Abstract
An effective route for the synthesis of aliphatic polyesters made from adipic or sebacic acid and alkanediols, using inorganic acid as a catalyst is reported. The monomer composition, reaction time, catalyst type, and reaction conditions were optimized to yield polyesters with weight average molecular weights of 23,000 for adipic acid and 85,000 for sebacic acid-based polyesters. The polymers melt at temperatures of 52–65°C and possess melt viscosity in the range of 5600–19,400cP. This route represents an alternative method for producing aliphatic polyesters for possible use in the preparation of degradable disposable medical supplies.
Keywords: catalyst, polyesters, synthesis
INTRODUCTION
Non-degradable plastics are durable and their disposal can be hazardous to the environment.[1] As means of overcoming the problems associated with the disposal of traditional plastic materials, the use of biodegradable polymers has been considered.[2,3] The preferred plastics are those that degrade into safe low molecular weight compounds in a relatively short time period by enzymes produced by microorganisms which are found in the environment, including bacteria, fungi, and algae. Biodegradable plastics eventually degrade to small molecules such as carbon dioxide and water.[4–6] Polyesters derived from aliphatic diols and aliphatic dicarboxylic acids are crystalline, biodegradable materials, which represent an alternative to biodegradable polyesters derived from lactones or cyclic diesters. Synthesis of polyesters by polycondensation of diacid with diol usually involves use of solvents, high vacuum, and temperature conditions for water removal. Aliphatic polyesters with number average molecular weights greater than 30,000 were difficult to produce synthetically using conventional polycondensation chemistry that avoids toxic catalysts.[4,6–9]
Kimura and colleague compared melt polycondensation with solid polycondensation of lactic acid. The high molecular weight PLA was achieved by solid polycondensation at carefully controlled temperature conditions.[10,11]
Polycondensation of diols with dicarboxylic acid dichlorides is limited by aliphatic dicarboxylic acid dichlorides’ high cost, sensitivity to hydrolysis, and occurrence of side reactions.[12] Azeotropic water removal by aromatic solvents in the presence of protic catalyst favor cyclization due to dilution and may yield high fractions of ether groups by proton-catalyzed reaction of two alcohols.[13,14] The limitation of chain growth by cyclization is a common problem when reactions in solution are concerned.[12]
Ahn et al. reported the synthesis of linear poly(butylene adipate) with molecular weight of Mn>20,000, Mw>40,000 using titanium (IV) isopropoxide (TIP) as a catalyst. The water was removed by applying high vacuum.[7] However, to reach these molecular weight values, very high temperature regime (230–240°C) was applied.[7] At the high temperature (up to 240°C) required for the transesterification of diols with dimethyl esters of aliphatic dicarboxylic acids, volatility of numerous monomers such as ethylene diol or dimethyl esters of succinic and adipic acid is a serious problem, and depending on the catalyst ether groups may be formed.[13] Another approach for polycondensation of diols with dicarboxylic acids is the use of nonspecific lipases.[15–18] However, rather large amounts (e.g. 1 wt%) of an expensive enzyme are required, and this catalyst needs to be separated from the reaction product at the end of the polycondensation.
The attempt to synthesize polyesters by condensation under mild conditions was reported by Takasu et al.[19,20] Mixtures of methyl succinic acid with various diols were condensed in bulk using scandium-triflate (Sc(OTf)3) as catalyst. Water was removed by applying vacuum for a period of 3 days. The evaluated temperatures were 35–80°C. However, these temperatures are too low for bulk reactions of most aliphatic dicarboxylic acid since they possess melting temperatures (Tm’s) of 50°C or higher. Adipic acid and sebacic acid have Tms of 152 and 131°C, respectively. Furthermore, number average molecular weights (Mn’s) lower then 14 000 Da were reported. However, when aliphatic polyesters are evaluated, SEC determinations overestimate the Mn’s by at least 50% above 104 Da.[21,22]
Recent studies focused on polycondensation of sebacic acid and 1,6 butanediol using bismuth triftalate. The reaction was conducted at 80°C. Vacuum of 1 mBar for 48 hr was applied. In this experiment, polymers with Mw<30,000 (uncorrected SEC data) were obtained.[23] Thereafter, the same experiment was performed using additional triftalates.
When lanthanide triflate was used, polyesters with Mw up to 65,000 (uncorrected SEC data) were received.[24] Still, the long reaction time is a drawback. Also, the reaction involves the use and removal of toxic organic solvent (dioxin), which can be problematic when the production of medical supplies is considered.
