Abstract
Our aim was to contribute to a better understanding of the pathophysiology of anemia in elderly, by studying how aging affects renal function, iron metabolism, erythropoiesis and the inflammatory response, using an experimental animal model. The study was performed in male Wistar, a group of young rats with 2 months age and an old one with 18 months age. Old rats presented a significant higher urea, creatinine, interferon (INF)-gamma, ferritin and soluble transferrin receptor serum levels, as well as increased counts of reticulocytes and RDW. In addition, these rats showed significant lower erythropoietin (EPO) and iron serum levels. Concerning gene expression of iron regulatory proteins, old rats presented significantly higher mRNA levels of hepcidin (Hamp), transferrin (TF), transferrin receptor 2 (TfR2) and hemojuvelin (HJV); divalent metal transporter 1 (DMT1) mRNA levels were significantly higher in duodenal tissue; EPO gene expression was significantly higher in liver and lower in kidney, and the expression of the EPOR was significantly higher in both liver and kidney. Our results showed that aging is associated with impaired renal function, which could be in turn related with the inflammatory process and with a decline in EPO renal production. Moreover, we also propose that aging may be associated with INF-gamma-induced inflammation and with alterations upon iron regulatory proteins gene expression.
Keywords: Anemia, older population, elderly, renal failure, inflammation, erythropoietic disturbances
Epidemiological studies show an increased occurrence of anemia with aging, with a prevalence of about 5% at 65 years, to more than 20% at the age of 85 years [1,2]. Previous studies showed that anemia, per se, is associated with higher morbidity, risk of hospitalization, and mortality rates [2,3]. When the identifiable causes of anemia are excluded, such as erythropoietic nutrient deficiencies, inflammatory conditions and chronic kidney disease (CKD), the prevalence of anemia is still high in the elderly population. In fact, several studies showed that as much as 1/3 of the anemia in older population is of unexplained cause [4–6]. Some possible explanations for the increased prevalence of anemia of unknown etiology in advancing age have been suggested [7–10]. Indeed, aging is characterized by a progressive mild pro-inflammatory status, as shown by the rising levels of pro-inflammatory markers [11]. This phenomenon can be partially explained by the increasing prevalence of chronic diseases; however, even when healthy older individuals were studied, they still had higher levels of pro-inflammatory cytokines and acute phase reactive proteins [11–14]. Additionally, a positive association between inflammation and erythropoietin (EPO) serum levels has been reported in old individuals without anemia [13]. These findings suggest that inflammation might hamper the response to EPO. Furthermore, it is known that increased levels of hepcidin (Hamp) are involved in the pathogenesis of the anemia of inflammation [10,11]. Nevertheless, it is still unclear whether the mild-pro-inflammatory state associated with aging, causes Hamp up-regulation and contributes to the development of anemia. More recently, it was reported that the anemia of chronic diseases is dependent on interferon-γ and that this cytokine reduces both the life span and the production of red blood cells [14]. This effect upon red blood cells has been attributed to interferon-γ capacity of inducing the expression of transcription factors of interferon regulatory factor-1 and PU.1 in human erythroid precursors, leading to inhibition of erythropoiesis.
Aging is also associated with physiological, functional and morphological changes in kidneys, which are similar to those found in CKD, though to a lesser extent [15]. Inflammation is also associated to aging-related diseases, and to progression of CKD [16]. The kidney is the major local of EPO synthesis, and one of the mechanisms proposed for the pathogenesis of the unexplained anemia in older people is an inadequate EPO production. In fact, it has been suggested that the ability of kidneys to secrete EPO in response to tissue hypoxia declines with aging. However, EPO levels in non-anemic older persons are conflicting, as some studies showed higher EPO levels, and other studies do not confirm that finding [17].
The aim of this work was therefore to contribute to a better understanding of the pathophysiology of anemia in elderly, by studying how aging affects renal function, iron metabolism and the inflammatory response, using for that purpose an experimental animal model; old male Wistar rats, aged 18 moths, were compared to young male Wistar rats, aged 2 months. The determinations included analytical blood studies (basic hematological study, reticulocyte count, glucose, urea and creatinine levels, C-reactive protein, interleukin-6, interferon-γ, iron, ferritin, transferrin, soluble transferrin receptor and erythropoietin concentrations) kidney histopathological studies and gene expression analysis of transferrin receptor 2 (TfR2), hepcidin (Hamp), ferroportin (SLC40A1), hemojuvelin (HJV), transferrin (TF), hemochromatosis (Hfe) in liver tissue; divalent metal transporter 1 (DMT1) gene expression in duodenal tissue, as well EPO and EPO receptor (EPOR) gene expression in both liver and kidney.
