Abstract
The antimicrobial activities of garlic and other plant alliums are primarily based on allicin, a thiosulphinate present in crushed garlic bulbs. We set out to determine if pure allicin and aqueous garlic extracts (AGE) exhibit antimicrobial properties against the Burkholderia cepacia complex (Bcc), the major bacterial phytopathogen for alliums and an intrinsically multiresistant and life-threatening human pathogen. We prepared an AGE from commercial garlic bulbs and used HPLC to quantify the amount of allicin therein using an aqueous allicin standard (AAS). Initially we determined the minimum inhibitory concentrations (MICs) of the AGE against 38 Bcc isolates; these MICs ranged from 0.5 to 3% (v/v). The antimicrobial activity of pure allicin (AAS) was confirmed by MIC and minimum bactericidal concentration (MBC) assays against a smaller panel of five Bcc isolates; these included three representative strains of the most clinically important species, B. cenocepacia. Time kill assays, in the presence of ten times MIC, showed that the bactericidal activity of AGE and AAS against B. cenocepacia C6433 correlated with the concentration of allicin. We also used protein mass spectrometry analysis to begin to investigate the possible molecular mechanisms of allicin with a recombinant form of a thiol-dependent peroxiredoxin (BCP, Prx) from B. cenocepacia. This revealed that AAS and AGE modifies an essential BCP catalytic cysteine residue and suggests a role for allicin as a general electrophilic reagent that targets protein thiols. To our knowledge, we report the first evidence that allicin and allicin-containing garlic extracts possess inhibitory and bactericidal activities against the Bcc. Present therapeutic options against these life-threatening pathogens are limited; thus, allicin-containing compounds merit investigation as adjuncts to existing antibiotics.
Introduction
There are spices and vegetables that can grow
Some are under the ground, some grow tall
Though they all have their qualities, this you should know
That the garlic is best of them all
Ruthie Gordon, The Garlic Waltz (1980)
The various medicinal properties of garlic (Allium sativum L.) and other alliums have long been recognised; nevertheless, these properties and their modus operandi remain enigmatic [1]. The antimicrobial activity derived from alliums was identified nearly 70 years ago and subsequently the chemical structure of allicin (2-propenylthiosulphinate, figure 1) and its properties elucidated over a series of papers by researchers at The Winthrop Chemical Company [2]–[5]. More recent analyses revealed that allicin accounts for approximately 75% of garlic-derived sulphinates [1], [6]–[9]. Amongst over 600 allium species, most attention has been paid to aqueous extracts of garlic which are particularly rich in allicin. In freshly prepared garlic homogenate, allicin is derived de novo by the action of the pyridoxal 5′-phosphate-containing enzyme, alliinase, on the non-protein amino acid, alliin (Figure 1) [10]. Unfortunately, the instability of allicin in the presence of other garlic-derived compounds has hampered attempts to distinguish between the antibacterial role of alliin, allicin and other sulfur-rich antibacterial compounds in plant extracts. In addition, most medicinal garlic supplements sold as garlic powder tablets or capsules show poor allicin release [7]. The mechanism(s) through which allicin and other garlic compounds inhibit or kill bacteria also remain unclear. Studies on inhibition of Salmonella typhimurium using allicin prepared from reacting alliin with alliin lyase suggested that inhibition of RNA synthesis is a primary target of allicin action [11]. Allicin and other thiosulphinates are also known to react with cysteine to abolish antimicrobial activity [12] and to inhibit acetyl-CoA synthases from plants, yeasts and mammals [13]. A recent review highlights the chemical and biological properties of allicin [14].
Figure 1. Chemical structure of allicin and mechanism of formation from alliin by the enzyme alliinase.
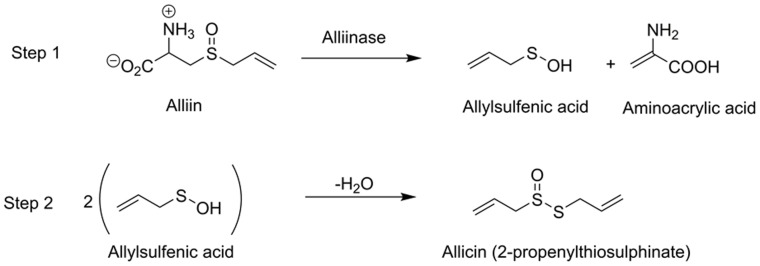
Step 1. Alliinase hydrolyses alliin to produce allylsulfenic acid which, in step 2, condenses spontaneously with the loss of water to produce allicin.
Most previous studies of the antibacterial activity of garlic extracts have focused on Escherichia coli and Staphylococcus aureus. Ajoene, a lipid-soluble allyl sulphide derivative of allicin, found in oil-derived garlic extracts has been reported to cause broad spectrum microbial growth inhibition [15]. Recently, ajoene has also been shown to inhibit quorum sensing in P. aeruginosa [16], [17]. Interestingly, we could find no published studies of the antimicrobial properties of garlic-related compounds on the Burkholderia cepacia complex (Bcc), a group of 17 closely-related species distributed widely in soil, water and the plant rhizosphere [18]. This is both surprising and ironic since as well as being important agents for bioremediation and biological control [19], [20] the Bcc are the major phytopathogens for allium species [21].
