Abstract
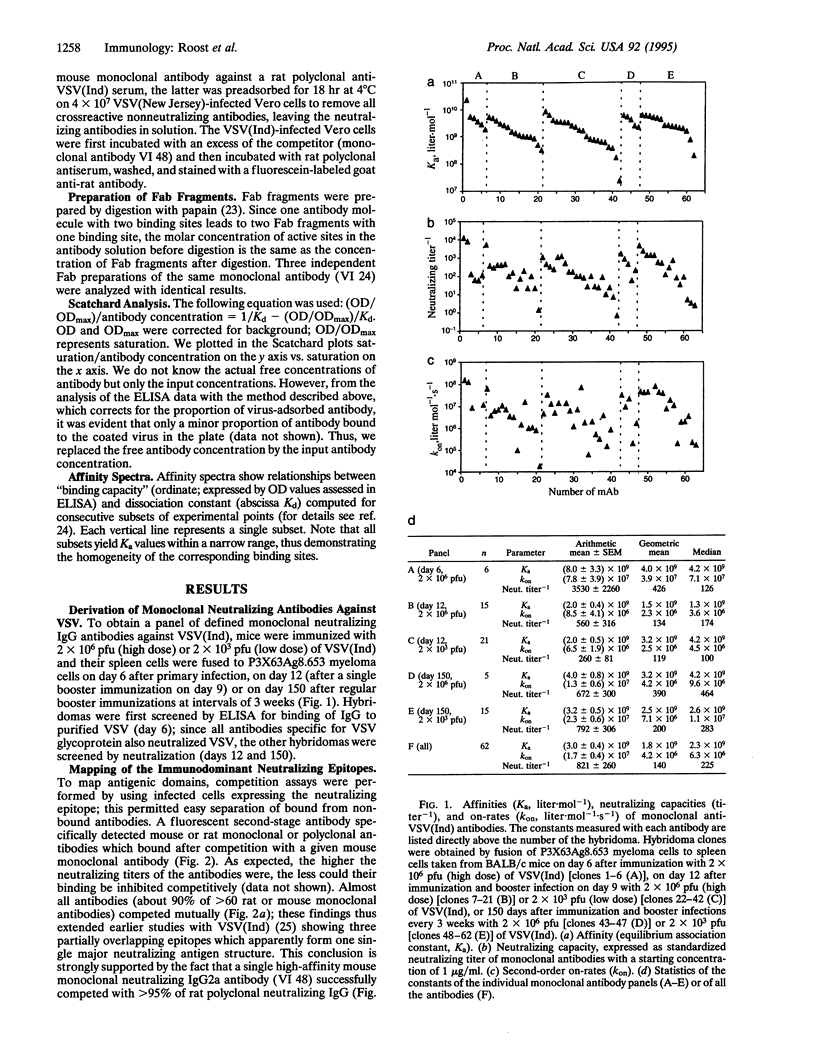
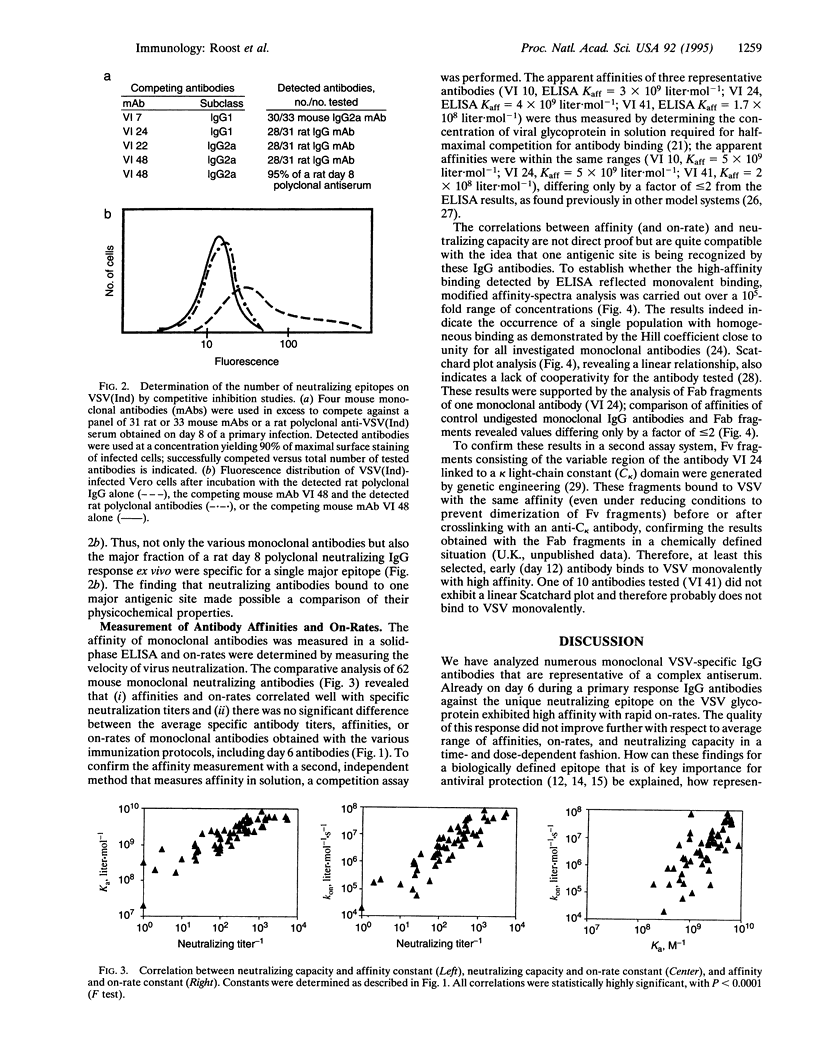
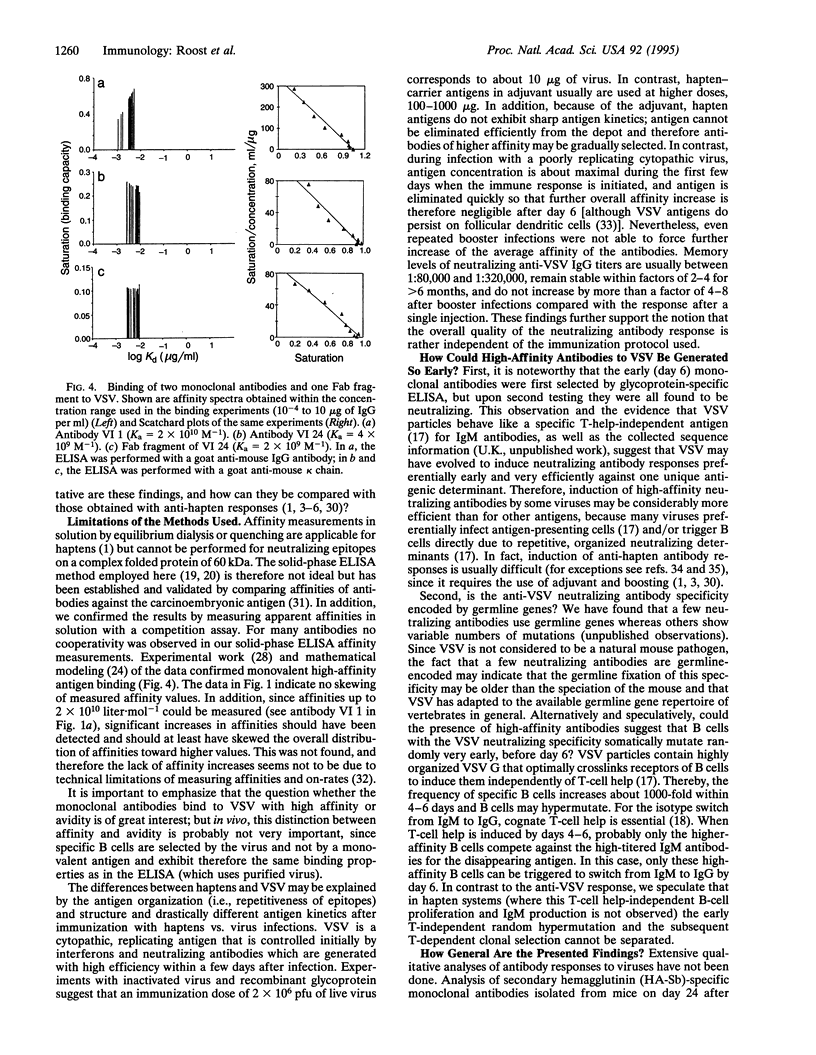
Affinity maturation of IgG antibodies in adaptive immune responses is a well-accepted mechanism to improve effector functions of IgG within 2 weeks to several months of antigen encounter. This concept has been defined mainly for IgG responses against chemically defined haptens. We have evaluated this concept in a viral system and analyzed neutralizing IgG antibody responses against vesicular stomatitis virus (a close relative of rabies virus) with a panel of monoclonal antibodies obtained early (day 6 or 12) and late (day 150) after hyperimmunization. These neutralizing IgG antibodies recognize a single major antigenic site with high affinities (Ka of 10(8)-10(10) liter.mol-1) and with rapid on-rates already on day 6 of a primary response and with no evidence for further antigen dose- and time-dependent overall improvement of affinity. This type of IgG response is probably representative for viruses or bacterial toxins which are crucially controlled by neutralizing antibodies.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azimzadeh A., Pellequer J. L., Van Regenmortel M. H. Operational aspects of antibody affinity constants measured by liquid-phase and solid-phase assays. J Mol Recognit. 1992 Mar;5(1):9–18. doi: 10.1002/jmr.300050103. [DOI] [PubMed] [Google Scholar]
- Azimzadeh A., Van Regenmortel M. H. Measurement of affinity of viral monoclonal antibodies by ELISA titration of free antibody in equilibrium mixtures. J Immunol Methods. 1991 Aug 9;141(2):199–208. doi: 10.1016/0022-1759(91)90146-7. [DOI] [PubMed] [Google Scholar]
- Bachmann M. F., Kündig T. M., Kalberer C. P., Hengartner H., Zinkernagel R. M. How many specific B cells are needed to protect against a virus? J Immunol. 1994 May 1;152(9):4235–4241. [PubMed] [Google Scholar]
- Bachmann M. F., Kündig T. M., Odermatt B., Hengartner H., Zinkernagel R. M. Free recirculation of memory B cells versus antigen-dependent differentiation to antibody-forming cells. J Immunol. 1994 Oct 15;153(8):3386–3397. [PubMed] [Google Scholar]