MATERIAL AND METHODS
Animals and groups
Two groups of male Wistar rats (Charles River Lab. Inc., Barcelona, Spain) were evaluated in this study: one of young rats, with only 2 months of age (n=8) and the other of natural aging rats, with 18 months of age (n=7). The rats were maintained in an air-conditioned room, subjected to 12 h dark/light cycles and given standard laboratory rat diet (IPM-R20, Letica, Barcelona, Spain) and free access to tap water. Animal experiments were conducted according to the European Communities Council Directives on Animal Care and to the National Authorities, and the study received approval from the Institutional Ethics Committee of the Faculty of Medicine from the University of Coimbra. Approval ID: FMUC/10/11.
During the entire experimental protocol, the body weight and the amount of beverage consumption were monitored.
Blood and tissue collection
At the end of the experimental protocol the rats were submitted to intraperitoneal anesthesia with 2.0 mg/kg of a 2:1 (v:v) 50.0 mg/mL ketamine (Ketalar®, Parke-Davis, Pfizer Laboratories Lda, Seixal, Portugal) solution in 2.5% chlorpromazine (Largatil®, Rhône-Poulenc Rorer, Vitória Laboratories, Amadora, Portugal). Blood samples were immediately collected by venepuncture from the jugular vein into vacutainer tubes with anticoagulant (EDTA) and without anticoagulant, in order to obtain whole blood, plasma and serum. Under anesthesia the rats were sacrificed by cervical dislocation, and the liver, heart, kidneys and duodenum were immediately removed, weighed and placed in ice-cold Krebs buffer; the organs were carefully cleaned from adherent fat and connective tissue. Two small portions of the liver, kidney and duodenum from each rat were prepared for histopathological analysis (further placed in formaldehyde) and for the genetic studies; to isolate total RNA, 0.2 g samples of liver, duodenum and kidney, from each rat, were immersed in RNA laterTM (Ambion, Austin, USA) and stored at 4 °C for 24h; afterwards, samples were frozen at −80°C. The body weight (BW) and the weight of kidneys (KW), heart weight (HW) and left ventricle weight (LVW) were used to calculate the tropism indexes (KW/BW, HW/BW, LVW/HW and LVW/BW).
Hematological data
Erythrocyte, leukocyte and platelet counts, hematocrit, hemoglobin concentration and hematimetric indices [mean cell volume (MCV), mean cell hemoglobin (MCH) and mean cell hemoglobin concentration (MCHC)] were measured by using an automatic blood cell counter (Sysmex K1000; Sysmex, Hamburg, Germany). Reticulocyte count was performed by microscopic counting on blood smears after vital staining with new methylene blue (reticulocyte stain; Sigma, St. Louis, Mo., USA).
Biochemical data
Serum levels of creatinine, urea and glucose concentrations were analyzed on a Hitachi 717 analyzer (Roche Diagnostics Inc., Massachuasetts, USA) using standard methods.
Serum iron concentration was determined using a colorimetric method (Iron, Randox Laboratories Ltd., North Ireland, UK), whereas serum ferritin and serum transferrin were measured by immunoturbidimetry (Ferritin, Laboratories Ltd., North Ireland, UK; Transferrin, Laboratories Ltd., North Ireland, UK). An enzyme-linked immunosorbent assay was used to measure plasma soluble transferrin receptors (s-TfR) (Human sTfR immunoassay, R&D systems, MN, USA). Serum levels of interleukin-6 (IL-6) and interferon-γ (IFN-γ), were measured by rat-specific Quantikine ELISA kits from R&D Systems (Minneapolis, USA). High-sensitive C-reactive protein (hsCRP) was determined by using a rat-specific Elisa kit from Alpha Diagnostic International (San Antonio, USA). Serum erythropoietin (EPO) concentrations were measured by rat ELISA kit (Eiaab Science Co. Ltd, Wuban, China). All assays were performed according to the manufacturers’ recommendations, in duplicate.
Kidney histopathological analysis
Renal tissue samples were fixed in Bock's fixative and embedded in paraffin wax; afterwards, 4 μm thick sections were stained with hematoxylin and eosin (HE) for routine histopathological analysis.
To quantify renal lesions we also performed a Periodic Acid of Schiff (PAS) staining. Renal tissue samples were fixed in 10% neutral formalin and embedded in paraffin wax; tissue sections, 4 μm thick, were prepared and mounted on slides that were immersed in water and subsequently treated with an aqueous solution of periodic acid (1%); slides were washed to remove any traces of the periodic acid, and, finally, were treated with Schiff's reagent. All samples were examined by light microscopy using a Microscope Zeiss Mod. Axioplan 2. The degree of injury was scored in a double-blinded fashion by two independent pathologists. Lesions were evaluated on the total tissue on the slide.