In the last few decades, the Bcc have also emerged as important opportunistic human pathogens, in particular as a cause of life-threatening lung infections in individuals with cystic fibrosis (CF) and chronic granulomatous disease [22], [23]. Although patient segregation and strict infection control have reduced the incidence of Bcc infections in individuals with CF, such infections remain an important clinical problem. At present, the most predominant Bcc species responsible for CF infections are B. cenocepacia and B. multivorans [22], [24]. Most transplant centres worldwide exclude individuals infected by B. cenocepacia from access to lung transplantation, the only proven treatment for severe CF lung disease. Thus, any new strategies that lead to the improved eradication of Bcc from an infected patient would be important. Unfortunately, a common feature of the Bcc is intrinsic resistance to most antibiotics [25]; hence antibiotic treatment presents a major challenge. To our knowledge, there have been only five case reports of successful antibiotic therapy for cepacia syndrome, the acute potentially fatal septicaemia and necrotising pneumonia caused by Bcc. These reports emphasise the need for prolonged treatment with IV and aerosolised antibiotic combinations which include ceftazidime, ciprofloxacin, tobramycin, temocillin and trimethoprim-sulphamethoxazole [26], [27]. At present, there is insufficient data to support the use of any specific antibiotic regimen against Bcc infection [28], [29]. There is an even greater need to have alternative therapies with the recent alarming report of a clinical trial of inhaled aztreonam that failed to treat CF patients with Burkholderia spp. infection [30], [31]. Thus, the availability of novel antimicrobial agents against Bcc would represent a major clinical advance for patients with Bcc infection.
Given the dearth of new antibiotics from conventional approaches within the pharmaceutical industry, the development of novel antimicrobial compounds derived from natural sources would be welcomed [32], [33]; this is particularly relevant to the management of lung infections which are the primary cause of morbidity and mortality in the CF population. As previously indicated, the therapeutic potential and complex chemistry of alliums is well documented but remains enigmatic [1], [6]. In our study, we have employed a multidisciplinary approach using both analytical chemistry and microbiology to investigate the antimicrobial properties of a pure aqueous allicin standard (AAS) and aqueous garlic extracts (AGE). Our data demonstrates that the antimicrobial potential of allicin-containing plant extracts is worth revisiting.
Materials and Methods
Quantitative RP-HPLC analyses
Allicin standard was purchased from LKT Laboratories, Minnesota as a preparation with 98% purity and was stored at −80°C until used. AGE preparations were prepared from 30 g crushed garlic bulb and 30 ml sterile, distilled water as described by Fujisawa et. al. 2008 [6]. We used a method based on that published by Lawson and Wang to analyse the allicin content in the AAS and AGE [7]. We prepared a 1000 µg/ml AAS stock solution and carried out serial dilutions to obtain standards that ranged from 8–1000 µg/mL to calibrate the RP-HPLC method (Column dimensions: 250×4.6 mm, 3.6 µ, C18 Aeris Peptide from Phenomenex). The elution conditions used were: isocratic elution with MeOH/water (60∶40, v/v) at 1 ml/min with UV detection at 240 nm, 25°C. An injection volume of 20 µL was used for all samples. The mass of the AAS was confirmed using direct infusion mass spectrometry. Typically, AAS was analysed at a concentration of 10 µM from a solution of water/acetonitrile (50∶50, v/v). Spectral data was collected on an electrospray micro-ToF mass spectrometer (Bruker) operating in the positive mode.
Antimicrobial assays
In a preliminary survey, we investigated the minimum inhibitory concentrations (MICs) of AGE by agar dilution against 38 Bcc isolates representing nine Bcc species and which included 30 isolates from an experimental Bcc strain panel [34]. The MICs and minimum bactericidal concentrations (MBCs) of allicin standard (AAS) against five members of the BCC strain panel were determined by the microtitre broth technique recommended by the NCCLS [35]. Time kill assays were then performed on a single Bcc isolate, B. cenocepacia C6433, with an initial bacterial inoculum of 106 CFU/mL and with AGE and AAS at concentrations ten times MICs. To provide further information on suitable screening assays, susceptibility to AGE was investigated by agar well diffusion and impregnated paper disc diffusion analysis [6]. Isosensitest agar (Oxoid Ltd., Basingstoke, UK) plates were flood seeded using a log phase 106 CFU/mL isosensitest broth culture of C6433. 10 µL undiluted AGE was added to a well cut in the agar or used to impregnate sterile paper discs. Antimicrobial activity was determined by a zone of inhibition after overnight incubation at 37°C (Figure S1).
Modification of recombinant B. cenocepacia BCP by AAS and AGE
The His-tagged B. cenocepacia J2315 bacterioferritin comigratory protein (BCP) peroxiredoxin (Prx), which is also referred to as “peroxidase”, was overexpressed in E. coli and purified and stored as described by Clarke et. al. [36] The purified BCP protein (10 µM) was incubated with AAS (0.1 mM and 1 mM) at 37°C for 1, 5, 30, 60, 120 and 180 mins. Each reaction was quenched with 0.1% formic acid and then analysed by electrospray ionisation Fourier transform ion cyclotron resonance mass spectrometry (ESI FT-ICR MS). Freshly-prepared AGE (3.38 mg/mL was passed through a PD-10 (Sephadex G-25M) desalting column, the low molecular weight fraction was collected and incubated with BCP (50 µM) for 1, 5, 30, 60, 120 and 180 mins at 37°C. To isolate the enzyme from the incubation mixture, the sample was then shaken with Ni-NTA resin for 50 mins followed by elution of the protein from the resin with 300 mM imidazole. Excess imidazole was removed from protein samples by PD-10 desalting which were then concentrated to 2 mL and analysed by ESI FT-ICR MS.