It was the objective of this study to develop alternative synthesis for the preparation of high molecular weight aliphatic polyesters. This report describes the synthesis of a range of polyesters made by polycondensation of adipic acid (AA)or sebacic acid (SA) with aliphatic diols to form high molecular weight polyesters. Synthetic conditions involved the use of inorganic acid (H3PO4 or H2SO4) as catalyst combined with mild temperatures and low vacuum. Synthesis conditions were validated using additional monomer such as ricinoleic acid or castor oil.
EXPERIMENTAL
Materials
Adipic acid (99% pure; Fluka, Buch, Switzerland), sebacic acid (99% pure; Sigma-Aldrich, Rehovot, Israel), butanediol (99% pure; Sigma-Aldrich, Rehovot, Israel), ethylene glycol (99% pure; Sigma-Aldrich, Rehovot, Israel), propylene glycol (99% pure; Sigma-Aldrich, Rehovot, Israel), ricinoleic acid (85% pure; Fluka, Buch, Switzerland), and castor oil (Eur. Ph; Haifa, Israel) were used. All the solvents were of analytical-grade from Biolab (Jerusalem, Israel) and were used without further purification.
Instrumentation
IR spectroscopy (2000 FTIR), Perkin Elmer) was performed on the prepolymer and polymer samples were cast on NaCl plates from a chloroform solution. Thermal analysis was performed on a Stuart Scientific SMP1 melting point heater. The molecular weights of the polyesters were estimated on a gel permeation chromatography (GPC) consisting of the a Waters 1515 isocratic HPLC pump with a Waters 2410 refractive-index detector and a Rheodyne (Coatati, CA) inject valve with a 20 µL Loop (Waters MA). The polyester samples were eluted with CHCl3 through a linear Ultrastyragel column (Waters; 500-Å pore size) at a flow rate of 1 ml/min. The molecular weights were determined relatively to polystyrene standards (Polyscience, Warrington, PA) with a molecular weight range of 1,110–17,300 or 9,100–355,000 with a breeze computer program. 1H NMR spectra (CDCl3) were obtained on a Varian 300 MHz spectrometer in tubes with 5 mm external diameter. CDCl3 containing tetramethylsilane served as solvent and shift reference.
The viscosity of polymers was determined by rotational method (Brookfield programmable rheometer, LV-DV-III). A cylindrical spindle LV4 was used.
Synthesis of aliphatic polyesters
All polyesters were synthesized using a three necked reaction vessel equipped with a mechanical stirrer on an oil heating bath. Adipic acid or sebacic acid (0.1 mol) were added into the vessel with different molar ratios of 1,4-butanediol/1,3 propyleneglycol/ethyleneglycol. The molar ratio between the dicarboxylic acid and the diol was investigated and optimized. The catalyst H3PO4/H2SO4 0.1–0.5–1% w/w was added to the reaction mixture prior to polymerization (the concentration of the catalyst was also optimized).
During dehydration, the temperature was slowly increased up to 190°C and the mixture was stirred for 2.5 hr (with open outlet to atmosphere). The polycondensation was conducted under low vacuum (15 mbar). The reaction was continued for additional 24–48 hr. The resulting polymers were evaluated by GPC and NMR. Because of a relatively narrow Mw/Mn distribution (p ≤ 2), no further purification steps were taken up. Polymers containing up to 20% w/w ricinoleic acid or castor oil were prepared similarly to produce non-crosslinked polymers.
All the polymers were characterized using NMR, IR, and GPC, melting point, and viscosity measurements.
Poly(butylene adipate) showed the main typical peaks at 1.649 ppm 8H (CH2)2 of the diol and the acid, 2.290 ppm 4H (CH2) acid, 4.05 ppm 4H (CH2) diol and 3.7 ppm terminal OH groups. Poly(butylene sebacate) showed typical peaks at 1.228 ppm 8H (CH2)4 aliphatic chain of the acid, 1.649 ppm 8H (CH2)2 of the diol and the acid, 2.217 ppm 4H (CH2) acid, 4.05 ppm 4H (CH2) diol and 3.7 ppm terminal OH groups. Poly(ethylene adipate) showed typical peaks at 1.649 ppm 4H (CH2)2 of the acid, 2.34 ppm 4H (CH2) acid, 4.253 ppm 4H (CH2) diol. Poly(propylene adipate) showed typical peaks at 1.12 ppm 3H methyl of the diol, 1.649 ppm 4H (CH2)2 of the acid, 2.280 ppm 4H (CH2) acid, 4.132 ppm 2H (CH2) diol, and 5.124 ppm H diol. Incorporation of ricinoleic acid showed typical peaks at 5.35 ppm of ricinoleic acid double bond and 4.8 ppm ricinoleic acid ester which indicate incorporation of ricinoleic acid in the polymer chain.