Glomerular damage was assessed by evaluating mesangial expansion, glomerular basement membrane and capsule of Bowman thickening, nodular sclerosis, glomerulosclerosis, atrophy, and hyalinosis of the vascular pole. Tubulointerstitial lesions were assessed by inflammatory infiltration, presence of hyaline cylinders, tubular basement membrane irregularity, tubular calcification, and by the association of interstitial fibrosis and tubular atrophy (IFTA). The evaluation of vascular lesions was concentrated on arteriolar hyalinosis, arteriolosclerosis and arteriosclerosis. A semi-quantitative rating for each slide, ranging from normal (or minimal) to severe (extensive damage), was assigned to each component. Severity was graded as absent/normal (0), mild (1), moderate (2), and severe (3). Scoring was defined according to the extension occupied by the lesion (number of capsules): normal: 0%; mild: <25%; moderate: 25–50%; severe: >50%. The final score for each sample was obtained by the average scores observed in individual glomeruli in the considered microscopic fields. Tubular calcification was evaluated and graded by the same semiquantitative method. Regarding vascular lesions, arteriosclerosis was scored as 0 if no intimal thickening was present, as 1 if intimal thickening was less than the thickness of the media, and as 2 if intimal thickening was more than the thickness of the media and considering the worst artery on the slide. Using PAS, the rating was set for intensity and extension of staining, ranging from 0 (no staining) to 3 (intense and extensive staining), respecting tissue specificity scoring when adequate.
Gene expression analysis
Tissue samples (50±10 mg) immersed in RNA laterTM were homogenized in 1 ml TRI® Reagent (Sigma, Sintra, Portugal), using a homogenizer, and total RNA was isolated as described in the TRI® Reagent Kit. To ensure inactivation of contaminating RNAses, metal objects and glassware were cleaned with detergent, immersed in RNAse-free water (0.2% diethyl pyrocarbonate) for 2 h and, finally, heated at 120°C for 1 h. RNA integrity (RIN, RNA Integrity Number) was analyzed using 6000 Nano ChipW kit, in Agilent 2100 bioanalyzer (Agilent Technologies, Walbronn, Germany) and 2100 expert software, following manufacturer instructions. The yield from isolation ranged from 0.5 to 1.5 μg; RIN values were 7.0–9.0 and purity (A260/A280) was 1.8–2.0. The concentration of the RNA preparations were confirmed with NanoDrop1000 (ThermoScientific, Wilmington, DE, USA). Possible contaminating remnants of genomic DNA were eliminated by treating these preparations with deoxyribonuclease I (amplication grade) prior to RT-qPCR amplification. Reverse transcription and relative quantification of gene expression were performed as previously described [18]. Real-time qPCR reactions were performed using the following primer sequences for EPO, EPOR, Transferrin receptor 2 (TfR2), Hepcidin (Hamp), Ferroportin (SLC40A1), Hemojuvelin (HJV), Transferrin (TF), Hemochromatosis (Hfe) and Divalent Metal Transporter 1 (DMT1), which were normalized in relation to the expression of beta-actin (Actb), and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH): EPO Forward: AGG GTC ACG AAG CCA TGA AG, EPO Reverse: GAT TTC GGC TGT TGC CAG TG; EPOR Forward: GCG ACT TGG ACC CTC TCA TC, EPOR Reverse: AGT TAC CCT TGT GGG TGG TG; TfR2 Forward: CAA GCT TCG CCC AGA AGG TA, TfR2 Reverse: CGT GTA AGG GTC CCC AGT TC; Hamp Forward: GAA GGC AAG ATG GCA CTA AGC, Hamp Reverse: CAG AGC CGT AGT CTG TCT CG; SLC40A1 Forward: CAG GCT TAG GGT CTA CTG CG, SLC40A1 Reverse: CCG AAA GAC CCC AAA GGA CA; HJV Forward: GCC TAC TTC CAA TCC TGC GT, HJV Reverse: GGT CAA GAA GAC TCG GGC AT; TF Forward: GGC ATC AGA CTC CAG CAT CA, TF Reverse: GCA GGC CCA TAG GGA TGT T; Hfe Forward: CTG GAT CAG CCT CTC ACT GC, Hfe Reverse: GTC ACC CAT GGT TCC TCC TG; DMT1 Forward: CAA CTC TAC CCT GGC TGT GG, DMT1 Reverse: GTC ATG GTG GAG CTC TGT CC; GAPDH Forward: CCA CTA AAG GGC ATC CTG GG, GAPDH Reverse: CAT TGA GAG CAA TGC CAG CC; Actb Forward: GAG ATT ACT GCC CTG GCT CC, Actb Reverse: CGG ACT CAT CGT ACT CCT GC. Results were analyzed with SDS 2.1 software (Applied Biosystems, Foster City, CA, USA) and the relative quantification was calculated by using the 2ΔΔCt method [19].