Protein Mass Spectrometry Analysis
Protein samples were analysed by LC-MS using an Ultimate 3000 HPLC system (Dionex Corporation, Sunnyvale, CA), equipped with a monolithic PS-DVB (500 µm×5 mm) analytical column (Dionex Corporation). Mobile phases A and B comprised 2∶97.95 and 80∶19.95 acetonitrile∶water with 0.05% formic acid respectively (v/v/v). Samples were injected onto the analytical column, washed with buffer A for 5 min, followed by a 20 min linear gradient elution (20 µl/min) into buffer B. MS data was acquired on a Bruker 12 Tesla Apex Qe FT-ICR (Bruker Daltonics, Billerica, MA). Desolvated ions were detected between m/z 600 and 2000 for 0.5 s to yield a broadband 512 Kword time-domain data. Fast Fourier Transforms and subsequent analyses were performed using DataAnalysis (Bruker Daltonics) software. All m/z spectra were deconvoluted using MaxEnt software in DataAnalysis (Bruker Daltonics).
Results
Quantification of allicin in AGE
The purity of AAS was confirmed using a published RP-HPLC method (Fig. 2A) [7]. Allicin eluted with a retention time of 4.1 min and an observed m/z of 185.0067 (consistent with the [M+Na]+ species; theoretical m/z 185.0065; [C6H10OS2+Na]+) (Fig. 2A inset). Using the same HPLC conditions, the elution profile of AGE contained several well-resolved peaks (Fig. 2B), and the peak eluting at 4.1 min was identified as allicin by mass spectrometry (m/z 185.0067; [M+Na]+). A calibration curve was obtained by analysing known concentrations of AAS (8–1000 µg/mL) and used to quantify the amount of allicin present in the fresh AGE preparations (Fig. 3). The peak area for allicin was linear over 0–20 g of allicin loaded onto the column (r2 = 0.9987 and gradient 0.57±0.01 mAU.min. µg−1) and the amount of allicin in freshly prepared AGE was determined as 67.6±7.9 µg in 20 µl, which equates to 3.38×103±0.39 µg/mL (allicin/AGE, w/v).
Figure 2. HPLC and MS analysis of aqueous allicin standard (AAS) and aqueous garlic extract (AGE).
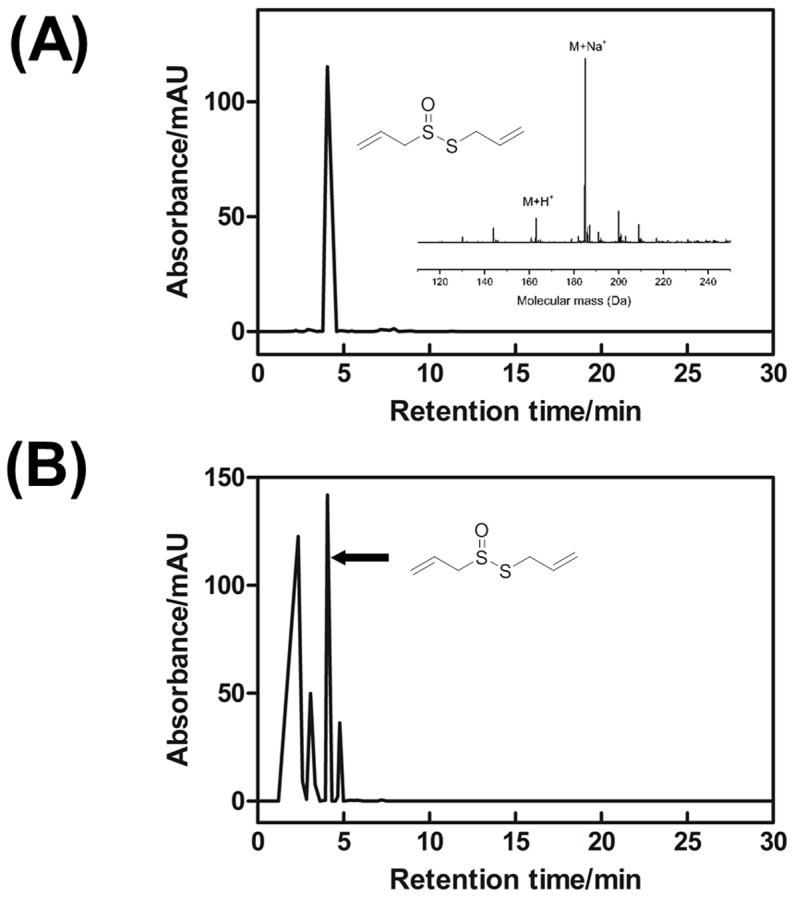
(A) AAS was analysed using a C18 reverse phase column with UV detection at 240 nm. The standard eluted at 4.1 minutes. AAS had an observed m/z of 185.0067 (consistent with the [M+Na]+ species; theoretical m/z 185.0065; [C6H10OS2+Na]+) (inset). (B) AGE was analysed by the same method as described for AAS. The peak at 4.1 minutes corresponds to the mass of allicin.