- Bachmann M. F., Rohrer U. H., Kündig T. M., Bürki K., Hengartner H., Zinkernagel R. M. The influence of antigen organization on B cell responsiveness. Science. 1993 Nov 26;262(5138):1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- Baer G. M., Cleary W. F. A model in mice for the pathogenesis and treatment of rabies. J Infect Dis. 1972 May;125(5):520–527. doi: 10.1093/infdis/125.5.520. [DOI] [PubMed] [Google Scholar]
- Beatty J. D., Beatty B. G., Vlahos W. G., Hill L. R. Method of analysis of non-competitive enzyme immunoassays for antibody quantification. J Immunol Methods. 1987 Jun 26;100(1-2):161–172. doi: 10.1016/0022-1759(87)90186-4. [DOI] [PubMed] [Google Scholar]
- Beatty J. D., Beatty B. G., Vlahos W. G. Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. J Immunol Methods. 1987 Jun 26;100(1-2):173–179. doi: 10.1016/0022-1759(87)90187-6. [DOI] [PubMed] [Google Scholar]
- Berek C., Griffiths G. M., Milstein C. Molecular events during maturation of the immune response to oxazolone. Nature. 1985 Aug 1;316(6027):412–418. doi: 10.1038/316412a0. [DOI] [PubMed] [Google Scholar]
- Charan S., Zinkernagel R. M. Antibody mediated suppression of secondary IgM response in nude mice against vesicular stomatitis virus. J Immunol. 1986 Apr 15;136(8):3057–3061. [PubMed] [Google Scholar]
- Clarke S., Rickert R., Wloch M. K., Staudt L., Gerhard W., Weigert M. The BALB/c secondary response to the Sb site of influenza virus hemagglutinin. Nonrandom silent mutation and unequal numbers of VH and Vk mutations. J Immunol. 1990 Oct 1;145(7):2286–2296. [PubMed] [Google Scholar]
- Dekker E. L., Porta C., Van Regenmortel M. H. Limitations of different ELISA procedures for localizing epitopes in viral coat protein subunits. Arch Virol. 1989;105(3-4):269–286. doi: 10.1007/BF01311363. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Tollis M., Lafon M., Wunner W. H., Koprowski H. Mechanisms of rabies virus neutralization by glycoprotein-specific monoclonal antibodies. Virology. 1987 Nov;161(1):29–36. doi: 10.1016/0042-6822(87)90167-x. [DOI] [PubMed] [Google Scholar]