Measurement of viscosity
Viscosity of polymers was measured using a Brookfield LVDV-III programmable viscometer coupled to a temperature-controlling unit. Cylindrical spindles were used. Temperature sensitivity test was performed starting at a temperature of 140°C and down to melt temperature (40–60°C) by applying constant rotational speed. Detection of rheological behavior was performed by measuring shear stress and/or viscosity at different shear rates, starting at 0.209 sec−1, for more viscous polymers and up to 8.3 sec−1 for less viscous polymers. All the experiments were performed in triplicate.
RESULTS AND DISCUSSION
Optimization of monomer ratio
The acid catalyzed polycondensation of diols and dicarbolylic acids favors cyclization when performed in solution due to dilution.[14] To reduce cyclization, the reactions were performed in bulk. To perform polymerization in bulk, the reaction temperature should be higher than the melting temperatures of the used dicarboxylic acids. Reaction temperatures of 230°C and higher are problematic due to the thermal stability of moderate polyesters.[25] In this work the reactions were performed at 190°C.
Due to high polymerization temperature, the excess of diol was optimized to compensate their loss due to volatility. The first set of experiments was performed on adipic acid and 1,4-butanediol at 1:1 ratio and with excess of diol. Similar experiments were performed with ethylene glycol and 1,3propylenediol.The results are shown in Table 1.
Table 1.
Effect of monomer composition on polyesters molecular weight
| Diacid/diol ratio |
1:1 |
1:1.25 |
1:1.5 |
|||
|---|---|---|---|---|---|---|
| Composition | Mn/Mwa | Melting point b (°C) | Mn/Mwa | Melting point b (°C) | Mn/Mwa | Melting point b (°C) |
| AABD | 3000 | 48 | 6100 | 55 | 4000 | 54 |
| 5500 | 8500 | 6700 | ||||
| AAEG | 2150 | 44 | 3100 | 45 | 4400 | 47 |
| 4000 | 5500 | 6700 | ||||
| AAPG | 1650 | Liquid at 4°C | 3700 | Liquid at 4°C | 3700 | Liquid at 4°C |
| 3200 | 6100 | 4650 | ||||
The reaction vessel was heated to 190°C and dried to atmosphere for 2.5 hr. The reaction was continued for 30 hr under vacuum (15 mBar).
Were established using Waters GPC system with CHCl3 as eluent (uncorrected SEC data).
Thermal analysis was determined on a Stuart Scientific SMP1 meting point heater.
AABD-poly(butylenes adipate).
AAEG-poly(ethylene adipate).
AAPG-poly(propylene adipate).
As can be seen, the molar ratio of 1:1.25 was optimal for 1,4 butanediol and 1,3 propyleneglycol, whereas for ethylene glycol, a lower boiling point monomer, optimal ratio for the monomers is 1:1.5.
As the reaction conditions were supposed to be suitable for a variety of monomers, additional fatty monomers were added. Adding long chain branch monomers should have resulted in more flexible polyesters as branched polymers are received. Addition of fatty monomers should also prolong degradation rates. Ricinoleic acid has carboxylic acid and hydroxyl functionalities. Castor oil has three hydroxyl groups on each molecule. These monomers readily undergo polycondensation reactions with different diacids and diols.[26,27] The prepared polymers are summarized in Table 2.
Table 2.
Incorporation of ricinoleic acid and castor oil into polyester chains
| Diacid/diol ratio |
AABD |
AAEG |
AAPG |
|||
|---|---|---|---|---|---|---|
| Composition | Mn/Mwa | Melting point b (°C) |
Mn/Mwa | Melting point b (°C) |
Mn/Mwa | Melting point b (°C) |
| No additives | 6100 | 55 | 4400 | 47 | 3700 | Liquid at 4°C |
| 8500 | 6700 | 6100 | ||||
| RA 20% | 5500 | 44 | 4400 | 37 | 1000 | Liquid at 4°C |
| 7500 | 6600 | 1300 | ||||
| CO 20% | 4500 | 38 | 3000 | 34 | 1600 | Liquid at 4°C |
| 6800 | 6200 | 2700 | ||||
The reaction vessel was heated to 190°C and dried to atmosphere for 2.5 hr. The reaction was continued for 30 hr under vacuum (15 mBar).