Figure 3. Calibration curve of aqueous allicin standard (AAS).
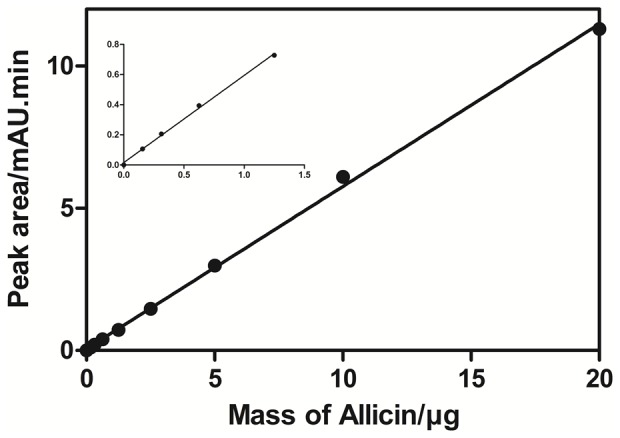
AAS of known concentrations (8–1000 µg/mL) were used to produce a calibration curve which was then used to quantify the allicin present in the fresh AGE preparations. The peak area for allicin was linear over 0–20 µg of allicin loaded onto the column (r2 = 0.9987 and gradient 0.57±0.01 mAU.min. µg−1).
Antimicrobial activity
AGE displayed antimicrobial activity against all of the 38 Bcc isolates (Table 1) with MICs ranging from 0.5 to 3%, where 100% equates to 10 g of garlic bulb homogenised in 10 ml of sterile, distilled water. Interestingly, comparison of the antimicrobial activity of four independent AGE preparations showed little batch-to-batch variation (data not shown). Activity also remained stable when AGE was stored at 4°C for seven days or for at least 6 months at −80°C. We noted that the epidemic, clinically-relevant isolate B. cenocepacia C6433 was sensitive (0.5%) to AGE. So in further experiments, the inhibitory activity of AGE was confirmed with impregnated paper discs and agar well diffusion assays using an inoculum of 10 µl (106 CFU/ml) per disc or well (Figure S1). Since the amount of pure allicin (AAS) available was limited, investigation of the antimicrobial activity of AAS was restricted to MIC and MBC assays using five Bcc isolates (table 2). MICs ranged from 8 to 62 µg/mL and MBCs 31 to 62 µg/mL. In time-kill assays, bactericidal activities of AGE and AAS against B. cenocepacia C6433 were 99.9% and 100% respectively within 12 h (data not shown). Since the MIC of AGE required for inhibition of B. cenocepacia C6433 is 0.5%, we used our quantification of allicin in garlic preparations (Fig. 3) to determine that this dilution of AGE contains ∼16.9 µg/mL allicin. This analysis now allows comparison with the MIC of pure allicin (AAS) against this B. cenocepacia isolate, which we determined as 4 µg/mL allicin. These observations suggest that, against this isolate, allicin is the major antibacterial species in the AGE preparation.
Table 1. MICs (µg/ml) of AGE for B. cepacia complex.
| Strain | Edinburgh Lab No. | LMG No. | *Strain Name | AGE (%) |
| B. cepacia | J673 | 1222 | ATCC 25416 | 2 |
| B. cepacia | J675 | 2161 | ATCC 2161 | 1 |
| B. cepacia | C2970 | 17997 | 0.5 | |
| B. cepacia | C3159 | 18821 | CEP509 | 0.5 |
| B. multivorans | C1576 | 16660 | 0.5 | |
| B. multivorans | C1962 | 16665 | 0.5 | |
| B. multivorans | C3160 | 18822 | C5393 | 1 |
| B. multivorans | C3161 | 13010 | 1 | |
| B. multivorans | C3162 | 18825 | CF-A1-1 | 1 |
| B. multivorans | C3163 | 18824 | JTC | 0.5 |
| B. multivorans | C3164 | 18823 | 249-2 | 0.5 |
| B. multivorans | J2511 | 17588 | ATCC 17616 | 1 |
| B. cenocepacia | J415 | 16654 | 1 | |
| B. cenocepacia | C1394 | 16659 | 1 | |
| B. cenocepacia | J2315 | 16656 | 0.5 | |
| B. cenocepacia | J2524 | 18832 | ATCC 17765 | 3 |
| B. cenocepacia | C3165 | 18826 | BC7 | 0.5 |
| B. cenocepacia | C3166 | 18863 | K56-2 | 1 |
| B. cenocepacia | C3167 | 18827 | C5424 | 0.5 |
| B. cenocepacia | C3168 | 18828 | C6433 | 0.5 |
| B. cenocepacia | C3169 | 18829 | PC184 | 0.5 |
| B. cenocepacia | C3170 | 18830 | CEP511 | 1 |
| B. stabilis | C3171 | 14294 | 0.5 | |
| B. stabilis | C3172 | 18870 | C7322 | 0.5 |
| B. stabilis | C3173 | 18888 | 1 | |
| B. stabilis | C3174 | 14086 | 1 | |
| B. vietnamiensis | C2978 | 16232 | 1 | |
| B. vietnamiensis | C3175 | 18835 | PC259 | 1 |
| B. vietnamiensis | C3176 | 18836 | FC441 | 3 |
| B. vietnamiensis | C3177 | 10929 | 1 | |
| B. dolosa | J3357 | 1 | ||
| B. dolosa | J3358 | 1 | ||
| B. ambifaria | J3361 | 2 | ||
| B. ambifaria | J3362 | 1 | ||
| B. anthina | J3364 | 1 | ||
| B. anthina | J3366 | 1 | ||
| B. pyrrocinia | J3368 | 2 | ||
| B. pyrrocinia | J3369 | 0.5 |
* We have included all codes to avoid confusion between Edinburgh strain collection numbers, LMG and ATCC codes.
