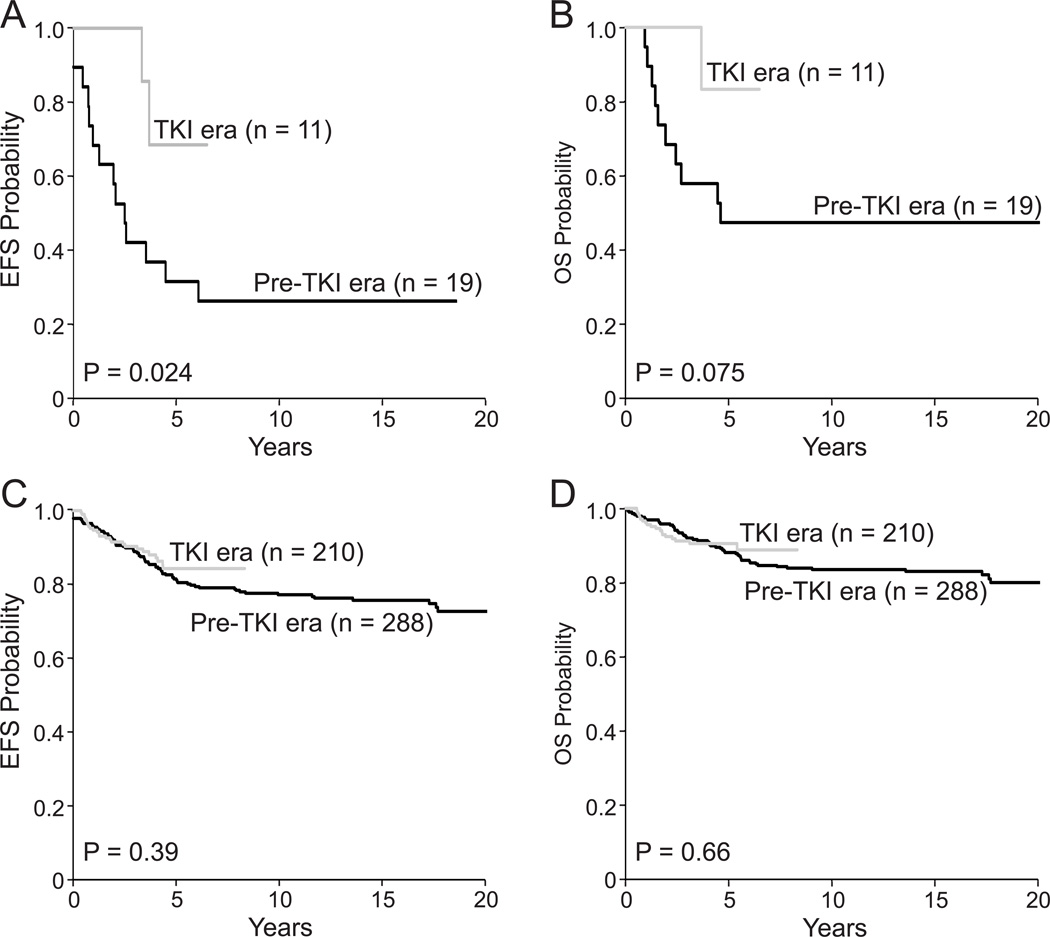
To ensure that the apparent benefits of TKIs were not simply related to their inclusion in the context of more contemporary treatment, we compared day 42 MRD levels among patients with standard/high risk Ph-negative ALL treated in the pre- and post-TKIs eras (MRD data available in 371 of the 498 patients): neither the prevalence of MRD nor its levels differ significantly between the two groups (Table 3). Gains in leukemia cytoreduction with TKIs were associated with a significantly better EFS (Fig. 2A); OS was also markedly superior although the difference failed to achieve statistical significance (Fig. 2B). This improvement in outcome is particularly noteworthy because only 2 of the 11 patients who received TKIs underwent HSCT versus 15 of the 19 treated in the pre-TKI era (P = 0.002). One patient (#17 in Table 2) was MRD-positive by both flow cytometry and PCR at the end of induction and received HSCT during maintenance therapy per physician preference while in morphologic remission and MRD negative by flow cytometry and by Ig/TCR gene PCR; this patient died while in remission 4 months post-HSCT. The second patient who received HSCT (#20 in Table 2) was MRD-negative at the end of induction and received HSCT per physician preference while in morphologic remission with negative MRD by both flow cytometry and Ig/TCR gene PCR; this patient is still in remission 74 months post-HSCT. In the last 5 years, no patient with Ph+ALL has received HSCT as physicians and families have become increasingly comfortable with omitting HSCT in the TKI era. The use of HSCT remained unchanged for standard/high Ph-negative ALL: of the 498 patients in this group, HSCT was applied in 20 of the 288 (7%) and 19 of the 210 (9%) of those treated in the pre- and post-TKIs eras, respectively.
Figure 2.