Were established using Waters GPC system with CHCl3 as eluent (uncorrected SEC data).
Thermal analysis was determined on a Stuart Scientific SMP1 meting point heater.
AABD-poly(butylenes adipate).
AAEG-poly(ethylene adipate).
AAPG-poly(propylene adipate).
RA20%–20% w/w ricinoleic acid as additional monomer.
CO20%–20% w/w castor oil as additional monomer.
When 1,4 butanediol and ethylene glycol were used, up to 20% w/w ricinoleic acid or castor oil were incorporated into the polymer chains, without reducing the molecular weight values. Copolymerization of these monomers with propylene glycol was not successful. The received polymers had very low molecular weights. Incorporation of ricinoleic acid/castor oil decreased the polymer’s melting temperature (Table 2). It was confirmed that these synthesis conditions are suitable for polycondensation of a variety of bi-functional monomers.
Catalyst selection
The effect of proton catalysis was investigated using H3PO4 and H2SO4. The catalysts at concentrations of 0.1–0.5–1% w/w were added to the polymerization vessel. The polyesters are summarized in Table 3.
Table 3.
Effect of H3PO4/H2SO2 as catalyst on polyesters molecular weight
| Catalyst |
H3PO4 Concentration (%w/w) |
H2SO2 Concentration (%w/w) |
|||||
|---|---|---|---|---|---|---|---|
| Composition | 0.1 | 0.5 | 1 | 0.1 | 0.5 | 1 | |
| Mn/Mwa | Mn/Mwa | Mn/Mwa | Mn/Mwa | Mn/Mwa | Mn/Mwa | ||
| AABDRA20% | 7500 | 9300 | 8600 | 6200 | 4300 | 3500 | |
| 9100 | 10,500 | 10,000 | 8200 | 6700 | 6200 | ||
| AAEG RA20% | 3400 | 4400 | 5000 | 6300 | 6000 | 5000 | |
| 5700 | 6700 | 7000 | 8400 | 8200 | 7200 | ||
| AAPG RA20% | 1800 | 2000 | 2600 | 5200 | 3500 | 4300 | |
| 3000 | 3500 | 4520 | 7500 | 5900 | 6700 | ||
The reaction vessel was heated to 190°C and dried to atmosphere for 2.5 h. The reaction was continued for 30 hr under vacuum (15 mBar).
Were established using Waters GPC system with CHCl3 as eluent (uncorrected SEC data).
AABD RA20%-poly(butylenes adipate co ricinoleic acid 20% w/w).
AAEG RA20%-poly(ethylene adipate co ricinoleic acid 20% w/w).
AAPG RA20%-poly(propylene adipate co ricinoleic acid 20% w/w).
The use of H2SO4 had no effect on poly(butylenes adipate co ricinoleic acid) molecular weight. The molecular weight of poly(ethylene adipate co ricinoleic acid) increased slightly. The significant increase in molecular weight was observed only for poly(propylene adipate co ricinoleic acid). The molecular weight increased from 1000 to 5200 for Mn and 1300 to 7500 for Mw. However, all polymers became brown. The use of H3PO4 was not effective for poly(ethylene adipate co ricinoleic acid) and poly(propylene adipate co ricinoleic acid). However, when poly(butylene adipate co ricinoleic acid) was synthesized using H3PO4 0.5% w/w as catalyst, molecular weight of the polymer increased significantly (Table 3, uncorrected SEC data). When adipic acid and butane diol without additional monomers were reacted, a dramatical increase in molecular weight was shown. Poly(butylenes adipate) reached Mw= 23,000, Mn= 16,000 and poly(butylene sebacate) reached Mw=85,000 and Mn=55,000 (uncorrected SEC data). Also, the use of H3PO4 0.5% reduced the diol excess and reaction time. Polymers showed kinetic pattern with a maximal Mw value at 24 hr (Fig. 1).
Figure 1.