Table 2. MICs and MBCs (µg/ml) of AAS for B. cepacia complex.
| Strain | Edinburgh Lab No. | LMG No. | MIC | MBC |
| B. cenocepacia | J2315 | 16656 | 8 | 31 |
| B. cenocepacia | C6433 | 18828 | 8 | 31 |
| B. cenocepacia | C3165 | 18826 | 16 | 31 |
| B. stabilis | C3172 | 18870 | 16 | 31 |
| B. cepacia | J673 | 1222 | 62 | 62 |
Interaction of BCP with allicin and AGE
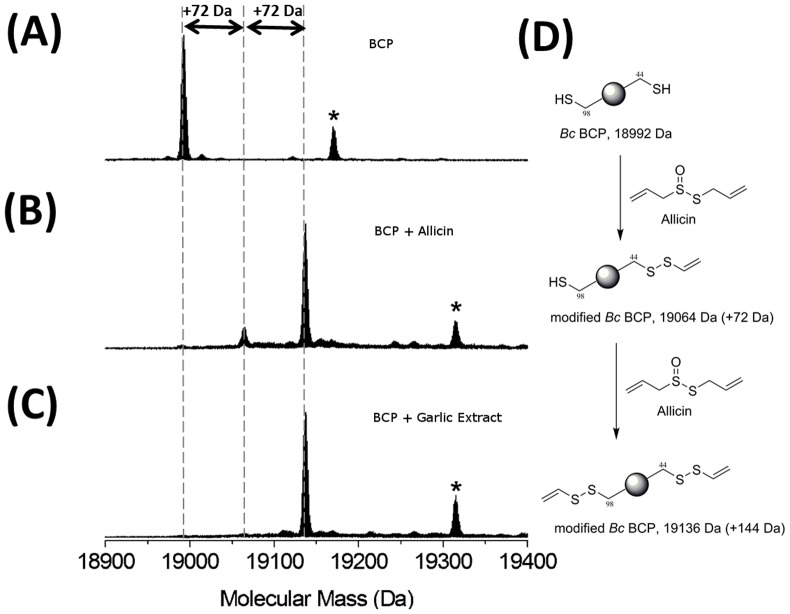
The exact chemical mechanism(s) by which allicin exerts its antibacterial activity has been the subject of numerous studies and it's chemical structure suggests it should react with free thiols. Indeed, allicin is known to covalently modify the free amino acid L-cysteine via formation of allyl-disulfide species [37]. Therefore, we postulate that the antimicrobial activity of allicin is derived from modification of cysteine residues found in key metabolic proteins within Burkholderia. In order to test this hypothesis we have studied the reaction of AAS and AGE with the enzyme BCP, a peroxiredoxin (Prx) which is known to contain catalytically important cysteine residues (Cys44 and Cys98) [36]. We use this protein as an example of cysteine-containing proteins within the B. cenocepacia proteome and not as the sole target of allicin within the organism. The interaction between AAS and the recombinant BCP was studied by mass spectrometry. The unmodified BCP has a mass of 18992 Da which confirmed that both cysteine residues are in the reduced (-SH) form (Fig. 4A). Upon incubation of the BCP with AAS (1 mM or 161 µg/ml) for 60 minutes we noted the appearance of two new species with masses of 19064 and 19136 Da (Fig. 4B). These observed increases in mass (Δ72 and Δ144 Da) are consistent with the addition of a single allyl thiol group (C3H5S) to either one or both cysteine residues (Fig. 4D). This result is not surprising as allicin is expected to react with the free thiols on BCP in a non-specific manner as shown by previous studies with cysteine, glutathione and papain [12], [37]. Importantly, similar mass increases were observed when we incubated the BCP with AGE for 60 minutes (Fig. 4C). This important experiment confirms that the AGE and the AAS react with the protein target in the same manner (Fig. 4D).
Figure 4. MS analysis of recombinantly produced Burkholderia cepacia BCP Prx incubated with AAS and AGE.
(A) Purified Bc BCP Prx has a mass of 18992 Da, consistent with its predicted mass based on the amino acid sequence (B) Bc BCP after incubation with 1 mM AAS for 1 h results in the formation of two covalently modified species of mass increases +72 Da and +144 Da. (C) Bc BCP Prx after incubation with AGE for 1 h results in the formation of a covalently modified species of mass increase +144 Da corresponding to the addition of two allicin adducts. * denotes enzyme species that undergo α-N-gluconoylation during protein expression. (D) Proposed mechanism of reaction of allicin with Bc BCP Prx.