- Dintzis H. M., Dintzis R. Z., Vogelstein B. Molecular determinants of immunogenicity: the immunon model of immune response. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3671–3675. doi: 10.1073/pnas.73.10.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dintzis R. Z., Middleton M. H., Dintzis H. M. Studies on the immunogenicity and tolerogenicity of T-independent antigens. J Immunol. 1983 Nov;131(5):2196–2203. [PubMed] [Google Scholar]
- EISEN H. N., SISKIND G. W. VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry. 1964 Jul;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- Foote J., Milstein C. Kinetic maturation of an immune response. Nature. 1991 Aug 8;352(6335):530–532. doi: 10.1038/352530a0. [DOI] [PubMed] [Google Scholar]
- Frankel M. E., Gerhard W. The rapid determination of binding constants for antiviral antibodies by a radioimmunoassay. An analysis of the interaction between hybridoma proteins and influenza virus. Mol Immunol. 1979 Feb;16(2):101–106. doi: 10.1016/0161-5890(79)90051-8. [DOI] [PubMed] [Google Scholar]
- French D. L., Laskov R., Scharff M. D. The role of somatic hypermutation in the generation of antibody diversity. Science. 1989 Jun 9;244(4909):1152–1157. doi: 10.1126/science.2658060. [DOI] [PubMed] [Google Scholar]
- Gobet R., Cerny A., Rüedi E., Hengartner H., Zinkernagel R. M. The role of antibodies in natural and acquired resistance of mice to vesicular stomatitis virus. Exp Cell Biol. 1988;56(4):175–180. doi: 10.1159/000163477. [DOI] [PubMed] [Google Scholar]
- Langman R. E., Cohn M. The E-T (elephant-tadpole) paradox necessitates the concept of a unit of B-cell function: the protection. Mol Immunol. 1987 Jul;24(7):675–697. doi: 10.1016/0161-5890(87)90050-2. [DOI] [PubMed] [Google Scholar]
- Lefrancois L. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J Virol. 1984 Jul;51(1):208–214. doi: 10.1128/jvi.51.1.208-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist T. P., Cobbold S. P., Waldmann H., Aguet M., Zinkernagel R. M. Functional analysis of T lymphocyte subsets in antiviral host defense. J Immunol. 1987 Apr 1;138(7):2278–2281. [PubMed] [Google Scholar]
- McKean D., Huppi K., Bell M., Staudt L., Gerhard W., Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984 May;81(10):3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims C. A., Tosolini F. A. Pathogenesis of lesions in lymphoid tissue of mice infected with lymphocytic choriomeningitis (LCM) virus. Br J Exp Pathol. 1969 Dec;50(6):584–592. [PMC free article] [PubMed] [Google Scholar]