Mw change during the reaction of adipic acid and 1,4 Butane diol at different mole ratios with or without H3PO4 as catalyst. The reaction vessel was heated at 190°C for 2.5 hr and continued for 48 hr under vacuum (15 mBar). Mw/Mn values were determined using Waters GPC system with CHCl3 as eluent. All tests were performed in triplicate. AABD 1:1.25-poly(butylene adipate) with feed ratio 1:1.5 mole diacid to diol. AABD 1:1.5-poly(butylene adipate) with feed ratio 1:1.5 mole diacid to diol.
These reaction conditions (0.5% H3PO4, 24 hr) were selected as optimal. Incorporation of ricinoleic acid up to 5% w/w did not affect molecular weights of poly(butylene adipate) and poly(-butylene sebacate) (Table 4). No coloring was observed. All the polymers were white solids. The polymers were not isolated or purified. The bulk polymers were removed from the vessel as poured melt. Small samples were dissolved in CH2Cl2 and analyzed by GPC (Fig. 2) and NMR (Fig. 3, A–D). This is an equilibrium polycondensation reaction and we would expect to see cyclic structures and oligomers. [28,29] Only a small peak at 4.1–4.2 ppm, which corresponds to cyclic structures, was seen. It might be suggested that the reaction was stopped before full conversion rates were achieved. On the other hand, it might be an artifact of the work-up and the SEC separation (the small molecular weight fraction was removed). Poly(butylene adipate co ricinoleic acid) (Fig. 3B) and poly(butylene sebacate co ricinoleic acid) (Fig. 3D) showed typical peaks at 5.35 ppm, ricinoleic acid double bond, and 4.8 ppm, ricinoleic acid ester, which indicates incorporation of ricinoleic acid in the polymer chain.
Table 4.
Summary of polyesters prepared under optimal polymerization conditions
| Composition |
Characterization |
|||||
|---|---|---|---|---|---|---|
| Diacid | Diol | Additional monomer |
Mna | Mwb | p | Tmc |
| AA | BD | — | 16,000 | 23,000 | 1.43 | 58 |
| AA | BD | RA 5% | 14,000 | 22,200 | 1.6 | 52 |
| SA | BD | — | 55,000 | 85,000 | 1.54 | 65 |
| SA | BD | RA 5% | 34,000 | 63,000 | 1.85 | 63 |
The reaction vessel was heated to 190°C and dried to atmosphere for 2.5 hr. The reaction was continued for 24 hr under vacuum (15 mBar). H3PO4 in concentration of 0.5% w/w was added as catalyst.
Were established using Waters GPC system with CHCl3 as eluent (uncorrected SEC data).
Were established using Waters GPC system with CHCl3 as eluent (uncorrected SEC data).
Thermal analysis was recorded by DSC at 10°C/min.
AA—adipic acid.
SA—sebacic acid.
BD—butanediol.
RA—ricinoleic acid.
CO—castor oil.
Figure 2.
GPC analysis of (A) poly(butylene adipate), (B) poly(butylene adipate co ricinoleic acid), (C) poly(butylene sebacate), and (D) poly(butylene sebacate co ricinoleic acid).
Figure 3.
1H-NMR spectra of (A) poly(butylene adipate), (B) poly(butylene adipate co ricinoleic acid), (C) poly(butylene sebacate), and (D) poly(butylene sebacate co ricinoleic acid).
Thermal analysis of the polyesters was performed by DSC (Fig. 4).
Figure 4.
Thermal analysis of polyesters. (A) poly(butylene adipate), (B) poly(butylene adipate co ricinoleic acid). (C) poly(butylene sebacate), and (D) poly(butylene sebacate co ricinoleic acid). This figure is available in colour online at wileyonlinelibrary.com/journal/pat
Viscosity of polymers
The viscosity of four polyesters, poly(butylene adipate), poly(-butylenes adipate co ricinoleic acid) (5%w/w r.a), poly(butylene adipate), and poly(butylene adipate co ricinoleic acid) (5%w/w r.a), was evaluated at a temperature range of 130–50°C, at which the polymers melted. Figs. 5 and 6 show the viscosities of the polymers at different shear rates.
Figure 5.
Viscosity of poly(butylenes adipate) and poly(butylene adipate co ricinoleic acid) as function of temperature at different shear rates. The viscosity was determined at shear rates between 8.28 and 1.035 sec−1. All tests were performed in triplicate. SD was lower than 15% for each polymer. AABD-poly(butylene adipate). AABDRA-poly(butylene adipate co ricinoleic acid).
Figure 6.