Discussion
Production of antimicrobial compounds by plant alliums which target Burkholderia species, their principal bacterial pathogen, is not surprising. However, what is surprising is that, to our knowledge; such activity has not been reported or investigated previously. We report the first evidence for the antimicrobial activity of allicin against the Bcc. The MICs and MBCs of allicin-containing preparations against BCC isolates were commensurate with breakpoint values for antibiotics in present clinical use. Encouragingly, killing activity includes B. cenocepacia, the most prevalent and transmissible Bcc species isolated from CF infections [22], [24]. In general, Bcc isolates exhibit intrinsic resistance to antimicrobial peptides and other antibiotics [25], [38], [39]. Thus, treatment of Bcc infections remains challenging and no particular antibiotic regimen is presently recognised [26], [40].
The chemical mechanisms involved in the bactericidal action of allicin are poorly understood, and in the case of the Bcc are unknown. Given previous evidence that allicin preferentially interacts with cysteine, our hypothesis is that the allylthio moiety of allicin allows it to react with cysteine-containing Burkholderia enzymes involved in key biosynthetic pathways. The exact targets of allicin are not known but we assume there will be many proteins that have the potential to be modified. As a relevant model protein, we used the Bcc-derived peroxidase enzyme, BCP peroxiredoxin, which we have previously shown plays a role in the detoxification of reactive oxygen species (ROS) within B. cenocepacia [36]. In that study we also identified that Cys44 and Cys98 are essential for the optimal catalytic activity of the enzyme, therefore any chemical modification of these residues will inactivate the enzyme. We chose mass spectrometry as the analytical tool for BCP/allicin analysis since BCP ionises well and mass spectrometry is ideally suited to measure covalently-bound adducts.
Our data strongly suggests that pure allicin (AAS) and, more importantly, the allicin within AGE react with both Cys44 and Cys98 residues of BCP to give modified isoforms of masses 19064 Da and 19136 Da. These observed mass increases of 72 and 144 Da correspond to the addition of one and two allylthio groups respectively by reaction with allicin (Fig. 4D). The proposed mechanism for the formation of the BCP-allythio derivative involves nucleophilic attack of a BCP cysteine thiol (either Cys44 or Cys98) on the more electrophilic sulfur of allicin to produce a new disulfide bond. A second molecule of allicin undergoes a similar reaction to add a second allylthio group. Reduction of the derivitised BCP with excess reducing agent (tris (2-carboxyethyl) phosphine, TCEP) returned the enzyme to the unmodified form (18992 Da) which suggests that the reaction of allicin with BCP is chemically reversible in the presence of strong reducing agents (data not shown). The results from the proteomic analysis of BCP with the AGE was very unexpected, as garlic extract is a complex mixture as observed from our HPLC analysis. This clearly-resolved data confirms that allicin is the main component of freshly prepared garlic that reacted with BCP (Fig. 4C). More importantly, this is the first study where AAS and AGE have been analysed by protein mass spectrometry with a relevant protein target from a bacterial pathogen. We think that this study opens the door to apply this powerful analytical tool for identifying many of the protein targets that are modified by allicin within the proteome of Burkholderia and other pthogens. It could also prove useful to study other similar allylthio-containing natural products (e.g. ajoene) derived from allicin.
Previous investigations of novel antimicrobial compounds against Bcc species have included mushroom extracts [41], polyketides [42], docosahexaenoic omega- 3 fatty acid [43] and microbe-derived volatile organic compounds [44]. Our results demonstrating the inhibitory activity of allicin-containing garlic extracts against Burkholderia species provide another potential line of research that is particularly relevant to Bcc infection. Evidence from animal and human studies have shown that allicin-containing garlic homogenates, delivered orally, intravenously or intraperitoneally, act as potent antioxidants leading to reduction of neutrophil-mediated lung damage, a major mechanism of Bcc lung infection [45]. The stability of the antimicrobial properties of AGE during storage at 4°C and −80°C coupled with the batch-to-batch reproducibility were notable. Similarly, the rapid therapeutic action of garlic homogenates in serum and plasma in the treatment of cardiovascular disorders [46], [47] could be a particular advantage in the treatment of the life-threatening septicaemia that is characteristic of cepacia syndrome. Our investigation suggests that further work is required to comprehensively describe the antimicrobial mechanisms of allicin and to assess allicin-containing formulations as adjuncts to conventional antimicrobial agents presently used against this challenging group of bacterial pathogens.
Supporting Information
Zones of inhibition against B. cenocepacia C6433 produced by AGE-impregnated disc (left) and AGE-containing agar well (right). Isosensitest agar plates were flood seeded using a 106 CFU/mL isosensitest broth culture of C6433. 10 µL undiluted AGE was added to a well cut in the agar or used to impregnate sterile paper discs. We note the lack of resistant mutants.
(TIF)
Acknowledgments
Part of this work was presented at the European Cystic Fibrosis Society Conference, Dublin, Ireland, 2012 (poster presentation 72) and Lisbon, Portugal 2013 (oral presentation WS17.3).
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors thank The University of Edinburgh for funding a PhD School of Chemistry Tercentenary studentship (DWR). The authors also thank the Biotechnology and Biological Sciences Research Council (BBSRC) UK for funding (award number BB/I013687/1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Block E (2010) Garlic and Other Alliums: The Lore and the Science. Cambridge, UK: The Royal Society of Chemistry.