Data analysis
For statistical analysis, we used the Statistical Package for Social Sciences (SPSS), version 19.0. Results are presented as mean ± s.e.m. Comparisons between groups were performed using Mann-Whitney U test. Significance was accepted at p less than 0.05.
RESULTS
The results were analyzed by comparing the two groups under study – young and old rat groups. The old rats presented a significant higher BW, in HW and KW, when compared with young rats. However, a significant decrease in all tissue trophy indexes was found for old rats group (table I).
Table 1.
Body and tissue weight, trophism indexes, hematological, biochemical and inflammatory data, iron metabolism and EPO levels in young and old rats
| Young rats (n=8) | Old rats (n=7) | |
|---|---|---|
| Body and tissue weights | ||
| BW (Kg) | 0.45±0.02 | 0.68±0.02* |
| HW (g) | 1.14±0.04 | 1.49±0.05* |
| LVW (g) | 0.51±0.02 | 0.59±0.03 |
| KW (g) | 1.15±0.02 | 1.37±0.09* |
| Tissue trophy indexes | ||
| HW/BW (g/Kg) | 2.57±0.11 | 2.21±0.06* |
| LVW/HW (g/g) | 0.45±0.02 | 0.40±0.02* |
| LVW/BW (g/Kg) | 1.17±0.07 | 0.88±0.04* |
| KW/BW (g/Kg) | 2.59±0.08 | 2.03±0.14* |
| Hematological data | ||
| RBC (X 109/L) | 7.50 ±0.09 | 7.39±0.18 |
| Hematocrit (%) | 42.16±0.42 | 41.34±0.94 |
| Hemoglobin (g/dL) | 14.48±0.11 | 14.22±0.25 |
| MCV (fL) | 56.00±0.78 | 56.57±0.69 |
| MCH (pg) | 19.30±0.23 | 19.41±0.23 |
| MCHC (g/dL) | 34.35±0.21 | 34.47±0.33 |
| Reticulocytes (X 109/L) | 246.60±48.70 | 154.00±24.10*** |
| RDW (%) | 12.30±0.18 | 12.91±0.17* |
| Platelets (X103/μL) | 696.75±19.45 | 682.86±26.82 |
| WBC (103/μL) | 3.89±0.84 | 3.46±0.41 |
| Biochemical data | ||
| Glucose (mg/dL) | 174.63±7.03 | 195.57±15.92 |
| Urea (mg/dL) | 34.00±1.66 | 43.26±2.31* |
| Creatinine (mg/dL) | 0.34±0.01 | 0.42±0.02* |
| Inflammatory markers | ||
| hsCRP (μg/mL) | 228.3±39.60 | 175.80±29.60 |
| IL-6 (pg/mL) | 136.69±1.45 | 130.16±3.14 |
| INF-γ (pg/mL) | 13.67±3.04 | 24.29±2.33* |
| Iron metabolism | ||
| Iron (μ.g/dL) | 189.75±6.76 | 172.00±5.02** |
| Ferritin (ng/mL) | 1254.00±249.16 | 1906.71±92.17* |
| Transferrin (mg/dL) | 100.75±2.81 | 111.29±3.01* |
| sTfR (nmol/L) | 5.11±0.24 | 3.75±0.90 |
| Erythropoietin | ||
| EPO (ng/mL) | 5.78±0.91 | 3.09±0.42* |
p<0.05 vs. young rats;
p=0.056 vs. young rats;
p=0.065 vs. young rats; results are presented as mean ± s.e.m. BW: body weight; HW: heart weight; LVW: left ventricle weight; KW: Kidney weight; RBC: red blood cell; MCV: mean cell volume; MCH: mean cell hemoglobin; MCHC: mean cell hemoglobin concentration; RDW: red blood cell distribution width; hsCRP: high sensitive C-reactive protein; IL-6: interleukin-6; INF-γ: interferon-gamma; sTfR: soluble transferrin receptor; EPO: erythropoietin.
The hematological study showed a trend towards a lower erythrocyte count, hematocrit and hemoglobin concentration in old rats, which was associated with a significant higher RDW and lower reticulocyte count, that almost achieved statistical significance (p= 0.065). A significant higher urea and creatinine serum levels was also found in old rats. Moreover, higher levels of INF-γ, ferritin and transferrin, as well as a significant decrease in EPO and lower iron serum levels were seen for old rats (table I).
Iron regulatory proteins gene expression, in liver tissue of old rats, showed a significant higher Hamp, TF, TfR2, and HJV expression; a trend towards a higher gene expression was also observed for SLC40A1 and HFE (Fig. 1). We also found in old rats duodenum, a higher DMT1 expression (Fig. 2). Additionally, EPO gene expression was also significantly up-regulated in liver, while down-regulated in kidney; expression of EPOR was significantly up-regulated in both liver and kidney tissues (Fig. 3).