Viscosity of poly(butylenes sebacate) and poly(butylene sebacate co ricinoleic acid) as function of temperature at different shear rates. The viscosity was determined at shear rates between 8.28 and 1.035 sec−1. All tests were performed in triplicate. SD was lower than 15% for each polymer. SABD-poly(butylene sebacate co ricinoleic acid). SABDRA-poly(butylene sebacate co ricinoleic acid).
It can be seen that the incorporation of ricinoleic acid into poly(butylene adipate) and poly (butylene sebacate) reduces the viscosity of the polymers and contributes to their liquidity. All polyesters show similar viscosity-temperature dependences.
The other aspect that was evaluated is the relationship between the shear stress (F) and the shear rate (dv/dr) which is expressed in the Newton equation:
F = η dv/dr
where the proportionality constant, η, is the coefficient of viscosity.
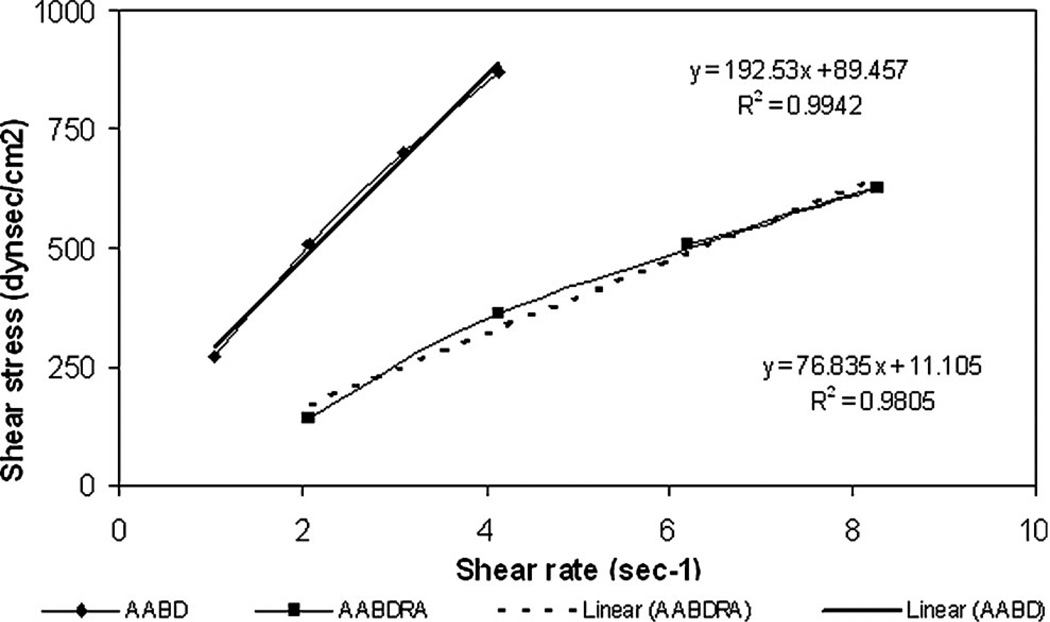
The results are shown in Figs. 7 and 8.
Figure 7.
Relationship of shear rate and shear stress for poly(butylene adipate) and poly(butylene adipate co ricinoleic acid) at 65°C temperature range. All tests were performed in triplicate. AABD-poly(butylene adipate). AABDRA-poly(butylene adipate co ricinoleic acid).
Figure 8.
Relationship of shear rate and shear stress of poly(butylene sebacate) and poly(butylene sebacate co ricinoleic acid). SABD-poly(butylene sebacate co ricinoleic acid). SABDRA-poly(butylene sebacate co ricinoleic acid).
All polyesters behave as Newtonian liquid. Their viscosity does not depend on shear stress applied, which is important for processing conditions of the polymers.
CONCLUSIONS
Effective synthesis of aliphatic polyesters with relatively high molecular weight has been developed. Different monomers, which can contribute to the desired qualities of the polyesters, can be incorporated into the polymer chains. Special attention was given to the safety of the polymers, so that they can be applied for the production of medical articles and disposable supplies.
Scheme 1.
Polyester formation.
Scheme 2.
Structures of castor oil and ricinoleic acid.
Acknowledgements
This work was supported by the US-Israel binational fund (BSF) awarded to Langer and Domb.