- 2. Cavallito C, Bailey J (1944) Allicin, the Antibacterial Principle of Allium sativum: Isolation, Physical Properties and Antibacterial Action. Journal of American Chemical Society 66:1950–1951. [Google Scholar]
- 3. Cavallito C, Buck J, Suter CM (1944) Allicin, the Antibacterial Principle of Allium sativum: Determination of the Chemical Structure. Journal of American Chemical Society 66:1952–1954. [Google Scholar]
- 4. Cavillito C, Bailey J, Buck J (1945) The Antibacterial Principle of Allium sativum: Its Precursor and Essential Oil of Garlic. Journal of American Chemical Society 67:1032–1033. [Google Scholar]
- 5. Small L, Bailey J, Cavallito C (1947) Alkyl Thiosulfinates. Journal of American Chemical Society 69:1710–1713. [DOI] [PubMed] [Google Scholar]
- 6. Fujisawa H, Suma K, Origuchi K, Kumagai H, Seki T (2008) Biological and chemical stability of garlic-derived allicin. Journal of Agriculture and Food Chemistry 56:4229–4235. [DOI] [PubMed] [Google Scholar]
- 7. Lawson L, Wang ZJ (2001) Low Allicin Release from Garlic Supplements: a Major problem Due to the Sensitivities of Allinase Activity. Journal of Agriculture and Food Chemistry 49:2592–2599. [DOI] [PubMed] [Google Scholar]
- 8. Kyung KH (2012) Antimicrobial properties of allium species. Curr Opin Biotechnol 23:142–147. [DOI] [PubMed] [Google Scholar]
- 9. Waag T, Gelhaus C, Rath J, Stich A, Leippe M, et al. (2010) Allicin and derivatives are cysteine protease inhibitors with parasitic activity. Bioorganic and Medicinal Chemistry letters 20:5541–5543. [DOI] [PubMed] [Google Scholar]
- 10. Shimon LJ, Rabinkov A, Shin I, Miron T, Mirelman D, et al. (2007) Two structures of alliinase from Alliium sativum L.: apo form and ternary complex with aminoacrylate reaction intermediate covalently bound to the PLP cofactor. J Mol Biol 366:611–625. [DOI] [PubMed] [Google Scholar]
- 11. Feldberg R, Chang S, Kotik A, Nadler M, Neuwirth Z (1988) In vitro mechanism of inhibition of bacterial cell growth by allicin. Antimicrobial Agents and Chemotherapy 32:1763–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujisawa H, Watanabe K, Suma K, Origuchi K, Matsufuji H (2009) Antimicrobial Potential of Garlic-Derived Allicin and Its Cancellation by Sulfydryl Compounds. Bioscience Biotechnology Biochemistry 73:1948–1955. [DOI] [PubMed] [Google Scholar]
- 13. Focke M, Feld A, Lichtenthaler H (1990) Allicin, a naturally occuring antibiotic from garlic, specifically inhibits acetyl-CoA synthase. FEBS Letters 261:106–108. [DOI] [PubMed] [Google Scholar]
- 14. Borlinghaus J, Albrecht F, Gruhlke MC, Nwachukwu ID, Slusarenko AJ (2014) Allicin: Chemistry and Biological Properties. Molecules 19:12591–12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naganawa R, Iwata N, Ishikawa K, Fukuda H, Fujino T, et al. (1996) Inhibition of Microbial Growth by Ajoene, a Sulfur-Containing Compound Derived from Garlic. Applied and Envirnmental Microbiology 62:4238–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smyth A, Cifelli P, Ortori C, Righetti K, Lewis S, et al. (2010) Garlic as an Inhibitor of Pseudomonas aeruginosa Pediatric Pulmonology. 45:356–362. [DOI] [PubMed] [Google Scholar]
- 17. Jakobsen T, Gennip MV, Phipps R, Shanmugham M, Christensen L, et al. (2012) Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum. Antimicrob Agents Chemother 56:2314–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coenye T, Vandamme P, Govan J, Lipauma J (2001) Taxanomy and Indentification of the Burkholderia cepacia complex. Journal of Clinical Microbiology 39:3427–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmes A, Govan J, Goldstein R (1998) Agricultural use of Burkholderia (Pseudomonas) cepacia: a threat to human health. Emerging Infectious Diseases 4. [DOI] [PMC free article] [PubMed]
- 20. Sullivan LA, Mahenthiralingam E (2005) Biotechnological potential within the genus Burkholderia. Letters in Applied Microbiology 41:8–11. [DOI] [PubMed] [Google Scholar]
- 21. Burkholder W (1950) Sour skin, a bacterial rot of onion bulbs. Phytopathology 40:115–117. [Google Scholar]
- 22. Govan J, Brown A, Jones A (2007) Evolving epidemiology of pseudomonas aeroginosa and the Burkholderia cepacia complex in cystic fibrosis lung infection. Future Microbiology 2:153–164. [DOI] [PubMed] [Google Scholar]
- 23. Greenberg D, Goldberg J, Stock F, Murray P, Holland S, et al. (2009) Recurrent Burkholderia infection in patients with chronic granulomatous disease. Clin Infect Dis 48:1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. LiPuma J (2010) The changing microbial epidemiology in cystic fibrosis. Clinical Microbiology Reviews 23:299–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nzula S, Vandamme P, Govan J (2002) Influence of taxonomic status on the in vitro antimicrobial suseptibility of the Burkholderia cepacia complex Journal of Antimicrobial Chemotherapy. 50:265–269. [DOI] [PubMed] [Google Scholar]