Figure 1.

Transferrin receptor 2 (TfR2), hepcidin (Hamp), ferroportin (SLC40A1), hemojuvelin (HJV), transferrin (TF), hemochromatosis (Hfe) gene expression in liver tissue for both groups of rats (young and old).
Figure 2.
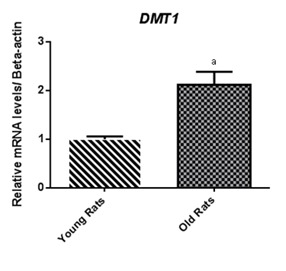
Divalent metal transporter 1 (DMT1) gene expression in duodenal tissues.
Figure 3.
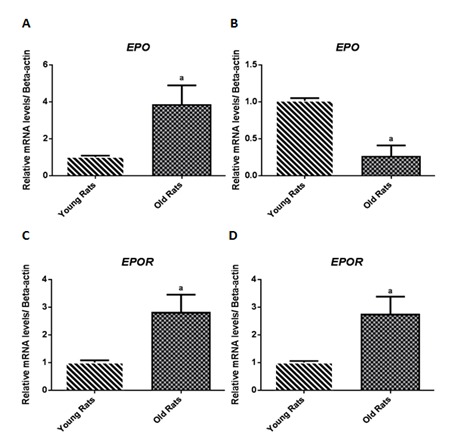
Erythropoietin (EPO) and erythropoietin receptor (EPOR) gene expression in liver (A and C) and kidney (B and D).
Concerning to kidney histomorphology (table II), we found that only old rats presented glomerular lesions (mild-to-moderate), namely thickening of CB (mild 57.1%; moderate 14.3%), hyalinosis of vascular pole (in the entire group), dilatation of the Bowman's Space (mild: 28.6%) (Fig.4A), and hypercellularity (mild 57.1%; moderate 14.3%); the entire group also presented mild to moderate tubular lesions (table II), such as IFTA, interstitial inflammatory infiltration, hyaline cylinders and vacuolar tubular degeneration. Regarding vascular lesions, old rats showed arteriosclerosis and arteriolosclerosis (Fig. 4B).
Table 2.
Scoring and distribution of glomerular and tubular lesions in the groups under study at the final time
|
Rat groups Young (n=8) Old (n=7) |
Scoring and distribution of glomerular and tubular lesions | ||||
|---|---|---|---|---|---|
| Absent | Mild (<25%) | Moderate (25–50%) | Severe (>50%) | ||
| Glomerular Lesions | |||||
| Thickening of CB | Young | 8 (100%) | 0 | 0 | 0 |
| Old | 2 (28,6%) | 4 (57,1%) | 1 (14,3%) | 0 | |
| Hyalinosis of the vascular pole | Young | 8 (100%) | 0 | 0 | 0 |
| Old | 0 (0 %) | 7 (100%) | 0 | 0 | |
| Glomerular atrophy | Young | 8 (100%) | 0 | 0 | 0 |
| Old | 7 (100%) | 0 | 0 | 0 | |
| Dilatation of the Bowman’s Space | Young | 8 (100%) | 0 | 0 | 0 |
| Old | 5 (71,4%) | 2 (28,6%) | 0 | 0 | |
| Hypercellularity | Young | 8 (100%) | 0 | 0 | 0 |
| Old | 2 (28,6%) | 4 (57,1%) | 1 (14,3%) | 0 | |
| Tubular Lesion | |||||
| Hyaline cylinders | Young | 8 (100%) | 0 | 2 (28,5%) | 0 |
| Old | 0 | 5 (71,4 %) | 0 | 0 | |
| TBM irregularity | Young | 8 (100%) | 0 | 0 | 0 |
| Old | 3 (42,9%) | 1 (14,3%) | 3 (42,9%) | 0 | |
| Tubular calcification | Young | 8 (100%) | 0 | 0 | 0 |
| Old | 7 (100%) | 0 | 0 | 0 | |
| Interstitial fibrosis and tubular atrophy (IFTA) | Young | 8 (100%) | 0 | 0 | 0 |
| Old | 0 | 7 (100%) | 0 | 0 | |
| Interstitial Inflammatory infiltration | Young | 8 (100%) | 0 | 0 | 0 |
| Old | 0 | 5(71,4%) | 2 (28,5%) | 0 | |
| Vacuolar Tubular Degeneration | Young | 0 | 8 (100%) | 0 | 0 |
| Old | 0 | 0 | 7 (100%) | 0 | |
Figure 4.