DISCUSSION
The results of this study demonstrate that incorporation of TKIs into remission induction therapy regimens for childhood Ph+ ALL produces a dramatic decrease in leukemia burden, leading to end of induction MRD levels that are similar to or lower than those seen among patients with Ph-negative ALL. Hence, none of the 11 Ph+ ALL patients who received TKIs in our study met the definition of very high-risk ALL based on our risk classification scheme, whereas 9 of 16 treated without TKIs met that definition. These results are in line with those of a study by Rives et al.(7), where most children with Ph+ ALL who received imatinib achieved molecular remission, although this was accomplished for most patients only after consolidation therapy. Collectively, these results suggest that early responses to TKIs are superior in pediatric Ph+ ALL patients, as 30%–50% of adults with Ph+ ALL never achieve molecular remission even when treated on regimens incorporating TKIs (11–13).
The best way to implement MRD-directed therapy in Ph+ ALL is unclear (12;13). We measured MRD by flow cytometry and/or PCR amplification of antigen-receptor genes. These methods produce largely equivalent results in our and others’ experience when a threshold of 0.01% was used to define MRD positivity (14–16), have been proven to be reliable MRD indicators in previous studies, and might provide a more accurate quantification of residual leukemic cells than BCR-ABL1 transcripts (17;18). The presence of BCR-ABL1 fusion transcripts after consolidation and before HSCT reportedly correlates with probability of relapse (11;19–21). MRD as measured by PCR amplification of antigen-receptor genes was associated with risk of relapse in children with Ph+ ALL in the pre TKIs era studies (22), but imatinib administered continuously after remission induction therapy containing no TKIs abrogated its adverse impact (5). In a study where adult patients with Ph+ALL received TKIs in combination with chemotherapy on days 1–14 of induction (noTKIs were given for the reminder of the induction cycle), MRD by flow cytometry and major molecular response measured by BCR-ABL1 transcripts were not significantly related with outcome upon achievement of morphologic remission, but they were prognostic at 3 months from starting therapy and beyond (13). Patient age and TKI schedule might have contributed to the discrepancy in the prognostic value of MRD at the end of remission induction between this study and ours.
Most adult patients with Ph+ ALL and, in some centers, children with suitable donors still receive HSCT regardless of MRD (5;7;23). Because we have used primarily MRD levels at completion of remission induction to guide risk stratification on frontline St Jude studies with excellent clinical outcome,(9;24) we applied similar principles to Ph+ALL, an approach that dramatically reduced the use of HSCT in our series. Our results indicate that high survival rates can be obtained without HSCT in patients with Ph+ALL who achieve MRD negativity at the end of remission induction therapy. MRD monitoring should be useful to identify patients who do not respond optimally to TKIs and might be candidates for HSCT and/or novel targeted agents. Because the relation between results of the most prevalent methods to monitor MRD in ALL (flow cytometry and PCR amplification of antigen receptor genes) and those of BCR-ABL1 transcript evaluation remains unclear, it might be worth applying these approaches in tandem until the value of each method in the management of patients with Ph+ALL is better established.
In summary, emerging data continue to demonstrate substantial benefit for patients with Ph+ALL who receive chemotherapy regimens incorporating TKIs earlier and in a more continuous fashion as opposed to an intermittent or alternating schedule.(5) TKIs were well tolerated when incorporated in a continuous fashion into Total therapy with no undue prolonged cytopenia. Addition of TKIs to induction chemotherapy should be considered as soon as the diagnosis of Ph+ALL is established. Although it is not yet clear whether chemotherapy plus TKIs can ultimately replace HSCT in children with Ph+ ALL, the results of our study indicate that MRD should be used to evaluate early response and guide transplant decisions in children with Ph+ ALL.
SUMMARY.
Patients with Ph+ ALL commonly have high levels of MRD at the end of remission induction chemotherapy. Incorporation of TKIs into induction therapy resulted in rapid MRD reduction which was associated with improved outcomes even if patients did not proceed to transplant.
Acknowledgments
Supported in part by grants P30 CA-21765 and Cancer Center Core Grant CA 21765 from the National Institutes of Health, a Center of Excellence grant from the State of Tennessee, and the American Lebanese Syrian Associated Charities (ALSAC). Dr. Pui is the American Cancer Society F.M. Kirby Professor.