- Pliska V., Heiniger J., Müller-Lhotsky A., Pliska P., Ekberg B. Binding of oxytocin to uterine cells in vitro. Occurrence of several binding site populations and reidentification of oxytocin receptors. J Biol Chem. 1986 Dec 25;261(36):16984–16989. [PubMed] [Google Scholar]
- Rajewsky K., Förster I., Cumano A. Evolutionary and somatic selection of the antibody repertoire in the mouse. Science. 1987 Nov 20;238(4830):1088–1094. doi: 10.1126/science.3317826. [DOI] [PubMed] [Google Scholar]
- Rath S., Stanley C. M., Steward M. W. An inhibition enzyme immunoassay for estimating relative antibody affinity and affinity heterogeneity. J Immunol Methods. 1988 Feb 10;106(2):245–249. doi: 10.1016/0022-1759(88)90204-9. [DOI] [PubMed] [Google Scholar]
- Roost H. P., Charan S., Zinkernagel R. M. Analysis of the kinetics of antiviral memory T help in vivo: characterization of short-lived cross-reactive T help. Eur J Immunol. 1990 Dec;20(12):2547–2554. doi: 10.1002/eji.1830201204. [DOI] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. The immunopathogenesis of HIV infection. Adv Immunol. 1989;47:377–431. doi: 10.1016/s0065-2776(08)60665-3. [DOI] [PubMed] [Google Scholar]
- Traunecker A., Oliveri F., Karjalainen K. Myeloma based expression system for production of large mammalian proteins. Trends Biotechnol. 1991 Apr;9(4):109–113. doi: 10.1016/0167-7799(91)90038-j. [DOI] [PubMed] [Google Scholar]
- Wagener C., Clark B. R., Rickard K. J., Shively J. E. Monoclonal antibodies for carcinoembryonic antigen and related antigens as a model system: determination of affinities and specificities of monoclonal antibodies by using biotin-labeled antibodies and avidin as precipitating agent in a solution phase immunoassay. J Immunol. 1983 May;130(5):2302–2307. [PubMed] [Google Scholar]
- Zinkernagel R. M., Hengartner H., Stitz L. On the role of viruses in the evolution of immune responses. Br Med Bull. 1985 Jan;41(1):92–97. doi: 10.1093/oxfordjournals.bmb.a072033. [DOI] [PubMed] [Google Scholar]