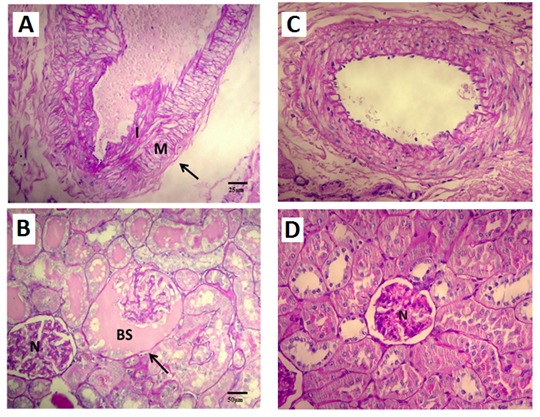
Renal histological changes associated with aging. (A) Example of atherosclerotic grade 1 lesions found in arteries of old rats (X40), intimal thickening (I) less than thickness of media (M). (B) Dilatation of the Bowman's Space (X20), N–normal glomerulus, BS – Bowman's Space. (C) Arteries of young rats without changes (X40). (D) Normal glomerulus.
DISCUSSION
Most of the studies performed to clarify the pathophysiology of anemia of the elderly, were performed in old humans. The frequent association of comorbidities and polypharmacy to advanced age, turns very difficult the study of the etiology and pathophysiology of this particular anemia. However, it would be important to understand how biological changes occurring with aging, underlie the development of an unexplained anemia, as occurs in one third of elderly patients with anemia. To achieve this, it would be necessary to analyze the crosstalk between anemia, inflammation and iron metabolism, by performing diverse studies at blood, tissue and molecular levels, in old individuals. Since these types of studies are not ethical in healthy old humans, we performed them using a natural aged rat model.
In this study, our model does not present yet anemia, since these rats are not very old. However, they presented already higher inflammatory markers and alterations in renal function and in iron metabolism, associated with mild erythropoietic disturbances. These data could be helpful to better understand the modifications that could lead to the development of anemia associated with aging. However, it seems important, in the future, to evaluate an older model (i.e. 24 months) that might highlight the erythropoietic, renal and inflammatory changes already observed in this model with “just” 18 months of age.
We found that our aged rat model has an impaired renal function, as shown by the significantly increased levels of urea and creatinine, associated with a significant decrease in its ability to secrete EPO; the kidney weight/body trophy index was significantly decreased and several histological changes in renal tissue were also observed. Indeed, concerning to glomerular lesions, the entire group showed mild hyalinosis of the vascular pole and the majority showed mild to moderate thickening of CB, dilation of the Bowman’s space and hypercellularity; in addition, all the old rats showed tubular lesions, presenting hyaline cylinders, TBM irregularity, IFTA, interstitial inflammatory inflammation and vacuolar tubular degeneration. None of the young rats presented such histological findings.
Age-related changes in renal structure and function are well documented in humans and in a wide range of animal species [15]. Actually, renal function has been proposed as one of the major predictors of longevity [20]. However, the etiology of renal function decline and structural changes with aging are poorly clarified and have been related with several factors, namely, with comorbidities associated to oxidative stress and inflammation.
The ability of the kidney to secrete EPO in response to hypoxia seems to decline with aging, despite the controversial data [21–23]. In fact, one of the proposed mechanisms for the pathogenesis of the unexplained anemia in elderly is an inadequate EPO production.
As already referred, we found that old rats present lower EPO levels and lower numbers of reticulocytes, associated to a slightly lower hemoglobin concentration, which suggests a decreased eryhtropoietic stimuli and, therefore, a reduced reticulocyte production; in accordance, we found that EPO gene was down-regulated in the kidneys; oppositely, we found an increased EPO expression in hepatic tissue, which may be the result of a compensatory mechanism to overcome the decrease in renal EPO production.
Besides the hematopoietic effect of EPO, it is known that EPO has a pleiotropic action, which might include renal, cardiovascular and nervous systems protection/regeneration [18, 24]. It has been reported that EPO has different intracellular signaling pathways in the kidney tissue, such as the PI3K/Akt pathway, a signaling pathway crucial for cell survival. In the present work, we found that old rats have an increased EPOR expression in both liver and kidney tissues, which might be a response to diminished EPO serum levels, in order to maximize the protective/regenerative effect of circulating EPO.
Several authors have previously proposed that the anemia of unexplained etiology in elderly could have an inflammatory component [4–6]. An underlying inflammatory process leads to increased Hamp levels, the pivotal iron-regulatory peptide. Hamp acts as a negative regulator of intestinal iron absorption and inhibits the release of iron to be used in hemoglobin synthesis, from macrophages and hepatocytes, through the degradation of ferroportin, localized at the membrane surface of enterocytes, hepatocytes and macrophages; Hamp seems to represent the molecular link between chronic inflammation, body iron status and anemia. A high Hamp production occurs in inflammatory and infectious diseases, leading to the development of anemia, known as the anemia of inflammation.