Footnotes
Authorship
Contributions: S.J., C.H.P., D.C. conceived and designed the study, analyzed and interpreted data, and wrote the manuscript; E.C.S. D.P., C.C., J.K.C., J.J., S.A.S.,S.R. analyzed and interpreted data; J.T.S., J.R., S.C.H., H.I., D.B., M.M., R.R. contributed patients; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests
Reference List
- 1.Heisterkamp N, Stephenson JR, Groffen J, et al. Localization of the c-ab1 oncogene adjacent to a translocation break point in chronic myelocytic leukaemia. Nature. 1983;306:239–242. doi: 10.1038/306239a0. [DOI] [PubMed] [Google Scholar]
- 2.Moorman AV, Harrison CJ, Buck GA, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109:3189–3197. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- 3.Arico M, Valsecchi MG, Camitta B, et al. Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. N. Engl. J. Med. 2000;342:998–1006. doi: 10.1056/NEJM200004063421402. [DOI] [PubMed] [Google Scholar]
- 4.Arico M, Schrappe M, Hunger SP, et al. Clinical outcome of children with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia treated between 1995 and 2005. J. Clin. Oncol. 2010;28:4755–4761. doi: 10.1200/JCO.2010.30.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children's oncology group study. J. Clin. Oncol. 2009;27:5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biondi A, Schrappe M, De LP, et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol. 2012;13:936–945. doi: 10.1016/S1470-2045(12)70377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rives S, Estella J, Gomez P, et al. Intermediate dose of imatinib in combination with chemotherapy followed by allogeneic stem cell transplantation improves early outcome in paediatric Philadelphia chromosome-positive acute lymphoblastic leukaemia (ALL): results of the Spanish Cooperative Group SHOP studies ALL-94, ALL-99 and ALL-2005. Br. J. Haematol. 2011;154:600–611. doi: 10.1111/j.1365-2141.2011.08783.x. [DOI] [PubMed] [Google Scholar]
- 8.Pui CH, Pei D, Sandlund JT, et al. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:371–382. doi: 10.1038/leu.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N. Engl. J. Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faham M, Zheng J, Moorhead M, et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012;120:5173–5180. doi: 10.1182/blood-2012-07-444042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribera JM. Optimal approach to treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: how to best use all the available tools. Leuk. Lymphoma. 2013;54:21–27. doi: 10.3109/10428194.2012.708753. [DOI] [PubMed] [Google Scholar]
- 12.Ravandi F, O'Brien S, Thomas D, et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116:2070–2077. doi: 10.1182/blood-2009-12-261586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravandi F, Jorgensen JL, Thomas DA, et al. Detection of MRD may predict the outcome of patients with Philadelphia chromosome-positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood. 2013;122:1214–1221. doi: 10.1182/blood-2012-11-466482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neale GA, Coustan-Smith E, Stow P, et al. Comparative analysis of flow cytometry and polymerase chain reaction for the detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia. 2004;18:934–938. doi: 10.1038/sj.leu.2403348. [DOI] [PubMed] [Google Scholar]
- 15.Kerst G, Kreyenberg H, Roth C, et al. Concurrent detection of minimal residual disease (MRD) in childhood acute lymphoblastic leukaemia by flow cytometry and real-time PCR. Br. J. Haematol. 2005;128:774–782. doi: 10.1111/j.1365-2141.2005.05401.x. [DOI] [PubMed] [Google Scholar]
- 16.Garand R, Beldjord K, Cave H, et al. Flow cytometry and IG/TCR quantitative PCR for minimal residual disease quantitation in acute lymphoblastic leukemia: a French multicenter prospective study on behalf of the FRALLE, EORTC and GRAALL. Leukemia. 2013;27:370–376. doi: 10.1038/leu.2012.234. [DOI] [PubMed] [Google Scholar]
- 17.Bruggemann M, Schrauder A, Raff T, et al. Standardized MRD quantification in European ALL trials: proceedings of the Second International Symposium on MRD assessment in Kiel, Germany, 18–20 September 2008. Leukemia. 2010;24:521–535. doi: 10.1038/leu.2009.268. [DOI] [PubMed] [Google Scholar]
- 18.Campana D. Minimal residual disease in acute lymphoblastic leukemia. Hematology. Am. Soc. Hematol. Educ. Program. 2010;2010:7–12. doi: 10.1182/asheducation-2010.1.7. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Kim DW, Cho B, et al. Risk factors for adults with Philadelphia-chromosome-positive acute lymphoblastic leukaemia in remission treated with allogeneic bone marrow transplantation: the potential of real-time quantitative reverse-transcription polymerase chain reaction. Br. J. Haematol. 2003;120:145–153. doi: 10.1046/j.1365-2141.2003.03988.x. [DOI] [PubMed] [Google Scholar]
- 20.Yanada M, Sugiura I, Takeuchi J, et al. Prospective monitoring of BCR-ABL1 transcript levels in patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia undergoing imatinib-combined chemotherapy. Br. J. Haematol. 2008;143:503–510. doi: 10.1111/j.1365-2141.2008.07377.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Kim DW, Cho BS, et al. Impact of minimal residual disease kinetics during imatinib-based treatment on transplantation outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 2012;26:2367–2374. doi: 10.1038/leu.2012.164. [DOI] [PubMed] [Google Scholar]
- 22.Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115:3206–3214. doi: 10.1182/blood-2009-10-248146. [DOI] [PubMed] [Google Scholar]
- 23.Ribera JM, Garcia O, Montesinos P, et al. Treatment of young patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia using increased dose of imatinib and deintensified chemotherapy before allogeneic stem cell transplantation. Br. J. Haematol. 2012;159:78–81. doi: 10.1111/j.1365-2141.2012.09240.x. [DOI] [PubMed] [Google Scholar]
- 24.Bhojwani D, Pei D, Sandlund JT, et al. ETV6-RUNX1-positive childhood acute lymphoblastic leukemia: improved outcome with contemporary therapy. Leukemia. 2012;26:265–270. doi: 10.1038/leu.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]