- 26.Grimwood K, Kidd TJ, Tweed M (2009) Successful treatment of cepacia syndrome. J Cyst Fibros. Netherlands. pp. 291–293. [DOI] [PubMed]
- 27. Gilchrist FJ, Webb AK, Bright-Thomas RJ, Jones AM (2012) Successful treatment of cepacia syndrome with a combination of intravenous cyclosporin, antibiotics and oral corticosteroids. J Cyst Fibros 11:458–460. [DOI] [PubMed] [Google Scholar]
- 28. Horsley A, Webb K, Bright-Thomas R, Govan J, Jones A (2011) Can early Burkholderia cepacia complex infection in cystic fibrosis be eradicated with antibiotic therapy? Front Cell Infect Microbiol 1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Horsley A, Jones AM (2012) Antibiotic treatment for Burkholderia cepacia complex in people with cystic fibrosis experiencing a pulmonary exacerbation. Cochrane Database Syst Rev 10:Cd009529. [DOI] [PubMed] [Google Scholar]
- 30. Tullis DE, Burns JL, Retsch-Bogart GZ, Bresnik M, Henig NR, et al. (2014) Inhaled aztreonam for chronic Burkholderia infection in cystic fibrosis: a placebo-controlled trial. J Cyst Fibros 13:296–305. [DOI] [PubMed] [Google Scholar]
- 31. Balfour-Lynn IM (2014) At last, Burkholderia spp. is one of the inclusion criteria–a negative (but published) randomised controlled trial. J Cyst Fibros 13:241–242. [DOI] [PubMed] [Google Scholar]
- 32. Fischbach M, Walsh C (2009) Antibiotics for Emerging Pathogens. Science 325:1089–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li J, Vederas J (2009) Drug Discovery and natural products. Science 325:161–165. [DOI] [PubMed] [Google Scholar]
- 34. Mahenthiralingam E, Coenye T, Chung J, Speert D, Govan J, et al. (2000) Diagnostically and experimentally useful panel of strains from the Burkholderia. J Clin Microbiol 38:910–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.NCCLS (1997) Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standard M2-M7. Wayne, PA, USA: National Committee for Clinical Laboratory Standards. pp. 1–182.
- 36. Clarke DJ, Ortega XP, Mackay CL, Valvano MA, Govan JR, et al. (2010) Subdivision of the bacterioferritin comigratory protein family of bacterial peroxiredoxins based on catalytic activity. Biochemistry 49:1319–1330. [DOI] [PubMed] [Google Scholar]
- 37. Miron T, Listowsky I, Wilchek M (2010) Reaction mechanisms of allicin and allyl-mixed disulfides with proteins and small thiol molecules. Eur J Med Chem 45:1912–1918. [DOI] [PubMed] [Google Scholar]
- 38. Taylor K, McCullough B, Clarke D, Langley R, Pechenick T, et al. (2007) Covalent dimer species of beta-defensin Defr1 display potent antimicrobial. Antimicrob Agents Chemother 51:1719–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Loutet SA, Valvano MA (2011) Extreme antimicrobial peptide and polymyxin B resistance in the genus Burkholderia. Front Cell Infect Microbiol 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Waters V, Ratjen F (2006) Multidrug-resistant organisms in cystic fibrosis: management and. Expert Rev Anti Infect Ther 4:807–819. [DOI] [PubMed] [Google Scholar]
- 41.Schwan W, Dunek C, Gebhardt M, Engelbrecht K, Klett T, et al.. (2010) Screening a mushroom extract library for activity against Acinetobacter baumannii. Ann Clin Microbiol Antimicrob 9. [DOI] [PMC free article] [PubMed]
- 42. Mahenthiralingam E, Song L, Sass A, White J, Wilmot C, et al. (2011) Enacyloxins are products of an unusual hybrid modular polyketide synthase encoded by a cryptic Burkholderia ambifaria Genomic Island. Chem Biol 18:665–677. [DOI] [PubMed] [Google Scholar]
- 43. Mil-Homens D, Bernardes N, Fialho A (2012) The antibacterial properties of docosahexaenoic omega-3 fatty acid against the. FEMS Microbiol Lett 328:61–69. [DOI] [PubMed] [Google Scholar]
- 44. Papaleo MC, Fondi M, Maida I, Perrin E, Lo Giudice A, et al. (2012) Sponge-associated microbial Antarctic communities exhibiting antimicrobial activity against Burkholderia cepacia complex bacteria. Biotechnol Adv 30:272–293. [DOI] [PubMed] [Google Scholar]
- 45. Ashry N, Gameil N, Suddek G (2013) Modulation of cyclophosphamide-induced early lung injury by allicin. Pharm Biol 51:806–811. [DOI] [PubMed] [Google Scholar]
- 46. Banerjee S, Maulik S (2002) Effect of garlic on cardiovascular disorders: a review. Nutr J 2002 1:4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang L, Wang E, Chen F, Yan H, Yuan Y (2013) Potential protective effects of oral administration of allicin on acrylamide-induced toxicity in male mice. Food Funct 4:1229–1236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Zones of inhibition against B. cenocepacia C6433 produced by AGE-impregnated disc (left) and AGE-containing agar well (right). Isosensitest agar plates were flood seeded using a 106 CFU/mL isosensitest broth culture of C6433. 10 µL undiluted AGE was added to a well cut in the agar or used to impregnate sterile paper discs. We note the lack of resistant mutants.
(TIF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.