In our model, the aged rats presented a decrease in iron serum levels, accompanied by an increase in ferritin and transferrin serum levels. These results suggest that aging is associated with a disturbance in iron metabolism, decreasing its iron availability for erythropoiesis, in spite of the increased iron storage. It is well documented that the gene encoding Hamp synthesis, in the liver, is regulated mainly by inflammatory cytokines, namely by IL-6 [25,26]. The old rats, as compared to young ones, presented an increased expression of Hamp gene; however, it is still unclear whether chronic inflammation and especially the mild-pro-inflammatory state associated with aging, causes Hamp up-regulation and contributes to anemia of aging. Indeed, IL-6 serum levels were not significantly different between young and old rats, suggesting that the mechanism underlying the up-regulation of Hamp gene expression is not related with IL-6 activating pathway. In accordance, we found that old rats presented increased hepatic TF, TfR2 and HJV gene expressions, which, are known to up-regulate Hamp expression [26,27]. Moreover, old rats presented increased ferritin serum levels, which are known to up-regulate Hamp gene expression in the liver [27]. In addition, as referred previously, kidney EPO gene expression and EPO circulating levels were decreased, which are known to up-regulate the Hamp expression.
INF-γ has been shown to have a direct suppressive effect on the formation of erythroid colonies in vitro [14]. However, the molecular mechanism by which INF-γ inhibits erythroid differentiation is still poorly clarified. Old rats presented a significant reduction in circulating reticulocytes, suggesting a reduction in erythropoiesis. Furthermore, sTfR are usually increased when iron is not available for erythroid cells, and decreased when erythropoiesis is reduced. The reduced mean value observed for sTfR in old rats seems to result, mainly, from a reduction in erythropoiesis process.
We also found in our aged rats an increase in DMT1 gene expression at duodenal level, probably, reflecting an attempt to increase duodenal iron absorption, to counteract the low iron serum levels.
Our results show that aging is associated with impaired renal function, which might be related to the inflammatory process and sclerosing modifications found in the renal tissue. These structural and functional kidney changes could lead to a decline in EPO renal production, reducing the erythropoietic stimuli. Moreover, we also showed that aging is associated with INFγ-induced inflammation and with changes in iron regulatory protein gene expression, including an increase in Hamp expression that will further alter erythropoiesis, by reducing iron absorption and mobilization. The increased hepatic EPO expression could be the result of a compensatory mechanism to overcome the decrease in renal EPO production. The increased EPOR expression, in both liver and kidney, might represent a protection/regenerating mechanism to counteract tissue aging.
In conclusion, the anemia of the elderly seems to result from the interplay between renal, inflammatory and erythropoietic disturbances. A better understanding of the pathophysiological mechanisms underlying this yet unexplained anemia will improve the possibility to develop therapeutics strategies directed to this condition, in order to reduce the high healthy and socioeconomic cost associated with the disease.
References
- [1].Patel K, Guralnik J. Epidemiology of anemia in older adults. In: Balducci L, Ershler W, de Gaetano G, editors. Blood Disorders in the Elderly. Cambridge, UK: Cambridge University Press; 2008. pp. 11–20. [Google Scholar]
- [2].Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–8. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- [3].Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747–50. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ferrucci L, Guralnik JM, Bandinelli S, Semba RD, Lauretani F, Corsi A, Ruggiero C, Ershler WB, Longo DL. Unexplained anaemia in older persons is characterized by low erythropoietin and low levels of pro-inflammatory markers. Br J Haematol. 2007;136:849–55. doi: 10.1111/j.1365-2141.2007.06502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zakai NA, Katz R, Hirsch C, Shlipak MG, Chaves PH, Newman AB, Cushman M. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: the Cardiovascular Health Study. Arch Intern Med. 2005;165:2214–20. doi: 10.1001/archinte.165.19.2214. [DOI] [PubMed] [Google Scholar]