REFERENCES
- 1.Lee BK, Ellenbecker MJ, Moure-Ersaso R. Waste Manag. 2004;24:243. doi: 10.1016/j.wasman.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Fritz J, Link U, Braun R. Starch/Sturke. 2001;53:105. [Google Scholar]
- 3.Gross RA, Kalra B. Science. 2002;297:803. doi: 10.1126/science.297.5582.803. [DOI] [PubMed] [Google Scholar]
- 4.Zhu CY, Zhang ZQ, Liu QP, Wang ZP, Jin J. J. Appl. Polym. Sci. 2003;90:982. [Google Scholar]
- 5.Shirahama H, Kawaguchi Y, Aludin MS, Yasuda H. J. Appl. Polym. Sci. 2001;80:340. [Google Scholar]
- 6.Saulnier B, Coudane J, Garreau H, Vert M. Polymer. 2006;47:1921. [Google Scholar]
- 7.Ahn BD, Kim SH, Kim YH, Yang JS. J. Appl. Polym. Sci. 2001;82:2808. [Google Scholar]
- 8.Mani R, Leriche C, Nie L, Bassi S. J. Polym. Sci.: Part A1. 2002;40:3232. [Google Scholar]
- 9.Han YK, Um JW, Im SS, Kim BC. J. Polym. Sci.: Part A1. 2001;39:2143. [Google Scholar]
- 10.Moon SI, Taniguchi I, Miyamoto M, Kimura Y, Lee CW. High Perform. Polym. 2001;13:S189. [Google Scholar]
- 11.Moon SI, Lee CW, Taniguchi I, Miyamoto M, Kimura Y. Polymer. 2001;42:5059. [Google Scholar]
- 12.Kricheldorf HR, Rabenstein M, Maskos M, Schmidt M. Macromolecules. 2001;34:713. [Google Scholar]
- 13.Kricheldorf HR, Behnken G, Schwarz G. Polymer. 2005;46:11219. [Google Scholar]
- 14.Ishii M, Okazaki M, Shibasaki Y, Ueda M, Teranishi T. Biomacromolecules. 2001;2:1267. doi: 10.1021/bm015576a. [DOI] [PubMed] [Google Scholar]
- 15.Gross RA, Kumar A, Kalra B. Chem. Rev. 2001;101:2097. doi: 10.1021/cr0002590. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi S, Uyama H, Kimura S. Chem. Rev. 2001;101:3793. doi: 10.1021/cr990121l. [DOI] [PubMed] [Google Scholar]
- 17.Olson DA, Sheares VV. Macromolecules. 2006;39:2808. [Google Scholar]
- 18.Shuai XT, Jedlinski Z, Kowalczuk M, Rydz J, Tan HM. Eur. Polym. J. 1999;35:721. [Google Scholar]
- 19.Takasu A, Ishii M, Inai Y, Hirabayashi T, Inomata K. Macromolecules. 2003;36:7055. [Google Scholar]
- 20.Takasu A, Oishi Y, Iio Y, Inai Y, Hirabayashi T. Macromolecules. 2003;36:1772. [Google Scholar]
- 21.Save M, Schappacher M, Soum A. Macromol. Chem. Phys. 2002;203:889. [Google Scholar]
- 22.Hiltunen K, Harkonen M, Seppala JV, Vaananen T. Macromolecules. 1996;29:8677. [Google Scholar]
- 23.Buzin P, Lahcini M, Schwarz G, Kricheldorf HR. Macromolecules. 2008;41:8491. [Google Scholar]
- 24.Garaleh M, Lahcini M, Kricheldorf HR, Weidner SM. J. Polym. Sci.: Part A1. 2009;47:170. [Google Scholar]
- 25.Chrissafis K, Paraskevopoulos KM, Bikiaris DN. Thermochim. Acta. 2006;440:166. [Google Scholar]
- 26.Slivniak R, Ezra A, Domb AJ. Pharm. Res. 2006;23:1306. doi: 10.1007/s11095-006-0140-x. [DOI] [PubMed] [Google Scholar]
- 27.Bruggeman JP, De Bruin BJ, Bettinger CJ, Langer R. Biomaterials. 2008;29:4726. doi: 10.1016/j.biomaterials.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kricheldorf HR, Langanke D, Spickermann J, Schmidt M. Macro-molecules. 1999;32:3559. [Google Scholar]
- 29.Kricheldorf HR, Lorenc A, Spickermann J, Maskos M. J. Polym. Sci.: Part A1. 1999;37:3861. [Google Scholar]