- [6].Riva R, Tettaman M, Mosconi P, Apolone G, Gandini F, Nobili A, Tallone MV, Detoma P, Giacomin A, Clerico M, Tempia P, Guala A, Fasolo G, Lucca U. Association of mild anemia with hospitalization and mortality in the elderly: the health and anemia population-based study. Haematologica. 2009;94:22–28. doi: 10.3324/haematol.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Beerman I, Maloney WJ, Weissmann IL, Rossi DJ. Stem cells and the aging hematopoietic system. Curr Opin Immunol. 2010;22:500–6. doi: 10.1016/j.coi.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kario K, Matsuo T, Kodama K, Nakao K, Asada R. Reduced erythropoietin secretion in senile anemia. Am J Hematol. 1992;41:252–7. doi: 10.1002/ajh.2830410406. [DOI] [PubMed] [Google Scholar]
- [9].Friedman J, Waalen J, Takeda A, Beutler E. Evidence for increased red cell turnover in unexplained anemia in patients over 70 years of age. Blood. 2008;112:3445. [Google Scholar]
- [10].Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ferrucci L, Semba RD, Guralnik JM, Ershler WB, Bandinelli S, Patel KV, Sun K, Woodman RC, Andrews NC, Cotter RJ, Ganz T, Nemeth E, Longo DL. Proinflammatory state, hepcidin, and anemia in older persons. Blood. 2010;115:3810–16. doi: 10.1182/blood-2009-02-201087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ferrucci L, Balducci L. Anemia of aging: the role of chronic inflammation and cancer. Semin Hematol. 2008;45:242–9. doi: 10.1053/j.seminhematol.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ferrucci L, Guralnik JM, Woodman RC, Bandinelli S, Lauretani F, Corsi AM, Chaves PH, Ershler WB, Longo DL. Proinflammatory state and circulating erythropoietin in persons with and without anemia. Am J Med. 2005;118:1288.e11–1288.e19. doi: 10.1016/j.amjmed.2005.06.039. [DOI] [PubMed] [Google Scholar]
- [14].Libregts SF, Gutiérrez L, de Bruin AM, Wensveen FM, Papadopoulos P, van Ijcken W, Ozgür Z, Philipsen S, Nolte MA. Chronic IFN-γ production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood. 2011;118:2578–88. doi: 10.1182/blood-2010-10-315218. [DOI] [PubMed] [Google Scholar]
- [15].Martin JE, Sheaff MT. Renal ageing. Journal of Pathology. 2007;211:198–205. doi: 10.1002/path.2111. [DOI] [PubMed] [Google Scholar]
- [16].Dukkipati R, Adler S, Mehrotra R. Cardiovascular implications of chronic kidney disease in older adults. Drugs Aging. 2008;25:241–53. doi: 10.2165/00002512-200825030-00006. [DOI] [PubMed] [Google Scholar]
- [17].Ble A, Fink JC, Woodman RC, Klausner MA, Windham BG, Guralnik JM, Ferrucci L. Renal function, erythropoietin, and anemia of older persons. Arch Intern Med. 2005;165:1714–20. doi: 10.1001/archinte.165.19.2222. [DOI] [PubMed] [Google Scholar]
- [18].Teixeira M, Rodrigues-Santos P, Garrido P, Costa E, Parada B, Sereno J, Alves R, Belo L, Teixeira F, Santos-Silva A, Reis F. Cardiac antiapoptotic and proproliferative effect of recombinant human erythropoietin in a moderate stage of chronic renal failure in the rat. J Pharm Bioallied Sci. 2012;4:76–83. doi: 10.4103/0975-7406.92743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real -time quanti-tative PCR and the 2-ΔΔCT. Method Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [20].Hediger MA. Kidney function: gateway to a long life? Nature. 2002;417:393–395. doi: 10.1038/417393a. [DOI] [PubMed] [Google Scholar]
- [21].Rowe JW, Andres R, Tobin JD, Norris AH, Shock NW. The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol. 1976;31:155–63. doi: 10.1093/geronj/31.2.155. [DOI] [PubMed] [Google Scholar]
- [22].Goodnough LT, Price TH, Parvin CA. The endogenous erythropoietin response and the erythropoietic response to blood loss anemia: the effects of age and gender. J Lab Clin Med. 1995;126:57–64. [PubMed] [Google Scholar]
- [23].Kario K, Matsuo T, Nakao K. Serum erythropoietin levels in the elderly. Gerontology. 1991;37:345–8. doi: 10.1159/000213283. [DOI] [PubMed] [Google Scholar]
- [24].Garrido P, Reis F, Costa E, Almeida A, Parada B, Teixeira LE, Santos P, Alves R, Sereno J, Pinto R, Tavares CA, Figueiredo A, Rocha-Pereira P, Belo L, Santos-Silva A, Teixeira F. Effect of Recombinant Human Erythropoietin in a Rat Model of Moderate Chronic Renal Failure – Focus on Inflammation, Oxidative Stress and Function/Renoprotection. The Open Drug Discovery Journal. 2010;2:25–32. [Google Scholar]
- [25].Fleming RE, Sly WS. Hepcidin: a putative iron-regulatory hormone relevant to hereditary hemochromatosis and the anemia of chronic disease. Proc Nath Acad USA. 2006;98:8160–2. doi: 10.1073/pnas.161296298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression trough STAT3. Blood. 2006;108:3204–09. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Feng Q, Migas MC, Waheed A, Britton RS, Fleming RE. Ferritin upregulates hepatic expression of bone morphogenetic protein 6 and hepcidin in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1397–404. doi: 10.1152/ajpgi.00020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


